Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) is a common disorder and becoming a leading cause of cirrhosis in the western world. The monitoring of the disease is challenging and the prognostic importance of α-fetoprotein (AFP) level elevation in NAFLD remains uncertain.
Methods
Eighty-four patients were evaluated in the study. Patients with evidence of fatty liver in an abdominal ultrasonography performed for any reason were enrolled in the study. Degree of liver steatosis was graded into three groups. As a control group, patients without fatty liver or other liver diseases were included. All patients and controls were asked about prior hepatic diseases, consumption of alcohol, smoking, drug use, and a physical examination, biochemical analyses including liver function tests, different components of the metabolic syndrome, and the homeostasis model assessment-estimated insulin resistance (HOMA-IR) score were also performed.
Results
Body mass index, aspartate aminotransferase, alanine aminotransferase, glucose, insulin, and HOMA-IR in patients with NAFLD were higher than in control group. Triglyceride, total cholesterol, low-density lipoprotein, and high-density lipoprotein cholesterol levels were higher in NAFLD group than in control group. A statistically significant increase in AFP levels was noted in patients with NAFLD (4.09 ± 1.68) when compared with healthy controls (2.95 ± 0.41) (P < 0.05). A statistically significant increase in AFP levels was noted in patients with grade 3 NAFLD (5.43 ± 1.51) when compared with grade 1 (2.92 ± 1.06) and grade 2 NAFLD groups (3.97 ± 1.45). Also, AFP was significantly higher in grade 2 NAFLD group than in grade 1 NAFLD group. AFP was correlated with NAFLD grade, but neither ALT nor AST showed correlation. According to multivariate analysis, correlation between NAFLD grade and serum AFP levels was independent from the other factors.
Conclusion
Patients with NAFLD have higher AFP levels than those without fatty liver changes. AFP levels rise as grade of liver steatosis increases. NAFLD should be among the differential diagnosis of elevated serum AFP levels.
Keywords: α-Fetoprotein, Liver, Steatosis, NAFLD
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a common disorder and becoming a leading cause of cirrhosis in the western world. It covers a histological spectrum that ranges from fat accumulation in hepatocytes lacking concomitant inflammation or fibrosis to hepatic steatosis with a necroinflammatory component may or may not have associated fibrosis. NAFLD appears to be most strongly associated with obesity and insulin resistance states including diabetes and with other features of the metabolic syndrome, such as high triglycerides and low high-density lipoprotein (HDL). In the community, the expected prevalence ranges from 3% to 24% [1].
The mechanism of NAFLD has not been entirely clarified. Insulin resistance is mostly offended situation. In this concept, NAFLD is frequently associated with both type 2 diabetes mellitus and metabolic syndrome. Although diabetes mellitus is responsible for all-cause death and liver-related mortality in NAFLD patients, metabolic syndrome mainly causes vascular complications [2].
α-Fetoprotein (AFP) is a glycoprotein that is normally generated during conception by the fetal liver and yolk sac. In clinical practice, AFP levels are elevated in various clinical situations, which include hepatocellular carcinoma, acute or chronic viral hepatitis, chronic liver disease, and gonadal tumors [3].
In this study, we evaluated serum AFP levels in NAFLD patients in comparison with normal subjects. To our knowledge, it is the first study in the literature and we demonstrated that serum AFP levels are elevated in NAFLD patients.
Materials and methods
Sixty-four NAFLD patients admitted to the gastroenterology outpatient clinic because of increased liver enzymes were evaluated together with 20 healthy control subjects in terms of fasting blood glucose, lipid profile, insulin resistance with homeostasis model assessment-estimated insulin resistance (HOMA-IR), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and AFP. Twenty-eight patients were men and 36 patients were women in the patient group and there were 9 men and 11 women in the control group. The mean age was 40.0 ± 5.8 years in the control group and 44.03 ± 10.81 years the in patient group.
NAFLD was diagnosed and its grade was assessed with the findings of liver ultrasound scan performed by the same radiologist. Scoring system described by Hamaguchi et al. [4] was used in this study. Ultrasonographic findings scored in this study included hepatorenal echo contrast, liver brightness, deep attenuation, and vascular blurring. Scores ranged from 1 to 3, with 1 representing either hepatorenal contrast or that bright liver was present, 2 representing that both were present, and a score 3 indicating in addition to hepatorenal echo contrast, bright liver was severe. Twenty-two patients had grade 1, 21 patients had grade 2, and 21 patients had grade 3 steatosis.
All patients’ serums were collected and ultrasound scanning was performed with patient consent after ethics committee approved this study.
Glucose, creatinin, urea, ALT, AST, total cholesterol, triglyceride, HDL cholesterol levels were studied with Roche Diagnostic kits in a Roche/Hitachi D2400 auto analyzer. Insulin levels were measured with Elecsys 1010 auto analyzer. AFP was studied with ELISA method. Low-density lipoprotein (LDL) cholesterol and very low-density lipoprotein (VLDL) cholesterol levels were calculated by Friedewald formula. VLDL (mg/dL) = Triglyceride (mg/dL)/5, LDL (mg/dL) = Total cholesterol (mg/dL) − [HDL (mg/dL) + VLDL (mg/dL)]. Insulin resistance was calculated by HOMA-IR formula (= fasting insulin value × fasting blood glucose/22.5). All the participants’ height and weight values were measured and their body mass index (BMI) was calculated.
Patients consuming more than 10 oz of alcohol per week were excluded from this category, as were any individuals with viral, autoimmune, and storage liver diseases or pregnancy.
Statistical analysis
The Statistical Package for Social Sciences (SPSS) 13.0 for Windows was used to analyze the data. All data were analyzed in terms of mean ± standard deviation (SD). For continuous variables, one-way analysis of variance and Student’s t test were used to analyze the variance among groups if appropriate. Chi-square test was used for comparison of categorical variables. Pearson correlation analyses were used to analyze the data. The value of P < 0.05 was considered statistically significant.
Results
Age and gender were similar in NAFLD and control groups (Table 1). BMI, AST, ALT, glucose, insulin, and HOMA-IR in patients with NAFLD were higher than in the control group. Triglyceride, total cholesterol, LDL cholesterol, and HDL cholesterol levels were higher in NAFLD group than in the control group (Table 1).
Table 1.
Demographics and laboratory findings of groups
| NAFLD group (N = 64) | Control group (N = 20) | P | |
|---|---|---|---|
| Age (mean) | 44.03 ± 10.81 | 40.05 ± 5.80 | 0.120 |
| Gender (female/male) | 36/28 | 11/9 | 0.922 |
| BMI (kg/m2) | 30.96 ± 5.65 | 25.47 ± 3.38 | 0.001 |
| Triglyceride (mg/dL) | 296.95 ± 105.25 | 155.35 ± 22.92 | 0.001 |
| Cholesterol (mg/dL) | 257.09 ± 57.19 | 160.95 ± 20.41 | 0.001 |
| LDL cholesterol (mg/dL) | 159.22 ± 37.11 | 89.05 ± 18.73 | 0.001 |
| HDL cholesterol (mg/dL) | 35.89 ± 6.37 | 40.90 ± 2.69 | 0.001 |
| Glucose (mg/dL) | 91.96 ± 9.04 | 83.65 ± 8.13 | 0.001 |
| AST (IU/L) | 65.93 ± 25.00 | 31.30 ± 6.15 | 0.001 |
| ALT (IU/L) | 84.59 ± 29.11 | 32.50 ± 6.46 | 0.001 |
| Insulin (mU) | 14.05 ± 4.59 | 8.30 ± 1.69 | 0.001 |
| HOMA-IR | 3.19 ± 1.12 | 1.71 ± 0.39 | 0.001 |
| α-Fetoprotein (ng/mL) | 4.09 ± 1.68 | 2.95 ± 1.41 | 0.008 |
BMI body mass index, AST aspartate aminotransferase, ALT alanine aminotransferase, HOMA-IR homeostasis model assessment-estimated insulin resistance, NAFLD nonalcoholic fatty liver disease
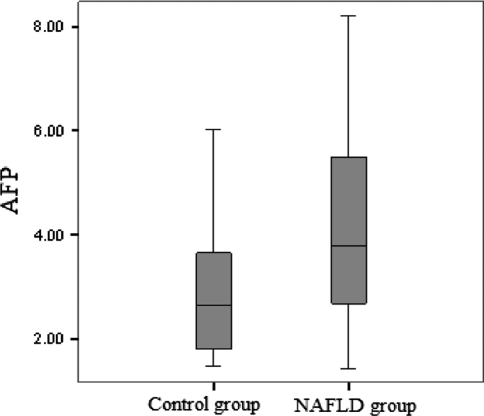
A statistically significant increase in AFP levels was noted in patients with NAFLD (4.09 ± 1.68) compared with healthy controls (2.95 ± 0.41) (P < 0.05; Table 1 and Fig. 1). Triglyceride, cholesterol, LDL cholesterol, HDL cholesterol, glucose, and ALT were not significantly different between NAFLD subgroups (Table 2).
Fig. 1.
α-Fetoprotein (AFP) levels in NAFLD and control groups
Table 2.
Demographics and laboratory findings of NAFLD subgroups
| Grade 1 NAFLD (n = 22) | Grade 2 NAFLD (n = 21) | Grade 3 NAFLD (n = 21) | P | |
|---|---|---|---|---|
| Age (mean ± SD) | 40.04 ± 12.52 | 42.76 ± 11.21 | 49.47 ± 5.25 | 0.011a |
| BMI (kg/m2) | 27.97 ± 4.97 | 31.60 ± 6.28 | 33.47 ± 4.29 | 0.004a |
| Triglyceride (mg/dL) | 265.95 ± 67.47 | 329.61 ± 98.41 | 296.76 ± 135.10 | 0.140 |
| Cholesterol (mg/dL) | 239.36 ± 37.75 | 271.57 ± 51.75 | 261.19 ± 74.47 | 0.169 |
| LDL cholesterol (mg/dL) | 149.57 ± 26.65 | 169.23 ± 34.57 | 159.33 ± 46.78 | 0.224 |
| HDL cholesterol (mg/dL) | 34.36 ± 5.83 | 35.33 ± 5.95 | 38.04 ± 6.99 | 0.148 |
| Glucose (mg/dL) | 88.95 ± 10.56 | 93.04 ± 7.28 | 94.04 ± 8.47 | 0.146 |
| AST (IU/L) | 55.77 ± 16.29 | 73.66 ± 27.95 | 68.85 ± 26.88 | 0.049b |
| ALT (IU/L) | 72.59 ± 26.29 | 92.09 ± 31.34 | 89.66 ± 26.78 | 0.053 |
| Insulin (mU) | 12.08 ± 3.78 | 13.65 ± 4.18 | 16.53 ± 4.81 | 0.004a |
| HOMA-IR | 2.63 ± 0.87 | 3.15 ± 1.08 | 3.81 ± 1.10 | 0.002a |
| α-Fetoprotein (ng/mL) | 2.92 ± 1.06 | 3.97 ± 1.45 | 5.43 ± 1.51 | 0.001a,b,c |
Abbreviations are explained in the footnote to Table 1
aThere is significant difference between grade 1 NAFLD and grade 3 NAFLD groups
bThere is significant difference between grade 1 NAFLD and grade 2 NAFLD groups
cThere is significant difference between grade 2 NAFLD and grade 3 NAFLD groups
There is significant difference between NAFLD subgroups for age, BMI, AST, insulin, HOMA score, and AFP. In the analysis of subgroups, age, BMI, insulin, and HOMA-IR in grade 3 NAFLD group were higher than in grade 1 NAFLD group. AST levels were higher in grade 2 NAFLD group than in grade 1 NAFLD group (Table 2).
A statistically significant increase in AFP levels was noted in patients with grade 3 NAFLD (5.43 ± 1.51) compared with grade 1 (2.92 ± 1.06) and grade 2 NAFLD groups (3.97 ± 1.45). Also, AFP level was significantly higher in grade 2 NAFLD group than in grade 1 NAFLD group (Table 2).
AFP was correlated with NAFLD grade (r = 0.6; P = 0.001), but neither ALT (r = 0.06; P = 0.656) nor AST had correlation (r = 0.05; P = 0.717). According to multivariate analysis, correlation between NAFLD grade and serum AFP levels was independent from the other factors (P = 0.001).
AFP did not correlate with BMI, triglyceride, cholesterol, glucose, ALT, insulin, and HOMA-IR in patient with NAFLD (P > 0.05).
Discussion
In this study, we have determined for the first time that serum AFP levels increase in patients with NAFLD. AFP levels rise further as the grade of liver steatosis increases.
The scale of NAFLD comprises simple hepatic steatosis, which eventually can progress to nonalcoholic steatohepatitis (NASH), with the ensuing advancement of fibrosis and cirrhosis. The generally agreed theory to describe the development of NAFLD and the progression to more advanced level is the “two-hit hypothesis.” The “first hit” is the amassing of lipids in the hepatocytes and insulin resistance is the important pathogenic factor for the maturity of hepatic steatosis. The “second hit” triggers hepatocyte damage, inflammation, and fibrosis. Factors commencing the second hit are oxidative stress, followed by lipid peroxidation, proinflammatory cytokines, adipokines, and mitochondrial dysfunction [5].
NAFLD is clearly linked with obesity, but body fat dispersal seems to play a more significant role in the pathogenesis of NAFLD. The increase in intra-abdominal lipid tissue specifically may be an important parameter in the pathogenesis of NAFLD because of its convincing relationship with insulin resistance [6]. As described in the literature, our patients have similar characteristics. Increased BMI and HOMA-IR score were also demonstrated in our NAFLD patients in comparison with normal subjects.
AFP is a glycoprotein normally synthesized by a developing fetus. A raised level of AFP indicates the existence of either primary liver cancer or a germ cell tumor of the ovary or testicle. Rarely, other kinds of cancer, for example, gastric cancer, are connected with an increased AFP level. Benign circumstances that may produce elevations of AFP include cirrhosis, hepatic necrosis, acute hepatitis, chronic active hepatitis, ataxia-telangiectasia, Wiskott-Aldrich syndrome, and pregnancy [7, 8]. Occasionally, a slight AFP elevation found in other cancers as a result of metastasis to the liver result in the regeneration of hepatic parenchyma. Very rarely, hereditary persistence of AFP may also be found in individuals with no obvious pathology. In our patients, we clearly ascertained that increased AFP levels might be associated with NAFLD. It increases as a result of ongoing inflammation, most probably secondary to cellular destruction or stimulation of AFP production by cytokines. Elevated serum AFP levels may also be due to altered hepatocyte–hepatocyte interaction and the loss of normal architectural arrangements [9]. The presence of hepatic inflammation and/or fibrosis may be the underlying cause of increased serum AFP levels in patients with severe fatty liver. Necrosis or active regeneration may also play a role in these patients. Unfortunately, we cannot speculate further since we did not have liver biopsy results showing the degree of inflammation. Degree of steatosis does not have correlation with degree of steatohepatitis. In addition, levels of liver transaminases do not always reflect the degree of inflammation. However, there are clear evidences that a score of 2 or more corresponded to steatohepatitis [4, 10]. Moreover, as demonstrated in the study, in grade 3 hepatosteatosis group, AFP level elevation is more striking than in the other groups, suggesting that AFP level is well correlated with the disease severity.
Ultrasonography is currently the preferred method for screening asymptomatic patients with elevated liver enzymes and suspected NAFLD. Several studies have demonstrated that the sensitivity, specificity, and positive predictive value of this technique to detect steatosis is as high as 80–100% [4, 10, 11]. However, there are several limitations as well, including operator dependency, less sensitivity in obese patients, and inability to diagnose steatohepatitis and hepatic fibrosis. Hence, attempts to generate an optimal scoring system continue to provide a noninvasive approach. We used scoring system described in the “Material and methods” section for a long time in our clinic. As we mentioned before, NAFLD could be present as simple steatosis or NASH. Taking into account for the ethical concerns, we could not perform liver biopsy of our patients. However, in the light of clinical and laboratory parameters, the diagnosis of NAFLD was clearly demonstrated. We need further studies to analyze the AFP levels in patients with biopsy-proven steatohepatitis.
This study has demonstrated for the first time that patients with NAFLD have a higher prevalence of elevated AFP levels than those without fatty liver changes. AFP level monitoring might help clinicians for the treatment of NAFLD. Elevated levels may herald the grave consequences in this slowly progressing disease.
Contributor Information
Başak Çakal, Email: basakcakal@hotmail.com.
Seyfettin Köklü, Phone: +90-312-3612568, Email: gskoklu@yahoo.com.
References
- 1.Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40(Suppl 1):S5–S10. doi: 10.1097/01.mcg.0000168638.84840.ff. [DOI] [PubMed] [Google Scholar]
- 2.Adams LA, Waters OR, Knuiman MW, Elliott RR, Olynyk JK. NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: an eleven-year follow-up study. Am J Gastroenterol. 2009;104:861–867. doi: 10.1038/ajg.2009.67. [DOI] [PubMed] [Google Scholar]
- 3.Collier J, Sherman M. Screening for hepatocellular carcinoma. Hepatology. 1998;27:273–278. doi: 10.1002/hep.510270140. [DOI] [PubMed] [Google Scholar]
- 4.Hamaguchi M, Kojima T, Itoh Y, Harano Y, Fujii K, Nakajima T, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol. 2007;12:2708–2715. doi: 10.1111/j.1572-0241.2007.01526.x. [DOI] [PubMed] [Google Scholar]
- 5.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/S0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 6.Cnop M, Landchild MJ, Vidal J, Havel PJ, Knowles NG, Carr DR, et al. The concurrent accumulation of intra-abdominal and subcutaneous fat explains the association between insulin resistance and plasma leptin concentrations: distinct metabolic effects of two fat compartments. Diabetes. 2002;51:1005–1015. doi: 10.2337/diabetes.51.4.1005. [DOI] [PubMed] [Google Scholar]
- 7.Brunicardi FC, editor. Schwartz’s principles of surgery. 8th ed. The McGraw-Hill Companies, Inc; 2004
- 8.Chen CH, Lin ST, Kuo CL, Nien CK. Clinical significance of elevated alpha-fetoprotein (AFP) chronic hepatitis C without hepatocellular carcinoma. Hepatogastroenterology. 2008;55:1423–1427. [PubMed] [Google Scholar]
- 9.Goldstein NS, Blue DE, Hankin R, Hunter S, Bayati N, Silverman AL. et al. Serum alpha-fetoprotein levels in patients with chronic hepatitis C. Relationships with serum alanine aminotransferase values, histologic activity index, and hepatocyte MIB-1 scores. Am J Clin Pathol. 1999;111:811–816. doi: 10.1093/ajcp/111.6.811. [DOI] [PubMed] [Google Scholar]
- 10.Liang RJ, Wang HH, Lee WJ, Liew PL, Lin JT, Wu MS, et al. Diagnostic value of ultrasonographic examination for nonalcoholic steatohepatitis in morbidly obese patients undergoing laparoscopic bariatric surgery. Obes Surg. 2007;17:45–56. doi: 10.1007/s11695-007-9005-6. [DOI] [PubMed] [Google Scholar]
- 11.Mishra P, Younossi ZM. Abdominal ultrasound for diagnosis of nonalcoholic fatty liver disease (NAFLD) Am J Gastroenterl. 2007;12:2716–2717. doi: 10.1111/j.1572-0241.2007.01520.x. [DOI] [PubMed] [Google Scholar]