Abstract
Background
Liver transplantation (LT) from Donation after Cardiac Death (DCD) donors is increasingly being used to address organ shortages. Despite encouraging reports, standard survival metrics have overestimated the effectiveness of DCD livers. We examined the mode, kinetics and predictors of organ failure and resource utilization to more fully characterize outcomes after DCD LT.
Methods
We reviewed the outcomes for 32 DCD and 237 Donation after Brain Death (DBD) LT recipients at our institution.
Results
Recipients of DCD livers had a 2.1 times greater risk of graft failure, a 2.5 times greater risk of re-listing, and a 3.2 times greater risk of re-transplantation compared to DBD recipients. DCD recipients had a 31.6% higher incidence of biliary complications and a 35.8% higher incidence of ischemic cholangiopathy (IC). IC was primarily implicated in the higher risk of graft failure observed after DCD LT. DCD recipients with IC experienced more frequent re-hospitalizations, longer lengths of stay, and required more invasive biliary procedures.
Conclusions
Related to higher complication rates, DCD recipients necessitated greater resource utilization. This more granular data should be considered in the decision to promote DCD LT. Modification of liver allocation policy is necessary to address those disadvantaged by a failing DCD graft.
Introduction
The supply of standard criteria donors does not meet the current needs for liver transplantation (LT). Critical organ shortages have led to the implementation of initiatives designed to increase the donor pool [1]. Extended criteria or “marginal” organs are increasingly utilized to reduce the discrepancy between the supply and demand of transplantable livers [2]. These include organs procured from deceased donors where donation occurs after cardiac death (DCD), as opposed to donation after brain death (DBD).
The National Transplant Collaborative and regulations from the Centers for Medicare and Medicaid Services (CMS) have mandated an increase in the number of DCD donors [3]. Organ Procurement Organizations (OPOs) have responded by increasing their efforts to promote DCD donation within their local donor hospitals [4]. As a result, the rate of DCD LT in the United States has grown more than ten-fold over the past decade [2, 5].
Early anecdotal reports touted the use of DCD livers [6, 7]. Unfortunately, these encouraging reports may have overstated the effectiveness of these organs. Conflicting data exists regarding inferior outcomes after DCD LT [8, 9]. Analysis of national [10] and single institution data [8, 11–14] have failed to identify significantly worse patient survival for DCD recipients. In contrast, worse graft survival has been widely reported [5, 9, 15–17]. Moreover, a higher incidence of biliary complications with DCD LT, most notably ischemic cholangiopathy (IC), has been described [9, 11, 12, 18, 19]. However, the full scope and burden of these complications upon the patient and health care system remain poorly characterized.
The purpose of this study was to analyze outcomes after DCD LT beyond patient and graft survival including the mode, kinetics, and predictors of organ failure necessitating re-listing and re-transplantation (re-LT). Also, we have provided a unique assessment of the impact of IC by evaluating long-term resource utilization which can negatively influence DCD recipients' quality of life.
Patients and Methods
After Institutional Review Board approval, we performed a retrospective review of LT performed at Northwestern Memorial Hospital between December, 2003 and May, 2008. Adult (age ≥ 18 years) deceased donor (DCD and DBD) LT recipients were included in the analysis. Live donor, split, multi-organ and re-LT were excluded. Donor and recipient parameters were collected for comparison. Primary outcome measures included patient and graft survival, rates of re-listing and re-LT, and vascular and biliary complications. IC was defined as diffuse intra-hepatic strictures identified at endoscopic retrograde percutaneous cholangio-pancreaticography (ERCP) or percutaneous transhepatic cholangiography (PTC) without concomitant hepatic artery thrombosis (HAT). Secondary outcomes included hospital and intensive care unit (ICU) length of stay (LOS), disposition at discharge, readmissions (at 30 days, 90 days, and 1 year post-transplant), and utilization of ERCP and PTC (performed in the first 2 years post-transplant). We also divided our experience into an early (2003–2005) and a later cohort (2006–2008) to assess the impact of experience with the DCD procurement procedure. Furthermore, we analyzed United Network for Organ Sharing (UNOS) data for a cohort of DCD LT recipients categorized by OPO (<50 to those with ≥50) to evaluate the impact of volume.
Organ Procurement and Preservation
All DCD organ retrievals occurred in a “controlled” manner. Withdrawal of ventilatory support took place in either the ICU or operating room, and subsequent death was declared after cessation of cardiopulmonary activity. This was followed by a mandatory 5 minute waiting period. Timing of systemic heparinization varied according to the procurement hospital's DCD protocol. All DCD procurements were performed by two surgeons. A midline sternotomy and laparotomy were created, and the aorta and superior mesenteric vein were cannulated for infusion of cold University of Wisconsin (UW) solution (Histidine-tryptophan-ketoglutarate (HTK) was used in 3 donors). After initiation of the cold flush, the organs were surface cooled with ice, and the aorta was then cross-clamped in the chest. The organs were rapidly removed from the abdomen, and the bile duct was cannulated and flushed with cold UW solution.
We used several parameters in order to decide whether the liver would be transplanted. First, warm ischemia could not exceed 30 minutes. Warm ischemia time was measured from drop in systolic blood pressure to < 50 mmHg or oxygen saturation <70% to cannulation and cold perfusion. Incision to cannulation time rarely exceeded 3 minutes. Second, we only accepted livers that flushed completely within 2–3 liters of preservation solution. Some livers appeared to have a high vascular resistance requiring 5–10 liters to obtain a clear effluent; these livers were not transplanted. Finally the same criteria used for DBD livers were applied in terms of medical history, laboratory values, and the appearance of the liver, both gross and microscopic. All patients were provided full informed consent including the fact that they were receiving a DCD liver.
Statistical Analyses
Student's t-test and chi-square tests were used to assess differences in donor and recipient parameters between DCD and DBD LT. Kaplan-Meier analysis with the log-rank test was used to compare patient and graft survival, re-list rates, and re-LT rates. Complications, readmission rates, LOS, utilization of biliary procedures, and other secondary outcomes were analyzed utilizing Mann Whitney U or Fisher's exact test. In addition, Cox proportional hazards models were utilized to evaluate predictors of graft failure. Factors first identified according to bivariate analysis (p<0.10) were evaluated in a multivariate model. Patient survival was evaluated according to the same multivariate analysis. Logistic regression was utilized to evaluate predictors of ischemic cholangiopathy. All analyses were conducted using STATA/SE 10.1 (StataCorp, College Station, TX). A p-value <0.05 was considered statistically significant.
Results
Donor and Recipient Characteristics
From December 2003 to May 2008, 32 DCD and 237 DBD LT were performed at our center. There were no significant differences in donor age, gender, race, or body mass index (BMI) between DCD and DBD donors (Table 1). The average donor age was 43.1 (± 17.6) for DCD and 44.7 (± 17.6) for DBD donors (p=0.63). Donor cause of death was different between the DCD and DBD groups (p<0.006). In particular, DCD donors less frequently had cerebrovascular accident (CVA; DCD 25% versus DBD 42%) and more frequently had “other” (DCD 21.9% versus DBD 5.5%) listed as the donor cause of death. Additionally, the usage of vasopressor agents was lower among DCD donors (DCD 9.4% versus DBD 74.7%; p<0.0001). Average warm ischemia time was 15.1 ± 4.6 minutes; none exceeded 30 minutes. The cold ischemia time was comparable for DCD (5.5 ± 1.5 hours) and DBD (5.2 ± 1.5 hours) LT (p=0.25). There were no differences in rates of regional and national sharing. Moreover, overall graft quality once DCD was excluded from the donor risk index (DRI)[20] calculation was comparable (DCD 1.41 ± 0.39 versus DBD 1.46 ± 0.37, p=0.43).
Table 1.
Donor characteristics for DCD and DBD livers.
| DCD (n=32) | DBD (n=237) | p-value | |
|---|---|---|---|
| Age (mean ± SD) | 43.1 ± 17.6 | 44.7 ± 17.6 | 0.63 |
| Male (n, %) | 20 (62.5%) | 132 (55.7%) | 0.47 |
| Race (n, %) | 0.13 | ||
| Non-Hispanic White | 24 (75.0%) | 130 (54.9) | |
| Non-Hispanic Black | 5 (15.6%) | 75 (31.6%) | |
| Hispanic | 2 (6.3%) | 27 (11.4%) | |
| Cause of Death (n, %) | 0.006 | ||
| Anoxia | 6 (18.8%) | 40 (16.9%) | |
| CVA | 8 (25.0%) | 100 (42.2%) | |
| Head trauma | 11 (34.4%) | 84 (35.4%) | |
| Other* | 7 (21.9%) | 13 (5.5%) | |
| Body mass index (mean ± SD) | 27.7 ± 6.5 | 26.6 ± 5.8 | 0.31 |
| Creatinine (mean ± SD) | 1.3 ± 1.6 | 1.8 ± 1.9 | 0.15 |
| Cold ischemia time (hours, mean ± SD) | 5.5 ± 1.5 | 5.2 ± 1.5 | 0.25 |
| Warm ischemia time (minutes, mean ± SD) | 15.8 ± 4.8 | NA | |
| Sharing | 0.23 | ||
| Local | 26 (81.3%) | 211 (89.0%) | |
| Regional | 6 (18.8%) | 23 (9.7%) | |
| National | 0 (0.0%) | 3 (1.3%) |
Cause of death was not trauma, stroke, or anoxia.
There were no significant differences in any recipient characteristics for DCD and DBD recipients (Table 2). The mean recipient age was 53.1 (± 12.8) years in the DCD group and 54.9 (± 10.0) in the DBD group (p=0.44). The average Model for End-Stage Liver Disease (MELD) scores were 22.9 (± 10.2) and 21.9 (± 10.1) among DCD and DBD recipients, respectively (p=0.57).
Table 2.
Recipient characteristics of DCD and DBD liver transplants.
| DCD (n=32) | DBD (n=237) | p-value | |
|---|---|---|---|
| Age (mean ± SD) | 53.1 ± 12.8 | 55.0 ± 10.0 | 0.44 |
| Male (n, %) | 26 (81.3%) | 157 (66.2%) | 0.09 |
| Race (n, %) | 0.50 | ||
| Non-Hispanic White | 29 (90.6%) | 185 (78.1%) | |
| Non-Hispanic Black | 1 (3.1%) | 24 (10.1%) | |
| Hispanic | 2 (6.3%) | 20 (8.4%) | |
| UNOS MELD (mean ± SD) | 26.56 ± 8.02 | 26.37 ± 6.29 | 0.90 |
| Calculated MELD (mean ± SD) | 22.94 ± 10.19 | 21.85 ± 10.12 | 0.57 |
| Etiology (n, %) | 0.45 | ||
| Fulminant | 3 (9.4%) | 10 (4.2%) | |
| Non-cholestatic | 19 (59.4%) | 125 (52.7%) | |
| Cholestatic | 2 (6.3%) | 16 (6.8%) | |
| Metabolic | 1 (3.1%) | 5 (2.1%) | |
| Malignant neoplasm | 7 (21.9%) | 80 (33.8%) | |
| Body mass index (mean ± SD) | 27.9 ± 5.2 | 29.5 ± 6.5 | 0.18 |
| HepCAb+ (n, %) | 15 (46.9%) | 115 (48.5%) | 0.86 |
| Hepatocellular carcinoma (n, %) | 10 (31.3%) | 88 (37.1%) | 0.52 |
| Medical condition (n, %) | 0.74 | ||
| ICU | 2 (6.3%) | 26 (11.0%) | |
| Hospital | 6 (18.8%) | 36 (15.2%) | |
| Not in hospital | 24 (75.0%) | 175 (73.8%) |
Post-Transplant Outcomes
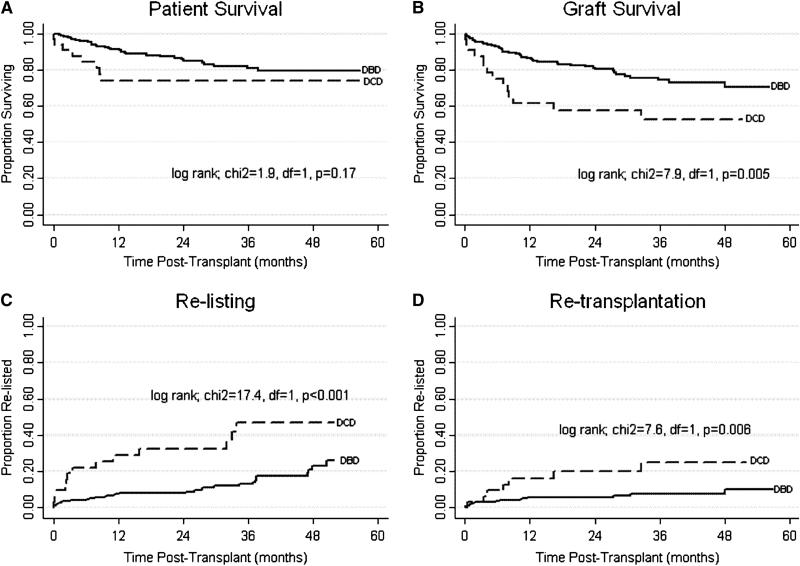
Patient survival (Figure 1A) for DCD and DBD recipients was not statistically different (p=0.17). One-year patient survival was 74.0% for DCD and 90.4% for DBD and three-year 74.0% for DCD and 80.7% for DBD. Graft survival (Figure 1B), however, was lower after DCD LT. One and three-year graft survival was 61.3% and 52.6% for DCD recipients and 85.2% and 74.2% for DBD recipients (p=0.005). Furthermore, we observed strikingly high re-list (40.6% versus 16.0%; p=0.003) and re-LT rates (21.9% versus 6.8%; p=0.01) among DCD compared to DBD recipients (Table 3). DCD livers exhibited a 2.1 times greater risk of graft failure, a 2.5 times greater risk of re-listing, and a 3.2 times greater risk of re-LT compared to DBD livers.
Figure 1A-1D.
Kaplan-Meier analysis: patient survival, graft survival, re-list and re-transplantation for DCD ans DBD liver transplanation.
Table 3.
Complications and natural history after DCD and DBD liver transplantation
| DCD (n=32) | DBD (n=237) | p-value | |
|---|---|---|---|
| Primary non-function (n, %) | 1 (3.1%) | 1 (0.4%) | 0.22 |
| Vascular complications (n, %) | |||
| (HAT, HAS, PVT) | 6 (18.8%) | 21 (8.9%) | 0.11 |
| Hepatic artery thrombosis (n, %) | 3 (9.4%) | 7 (3.0%) | 0.10 |
| Biliary complications (n, %) | 17 (53.1%) | 51 (21.5%) | <0.001 |
| Ischemic Cholangiopathy (n, %) | 12 (37.5%) | 4 (1.7%) | <0.001 |
| Re-list (n, %) | 13 (40.6%) | 38 (16.0%) | 0.003 |
| Re-transplant (n, %) | 7 (21.9%) | 16 (6.8%) | 0.01 |
| Transplant to re-list (days) (mean ± SD) | 167 ± 115 | 266 ± 315 | 0.14 |
| Transplant to ERCP (days) (mean ± SD) | 79 ± 57 | -- | -- |
| ERCP to re-list (days) (mean ± SD) | 88 ± 119 | -- | -- |
| Re-list to re-transplant (days) (mean ± sd) | 22 ± 17 | 68 ± 124 | 0.25 |
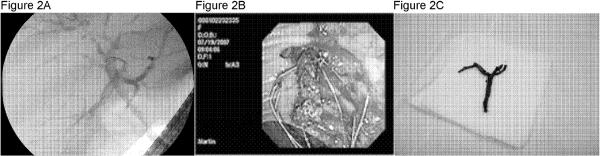
There were no significant differences in the rates of primary non-function (PNF) or vascular complications which included HAT, hepatic artery stenosis (HAS) and portal vein thrombosis (PVT) (p=NS). The majority of re-listing (69.2%) and re-LT (71.4%) in the DCD group were a consequence of biliary complications. The development of biliary complications was more prevalent among DCD (53.1%) compared to DBD (21.5%) recipients (p<0.001). IC was identified in 12 (37.5%) DCD, but only 4 (1.7%) DBD recipients (p<0.001). Figure 2 illustrates an ERCP from a patient with persistent fevers and elevated liver function tests six months after DCD LT. Figure 2A depicts a cholangiogram with pruning and beading of the biliary tree consistent with IC. Figure 2B shows the endoscopic retrieval of a typical occlusive bile duct cast which is then shown ex vivo in figure 2C.
Figure 2.
illustrates a representative example of an endoscopic retrograde cholangio-pancreatogram performed on a patient with persistend fevers and elevated liver function teste 6 months post transplant. Figure 2A depicts the cholangiogram showing diffuse ischemic cholangiogram. Figure 2B shows the endoscopic of an occlusive bile duct cast which is then shown ex vivo in figure 2C.
The natural history of DCD livers demonstrates a rapid deterioration (Table 3), characterized by a mean of 79 days from transplant to first ERCP, 88 days from first ERCP to re-listing, and 22 days from re-listing to re-LT. The mean transplant to re-list time was 167 days for DCD compared to 266 days for DBD livers; the mean time from re-listing to re-LT was 22 days for DCD compared to 68 days for DBD livers. Although there was a tendency for DCD recipients to more quickly progress to re-listing and re-LT, neither interval reached statistical significance. The average MELD scores at re-listing and re-LT were lower for DCD (25.7 ± 8.9 and 20.6 ± 6) compared to DBD (31.3 ± 8 and 26.7 ± 5.5) recipients, respectively (re-list p=0.09 and re-LT p=0.03). Consequently, the DRI scores of grafts at re-LT were higher for the DCD group, but the difference was not statistically significant (DCD 1.56 ± 0.74 versus DBD 1.36 ± 0.38; p=0.45).
Resource Utilization
Next, we evaluated post-transplant LOS, re-admission rates and frequency of invasive biliary procedures (Table 4). There was a trend towards a greater proportion of patients re-admitted within 30 and 90 days post-transplant among DCD (34% and 47%, respectively) compared to DBD (28% and 36%, respectively) recipients (p=0.25 and 0.53). Similarly, the number of re-admissions in the first year tended to be higher in the DCD group (1.6 versus 1.1; p=0.09). The average LOS for readmissions was 5.1 days for DCD and 3.4 days for DBD patients (p=0.09). Of the 21 DCD recipients who required re-admission, 13 (61.9%) were related to biliary complications. We found significant increases in the re-admission rates (IC 2.6 versus No IC 1.1; p<0.001) and LOS (IC 7.4 days versus No IC 3.2 days; p<0.01) among patients with IC.
Table 4.
Resource utilization after DCD and DBD liver transplantation
| DCD (n=32) | DBD (n=237) | p-value | |
|---|---|---|---|
| Total length of stay after transplant (days) (mean ± sd) | 7.6 ± 4.0 | 7.8 ± 7.9 | 0.11 |
| ICU length of stay after transplant (days) (mean ± sd) | 3.5 ± 3.4 | 4.0 ± 7.6 | 0.20 |
| Disposition | 0.56 | ||
| Home (n, %) | 22 (68.8%) | 171 (72.2%) | |
| Skilled nursing facility (n, %) | 4 (12.5%) | 35 (14.8%) | |
| Acute care facility (n, %) | 4 (12.5%) | 26 (11.0%) | |
| Dead | 2 (6.3%) | 5 (2.1%) | |
| 30 day readmissions (n, %) | 11 (34.4%) | 67 (28.3%) | 0.48 |
| 90 day readmissions (n, %) | 15 (46.9%) | 85 (35.9%) | 0.25 |
| Readmissions in first year (mean ± sd) | 1.6 ± 1.9 | 1.1 ± 1.5 | 0.09 |
| Length of stay for readmissions (days) (mean ± sd) | 5.1 ± 7.5 | 3.2 ± 5.3 | 0.09 |
| Patients undergoing ERCP/PTC within 6 mo.s (n, %) | 15 (50.0%) | 49 (20.9%) | 0.001 |
| Procedures (ERCP/PTC) per year (mean ± sd) | 1.0 ± 2.2 | 0.4 ± 0.7 | 0.04 |
| IC (N=16) | No IC (N=250) | p-value | |
|---|---|---|---|
| Readmissions in first year (mean ± sd) | 2.6 ± 2.1 | 1.1 ± 1.5 | 0.0007 |
| Length of stay for readmissions (days) (mean ± sd) | 7.4 ± 9.1 | 3.2 ± 5.3 | 0.003 |
| Procedures (ERCP/PTC) per year (mean ± sd) | 2.4 ± 2.8 | 0.3 ± 0.6 | <0.0001 |
Moreover, the proportion of DCD recipients requiring invasive biliary procedures within 6 months post-transplant was higher (DCD 50% versus DBD 21%, p=0.001). The average number of procedures per year was 1.0 ± 2.2 for DCD recipients compared with 0.4 ± 0.7 for DBD recipients (p=0.04). Those patients with IC underwent an average of 2.4 (range 1.0–10.5) biliary procedures per year during the initial two years post-transplant.
Predictors of Graft Failure, Patient Survival and Ischemic Cholangiopathy
DCD was the primary predictor of graft failure (HR 2.45; 95%CI 1.35–4.44). Four other recipient factors (age, African-American race, Hepatocellular carcinoma (HCC), and MELD score) were also marginally predictive of graft failure in bivariate analysis (p<0.10). In a multivariate model, only DCD (HR 2.64, 95% CI 1.43–4.88) and recipient age (HR 1.35 per 10 year increase, 95%CI 1.04–1.75) remained significant predictors of graft failure. Factors leading to patient survival were analyzed according to the same multivariate adjustment. DCD was not predictive of patient survival in bivariate (p=0.17) or multivariate analysis (p=0.12). Only recipient age remained a predictor of patient survival (HR 1.50 per 10 year increase; 95%CI 1.09–2.06) in multivariate analysis.
We then examined predictors of IC using logistic regression modeling. Donor age >40 was associated with a 9.3 increased odds of IC (95% CI 1.2–71.6, p=0.03). We also evaluated the relationship between donor and recipient height representing a possible size mismatch. Specifically, donor height / recipient height × 100 had an OR 0.91 (95% CI 0.84–0.98, p=0.03). In other words, there is a 9% reduction in the risk of IC conferred for each unit increase in the percentage of donor height divided by recipient height.
Impact of OPO/Transplant Center Experience
To study the impact of experience, we examined if patient and graft outcomes with DCD livers differed between the earlier (2003–2005, n=18) and later cohorts (2006–2008, n=14). Patients in both cohorts demonstrated similar patient and graft survival, re-list, and re-LT rates (data not shown). We also evaluated UNOS data categorized by OPO with <50 and ≥50 DCD transplants excluding our center (n=1,019 for DCD and n=19,260 for DBD). The UNOS data demonstrated that patient and graft survival rates were similar regardless of OPO volume (data not shown).
Discussion
National initiatives aimed at augmenting organ donation rates in the United States have succeeded in increasing both the number of donors as well as the number of organs transplanted per donor, but increases in standard criteria donors appear to have reached a plateau [2]. LT continues to face a substantial imbalance between organ supply and demand. In 2003, our OPO began heavily promoting DCD donors and subsequently more DCD livers became available. We observed an increase in DCD livers in the context of a relative decline in DBD livers. Our institution adopted a policy whereby DCD livers were offered to patients with MELD scores high enough to warrant LT, but too low to reach priority on the waiting list. Additionally, DCD livers were offered to patients with tumors beyond Milan criteria without living donors.
Initially, increased rates of PNF were a major concern after DCD LT. Our results and others [7–9, 21] have confirmed that the PNF rate after DCD LT is comparable to DBD LT. Likewise, we have demonstrated a similar incidence of vascular complications for DCD and DBD recipients consistent with other published series [8, 12, 21, 22].
Our data exhibit inferior 1 year-patient (74.0% vs. 90.4%) and graft (61.3% vs. 85.2%) survival of DCD compared to DBD livers. Similarly, Foley et al. reported significantly reduced patient and graft survival after DCD LT in their analysis of 36 DCD and 553 DBD liver recipients at the University of Wisconsin [9]. Furthermore, several analyses of the UNOS database have documented significantly worse graft survival for DCD livers [5, 10, 16, 17, 23].
In contrast, other reports have failed to identify a significant reduction in patient and graft survival among DCD livers [8, 11, 12]. For instance, Fujita et al. showed comparable survival between 24 DCD and 1,209 DBD LT [8]. Moreover, Abt et al. reported favorable patient and graft survival at 1 and 3 years for 15 DCD LT performed at the University of Pennsylvania [11]. In addition, Chan et al. found no difference in patient or graft survival for 52 LT from DCD donors [12]. As such, survival metrics for DCD compared to DBD livers have remained a contentious issue. However, single institution studies, while important, are marred by limited sample sizes restricting their power to detect differences in survival. Moreover, the generalizability of these findings to other OPOs and/or transplant centers remains questionable given the variability in policies and procedures employed across the country.
Another concern regarding the utilization of DCD livers involves the high incidence of biliary complications. In our cohort, biliary complications were directly implicated in the reduced graft survival observed for DCD livers. Similarly, D'Alessandro et al. documented a substantial rate of biliary strictures in DCD livers in their study [15]. Another report from Maheshwari et al. identified up to a 60% biliary complication rate among twenty recipients of DCD livers [18]. The incidence of biliary complications was markedly lower (15.5%) among DBD livers. Most strikingly, the majority of DCD recipients who experienced biliary complications required repeat interventions or re-LT [18]. Interestingly, Abt et al. [11] and Chan et al. [12] both have demonstrated an increased incidence of biliary complications amongst DCD livers in the face of practically identical patient and graft survival. Arguably, the impact of biliary complications following DCD LT can be profound, and yet not immediately reflected by patient or graft survival. Hence, the use of survival metrics alone to determine the efficacy of DCD LT may be inadequate. A more rigorous examination of the impact of biliary complications is essential.
The impact of biliary complications in general and IC in particular on the need for re-listing and re-LT is profound. Herein, we have noted that DCD LT was associated with a 40.6% re-list rate and a 21.9 % re-LT rate. IC was the reason for re-listing and re-LT in 69.2% and 71.4% of patients respectively. Moreover, DCD recipients who developed IC (79 days from transplant to diagnosis) experienced a fairly abrupt clinical deterioration (167 days to re-listing) necessitating early re-transplant (22 days from re-list to re-transplant). Selck et al. identified a similar early (within 180 days) failure pattern of DCD livers in their study [23]. After LT the development of IC is most commonly precipitated by the occlusion of hepatic arterial flow [24]. However, after DCD LT, warm ischemia, preservation and reperfusion injury involving the peribiliary plexus have been implicated. Endothelial activation triggers a cascade of events leading to microvascular thrombosis and ischemia resulting in stricture formation, biliary necrosis and cholangitis culminating in progressive graft failure. In this analysis we have reported IC rates based upon only those patients without concomitant HAT. In our DCD cohort one recipient developed IC related to HAT and was excluded from the analysis.
Although there appears to be a dichotomous approach to the management of IC after DCD LT among transplant centers, the decision to re-list and ultimately re-transplant patients in our experience seems justified by the severity of their liver disease. Notably, the average MELD scores at re-listing and re-LT were substantial for DCD recipients (MELD 25.6 and 20.6 respectively). These MELD scores correspond to a substantial (~10%) 90 day mortality risk on the wait-list [3]. Nonetheless, MELD scores at re-LT were lower for DCD compared to DBD recipients. Our data and others' [23] have identified substantial impediments to re-LT. Despite our institutional practice to avoid re-LT with a DCD graft, DCD recipients who required re-LT received grafts with a relatively higher DRI (DCD DRI = 1.56 ± 0.74 versus DBD DRI 1.36 ± 0.38; p=0.45). However, mortality after re-LT was comparable between DCD and DBD recipients (data not shown). Similarly, those with IC did not experience worse survival after re-LT. Taken together, modification of liver allocation policy is necessary to address those disadvantaged by a failing DCD graft. A priori allocation of DCD livers to “desperate” patients (low MELD with significant disease burden) would seem prudent. Additionally, allowances for MELD score upgrades which more accurately reflect the mortality and morbidity of a failing DCD liver should be considered.
No study to date has measured quality of life in recipients of DCD livers or patients with IC. It is our contention that recipients of DCD livers who develop IC experience poor quality of life and pose a significant burden to the health care system. Based on our observations that DCD recipients necessitated frequent re-admissions, repeated biliary tract instrumentation and chronic suppressive anti-microbial therapy, we hypothesized that these factors are associated with worse quality of life. While the economic impact of the development of IC after DCD LT has not been delineated, biliary complications clearly drive up the cost of LT [25]. Not surprisingly, increases in readmissions, lengths of stay, and rates of ERCP/PTC were even more significant among patients with IC. These factors are not explicitly reflected in the typical survival metrics, yet they have significant economic and quality of life implications.
We hypothesized that DCD liver transplant outcomes would improve with experience. In our dataset, we compared outcomes from an early (2003–2005) and late (2006–2008) cohort. We saw no differences in patient or graft survival and in re-list or re-transplant rates between the two time periods. Furthermore, we evaluated UNOS data categorized by OPO divided into those with <50 and ≥50 DCD cases. We found no differences between high or low-volume OPOs in any of the defined outcomes at one-year. This suggests that increasing experience does not correlate with improved outcomes for DCD LT.
Our data and others [10, 12, 13, 17, 20, 23, 26] have identified multiple donor and recipient variables conferring greater risk following DCD LT. DCD (HR 2.64) was highly predictive of graft failure in the multivariate analysis. In addition, recipient age (HR 1.35 per 10 year increase) predicted graft failure. DCD was not a predictor of patient survival. Donor age >40 (OR 9.3) and donor to recipient height ratio (OR 0.91) were predictors of IC. In our experience, cold (<8 hours) and warm (<30 min) ischemia times were kept well below the parameters that have been implicated in graft failure [10, 13, 17, 26] and the development of IC [12]. Moreover, we identified no difference in graft survival in DCD livers with <15 minutes and ≥15 minutes of warm ischemia time (data not shown). While the majority of DCD livers in our cohort were procured utilizing UW solution (n=29), 3 were procured with HTK. We found no association between preservative solution and graft failure or IC in our limited sample size. However, a recent report has demonstrated inferior graft survival with use of HTK preservation of DCD livers [26].
Our data and other studies suggest that the optimal use of DCD livers might involve the application of stringent limits. Specifically, donor age >35–45 has been associated with increased risk of graft failure and IC [9, 13, 17]. Warm and cold ischemia times should be limited to <15–30 minutes and <10 hours respectively [10, 11, 13, 17]. Our analysis also demonstrated the importance of graft size as approximated by donor height. We observed that smaller donors and more notably larger discrepancies between donor and recipient height were associated with a greater risk for IC. Our hypothesis is that small DCD livers are more prone to microvascular thrombosis. Failure of the peribiliary microcirculation exaggerates ischemia-reperfusion injury of the sensitive biliary epithelium causing stricture formation. In contrast, Chan and colleagues identified ischemic cholangiopathy in 3 of 4 donors >100 kg. However, steatosis was implicated in 2 of these. In our series, steatotic livers were not transplanted and 5 of 8 DCD recipients from donors >100 kg did not develop IC [12]. The question of graft size as estimated by donor height or weight requires further clarification from a larger dataset. Nevertheless, by limiting selection of DCD livers according to these criteria, risks of IC and graft failure may be minimized.
Limitations
This study has several limitations. First, it is not a randomized study and therefore our results may be affected by unmeasured selection biases. Second, the “quality” of DCD grafts may vary as a consequence of unmeasurable factors due to differing OPO, procurement hospital, and transplant center policies. These variables include whether donors are extubated in the operating room or elsewhere, the time period of inadequate perfusion and oxygenation of the donor organs following cessation of life-support, the time of heparinization, and many other variables that are exceedingly difficult to control. Differences in institutional and OPO policies and practice raise further questions regarding generalizability. Furthermore, we recognize fully that there may be recipient selection bias in allocating DCD and DBD livers affecting post-transplant outcomes. Nonetheless, we feel that the observations provided in this study are both valuable and important particularly in the current heightened environment of disclosure and transparency.
Conclusions
Our findings suggest that DCD livers should not be viewed as equivalent to DBD livers. Any effort to promote increases in DCD livers should include precautions against cannibalization of DBD livers. Rates of re-listing, re-transplant and resource utilization approximate the incidence of chronic graft problems and IC but also delineate the burden incurred upon patients and society. We view frequent and prolonged hospitalizations, requirement for multiple endoscopic/percutaneous interventions, and chronic suppressive antimicrobial therapy as an unacceptable outcome after LT. Modification of current liver allocation policy to address the shortcomings of mortality and morbidity associated with failing DCD livers are justified. Furthermore, application of stringent selection criteria can guide utilization of these marginal grafts without compromising patient outcomes.
Table 5.
Bivariate and multivariate analysis of graft and patient survival.
| Variable | Bivariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR [exp(β)] | 95% CI | p-value* | HR [exp(β)] | 95% CI | p-value* | |
|
Graft Survival |
||||||
| DCD | 2.45 | 1.35–4.44 | 0.007 | 2.64 | 1.43–4.88 | 0.002 |
| Recipient age (per 10 years) | 1.25 | 0.97–1.62 | 0.09 | 1.35 | 1.04–1.75 | 0.02 |
| Recipient race/ethnicity | ||||||
| White (reference) | 1.00 | NA | NA | 1.00 | NA | NA |
| African-American | 2.06 | 0.97–4.37 | 0.06 | 2.19 | 0.99–4.81 | 0.051 |
| Other (hispanic and asian) | 0.86 | 0.31–2.39 | 0.78 | 0.65 | 0.24–1.82 | 0.42 |
| Hepatocelluar carcinoma | 0.62 | 0.36–1.07 | 0.08 | 0.60 | 0.30–1.22 | 0.16 |
| MELD (calculated) | 1.02 | 0.96–1.04 | 0.10 | 1.004 | 0.97–1.04 | 0.81 |
|
Patient Survival** |
||||||
| DCD | 1.86 | 0.86–4.01 | 0.12 | 1.75 | 0.80–3.84 | 0.17 |
| Recipient age (per 10 years) | 1.39 | 1.006–1.92 | 0.05 | 1.50 | 1.09–2.06 | 0.01 |
| Recipient race/ethnicity | ||||||
| White (reference) | 1.00 | NA | NA | 1.00 | NA | NA |
| Afrincan-American | 1.15 | 0.41–3.24 | 0.79 | 0.99 | 0.34–2.87 | 0.99 |
| Other (hispanic and asian) | 0.41 | 0.10–1.72 | 0.23 | 0.43 | 0.10–1.78 | 0.24 |
| Hepatocelluar carcinoma | 0.70 | 0.36–1.35 | 0.28 | 0.90 | 0.39–2.10 | 0.81 |
| MELD (calculated)*** | 1.03 | 1.00–1.06 | 0.05 | 1.03 | 0.99–1.07 | 0.10 |
In bivariate analysis, p-value <0.10 was considered significant; p<0.05 significant in multivariate model.
Patient survival adjusted according to same multivariate model applied to graft survival.
Hazard ratios reported per unit increase in MELD score.
List of Abbreviations
- LT
Liver Transplantation
- DCD
Donation after Cardiac Death
- DBD
Donation after Brain Death
- CMS
Centers for Medicare and Medicaid Services
- OPO
Organ Procurement Organization
- IC
Ischemic Cholangiopathy
- Re-LT
Re-transplantation
- ERCP
Endoscopic Retrograde Cholangio-Pancreatography
- PTC
Percutaneous Transhepatic Cholangiography
- HAT
Hepatic Artery Thrombosis
- ICU
Intensive Care Unit
- LOS
Length of Stay
- UNOS
United Network for Organ Sharing
- UW
University of Wisconsin
- HTK
Histidine-tryptophan-ketoglutarate
- BMI
Body Mass Index
- CVA
Cerebrovascular Accident
- DRI
Donor Risk Index
- MELD
Model for End-Stage Liver Disease
- PNF
Primary non-function
- HAS
Hepatic Artery Stenosis
- PVT
Portal Vein Thrombosis
- HCC
Hepatocellular Carcinoma
- HR
Hazard ratio
- OR
Odds ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Research for this paper was done while the author was a National Research Service Award postdoctoral fellow with the Division of Organ Transplantation at Northwestern University, Feinberg School of Medicine under an institutional award from the Agency for Healthcare Research and Quality, 5 T32 DK077662-02 (PI: Michael Abecassis, MD MBA).
References
- 1.Pomfret EA. Solving the organ shortage crisis: the 7th annual American Society of Transplant Surgeons' State-of-the-Art Winter Symposium. Am J Transplant. 2008;8(4):745–52. doi: 10.1111/j.1600-6143.2007.02146.x. [DOI] [PubMed] [Google Scholar]
- 2.Freeman RB, Jr., et al. Liver and intestine transplantation in the United States, 1997–2006. Am J Transplant. 2008;8(4 Pt 2):958–76. doi: 10.1111/j.1600-6143.2008.02174.x. [DOI] [PubMed] [Google Scholar]
- 3.Medicare and Medicaid programs; conditions for coverage for organ procurement organizations (OPOs). Final rule. Fed Regist. 2006;71(104):30981–1054. [PubMed] [Google Scholar]
- 4.Whiting JF, et al. Clinical results of an organ procurement organization effort to increase utilization of donors after cardiac death. Transplantation. 2006;81(10):1368–71. doi: 10.1097/01.tp.0000179641.82031.ea. [DOI] [PubMed] [Google Scholar]
- 5.Merion RM, et al. Donation after cardiac death as a strategy to increase deceased donor liver availability. Ann Surg. 2006;244(4):555–62. doi: 10.1097/01.sla.0000239006.33633.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Alessandro AM, et al. Successful extrarenal transplantation from non-heart-beating donors. Transplantation. 1995;59(7):977–82. doi: 10.1097/00007890-199504150-00009. [DOI] [PubMed] [Google Scholar]
- 7.Casavilla A, et al. Experience with liver and kidney allografts from non-heart-beating donors. Transplantation. 1995;59(2):197–203. doi: 10.1097/00007890-199501000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujita S, et al. Liver transplantation from donation after cardiac death: a single center experience. Transplantation. 2007;84(1):46–9. doi: 10.1097/01.tp.0000267424.88023.7b. [DOI] [PubMed] [Google Scholar]
- 9.Foley DP, et al. Donation after cardiac death: the University of Wisconsin experience with liver transplantation. Ann Surg. 2005;242(5):724–31. doi: 10.1097/01.sla.0000186178.07110.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abt PL, et al. Survival following liver transplantation from non-heart-beating donors. Ann Surg. 2004;239(1):87–92. doi: 10.1097/01.sla.0000103063.82181.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abt P, et al. Liver transplantation from controlled non-heart-beating donors: an increased incidence of biliary complications. Transplantation. 2003;75(10):1659–63. doi: 10.1097/01.TP.0000062574.18648.7C. [DOI] [PubMed] [Google Scholar]
- 12.Chan EY, et al. Ischemic cholangiopathy following liver transplantation from donation after cardiac death donors. Liver Transpl. 2008;14(5):604–10. doi: 10.1002/lt.21361. [DOI] [PubMed] [Google Scholar]
- 13.Lee KW, et al. Factors affecting graft survival after liver transplantation from donation after cardiac death donors. Transplantation. 2006;82(12):1683–8. doi: 10.1097/01.tp.0000250936.73034.98. [DOI] [PubMed] [Google Scholar]
- 14.Manzarbeitia CY, et al. Long-term outcome of controlled, non-heart-beating donor liver transplantation. Transplantation. 2004;78(2):211–5. doi: 10.1097/01.tp.0000128327.95311.e3. [DOI] [PubMed] [Google Scholar]
- 15.D'Alessandro AM, et al. Donation after cardiac death: the University of Wisconsin experience. Ann Transplant. 2004;9(1):68–71. [PubMed] [Google Scholar]
- 16.Doshi MD, Hunsicker LG. Short- and long-term outcomes with the use of kidneys and livers donated after cardiac death. Am J Transplant. 2007;7(1):122–9. doi: 10.1111/j.1600-6143.2006.01587.x. [DOI] [PubMed] [Google Scholar]
- 17.Mateo R, et al. Risk factors for graft survival after liver transplantation from donation after cardiac death donors: an analysis of OPTN/UNOS data. Am J Transplant. 2006;6(4):791–6. doi: 10.1111/j.1600-6143.2006.01243.x. [DOI] [PubMed] [Google Scholar]
- 18.Maheshwari A, et al. Biliary complications and outcomes of liver transplantation from donors after cardiac death. Liver Transpl. 2007;13(12):1645–53. doi: 10.1002/lt.21212. [DOI] [PubMed] [Google Scholar]
- 19.Kaczmarek B, et al. Ischemic cholangiopathy after liver transplantation from controlled non-heart-beating donors-a single-center experience. Transplant Proc. 2007;39(9):2793–5. doi: 10.1016/j.transproceed.2007.08.081. [DOI] [PubMed] [Google Scholar]
- 20.Feng S, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6(4):783–90. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 21.Reich DJ, et al. Controlled non-heart-beating donor liver transplantation: a successful single center experience, with topic update. Transplantation. 2000;70(8):1159–66. doi: 10.1097/00007890-200010270-00006. [DOI] [PubMed] [Google Scholar]
- 22.Yagci G, et al. The impact of donor variables on the outcome of orthotopic liver transplantation for hepatitis C. Transplant Proc. 2008;40(1):219–23. doi: 10.1016/j.transproceed.2007.11.058. [DOI] [PubMed] [Google Scholar]
- 23.Selck FW, et al. Utilization, outcomes, and retransplantation of liver allografts from donation after cardiac death: implications for further expansion of the deceased-donor pool. Ann Surg. 2008;248(4):599–607. doi: 10.1097/SLA.0b013e31818a080e. [DOI] [PubMed] [Google Scholar]
- 24.Deltenre P, Valla DC. Ischemic cholangiopathy. J Hepatol. 2006;44(4):806–17. doi: 10.1016/j.jhep.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Englesbe MJ, et al. Who pays for biliary complications following liver transplant? A business case for quality improvement. Am J Transplant. 2006;6(12):2978–82. doi: 10.1111/j.1600-6143.2006.01575.x. [DOI] [PubMed] [Google Scholar]
- 26.Stewart ZA, et al. Histidine-Tryptophan-Ketoglutarate (HTK) is associated with reduced graft survival in deceased donor livers, especially those donated after cardiac death. Am J Transplant. 2009;9(2):286–93. doi: 10.1111/j.1600-6143.2008.02478.x. [DOI] [PubMed] [Google Scholar]