Abstract
ATP-sensitive K+ (KATP) channels regulate many cellular functions by linking cell metabolism to membrane potential. We have generated KATP channel-deficient mice by genetic disruption of Kir6.2, which forms the K+ ion-selective pore of the channel. The homozygous mice (Kir6.2−/−) lack KATP channel activity. Although the resting membrane potential and basal intracellular calcium concentrations ([Ca2+]i) of pancreatic beta cells in Kir6.2−/− are significantly higher than those in control mice (Kir6.2+/+), neither glucose at high concentrations nor the sulfonylurea tolbutamide elicits a rise in [Ca2+]i, and no significant insulin secretion in response to either glucose or tolbutamide is found in Kir6.2−/−, as assessed by perifusion and batch incubation of pancreatic islets. Despite the defect in glucose-induced insulin secretion, Kir6.2−/− show only mild impairment in glucose tolerance. The glucose-lowering effect of insulin, as assessed by an insulin tolerance test, is increased significantly in Kir6.2−/−, which could protect Kir6.2−/− from developing hyperglycemia. Our data indicate that the KATP channel in pancreatic beta cells is a key regulator of both glucose- and sulfonylurea-induced insulin secretion and suggest also that the KATP channel in skeletal muscle might be involved in insulin action.
Keywords: sulfonylurea receptor/inward rectifier/gene targeting
KATP channels couple cell metabolism to membrane potential in many tissues (1–7). Classical KATP channels comprise two subunits: a receptor [SUR1 (8), SUR2A (9), or SUR2B (10)] of sulfonylureas such as glibenclamide and tolbutamide, widely used to treat noninsulin-dependent diabetes mellitus, and an inward rectifier K+ channel member, Kir6.2 (11, 12). The pancreatic beta cell KATP channel comprises SUR1 and Kir6.2 (11, 12), while the skeletal muscle and cardiac KATP channel comprises SUR2A and Kir6.2 (9). The pancreatic beta cell KATP channels, as ATP and ADP sensors, have been thought to play a critical role in the regulation of glucose- and sulfonylurea-induced insulin secretion (13). In fact, mutations of the SUR1 or Kir6.2 gene are known to cause familial hypoglycemia associated with unregulated insulin secretion (14–18). However, recent studies suggest that both glucose and the sulfonylureas might have additional effects distal to those on the KATP channels (19–21). In addition, although different roles of the KATP channels in the various tissues, including cytoprotection in heart and brain ischemia and excitability of muscles and neurons, have been proposed (22, 23), no direct evidence has been available. To clarify the physiological roles of KATP channels in various cellular functions directly, we generated KATP channel-deficient mice by disruption of the Kir6.2 gene. In the present study, we have focused on the role of the KATP channels in pancreatic beta cell function. Our data clearly demonstrate that both glucose- and sulfonylurea-induced insulin secretion depend critically on KATP channel-dependent pathway, and also suggest that the KATP channels in skeletal muscle are involved in insulin action.
MATERIALS AND METHODS
Targeting the Kir6.2 Gene.
The Kir6.2 gene was cloned from a 129/Sv mouse genomic DNA library (Stratagene) by using its cDNA probe. A targeting vector was constructed by inserting the neomycin-resistance gene at the XhoI site in Kir6.2. The herpes simplex virus thymidine kinase gene was inserted downstream (Fig. 1A). The targeting vector was introduced into E14 embryonic stem (ES) cells by electroporation. The homologous recombinant clone was identified by Southern blot analysis, and homozygous mice (Kir6.2−/−) were generated by the standard procedures.
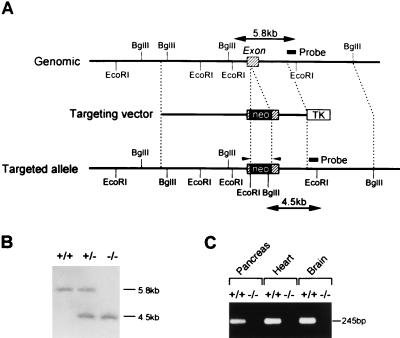
Figure 1.
(A) Schematic representation of the mouse Ki6.2 gene, targeting vector, and targeted allele. The exon is indicated by shaded boxes. Neo and TK indicate a neomycin-resistant gene and a herpes simplex virus thymidine kinase gene, respectively. Restriction sites are indicated. The probe used for Southern blot analysis is shown. Primers used for reverse transcription–PCR (RT-PCR) are indicated by arrowheads. (B) Southern blot analysis of F2 offspring. Genomic DNA was digested with EcoRI and BglII and hybridized with the probe. Lanes: +/+, wild type; +/−, heterozygote; −/−, homozygote. (C) RT-PCR analysis of pancreas, heart, and brain of Kir6.2+/+ and Kir6.2−/−. cDNAs were synthesized from total RNA (10 μg) from the tissues of Kir6.2+/+ and Kir6.2−/−. The expected size of the PCR product (245 bp) is indicated. Lanes: +/+, heterozygote; −/−, homozygote. The Kir6.2 transcript was not detected in tissues of Kir6.2−/−.
Electrophysiology and Measurements of Intracellular Calcium Concentrations ([Ca2+]i).
Pancreatic islets were isolated by collagenase digestion method (24), and dispersed islet cells were cultured in DMEM supplemented with 10% fetal bovine serum, plated into 3.5-cm dishes containing Cellocate Coverslips (Eppendorf), and incubated at 37°C for 24–72 hr before experiments. The whole-cell recordings, single-channel recordings, and measurements of [Ca2+]i in single pancreatic beta cells were performed as described previously (25). After the experiments, pancreatic beta cells were identified by immunostaining with the antiinsulin antibody.
Batch Incubation Experiments.
Batch incubation was performed by the method of Okamoto et al. (26) with slight modifications. Briefly, pancreatic islets (10 in each tube) were preincubated at 37°C for 60 min in Hepes-Krebs buffer containing 118.4 mM NaCl/4.7 mM KCl/1.2 mM KH2PO4/2.4 mM CaCl2/1.2 mM MgSO4/20 mM NaHCO3/2.8 mM glucose/10 mM Hepes, supplemented with 0.2% (wt/vol) BSA. The pancreatic islets then were incubated for 30 min in 400 μl of the buffer containing various stimuli of insulin secretion.
Perifusion Experiments.
Perifusion of pancreatic islets was performed according to the method of Wollheim et al. (24) with slight modifications. Briefly, 150 islets were placed in a column with Bio-Gel P-2 (Bio-Rad) and continuously perifused with gassed Hepes-Krebs at a constant flow rate of 0.5 ml/min at 37°C. After preincubation for 60 min, various stimuli of insulin secretion were applied.
Measurements of Blood Glucose and Insulin Levels.
Serum insulin levels were determined by ELISA kit (Morinaga, Yokohama, Japan). Blood glucose levels were measured in whole blood with Antosense Glucose II (Sankyo). Intraperitoneal glucose tolerance test was performed on male mice that were fasted for 16 hr at 10–16 weeks of age, and glucose (1 g/kg) was injected into the intraperitoneal space after anesthesia with sodium pentobarbital (75 mg/kg). In the insulin tolerance test, human insulin (0.1 units/kg) was injected intraperitoneally in anesthetized adult mice (10–16 weeks) after fasting for 16 hr. Blood samples were taken from the tail vein.
RESULTS AND DISCUSSION
Since the Kir6.2 subunit forms the K+ ion-selective pore (11, 12, 25), we assumed that KATP channel-deficient mice could be generated by disruption of the Kir6.2 gene (Fig. 1A). Homozygous mice (Kir6.2−/−) were generated by interbreeding heterozygous mice (Kir6.2+/−) (Fig. 1B). The absence of expression of the Kir6.2 transcript in pancreas, heart, and brain of Kir6.2−/− was confirmed by reverse transcription–PCR (Fig. 1C). Although Kir6.2−/− show a transient hypoglycemia in neonates [Kir6.2−/−, 31.6 ± 5.0 mg/dl (n = 8); Kir6.2+/+, 65.9 ± 4.4 mg/dl (n = 19), P < 0.0001], they develop normally and are fertile, and there are no apparent abnormalities in general appearance or behavior. No significant differences in body weight were found between age-matched Kir6.2−/−and Kir6.2+/+.
To confirm the absence of functional KATP channels in Kir6.2−/−, we compared whole-cell membrane currents in Kir6.2+/+ and Kir6.2−/− pancreatic beta cells (Fig. 2A). When Kir6.2+/+ beta cells were dialyzed intracellularly with the ATP-free pipette solution, a progressive increase in membrane conductance was observed, and the increase in conductance was inhibited by tolbutamide, properties characteristic of the pancreatic beta cell KATP channel, but there was no increase in K+ membrane conductance in Kir6.2−/− beta cells. Complete loss of KATP channel activity in Kir6.2−/− was confirmed further by single-channel analysis. Unitary KATP channel currents were detected in 39/40 patches (97.5%) and 0/40 patches (0%) in Kir6.2+/+ and Kir6.2−/−, respectively. The absence of KATP channel activity in Kir6.2−/− was confirmed also in skeletal muscle (data not shown). These results indicate that Kir6.2 is an essential subunit of KATP channels.
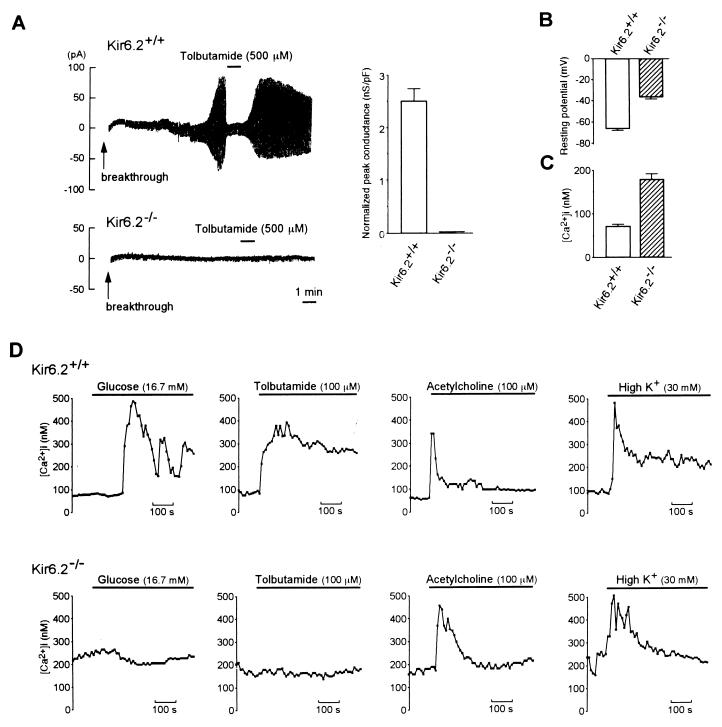
Figure 2.
(A Left) Representative traces of whole-cell recordings of pancreatic beta cells in Kir6.2+/+ and Kir6.2−/−. The holding potential was −70 mV, and alternate voltage pulses of ±10 mV and 200-ms duration every 2 s were applied. In Kir6.2+/+ beta cells (Upper), dialysis of the beta cells intracellularly with the ATP-free pipette solution (breakthrough) caused a progressive increase in K+ conductance, and addition of 500 μM tolbutamide promptly inhibited this conductance. In contrast, no increase in K+ conductance was observed in Kir6.2−/− beta cells (Lower). (A Right) Normalized peak KATP channel conductance of pancreatic beta cells in Kir6.2+/+ and Kir6.2−/−. Since the membrane area of each beta cell varied, ATP-sensitive conductance was normalized by dividing by the membrane capacitance for each cell. The KATP channel conductance in Kir6.2+/+ beta cells was 2.50 ± 0.25 nS/pF (n = 36) and was completely lost in Kir6.2−/− (0 nS/pF, n = 38). (B) Resting membrane potential of Kir6.2+/+ and Kir6.2−/− beta cells. (C) Basal [Ca2+]i in Kir6.2+/+ and Kir6.2−/− beta cells. (D) Effects of various insulin secretagogues on [Ca2+]i in Kir6.2+/+ and Kir6.2−/− beta cells. The secretagogues used were glucose (16.7 mM), tolbutamide (100 μM), acetylcholine (100 μM), and K+ (30 mM). In Kir6.2+/+ beta cells, these secretagogues all increased [Ca2+]i. In contrast, in Kir6.2−/− beta cells, acetylcholine or K+ increased [Ca2+]i, but neither glucose nor tolbutamide increased [Ca2+]i. Horizontal bars indicate application periods of the agents. Representative examples are shown.
The resting membrane potential in Kir6.2−/− beta cells was significantly higher than in Kir6.2+/+ beta cells [−36.1 ± 1.88 mV in Kir6.2−/− (n = 59) and −65.5 ± 1.99 in Kir6.2+/+ (n = 59) (P < 0.001)] (Fig. 2B). In the presence of 2.8 mM glucose, repetitive bursts of action potential were found frequently in Kir6.2−/− beta cells (data not shown). The basal [Ca2+]i at 2.8 mM glucose in single Kir6.2−/− beta cells fluctuated spontaneously, but the mean value of basal [Ca2+]i was elevated significantly in Kir6.2−/− beta cells (Kir6.2−/−, 179.1 ± 13.1 nM, n = 34; Kir6.2+/+, 70.9 ± 4.31, n = 16, P < 0.001) (Fig. 2C). In contrast to Kir6.2+/+ beta cells in which glucose at high concentrations (16.7 mM) induced depolarization of the membrane, 16.7 mM glucose did not alter membrane potential in Kir6.2−/− beta cells (data not shown). These results show the resting membrane potential and basal [Ca2+]i of pancreatic beta cells to be determined primarily by the KATP channels.
Since glucose and tolbutamide close the KATP channels in pancreatic beta cells, causing membrane depolarization and calcium influx, changes in [Ca2+]i in the beta cells in response to various stimuli were examined. Neither 16.7 mM glucose nor 100 μM tolbutamide elicited any change in [Ca2+]i in Kir6.2−/− beta cells (Fig. 2D). This indicates that the rise of [Ca2+]i in normal pancreatic beta cells that is elicited by both glucose and tolbutamide requires closure of the KATP channels. In contrast, 100 μM acetylcholine or 30 mM K+ stimulation increased [Ca2+]i in Kir6.2−/− beta cells (Fig. 2D), to levels comparable to those in Kir6.2+/+ beta cells, confirming that the intracellular calcium mobilization from inositol 1,4,5 triphosphate [Ins(1,4,5)P3]-sensitive Ca2+ stores and Ca2+ influx through voltage-dependent calcium channels are normal in Kir6.2−/− beta cells.
The insulin secretory responses to glucose and tolbutamide were determined in vitro by batch incubation and perifusion of pancreatic islets of adult mice (Fig. 3 A and B). Despite the elevation of basal levels of [Ca2+]i in Kir6.2−/− beta cells, basal levels of insulin secretion (at 2.8 mM glucose) from batch-incubated pancreatic islets in Kir6.2−/− were not different from those in Kir6.2+/+ (Fig. 3A). Neither 16.7 mM glucose nor 100 μM tolbutamide elicited a significant insulin secretion in Kir6.2−/− (Fig. 3A). In contrast, both 100 μM acetylcholine and 60 mM K+ in the presence of 16.7 mM glucose elicited insulin secretion (data not shown).
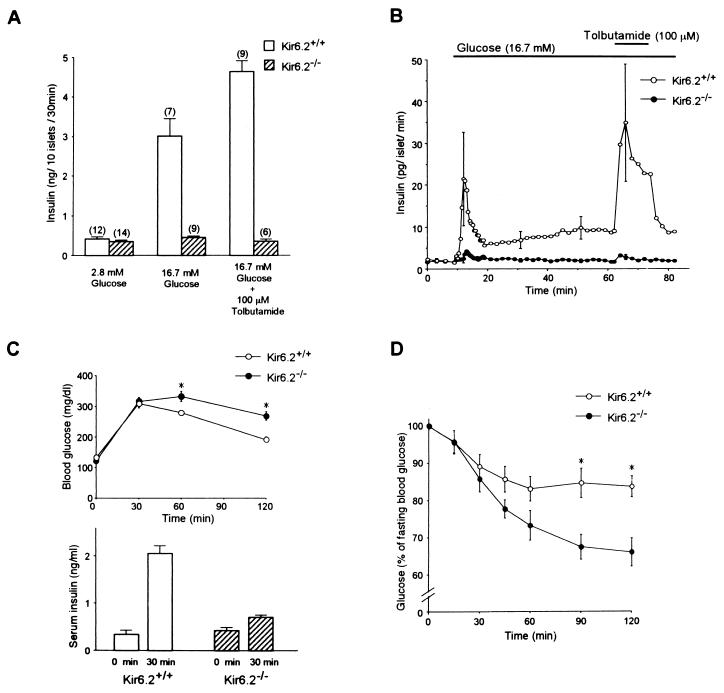
Figure 3.
(A) Insulin secretion in batch-incubated pancreatic islets of Kir6.2+/+ and Kir6.2−/−. There was no significant difference in the basal levels of insulin secretion in the presence of 2.8 mM glucose (Kir6.2+/+, 0.42 ± 0.05 ng/10 islets per 30 min; Kir6.2−/−, 0.35 ± 0.04 ng/10 islets per 30 min). In the islets of Kir6.2+/+, there was a 7.2-fold increase in insulin secretion in response to 16.7 mM glucose, and the secretion was further increased by addition of 100 μM tolbutamide. In contrast, in the islets of Kir6.2−/−, there was no increase in insulin secretion in response to 16.7 mM glucose, and addition of 100 μM tolbutamide did not elicit any insulin secretion. The number of experiments is indicated above each bar. (B) Insulin secretory responses to glucose and tolbutamide in perifused pancreatic islets of Kir6.2+/+ and Kir6.2−/−. There was no significant difference in the basal levels of insulin secretion in the presence of 2.8 mM glucose (Kir6.2+/+, 2.04 ± 0.50 pg/islet per min; Kir6.2−/−, 1.92 ± 0.41 pg/islet per min). Only a trace in the first phase of the insulin secretory response to 16.7 mM glucose was detected in Kir6.2−/−. There was no insulin response in the second phase in Kir6.2−/−. Tolbutamide (100 μM) in the presence of 16.7 mM glucose did not stimulate insulin secretion in Kir6.2−/−. For each experiment, pancreatic islets were isolated from three to five Kir6.2+/+ or Kir6.2−/−. The mean values ± SE were obtained from four and five independent experiments for Kir6.2+/+ and Kir6.2−/−, respectively. Horizontal bars indicate application periods of the agents. (C Upper) Intraperitoneal glucose tolerance test. Fasting blood glucose levels in Kir6.2+/+ and Kir6.2−/− were 132 ± 6 mg/dl (n = 11) and 121 ± 8 mg/dl (n = 11), respectively. The blood glucose levels of Kir6.2−/− 60 and 120 min after glucose load were slightly but significantly higher than those of Kir6.2+/+ (332 ± 16 mg/dl to 279 ± 8 mg/dl at 60 min) (n = 11) (268 ± 14 mg/dl to 192 ± 8 mg/dl at 120 min) (n = 11). ∗, P < 0.01. (C Lower) Insulin response to glucose load. The serum insulin concentrations before and 30 min after glucose load increased from 0.34 ± 0.09 to 2.05 ± 0.17 ng/ml in Kir6.2+/+ (n = 6, P < 0.001) and from 0.42 ± 0.07 to 0.70 ± 0.05 ng/ml in Kir6.2−/− (n = 6, P < 0.01). (D) Insulin tolerance test. The blood glucose levels of Kir6.2−/− (n = 10) 90 and 120 min after glucose load were significantly lower than those of Kir6.2+/+ (n = 10) (67.4 ± 3.4% to 84.7 ± 3.9% at 90 min; 66.0 ± 3.8% to 83.7 ± 2.8% at 120 min). ∗, P < 0.01.
These results show that a rapid rise in [Ca2+]i, rather than a continuous elevation of [Ca2+]i, is important for both glucose-induced and tolbutamide-induced insulin secretion. Only a trace of insulin secretion in response to glucose in the first phase and no secretion at all in the second phase were found in perifused pancreatic islets of Kir6.2−/− (Fig. 3B), suggesting a critical role of the KATP channels in both the first and second phase of glucose-induced insulin secretion. Only a very small insulin response to glucose was found in vivo in Kir6.2−/− (Fig. 3C).
Surprisingly, despite the defect in glucose-induced insulin secretion, glucose tolerance was found to be impaired only slightly in Kir6.2−/− (Fig. 3C). In addition, blood glucose levels at random measurements in Kir6.2−/− (at 12 weeks) were not different from those in age-matched Kir6.2+/+ [Kir6.2−/−, 191 ± 6.4 mg/dl (n = 33); Kir6.2+/+, 188 ± 3.8 mg/dl (n = 29)]. Since an enhanced glucose-lowering effect of insulin might account for these results in Kir6.2−/−, we injected them with a relatively low dose of insulin (0.1 units/kg) and examined changes in blood glucose levels. Indeed, the glucose-lowering effect of insulin is increased significantly in Kir6.2−/−, compared with Kir6.2+/+ (Fig. 3D).
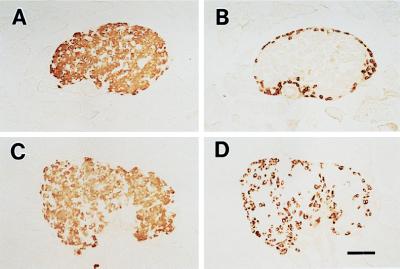
Histological examination shows that glucagon-positive alpha cells, which are present primarily in the periphery in islets of normal mice, appear also in the central region in islets of Kir6.2−/− (Fig. 4). This finding is similar to that in the transgenic (Kir6.2G132S Tg) mice, which we have generated recently by targeted expression of a dominant-negative form of the Kir6.2 subunit in pancreatic beta cells (25).
Figure 4.
Histology of pancreatic islets in Kir6.2+/+ and Kir6.2−/−. Pancreatic beta cells (A and C) and alpha cells (B and D) were stained by using guinea pig antiinsulin and rabbit antiglucagon antibodies, respectively, as described previously (25). In the islets of Kir6.2−/−, glucagon-positive alpha cells (D), which are present in the periphery of the islets of Kir6.2+/+ (B), are seen also in the central region of the islets. The beta cell population in Kir6.2−/− (C) is not different from that in Kir6.2+/+ (A). (Bar = 100 μm.)
Recent studies suggest the importance of KATP channel-independent pathways in glucose-induced insulin secretion (19, 20, 27). In addition, Elliasson et al. (21) have proposed that tolbutamide acts directly on the exocytotic process in pancreatic beta cells. However, we have found in the present study that glucose at high concentrations and tolbutamide even in the presence of high concentrations of glucose does not stimulate insulin secretion in Kir6.2−/−. This indicates that glucose metabolism itself is insufficient for glucose-induced and sulfonylurea-induced insulin secretion, both of which require the rapid rise in [Ca2+]i caused by closure of the KATP channels, and strongly suggests that direct effects of both glucose and sulfonylureas on the exocytotic process are of little physiological significance, or that they are also mediated by the KATP channels, possibly in the secretory granules themselves.
The present data indicate that the disruption of the KATP channels increases insulin-induced glucose disposal in vivo, which could account for the only mild impairment of glucose tolerance seen in Kir6.2−/−. In contrast, Kir6.2G132S Tg mice, in which the beta cell KATP channels are impaired while the skeletal muscle KATP channels are normal, develop marked hyperglycemia as adults (25). Since glucose-induced insulin secretion is defective in both Kir6.2G132S Tg mice and Kir6.2−/−, the difference in the severity of the impairment of glucose tolerance between Kir6.2G132S Tg mice and Kir6.2−/− could be explained at least in part by the difference in insulin action. In addition to stimulating insulin secretion, sulfonylureas have been found to potentiate insulin-stimulated glucose transport in skeletal muscle (28, 29), the major site of glucose disposal (30), suggesting also that the KATP channels in skeletal muscle might be involved in glucose transport. In addition, the responses of glucose-dependent insulinotropic hormones (incretin) such as gastric inhibitory polypeptide (GIP) and glucagon-like peptide 1 (GLP-1) to glucose and meal might be enhanced in Kir6.2−/−, which could also contribute to the prevention of hyperglycemia in these mice.
In conclusion, the KATP channel in pancreatic beta cells is a key regulator of both glucose- and sulfonylurea-induced insulin secretion. Further studies are needed to clarify the mechanism of the enhanced insulin action in Kir6.2−/−, including the insulin-stimulated glucose transport in skeletal muscle.
Acknowledgments
We thank N. Inagaki, H. Yano, M. Dunne, and P. Beguin for helpful advice during the course of the study and Y. Komagata and K. Sakai for technical assistance in generating Kir6.2−/−. This work was supported by Grant-in-Aid for Creative Basic Research from the Ministry of Education, Science, Sports, and Culture and from the Ministry of Health and Welfare, Japan; a grant from Uehara Memorial Foundation; a grant from the Naito Foundation; a grant from Novo Nordisk Pharma Ltd.; a grant for studies on the pathophysiology and complications of diabetes from Tsumura Pharma Ltd.; and a grant from Yamanouchi Foundation for Research on Metabolic Disorders.
ABBREVIATION
- ES
embryonic stem
References
- 1.Noma A. Nature (London) 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 2.Cook D L, Hales C N. Nature (London) 1984;310:271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- 3.Ashcroft F M, Harrison D E, Ashcroft S J H. Nature (London) 1984;312:446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- 4.Spruce A E, Standen N B, Stanfield P R. Nature (London) 1985;316:736–738. doi: 10.1038/316736a0. [DOI] [PubMed] [Google Scholar]
- 5.Standen N B, Quayle J M, Davies N W, Brayden J E, Huang Y, Nelson M T. Science. 1989;245:177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- 6.Amoroso S, Schmid-Antomarchi H, Fosset M, Lazdunski M. Science. 1990;247:852–854. doi: 10.1126/science.2305257. [DOI] [PubMed] [Google Scholar]
- 7.Spanswick D, Smith M A, Groppi V E, Logan S D, Ashford M L J. Nature (London) 1997;390:521–525. doi: 10.1038/37379. [DOI] [PubMed] [Google Scholar]
- 8.Aguilar-Bryan L, Nichols C G, Wechsler S W, Clement J P, IV, Boyd A E, III, González G, Herrera-Sosa H, Nguy K, Bryan J, Nelson D A. Science. 1995;268:423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- 9.Inagaki N, Gonoi T, Clement J P, IV, Wang C-Z, Aguilar-Bryan L, Bryan J, Seino S. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- 10.Isomoto S, Kondo C, Yamada M, Matsumoto S, Higashiguchi O, Hoyio Y, Matsuzawa Y, Kurachi Y. J Biol Chem. 1996;271:24321–24324. doi: 10.1074/jbc.271.40.24321. [DOI] [PubMed] [Google Scholar]
- 11.Inagaki N, Gonoi T, Clement J P, IV, Namba N, Inazawa J, Gonález G, Aguilar-Bryan L, Seino S, Bryan J. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- 12.Sakura H, Ämmälä C, Gribble P A, Ashcroft F M. FEBS Lett. 1995;377:338–344. doi: 10.1016/0014-5793(95)01369-5. [DOI] [PubMed] [Google Scholar]
- 13.Cook D L, Satin L S, Ashford M L J, Hales C N. Diabetes. 1988;37:495–498. doi: 10.2337/diab.37.5.495. [DOI] [PubMed] [Google Scholar]
- 14.Thomas P M, Cote G J, Wohllk N, Haddad B, Mathew P M, Rabl W, Aguilar-Bryan L, Bryan J. Science. 1995;268:426–429. doi: 10.1126/science.7716548. [DOI] [PubMed] [Google Scholar]
- 15.Kane C, Shepherd R M, Squires P E, Johnson P R V, James R F L, Milla P J, Aynsley-Green A, Lindley K J, Dunne M J. Nat Med. 1996;2:1344–1347. doi: 10.1038/nm1296-1344. [DOI] [PubMed] [Google Scholar]
- 16.Nichols C G, Shyng S L, Nestorowicz A, Glaser B, Clement J P, IV, González G, Aguilar-Bryan L, Permutt M A, Bryan J. Science. 1996;272:1785–1787. doi: 10.1126/science.272.5269.1785. [DOI] [PubMed] [Google Scholar]
- 17.Dunne M J, Kane C, Shepherd R M, Sanchez J A, James R F L, Johnson P R V, Aynsley-Green A, Lu S, Clement J P, IV, Lindley K J, et al. N Engl J Med. 1997;336:703–706. doi: 10.1056/NEJM199703063361005. [DOI] [PubMed] [Google Scholar]
- 18.Nestorowicz A, Inagaki N, Gonoi T, Schoor K P, Wilson B A, Glaser B, Landau H, Stanley C A, Thornton P S, Seino S, et al. Diabetes. 1997;46:1743–1748. doi: 10.2337/diab.46.11.1743. [DOI] [PubMed] [Google Scholar]
- 19.Gembal M, Detimary P, Gilon P, Gao Z Y, Henquin J C. J Clin Invest. 1993;91:871–880. doi: 10.1172/JCI116308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komatsu M, Schermerhorn T, Aizawa T, Sharp G W G. Proc Natl Acad Sci USA. 1995;92:10728–10732. doi: 10.1073/pnas.92.23.10728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eliasson L, Renström E, Ämmälä C, Berggren P O, Bertorello A M, Bokvist K, Chibalin A, Deeney J T, Flatt P R, Gäbel J, et al. Science. 1996;271:812–815. doi: 10.1126/science.271.5250.813. [DOI] [PubMed] [Google Scholar]
- 22.Terizic A, Jahangir A, Kurachi Y. Am J Physiol. 1995;269:C525–C545. doi: 10.1152/ajpcell.1995.269.3.C525. [DOI] [PubMed] [Google Scholar]
- 23.Heurteaux C, Lauritzen I, Widmann C, Lazdunski M. Proc Natl Acad Sci USA. 1995;92:4666–4670. doi: 10.1073/pnas.92.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wollheim C, Meda P, Halban P A. In: Methods in Enzymology. Fleischer S, Fleischer B, editors. Vol. 192. San Diego: Academic; 1990. pp. 188–223. [DOI] [PubMed] [Google Scholar]
- 25.Miki T, Tashiro F, Iwanaga T, Nagashima K, Yoshitomi H, Aihara H, Nitta Y, Gonoi T, Inagaki N, Miyazaki J-I, et al. Proc Natl Acad Sci USA. 1997;94:11969–11973. doi: 10.1073/pnas.94.22.11969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okamoto Y, Ishida H, Taminato T, Tsuji K, Kurose T, Tsuura Y, Kato S, Imura H, Seino Y. Diabetes. 1992;41:1555–1561. doi: 10.2337/diab.41.12.1555. [DOI] [PubMed] [Google Scholar]
- 27.Takasawa S, Nata K, Yonekura H, Okamoto H. Science. 1993;259:370–373. doi: 10.1126/science.8420005. [DOI] [PubMed] [Google Scholar]
- 28.Wang P H, Moller D, Flier J S, Nayak R C, Smith R J. J Clin Invest. 1989;84:62–67. doi: 10.1172/JCI114170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pulido N, Casla A, Suarez A, Casanova B, Arrieta F J, Rovira A. Diabetologia. 1996;39:22–27. doi: 10.1007/BF00400409. [DOI] [PubMed] [Google Scholar]
- 30.Simpson I A, Cushman S W. Annu Rev Biochem. 1986;55:1059–1089. doi: 10.1146/annurev.bi.55.070186.005211. [DOI] [PubMed] [Google Scholar]