Abstract
Objective
To examine associations between adult weight gain and diabetes among African Americans and whites.
Method
Cross-sectional interview data from 19,589 African American men, 6,202 white men, 27,021 African American women, and 11,623 white women enrolled in the Southern Community Cohort Study in the southeastern USA from 2002–2009 were analyzed in multivariate logistic regression models to examine odds ratios (OR) and 95% confidence intervals (CI) between self-reported diabetes and weight change from age 21.
Results
Diabetes odds rose with increasing weight gain and effects varied somewhat by race and gender; ORs (95% CI) for diabetes associated with weight gain of 40+ kilograms compared to stable weight (change <5 kg) were 3.3 (2.8–4.0) for African American males, 3.6 (2.7–4.8) for white males, 2.6 (2.3–3.1) for African American females, and 4.0 (3.2–4.9) for white females. Among women, significantly increased diabetes odds (41% for whites and 21% for African Americans) were observed even for weight gain of 5–10 kg. Relative increases in odds ratios for diabetes were most pronounced among individuals who had a healthy BMI (18.5–24.9 kg/m2) at age 21 compared to those already overweight.
Conclusion
Adult weight gain is strongly associated with diabetes across gender and race groups indicating that a uniform prevention message can be presented, even to those of healthy weight.
Keywords: Diabetes mellitus, weight gain, African Americans, epidemiology
INTRODUCTION
Obesity is a well-known risk factor for type II diabetes (hereafter called ‘diabetes’) (Eckel et al., 2004). In the United States, diabetes affects nearly 24 million people (National Institute of Diabetes and Digestive and Kidney Diseases, 2008). For reasons that remain unclear, diabetes prevalence is higher among African Americans than whites (National Institute of Diabetes and Digestive and Kidney Diseases, 2008), and work continues to determine the relative contributions of lifestyle factors (such as obesity), socioeconomic status, and genetic susceptibility to this disparity (Dagogo-Jack, 2003, Signorello et al., 2007).
While obesity measured by body mass index (BMI) has been positively associated with diabetes in numerous large studies (Colditz et al., 1990, Chan et al., 1994, Wilson et al., 2002, Nguyen et al., 2008, Nemesure et al., 2008), gaps in the literature exist regarding the specific effect of weight gain on diabetes risk, especially among African Americans. Research defining the association between weight gain and diabetes remains important because of the growing prevalence of obesity overall, and particularly among African American women (Flegal et al., 2002). Positive associations between weight gain, even at modest levels, and diabetes in studies of white adults have been reported (Brancati et al., 1999, Colditz et al., 1995, Cowie et al., 1993, Ford et al., 1997, Hanson et al., 1995, Holbrook et al., 1989, Koh-Banerjee et al., 2004, Krishnan et al., 2007, Oguma et al., 2005, Schienkiewitz et al., 2006, Wannamethee et al., 2005). Only a few studies of adult weight gain have included African Americans and have been limited by relatively small sample sizes or an inability to adequately compare African Americans and whites of both genders (Cowie et al., 1993, Ford et al., 1997, Krishnan et al., 2007, Resnick et al., 2000). Using data from the Southern Community Cohort Study (SCCS), which includes large numbers of both African American and white men and women drawn from similar communities in the southeastern US, we had a unique opportunity to evaluate the relationship between adult weight gain and diabetes in a context that facilitated comparability of findings across race and gender groups and that could provide robust estimates of the effect of weight gain across a broad spectrum of exposure.
METHODS
This study was approved by the Institutional Review Boards at Vanderbilt University and Meharry Medical College.
Data Collection
Study participants enrolled in-person in the SCCS, a prospective epidemiologic cohort study (Signorello et al., 2005), starting in March 2002 at community health centers (CHCs) in twelve southeastern states. CHCs are government-funded health care facilities that provide health services primarily to low-income individuals (Hargreaves et al., 2006). Thus, the SCCS is unique in that the majority of the cohort is low-income (Signorello et al., 2005). Cohort members are required to be age 40–79, English-speaking and not have undergone treatment for cancer within the past year.
Participants provided written informed consent and completed a comprehensive, in-person, interview covering various aspects of health and behavior. During the interview, participants were asked: “Has a doctor ever told you that you have had diabetes or high blood sugar?” Participants responding “yes” were asked follow-up questions regarding their age at first diagnosis and use of diabetes medications. Women were specifically asked not to include gestational diabetes.
Current weight, weight at age 21, the most ever weighed (not counting weight during pregnancy), and current height were all self-reported by participants enrolled through October 2007. Also during this time, measured height and weight were abstracted from patients’ medical records at the CHC if these measurements were taken during a medical visit on the day of the interview (approximately 25% of the cohort). Starting in October 2007, all participants were measured by a trained interviewer for current height and weight using a standardized protocol and calibrated equipment. Adult weight change was examined in two ways in this analysis. First, it was defined as (current weight reported or measured at the baseline interview minus weight at age 21) and second, as (the most the participant ever reported weighing minus weight at age 21).
Statistical analyses
For the present analysis, we included cohort members who enrolled between March 2002 and February 2009 at CHCs and identified themselves as either only Black/African American or only White (N=67,895, or 96% of the total current cohort). We excluded participants with missing diabetes status (N=374) or weight or height (N=2,557) as well as those with age at diabetes diagnosis ≤ 21 (N=529) leaving 64,435 participants in our study population.
Descriptive characteristics for males and for females were compared between race groups using Chi-square tests. Odds ratios (OR) for diabetes with 95% confidence intervals (95% CI) associated with weight gain were estimated using logistic regression. Participants with stable weight (gain or loss < 5 kg) were used as the referent category. Potential confounders were determined from the literature and included age at interview, BMI at age 21, educational attainment (<9 years, 9–11 years, high school, some college, college graduate+), total household income (<$15K, $15-24K, $25-49K, $50K+), current cigarette smoking status (current/former/never), self-reported hypertension (yes/no), marital status (married/divorced/widowed/never married), and physical activity level (metabolic equivalent (MET)-hours for all vigorous activity (occupational, household, and sports) currently and during the participants’ thirties, modeled as continuous variables).
Logistic regression models were run separately for males and females. Effect modification by race was evaluated by comparing models with and without interaction terms for race*weight change using the likelihood ratio test (LRT). The LRT was found to be significant (p<0.1, determined a priori) for weight change determined using the ‘weight at 21 to the most weight’ metric (p=0.096 for males and p<0.0001 for females) and for females only using the ‘weight at 21 to the current weight’ metric (p<0.0001, males p-value=0.31). Thus models were stratified by race as well as by gender for this report.
RESULTS
The proportion of participants who were overweight or obese (BMI ≥ 25 kg/m2) at enrollment was higher for women than for men and highest overall among African American women (Table 1). At age 21, a much higher proportion of the participants had a healthy BMI (<25 kg/m2) compared to the BMI distribution at study enrollment. Nearly 21% of the participants reported a diagnosis of diabetes with African American males having the lowest prevalence (16.7%) and African American females having the highest prevalence (23.7%).
Table 1.
Descriptive statistics for age, body size measures, and diabetes among adults from southeastern communities in the US, 2002–2009, stratified by sex and race (n=64,435)
| Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | White N=6,202 | African American N=19,589 | White N=11,623 | African American N=27,021 | ||||
| n | % | n | % | n | % | n | % | |
| Age at interview (yrs) | ||||||||
| 40–44 | 1,453 | 23.4 | 5,547 | 28.3 | 2,232 | 19.2 | 6,919 | 25.6 |
| 45–49 | 1,387 | 22.4 | 4,992 | 25.5 | 2,398 | 20.6 | 6,311 | 23.4 |
| 50–54 | 1,148 | 18.5 | 4,124 | 21.1 | 2,153 | 18.5 | 5,371 | 19.9 |
| 55–59 | 816 | 13.2 | 2,377 | 12.1 | 1,823 | 15.7 | 3,536 | 13.1 |
| 60–64 | 634 | 10.2 | 1,357 | 6.9 | 1,430 | 12.3 | 2,283 | 8.5 |
| 65–69 | 397 | 6.4 | 674 | 3.4 | 833 | 7.2 | 1,376 | 5.1 |
| 70+ | 367 | 5.9 | 518 | 2.6 | 754 | 6.5 | 1,225 | 4.5 |
| p-value | <0.0001 | <0.0001 | ||||||
| Current body mass index (kg/m2)a | ||||||||
| <18.5 | 75 | 1.2 | 274 | 1.4 | 212 | 1.8 | 301 | 1.1 |
| 18.5 – 24.9 | 1,821 | 29.4 | 7,111 | 36.3 | 2,666 | 22.9 | 4,298 | 15.9 |
| 25.0 – 29.9 | 2,087 | 33.7 | 6,753 | 34.5 | 3,033 | 26.1 | 6,853 | 25.4 |
| 30.0 – 34.9 | 1,280 | 20.6 | 3,395 | 17.3 | 2,623 | 22.6 | 6,713 | 24.8 |
| 35.0 – 39.9 | 548 | 8.8 | 1325 | 6.8 | 1,510 | 13.0 | 4,464 | 16.5 |
| 40.0+ | 391 | 6.3 | 731 | 3.7 | 1,579 | 13.6 | 4,392 | 16.3 |
| p-value | <0.0001 | <0.0001 | ||||||
| Highest body mass index (kg/m2)b | ||||||||
| <18.5 | 10 | 0.2 | 48 | 0.3 | 36 | 0.3 | 70 | 0.3 |
| 18.5 – 24.9 | 881 | 14.2 | 3,848 | 19.6 | 1,593 | 13.7 | 2,494 | 9.2 |
| 25.0 – 29.9 | 1,905 | 30.7 | 6,908 | 35.3 | 2,723 | 23.4 | 5,537 | 20.5 |
| 30.0 – 34.9 | 1,733 | 27.9 | 4,970 | 25.4 | 2,696 | 23.2 | 7,075 | 26.2 |
| 35.0 – 39.9 | 912 | 14.7 | 2,331 | 11.9 | 1,883 | 16.2 | 5,148 | 19.1 |
| 40.0+ | 757 | 12.2 | 1,473 | 7.5 | 2,686 | 23.1 | 6,656 | 24.6 |
| Unknown | 4 | 0.1 | 11 | 0.1 | 6 | 0.1 | 41 | 0.2 |
| p-value | <0.0001 | <0.0001 | ||||||
| Body mass index at age 21 (kg/m2) | ||||||||
| <18.5 | 447 | 7.2 | 1,444 | 7.4 | 1,883 | 16.2 | 3,852 | 14.3 |
| 18.5 – 24.9 | 3,623 | 58.4 | 11,964 | 61.1 | 7,325 | 63.0 | 16,437 | 60.8 |
| 25.0 – 29.9 | 1,608 | 25.9 | 4,649 | 23.7 | 1,354 | 11.7 | 4,267 | 15.8 |
| 30.0 – 34.9 | 363 | 5.9 | 1,093 | 5.6 | 621 | 5.3 | 1,541 | 5.7 |
| 35.0 – 39.9 | 98 | 1.6 | 278 | 1.4 | 247 | 2.1 | 532 | 2.0 |
| 40.0+ | 63 | 1.0 | 161 | 0.8 | 193 | 1.7 | 392 | 1.5 |
| p-value | 0.0023 | <0.0001 | ||||||
| Weight change [current – age 21] (kg) | ||||||||
| Lost 10.0+ kg | 292 | 4.7 | 1,250 | 6.4 | 320 | 2.8 | 679 | 2.5 |
| Lost 5.0 – 9.9 kg | 226 | 3.6 | 1,153 | 5.9 | 189 | 1.6 | 513 | 1.9 |
| Stablec | 1,261 | 20.3 | 4,431 | 22.6 | 1,289 | 11.1 | 2,129 | 7.9 |
| Gained 5.0 – 9.9 kg | 829 | 13.4 | 2,928 | 15.0 | 1,112 | 9.6 | 2,038 | 7.5 |
| Gained 10.0 – 19.9 kg | 1,450 | 23.4 | 4,407 | 22.5 | 2,735 | 23.5 | 5,838 | 21.6 |
| Gained 20.0 – 29.9 kg | 1,068 | 17.2 | 2,824 | 14.4 | 2,556 | 22.0 | 6,090 | 22.5 |
| Gained 30.0 – 39.9 kg | 503 | 8.1 | 1,292 | 6.6 | 1,576 | 13.6 | 4,152 | 15.4 |
| Gained 40.0+ kg | 573 | 9.2 | 1,304 | 6.7 | 1,846 | 15.9 | 5,582 | 20.7 |
| p-value | <0.0001 | <0.0001 | ||||||
| Weight change [most – age 21] (kg) | ||||||||
| Stable c | 660 | 10.6 | 3,389 | 17.3 | 643 | 5.5 | 1,582 | 5.9 |
| Gained 5.0 – 9.9 kg | 623 | 10.1 | 2,579 | 13.2 | 759 | 6.5 | 1,398 | 5.2 |
| Gained 10.0 – 19.9 kg | 1,442 | 23.3 | 4,919 | 25.1 | 2,266 | 19.5 | 4,555 | 16.9 |
| Gained 20.0 – 29.9 kg | 1,488 | 24.0 | 4,082 | 20.8 | 2,726 | 23.5 | 6,413 | 23.7 |
| Gained 30.0 – 39.9 kg | 882 | 14.2 | 2,190 | 11.2 | 2,049 | 17.6 | 4,916 | 18.2 |
| Gained 40.0+ kg | 1,103 | 17.8 | 2,419 | 12.4 | 3,174 | 27.3 | 8,116 | 30.0 |
| Unknown | 4 | 0.1 | 11 | 0.1 | 6 | 0.1 | 41 | 0.2 |
| p-value | <0.0001 | <0.0001 | ||||||
| Family History of diabetesd | ||||||||
| No | 3,706 | 59.8 | 10,898 | 55.6 | 5,980 | 51.5 | 12,420 | 46.0 |
| Yes | 2,332 | 37.6 | 8,378 | 42.8 | 5,419 | 46.6 | 14,248 | 52.7 |
| Missing | 164 | 2.6 | 313 | 1.6 | 224 | 1.9 | 353 | 1.3 |
| p-value | <0.0001 | <0.0001 | ||||||
| Diabetes (diagnosed after age 21)e | ||||||||
| No | 5,043 | 81.3 | 16,287 | 83.1 | 9,192 | 79.1 | 20,612 | 76.3 |
| Yes | 1,159 | 18.7 | 3,302 | 16.9 | 2,431 | 20.9 | 6,409 | 23.7 |
| p-value | 0.0009 | <0.0001 | ||||||
Current body mass index calculated using weight at time of SCCS baseline interview
Highest body mass index calculated using self-report of most ever weighed
Stable weight change defined as weight at age 21 within 5.0 kg of weight at the time of the SCCS baseline interview
Family history of diabetes is yes if participant reported birth mother, birth father, full sister, or full brother having diabetes
Self-reported using the question: “Has a doctor ever told you that you have had diabetes or high blood sugar?” Participants who reported age at first diabetes diagnosis age 21 or younger were excluded from analysis
Most (79%) of the participants reported gaining weight since age 21. As weight gain increased (measured as the difference between current weight and weight at age 21), the odds of diabetes increased across all race and gender strata in multivariate models (Table 2). The magnitude of the association was similar for white and African American males for the highest category of weight gain (40+ kg) compared to men with stable weight. A larger difference in odds ratios was seen for weight gain of 40+ kg between white and African American women. Among all groups except white males, weight loss of more than 10 kg was also associated with increased odds of diabetes. However, both males and females who lost more than 10 kg weighed on average 91 kg (200 lbs) at age 21 and were substantially heavier at age 21 than those with stable weight (26 kg heavier for white males, 24 kg for African American males, 37 kg for white females, and 32 kg for African American females).
Table 2.
Multivariate-adjusteda odds ratios (95% confidence intervals) for diabetes by category of adult weight change among adults from southeastern communities in the US, 2002–2009, stratified by sex and race
| Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|
| White | African American | White | African American | |||||
| OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | |
| Weight change [current – age 21] (kg) | ||||||||
| Lost 10.0+ kg | 0.95 | (0.65–1.39) | 1.57 | (1.30–1.90) | 1.45 | (1.03–2.05) | 1.57 | (1.24–1.98) |
| Lost 5.0 – 9.9 kg | 1.21 | (0.77–1.88) | 1.12 | (0.91–1.39) | 1.45 | (0.94–2.25) | 1.39 | (1.07–1.81) |
| Stable b | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref |
| Gained 5.0 – 9.9 kg | 1.01 | (0.74–1.37) | 1.15 | (0.98–1.36) | 1.41 | (1.08–1.84) | 1.21 | (1.01–1.46) |
| Gained 10.0 – 19.9 kg | 1.36 | (1.06–1.75) | 1.44 | (1.25–1.66) | 1.56 | (1.24–1.95) | 1.47 | (1.26–1.71) |
| Gained 20.0 – 29.9 kg | 1.57 | (1.21–2.04) | 2.02 | (1.74–2.34) | 2.21 | (1.77–2.76) | 1.69 | (1.46–1.97) |
| Gained 30.0 – 39.9 kg | 2.71 | (2.02–3.62) | 2.49 | (2.09–2.96) | 2.59 | (2.05–3.26) | 2.02 | (1.73–2.36) |
| Gained 40.0+ kg | 3.60 | (2.72–4.77) | 3.33 | (2.81–3.95) | 3.95 | (3.16–4.95) | 2.62 | (2.26–3.05) |
| Weight change [most – age 21] (kg) | ||||||||
| Stable b | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref |
| Gained 5.0 – 9.9 kg | 1.07 | (0.70–1.65) | 1.01 | (0.84–1.22) | 0.91 | (0.61–1.34) | 0.86 | (0.68–1.08) |
| Gained 10.0 – 19.9 kg | 1.60 | (1.14–2.27) | 1.24 | (1.06–1.45) | 1.26 | (0.93–1.70) | 1.11 | (0.93–1.32) |
| Gained 20.0 – 29.9 kg | 1.89 | (1.35–2.65) | 2.04 | (1.75–2.37) | 1.68 | (1.25–2.25) | 1.52 | (1.28–1.80) |
| Gained 30.0 – 39.9 kg | 3.26 | (2.31–4.61) | 2.64 | (2.24–3.11) | 2.78 | (2.07–3.72) | 2.03 | (1.71–2.40) |
| Gained 40.0+ kg | 5.89 | (4.22–8.23) | 4.18 | (3.57–4.90) | 4.41 | (3.32–5.86) | 2.91 | (2.47–3.43) |
Adjusted for age, BMI at age 21, education (<9 yrs, 9–11 yrs, High School, Some college, College+), income (<$15K, $15-25K, $25-50K, $50K+), cigarette smoking status (current/former/never), total MET-hours of vigorous physical activity currently (continuous), total MET-hours of vigorous physical activity during 30s (continuous), hypertension (Y/N), and marital status (Married, Separated/Divorced, Widowed, Single) as categorized in table 1 unless otherwise specified.
Stable weight change is gain or loss of less than 5 kg
For the ‘age 21 to highest lifetime weight’ metric, the odds ratios for diabetes also increased as weight gain increased (Table 2). The magnitude of effect at the highest categories of weight gain was larger in males than in females within each race group. White participants also had higher odds ratios for diabetes than did African American participants at comparable categories of weight gain for both genders.
We considered that overweight or obese participants may have received heavier screening for diabetes, possibly confounding the associations observed between diabetes and weight gain. However, additional adjustment for time since last physician visit (as a surrogate for increased clinical attention) did not appreciably alter the weight change ORs (data not shown).
In order to examine whether the association between diabetes and weight change was modified by body size in early adulthood, we repeated our analysis after stratifying by BMI at age 21 (18.5–24.9, 25–29.9, and 30+ kg/m2) (Figure 1). In all race and gender groups, the relative effect of weight gain was stronger for participants who were not obese at age 21 and was particularly strong for those who were at a healthy weight (BMI 18.5–24.9 kg/m2) at age 21.
Figure 1.
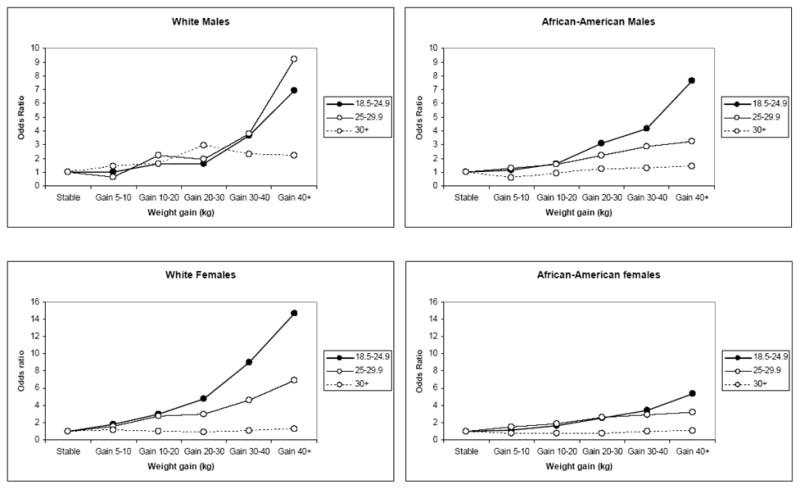
Multivariate odds ratios for diabetes by category of adult weight change (most weight - weight at age 21), stratified by race, gender, and BMI at age 21 (18.5–24.9, 25–29.9 and 30+ kg/m2) among adults from southeastern communities in the US, 2002–2009.
Still, although the odds ratios associated with weight gain were stronger for the leaner groups, the higher rate of diabetes in the obese group compared to the leaner groups (not shown in the figure) placed obese gainers of 40+ kg clearly at the highest overall risk (OR=11.9, 95%CI 9.50–14.8 compared to those who were healthy weight at age 21 and maintained a stable weight). Particularly among women, for those who were already obese at age 21, additional adult weight gain did not appreciably impact their subsequent odds of diabetes.
To address concerns about self-reported anthropometrics, we re-ran the analysis using the ‘age 21 to current weight’ metric limited to the 9,287 individuals who joined the SCCS after October 2007 when measured current height and weight values replaced self-reported values. The patterns that were observed in the entire cohort were essentially unchanged in the subset of individuals with measured values; the ORs for diabetes for a gain of 40+ kg in the subset with measured values versus the entire cohort were 3.09 v. 3.33 for African American males, 3.74 v. 3.60 for white males, 2.52 v. 2.63 for African American females, and 4.23 v. 3.95 for white females.
We additionally examined models stratified by time between diabetes diagnosis and the baseline SCCS interview (<5, 5–9, and 10+ years) in order to address the cross-sectional nature of our dataset. Each subset of diabetic participants was compared to the entire non-diabetic population in separate models using the same set of covariates as in previous models with additional adjustment for race and gender. For both metrics of weight change, weight gain was associated with increased odds for diabetes in each subset (Table 3); ORs were highest in the patients diagnosed <5 years ago, intermediate for those diagnosed 5–9 years ago, and lowest for those diagnosed 10+ years ago (Table 3).
Table 3.
Multivariatea odds ratios (95% confidence intervals) for diabetes stratified by time between diagnosis and baseline interview compared to non-diabetics among adults from southeastern communities in the US, 2002–2009
| Time between diabetes diagnosis and baseline SCCS interview | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No diabetes | < 5 years | 5 – 9 years | 10+ years | |||||||
| n | n | OR | (95% CI) | n | OR | (95% CI) | n | OR | (95% CI) | |
| Weight change [current – age 21] (kg) | ||||||||||
| Lost 10.0+ kg | 1,855 | 181 | 1.14 | (0.93–1.40) | 171 | 1.54 | (1.23–1.93) | 298 | 1.86 | (1.54–2.25) |
| Lost 5.0 – 9.9 kg | 1,737 | 122 | 1.23 | (0.99–1.54) | 75 | 1.17 | (0.89–1.53) | 122 | 1.27 | (1.01–1.60) |
| Stablec | 8,154 | 351 | 1.0 | Ref | 228 | 1.0 | Ref | 322 | 1.0 | Ref |
| Gained 5.0 – 9.9 kg | 6,044 | 329 | 1.24 | (1.06–1.45) | 213 | 1.27 | (1.04–1.54) | 279 | 1.13 | (0.95–1.34) |
| Gained 10.0 – 19.9 kg | 12,015 | 977 | 1.69 | (1.49–1.93) | 546 | 1.44 | (1.22–1.70) | 759 | 1.28 | (1.10–1.47) |
| Gained 20.0 – 29.9 kg | 9,809 | 1153 | 2.26 | (1.99–2.57) | 631 | 1.86 | (1.58–2.19) | 805 | 1.51 | (1.30–1.74) |
| Gained 30.0 – 39.9 kg | 5,472 | 883 | 2.86 | (2.50–3.28) | 476 | 2.28 | (1.92–2.71) | 572 | 1.70 | (1.45–1.98) |
| Gained 40.0+ kg | 6,048 | 1,465 | 3.92 | (3.44–4.47) | 717 | 2.80 | (2.38–3.31) | 900 | 2.24 | (1.93–2.60) |
| Weight change [most – age 21] (kg) | ||||||||||
| Stablec | 5,518 | 267 | 1.0 | Ref | 179 | 1.0 | Ref | 264 | 1.0 | Ref |
| Gained 5.0 – 9.9 kg | 4,862 | 182 | 0.91 | (0.75–1.11) | 102 | 0.80 | (0.62–1.03) | 187 | 1.12 | (0.91–1.38) |
| Gained 10.0 – 19.9 kg | 11,550 | 644 | 1.27 | (1.09–1.48) | 393 | 1.26 | (1.04–1.52) | 516 | 1.18 | (1.00–1.40) |
| Gained 20.0 – 29.9 kg | 12,076 | 1,072 | 1.89 | (1.63–2.19) | 597 | 1.70 | (1.41–2.03) | 810 | 1.59 | (1.36–1.87) |
| Gained 30.0 – 39.9 kg | 7,547 | 1,060 | 2.78 | (2.40–3.22) | 591 | 2.49 | (2.07–3.00) | 719 | 2.13 | (1.80–2.51) |
| Gained 40.0+ kg | 9,532 | 2,231 | 4.11 | (3.57–4.74) | 1,194 | 3.51 | (2.95–4.19) | 1,555 | 3.24 | (2.76–3.79) |
Adjusted for race, gender, age, BMI at age 21, education (<9 yrs, 9–11 yrs, High School, Some college, College+), income (<$15K, $15-25K, $25-50K, $50K+), cigarette smoking status (current/former/never), total MET-hours of vigorous physical activity currently (continuous), total MET-hours of vigorous physical activity during 30s (continuous), hypertension (Y/N), and marital status (Married, Separated/Divorced, Widowed, Single) as categorized in table 1 unless otherwise specified.
DISCUSSION
In this cross-sectional analysis using a large population of African American and white individuals, we observed a strong positive relationship between weight gain since age 21 and diabetes. There was also no apparent threshold, as significantly elevated ORs were observed for weight gain as low as 5–10 kg and as high as 40+ kg over a period of at least 20 years. While all groups were at increased risk of diabetes at higher levels of weight gain, the magnitude of effect varied somewhat by race and gender. The odds ratios for diabetes were typically higher among males compared to females at the highest levels of weight gain, and among women, were higher for whites compared to African Americans at each level of weight gain.
The patterns observed in our study are largely comparable to the existing literaturewhich have found increased diabetes risk as adult weight gain increased (Brancati et al., 1999, Colditz et al., 1995, Hanson et al., 1995, Holbrook et al., 1989, Koh-Banerjee et al., 2004, Oguma et al., 2005, Schienkiewitz et al., 2006, Wannamethee et al., 2005). Gender differences were observed in some studies (Hanson et al., 1995, Cowie et al., 1993) but not in others (Ford et al., 1997, Holbrook et al., 1989, Resnick et al., 2000, Schienkiewitz et al., 2006). While smaller, previous studies of African American participants have generally found positive associations between weight gain and diabetes risk (Cowie et al., 1993, Ford et al., 1997, Resnick et al., 2000), our study is the first to include large numbers of both white and African American men and women of comparable geographic and socioeconomic backgrounds; Using NHANES Epidemiologic Follow-up data, positive associations between weight gain and incident diabetes were observed overall but no significant interactions with race or gender were detected (Cowie et al., 1993, Ford et al., 1997). The Black Women’s Health Study (BWHS) reported increased relative risks for diabetes in relation to weight gain, including rate ratios as high as 11 for weight gain of more than 40 kg (Krishnan et al., 2007). Unlike many previous studies, we also found a significant positive association between weight loss of 10+ kg and diabetes. Reasons for this finding are uncertain but are likely an artifact reflecting weight change after a diagnosis of diabetes such as weight loss due to dietary management of the disease.
Study limitations and strengths
Several study limitations should be noted. This analysis was cross-sectional and thus temporality could not be assessed for the relationship between diabetes and adult weight gain. In order to address this limitation and evaluate the potential for weight changes after diabetes diagnosis to obscure the results, we performed analyses stratified by time between diabetes diagnosis and the baseline SCCS interview. We hypothesized that those individuals who were diagnosed most recently were the most likely to have gained weight prior to their diagnosis whereas those who were the furthest out from their diagnosis were more likely to have had their weight affected by diabetes diagnosis or its treatment. The positive association between weight gain and diabetes was strongest for patients who were recently diagnosed (<5 years) and thus most likely to have gained weight prior to their diabetes diagnosis in comparison to those diagnosed 5–9 years prior and even more so compared to those diagnosed 10+ years ago. While not as robust a confirmation of an etiologic relationship as could be found using a longitudinal study design, this analysis supports the hypothesis that weight gain may be a precipitating cause of diabetes.
Additionally, diabetes status was self-reported rather than determined via gold standard blood glucose testing. However, in a sample of nearly 800 SCCS participants, 94% of those self-reporting diabetes had an elevated HbA1c (>6.1%) or were currently taking antiglycemic medication (Huizinga et al, manuscript in preparation). Depending upon the HbA1c cutoff used to define diabetes, misclassification of individuals with undiagnosed diabetes may be a larger problem. Under the scenario with the greatest number of undetected cases (HbA1c > 6.1%), we observed that those with undiagnosed diabetes were more likely to have lower BMI values. If these undiagnosed cases were reclassified as diabetics, the percentages with low BMI would increase in the diabetes group and decrease in the non-diabetes group, likely attenuating the odds ratios we observed.
Using self-reported height and weight measures is another potential limitation of this analysis. While older literature indicates a high concordance for measured and self-reported values (Stewart, 1982), a more recent review indicates that height tends to be over-reported while weight tends to be under-reported (Gorber et al., 2007). However, in the SCCS, it is expected that the in-person nature of the interview was a deterrent for gross under- or over-reporting. Additionally, weight and height measured in the CHC during a medical visit on the day of the interview for approximately 25% of the study population were highly correlated with self-reported values (Pearson correlation >0.95 for BMI values calculated from self-report versus medical record). When participants were stratified by BMI category (based on self-reported values), the differences between self-reported and measured weights show no indication that overweight or obese participants were misreporting weight more so than healthy weight individuals. Differences between self-reported and measured weights also did not vary by race, education, or income categories. In our analysis limited to the 9,287 participants who had interviewer-measured height and weight values, we obtained similar results as those observed in the full population. We had no means by which to validate recalled weight at age 21, but reports in the literature indicate that recalled weight in middle to later age is generally quite good (Casey et al., 1991, Must et al., 1993, Stevens et al., 1990).
This study also has several notable strengths. First, although the SCCS is not reflective of the SES or racial distributions in the general US population, it is a unique cohort in which to study health effects in different racial groups because of the large number of African Americans and the comparability of SES across racial groups. The within-population comparisons described here are valid regardless of the generalizability of the SCCS to the general population and further, there is little reason to postulate that the association between weight change and diabetes would differ for groups of different SES. A further strength of this study is that the study population is the largest-to-date to examine diabetes in relation to weight gain among both African Americans and whites of both genders. In addition, a broad range of weight gain is represented within the study population that allowed us to examine categories of weight gain over a large spectrum. The SCCS population also has a broad range of weight at age 21 which permitted the examination of weight change according to different baselines of early adult BMI.
CONCLUSIONS
The observation that weight gain is associated with odds of diabetes even at modest levels of weight gain over many years and particularly among adults who were of a healthy weight in early adulthood is a powerful public health message. In addition to diabetes prevention messages aimed at already overweight or obese patients, these results imply that medical practitioners should convey equally strong messages to healthy weight patients that maintaining a healthy body weight over time may help reduce the risk of developing diabetes. In a study population with high comparability across race and gender groups, our findings also suggest that the link between weight gain and diabetes holds regardless of race and gender, and thus, prevention strategies may be uniformly applied.
Acknowledgments
The Southern Community Cohort Study is supported by grant R01 CA92447 from the National Cancer Institute. Ms. Cohen also received support from NCI Training Grants T32 CA09330-26 and 5-R25-CA057726. The authors are grateful to Charles Li and Heather Munro at the International Epidemiology Institute for performing a statistical review.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brancati FL, Wang NY, Mead LA, Liang KY, Klag MJ. Body weight patterns from 20 to 49 years of age and subsequent risk for diabetes mellitus: the Johns Hopkins Precursors Study. Arch Intern Med. 1999;159:957–63. doi: 10.1001/archinte.159.9.957. [DOI] [PubMed] [Google Scholar]
- Casey VA, Dwyer JT, Berkey CS, Coleman KA, Gardner J, Valadian I. Long-term memory of body weight and past weight satisfaction: a longitudinal follow-up study. Am J Clin Nutr. 1991;53:1493–8. doi: 10.1093/ajcn/53.6.1493. [DOI] [PubMed] [Google Scholar]
- Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17:961–9. doi: 10.2337/diacare.17.9.961. [DOI] [PubMed] [Google Scholar]
- Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122:481–6. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- Colditz GA, Willett WC, Stampfer MJ, Manson JE, Hennekens CH, ARKY RA, SPEIZER FE. Weight as a risk factor for clinical diabetes in women. Am J Epidemiol. 1990;132:501–13. doi: 10.1093/oxfordjournals.aje.a115686. [DOI] [PubMed] [Google Scholar]
- Cowie CC, Harris MI, Silverman RE, Johnson EW, Rust KF. Effect of multiple risk factors on differences between blacks and whites in the prevalence of non-insulin-dependent diabetes mellitus in the United States. Am J Epidemiol. 1993;137:719–32. doi: 10.1093/oxfordjournals.aje.a116732. [DOI] [PubMed] [Google Scholar]
- Dagogo-Jack S. Ethnic disparities in type 2 diabetes: pathophysiology and implications for prevention and management. J Natl Med Assoc. 2003;95:774, 779–89. [PMC free article] [PubMed] [Google Scholar]
- Eckel RH, York DA, Rossner S, Hubbard V, Caterson I, St Jeor ST, Hayman LL, Mullis RM, Blair SN. Prevention Conference VII: Obesity, a worldwide epidemic related to heart disease and stroke: executive summary. Circulation. 2004;110:2968–75. doi: 10.1161/01.CIR.0000140086.88453.9A. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. Jama. 2002;288:1723–7. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- Ford ES, Williamson DF, Liu S. Weight change and diabetes incidence: findings from a national cohort of US adults. Am J Epidemiol. 1997;146:214–22. doi: 10.1093/oxfordjournals.aje.a009256. [DOI] [PubMed] [Google Scholar]
- Gorber SC, Tremblay M, Moher D, Gorber B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev. 2007;8:307–26. doi: 10.1111/j.1467-789X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- Hanson RL, Narayan KM, Mccance DR, Pettitt DJ, Jacobsson LT, Bennett PH, Knowler WC. Rate of weight gain, weight fluctuation, and incidence of NIDDM. Diabetes. 1995;44:261–6. doi: 10.2337/diab.44.3.261. [DOI] [PubMed] [Google Scholar]
- Hargreaves MK, Arnold C, Blot WJ. Community health centers: Their role in the treatment of minorities and in health disparities research. In: SATCHER D, PAMIES R, editors. Multicultural Medicine and Health Disparities. New York: McGraw-Hill; 2006. [Google Scholar]
- Holbrook TL, Barrett-Cconnor E, Wingard DL. The association of lifetime weight and weight control patterns with diabetes among men and women in an adult community. Int J Obes. 1989;13:723–9. [PubMed] [Google Scholar]
- Koh-Banerjee P, Wang Y, Hu FB, Spiegelman D, Willett WC, Rimm EB. Changes in body weight and body fat distribution as risk factors for clinical diabetes in US men. Am J Epidemiol. 2004;159:1150–9. doi: 10.1093/aje/kwh167. [DOI] [PubMed] [Google Scholar]
- Krishnan S, Rosenberg L, Djousse L, Cupples LA, Palmer JR. Overall and central obesity and risk of type 2 diabetes in U.S. black women. Obesity (Silver Spring) 2007;15:1860–6. doi: 10.1038/oby.2007.220. [DOI] [PubMed] [Google Scholar]
- Must A, Willett WC, Dietz WH. Remote recall of childhood height, weight, and body build by elderly subjects. Am J Epidemiol. 1993;138:56–64. doi: 10.1093/oxfordjournals.aje.a116777. [DOI] [PubMed] [Google Scholar]
- National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics, 2007 fact sheet. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health; 2008. [Google Scholar]
- Nemesure B, Wu SY, Hennis A, Leske MC. The relationship of body mass index and waist-hip ratio on the 9-year incidence of diabetes and hypertension in a predominantly African-origin population. Ann Epidemiol. 2008;18:657–63. doi: 10.1016/j.annepidem.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen NT, Magno CP, Lane KT, Hinojosa MW, Lane JS. Association of hypertension, diabetes, dyslipidemia, and metabolic syndrome with obesity: findings from the National Health and Nutrition Examination Survey, 1999 to 2004. J Am Coll Surg. 2008;207:928–34. doi: 10.1016/j.jamcollsurg.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Oguma Y, Sesso HD, Paggenbarger RS, JR, Lee IM. Weight change and risk of developing type 2 diabetes. Obes Res. 2005;13:945–51. doi: 10.1038/oby.2005.109. [DOI] [PubMed] [Google Scholar]
- Resnick HE, Valsania P, Halter JB, Lin X. Relation of weight gain and weight loss on subsequent diabetes risk in overweight adults. J Epidemiol Community Health. 2000;54:596–602. doi: 10.1136/jech.54.8.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schienkiewitz A, Schulze MB, Hoffmann K, Kroke A, Boeing H. Body mass index history and risk of type 2 diabetes: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr. 2006;84:427–33. doi: 10.1093/ajcn/84.1.427. [DOI] [PubMed] [Google Scholar]
- Signorello LB, Hargreaves MK, Steinwandel MD, Zheng W, Cai Q, Sschlundt DG, Buchowski MS, Arnold CW, McLaughlin JK, Blot WJ. Southern community cohort study: establishing a cohort to investigate health disparities. J Natl Med Assoc. 2005;97:972–9. [PMC free article] [PubMed] [Google Scholar]
- Signorello LB, Schlundt DG, Cohen SS, Steinwandel MD, Buchowski MS, McLaughlin JK, Hargreaves MK, Blot WJ. Comparing diabetes prevalence between African Americans and Whites of similar socioeconomic status. Am J Public Health. 2007;97:2260–7. doi: 10.2105/AJPH.2006.094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J, Keil JE, Waid LR, Gazes PC. Accuracy of current, 4-year, and 28-year self-reported body weight in an elderly population. Am J Epidemiol. 1990;132:1156–63. doi: 10.1093/oxfordjournals.aje.a115758. [DOI] [PubMed] [Google Scholar]
- Stewart AL. The reliability and validity of self-reported weight and height. J Chronic Dis. 1982;35:295–309. doi: 10.1016/0021-9681(82)90085-6. [DOI] [PubMed] [Google Scholar]
- Wannamethee SG, Shaper AG, Walker M. Overweight and obesity and weight change in middle aged men: impact on cardiovascular disease and diabetes. J Epidemiol Community Health. 2005;59:134–9. doi: 10.1136/jech.2003.015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson PW, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162:1867–72. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]


