Abstract
INTRODUCTION
Recent work has suggested the transected ACL can heal and support reasonable loads if repaired with sutures and a bioactive scaffold; however, use of a traditional suture configuration results in knees with increased AP laxity. The objective was to determine whether one of five different suture repair constructs when performed at two different joint positions would restore normal AP knee laxity.
METHODS
AP laxity of the porcine knee at 60° of flexion was evaluated for five suture repair techniques. Femoral fixation for all repair techniques utilized a suture anchor. Primary repair was to either the tibial stump, one of three bony locations in the ACL footprint, or a hybrid bony fixation. All five repairs were tied with the knee in first 30° and then 60° of flexion for a total of 10 repair constructs.
RESULTS
Suture repair to bony fixation points within the anterior half of the normal ACL footprint resulted in knee laxity values within 0.5 mm of the ACL-intact joint when the sutures were tied with the knee at 60° flexion. Suture repair to the tibial stump, or with the knee at 30° of flexion, did not restore normal AP laxity of the knee.
CONCLUSIONS
Three specific suture repair techniques for the transected porcine ACL restored the normal AP laxity of the knee at the time of surgery. Additional studies defining the changes in laxity with cyclic loading and in vivo healing are indicated.
INTRODUCTION
Recent advances in tissue engineering suggest suture repair and healing of the anterior cruciate ligament (ACL) may be feasible if a biologic boost using a tissue engineered scaffold is provided at the wound site. Primary suture repair of the ACL was pioneered by John Marshall in the 1960’s.1,2 Favor for this technique was lost due to the high re-rupture rate,3 and the inconsistent improvement in the anterior-posterior (AP) laxity. Since that time, advances in the understanding of the biology of healing in the synovial joint have shown that healing of the ACL can be stimulated if the suture repair is supplemented with a bioengineered scaffold containing growth factors.4-6 However, while in these animal models the healing ACL supports a reasonable load before failure, the AP laxity of the knees with a repaired ACL is higher than knees with an intact ACL but similar to those seen in ACL reconstructed knees.7-10 As restoration of normal AP laxity is likely to be important in preserving the long-term function of the joint, it seems reasonable to assume that a suitable repair technique must, at a minimum, restore normal AP laxity of the joint at the time of surgery as it is unlikely that abnormal AP laxity would self-correct over time.
Since Marshall’s initial description of primary repair,1,2 there has been no published work comparing different suturing techniques, and it is not known if any suture technique is even capable of restoring the initial normal sagittal plane laxity of the knee. This is in stark contrast to the numbers of papers published each year on ACL reconstruction techniques, in which 400 references on “ACL Reconstruction and Fixation” alone were identified on a 2007 PubMed search. Assuming that biology alone will not be capable of reducing AP laxity if the laxity condition is not normal at the time of surgery, there is a need to determine how AP laxity will be affected by the suture repair technique selected. With the recent renewed interest in primary repair, a study is needed to compare the initial AP laxity response of the knee using different suturing techniques to determine whether it is possible to restore normal laxity to the joint, even at the time of surgery. The surgical variables that are controllable in the operating room and that one would anticipate could affect joint laxity in ACL repair include in part: 1) soft-tissue versus bone fixation, 2) the location of the tibial attachment for bone fixation, and 3) the knee flexion angle at which the sutures are tensioned and tied. Having determined the effect of these variables on the AP laxity of the knee at the time of surgery, future studies then could be optimized using the best technique established here to evaluate the healing response of “enhanced” ACL repair in an animal model.
The objective of this study was to determine whether one of five suture repair constructs would restore normal AP knee laxity when measured at 60° of flexion. Each of the five suture repair constructs was performed with the knee at 30° and 60° for a total of 10 repair techniques under study. Two hypotheses were tested: 1) a suture repair technique that is anchored to both the tibia and femur can restore the normal AP laxity of the knee at the time of surgery unlike the traditional Marshall technique, and 2) the knee flexion angle at which the suture repair is tied significantly affects the AP laxity of the repaired joint. This second hypothesis is based on current surgical technique for ACL reconstruction where surgeons typically vary the knee flexion angle at the time of graft fixation to obtain the desired graft tension.11
MATERIALS AND METHODS
Specimens
Six knees from six 3-month old female Yorkshire pigs were harvested for this study. The knees were obtained from an IACUC approved study at the time of euthanasia and frozen at −20° C until the time of testing. The sealed limbs were thawed to room temperature on the morning of testing. The knees were isolated by sectioning the femur just below the lesser trochanter and the tibia 2 cm above the ankle joint. The muscular attachments to the tibia and femur were removed leaving the knee joint capsule intact. The tibia and femur were potted in 5cm diameter polyvinyl chloride tubes using a potting material (SmoothCast 300; Smooth-On Inc; Easton, PA) to facilitate attachment to the AP laxity test fixture. Prior to potting the specimens, drywall screws were placed at intervals along the femur and tibia to augment the purchase of bone within the potting material. The knees were wrapped in towels moistened with normal saline until testing.
AP Laxity Test Fixture
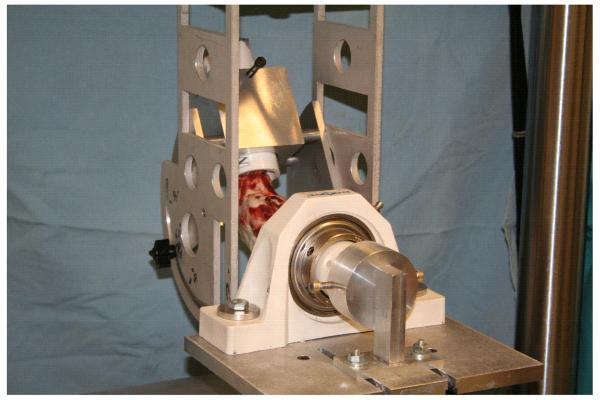
AP laxity testing was performed using a customized fixture that was mounted on a material test system (MTS 810; MTS, Prairie Eden, MN) (Fig. 1).12 The femoral support was directly connected to the actuator of the test system. The fixture allowed for positioning the knee at 60° of flexion. Since full extension in the pig occurs at 30° of flexion, the 60° angle was selected to simulate the Lachman test which is typically performed with the knee flexed 30° from full extension in humans. The tibia was supported on an X-Y platform that allowed unconstrained translations of the tibia in the coronal plane. Varus-valgus angulation was unconstrained during testing. Axial rotation of the tibia was unconstrained when the joint was positioned in the fixture, but locked in the neutral position just prior to laxity testing. Care was taken to ensure that the epicondylar axis of the femur was aligned with the axis of rotation of the femoral support, and that the tibia was locked in the neutral position to standardize the orientation of the AP axis across specimens.
Fig. 1.
AP laxity fixture assembled in the material test system. The femoral shaft is secured in the upper fixture which can be rotated to place the knee between 0° and 90° of flexion for testing. All testing for this experiment was performed with the knee at 60° of flexion to simulate the animal equivalent of the Lachman test. During the test, axial rotation of the tibia was locked in the neutral position. The shear loads were applied perpendicular to the long-axis of the tibia in the mid-sagittal plane of the tibia.
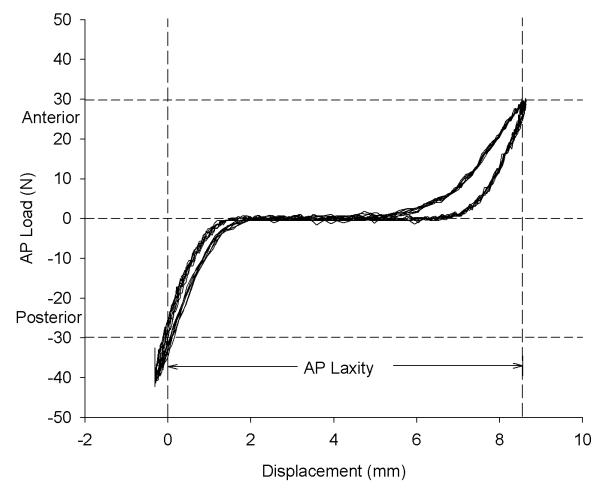
AP laxity was defined as the total AP displacement between the AP shear load limits of ±30 N (Fig. 2). The AP loads and displacements were directly measured from the load cell (resolution: <0.1 N) and LVDT (resolution: 0.14 mm) of the material testing system. 12 Although the shear loads were directly applied to the femur via the material test system, the laxity values were reported as motion of the tibia relative to the femur to be consistent with the clinical standard. AP displacements were measured for ten cycles of the applied shear loads at 100 Hz to produce the load-displacement curves. The residual displacement of the AP laxity fixture under these load limits was 0.57 mm. This value was subtracted from the measured AP laxity displacements to get the total AP laxity value.
Fig. 2.
Sample graph of the AP laxity load versus displacement data. This test was for the sutures tied through both the anterior and middle tunnels (ANT-MID) with the knee flexed 30°. The resulting AP laxity for this specimen is 8.7 mm and represents the distance between ±30N on the anterior loading cycle of the curve. The selected load limits are sufficient since they are well within the linear portions of the anterior and posterior loading curves.
Experimental Protocol
The AP laxity of each knee was ascertained with the knee capsule and ACL intact (INTACT). Dissection was then performed to open the capsule, and to remove the patella and patellar tendon (OPEN). AP laxity testing of the OPEN joint was repeated to ensure that the incisions required to perform the repairs would not affect the AP laxity values. The ACL was then transected with a scalpel in the midsubstance, and testing was repeated.
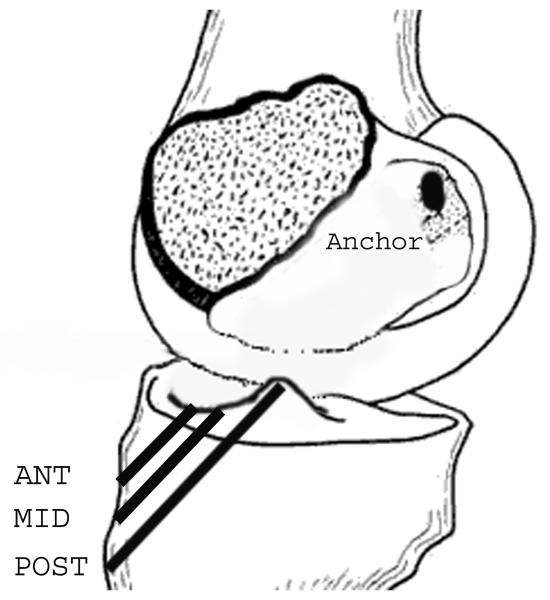
The knees were then prepared for the ten primary repair conditions: 5 suture constructs each tied with the knee at 60° and 30° of flexion. For all repair constructs, a 3.5 mm titanium anchor (TwinFix AB 5.0 Suture Anchor with DuraBraid Sutures; Smith and Nephew, Andover MA) was placed in the posterolateral notch of the femur, at the 11:00 position for the right knees and the 1:00 position for the left knees. The two Durabraid sutures were passed through the anchor eyelet, resulting in four strands available for each repair. First, the traditional Marshall technique was performed using sutures placed at variable depths to secure the tibial stump (MARSHALL) at both the 30° and 60° angles. Next, three repair constructs utilizing a 2.4 mm bone tunnel in one of three regions of the ACL tibial insertion (the anterior (ANT), central (MID), and posterior (POST) regions) were evaluated. For these three constructs, all four suture strands were passed through the respective bone tunnel and then tied over an Endobutton (Smith-Nephew, Inc, Andover, MA) to secure them (Fig. 3). The fifth construct utilized both the ANT and MID tunnels in which two suture strands coursed from the femur down through each of these tunnels. For the tunnel- based constructs, bone-to-bone fixation was obtained and sutures were not placed in the tibial stump. All bone tunnels were created with a K-wire directed at the specified location using a commercial drill guide system (ACUFEX elbow aimer, Smith and Nephew, Inc, Andover MA). Care was taken to maintain a minimum of 5 mm between each of the drill sites on the anteromedial tibia. The sutures for the ANT-MID technique were tied over the bony bridge at the front of the tibia. All five suture repair techniques were performed with the knee at 30° and 60° of flexion by one surgeon (MMM) after applying maximum manual tension to the sutures.
Fig. 3.
Diagram illustrating the location of the tibial bone tunnels in the anterior (ANT), central (MID) and posterior (POST) positions. Note the anchor located in the femoral insertion site of the ACL for proximal fixation that was used for all repair constructs.
The AP laxity measurements for each tunnel-based repair technique tied at 30° of flexion were obtained. Each repair was then repeated with the sutures tied while the knee was maintained at 60° of flexion and reevaluated for AP laxity. After each test, the knee was inspected to make sure the suture anchor was secure. There was no evidence of suture anchor pullout or tunnel widening for any of the tests.
Statistical Analysis
Two way mixed model analysis of variance (ANOVA) using repeated measures for location (7 levels) and knee flexion angle (2 levels) was performed to compare laxity between treatments and to assess the effects of flexion angle. A compound symmetry covariance structure was used as the within-subjects covariance matrix and showed good model fit to the data as judged by Akaike’s information criterion (AIC). Mean differences in laxity between intact and individual repair technique cases were calculated with 95% confidence intervals for each level of flexion (30° or 60°) at the time of repair. Two-tailed values of p<0.05 were considered statistically significant with Fisher’s least significant difference (LSD) procedure to make multiple comparisons between each repair construct relative to the INTACT condition.
RESULTS
The AP laxity of the knee after suture repair was significantly affected by the suture repair technique (p<0.001) and the angle of the knee when the sutures were tied (p<0.001). There was no significant interaction between location and degree of flexion in this analysis (p=0.674), indicating that the effect of flexion angle on AP laxity was consistent for each location.
The intact knees had an AP laxity of 4.3±0.6 mm (mean±SEM) (Table 1). Removal of the patella, patellar tendon, ligamentum mucosum and fat pad (the OPEN case) had a negligible effect on the AP laxity values of the knee (Table 1). Values for the OPEN group were 4.6±0.6 mm and not significantly different from the intact knees (p=0.802). When the ACL was sectioned, the laxity of the knee exceeded the maximum level permitted by the material test system (32 mm). Therefore, this group was assigned the maximum measured displacement of 32 mm.
Table 1.
AP laxity for the ACL intact and suture repair constructs. INTACT=ACL-intact, capsule intact; OPEN=ACL-intact capsule opened; ANT=Anterior tibial tunnel; MID=Central tibial tunnel; POST=Posterior tibial tunnel; ANT-MID=Anterior & central tunnel combined.
| Location | Knee Flexion (deg) |
n | Mean AP Laxity (mm) |
95% Confidence Interval | |
|---|---|---|---|---|---|
| Lower Bound |
Upper Bound |
||||
| INTACT | N/A | 6 | 4.3 | 2.9 | 5.8 |
| OPEN | N/A | 6 | 4.6 | 3.1 | 6.1 |
| MARSHALL | 30° | 6 | 13.1* | 11.9 | 14.4 |
| 60° | 6 | 10.5* | 9.3 | 11.8 | |
| ANT | 30° | 6 | 7.1* | 5.8 | 8.3 |
| 60° | 6 | 4.6 | 3.3 | 5.8 | |
| MID | 30° | 6 | 5.2 | 3.9 | 6.4 |
| 60° | 6 | 4.0 | 2.8 | 5.3 | |
| POST | 30° | 6 | 8.4* | 7.2 | 9.7 |
| 60° | 6 | 5.7 | 4.4 | 6.9 | |
| ANT-MID | 30° | 6 | 6.1† | 4.9 | 7.4 |
| 60° | 6 | 4.3 | 3.1 | 5.6 | |
Statistically different from the ACL INTACT knees (p<0.001).
Statistically different from the ACL INTACT knees (p<0.05).
Suture Repair Techniques
When pooled across the knee flexion angles at which the sutures were tied, primary repair using the MARSHALL technique did not restore the normal AP laxity of the knee when compared to the INTACT case (Table 1, p<0.001); however, it resulted in improved laxity in comparison with the ACL-deficient knee (11.8±0.5 mm for the Marshall versus >32 mm for the ACL-deficient conditions).
Sutures placed in the middle bone tunnel (MID) restored AP laxity to that of the INTACT knees (4.6±0.4 mm versus 4.3±0.6 mm, p=0.779; Table 1). Placement of the suture in a more posterior location (POST) on the tibial spine resulted in knees with significantly greater AP laxity than the knees with an intact ACL (7.0±0.5 mm; p=0.004). Placement of the suture in a more anterior location (ANT) or the hybrid tunnel combination (ANT-MID) produced AP laxity values that were not significantly different from the INTACT condition (5.8±0.5 mm; p=0.115 and 5.2±0.5 mm; p=0.344, respectively).
Knee Flexion Angle Effects
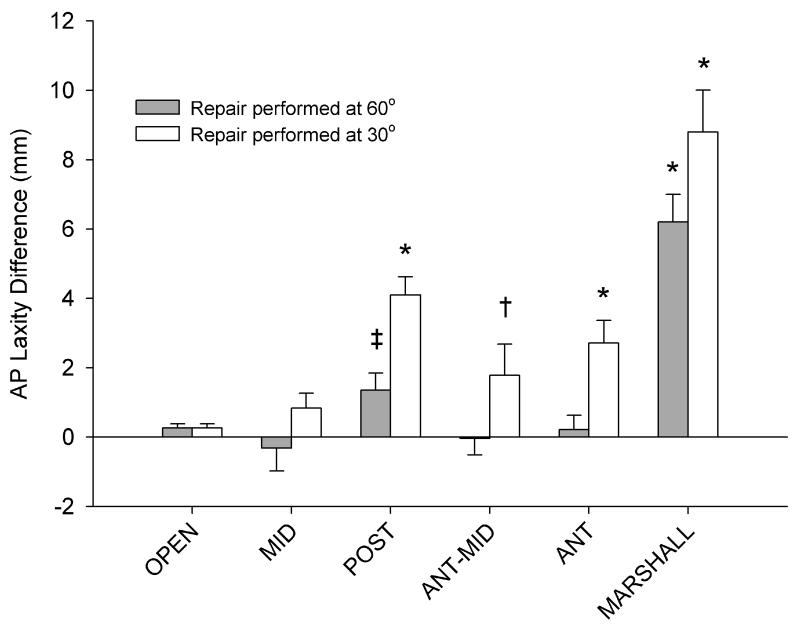
On average, the laxity values were greatest when the repairs were tied at 30° of flexion, with less laxity consistently noted when the repairs were tied at 60° (p<0.001) (Fig. 4). However, when the sutures were passed through the MID tunnel, no significant difference was noted between the two knee flexion angles (p=0.197) at which the sutures were tied.
Fig. 4.
Mean differences from the intact AP laxity of the knee for all repair conditions (Repair – INTACT). N=6 for all groups. The error bars represent the standard error of the mean. Asterisks and daggers represent groups that are statistically different from the INTACT case (*p<0.001, †p<0.05). The POSTERIOR location tied at 60° approached, but did not reach, a significant difference from the INTACT case (‡p=0.101). For all other groups tied at 30°, p>0.30; and for repairs ties at 60°, p>0.10 for comparison with the INTACT group.
DISCUSSION
The results of this study support the first hypothesis; namely, that ACL repair techniques that are anchored directly to the tibia and femur can restore the normal AP laxity of the knee at the time of surgery. In addition, the angle of knee flexion at the time of suture fixation was also found to significantly influence the post-operative laxity, and thus the second hypothesis was also verified. Normal AP laxity of the knee was restored when the tibial fixation was to bone and located in the anterior half of the ACL footprint and the sutures were tied with the knee in 60° of flexion.
Traditional suture repair to the tibial stump (MARSHALL) did not restore normal AP laxity of the knee, an important finding that provides a rationale as to why use of this technique resulted in a large percentage of patients having abnormal knee laxity post-operatively.13,14 This technique likely fails in part due to the difficulty in placing a stitch in a short ligament stump that is composed primarily of longitudinal fibers. The inability of the suture to sufficiently grasp these fibers leads to a high probability that the sutures will slide along the fascicles with repetitive stress, leading to premature laxity and failure of the repair. For this study, we selected the Marshall technique as the standard repair since it utilizes variable depth sutures to provide optimal purchase within the fascicular ligament tissue.1 We anticipate that other suture techniques through the tibial stump would provide similar results due to the soft tissue-suture interface, however other specific techniques were not examined and further study is required.
The suture repair techniques that were anchored directly to the femur and tibia reduced AP laxity values when compared to the traditional Marshall repair (Fig. 4). These constructs provided a stent that transferred the load between the tibia and the femur, bypassing the injured ligament, and eliminating the soft tissue repair interface of the Marshall procedure. For these configurations, sutures were not used to align the ligament remnants. Whether this needs to be done with additional sutures to augment healing in vivo remain to be studied. In the past, large stents, such as the carbon fiber graft or Ligament Augmentation Device (LAD), and even the middle third of the patellar tendon, have been used to protect repair of the ACL,14-16 however, these procedures all involved placement of a relatively large amount of material in the same space as the ACL, thus leaving less room for formation of a hypertrophic scar and also resulting in extensive disruption to the native ACL insertion sites. The technique proposed here could possibly provide a means to modulate load and protect the healing ACL, particularly during initial healing17,18, while still allowing preservation of the insertion sites and scar volume. An additional advantage with this technique is that the sutures could be selected or designed to support the repair for a specified period of time and then release or resorb, to minimize any potential complication of stress shielding by the suture-stent. As the sutures also require minimal volume, the stent technique could be combined with additional sutures designed not to support mechanical load, but to simply re-approximate the torn ends of the ACL.
In this study, the laxity values were closer to normal when the sutures were tied with the knees in greater flexion (60° as compared to 30° of flexion). This result is consistent with prior studies that have shown the length of the ACL to be greatest in extension;19,20 thus, tightening an ACL graft or suture repair in greater extension leads to a repair with greater length (and thus greater laxity) and conversely, tightening a repair in flexion will result in a tighter repair. As the porcine knee joint is thought to be similar to the human knee in terms of biomechanical behavior,21 this result is also consistent with the prior ACL graft literature.19
AP laxity is an important variable to consider since it provides an overall assessment of ACL integrity,22,23 it is easy to perform intra-operatively,24 and it has been shown to be inversely proportional to the strength and stiffness of a healing ACL graft.25,26. Again, as the porcine knee has a maximum extension at 30° of flexion, the 60° case is analogous to testing AP laxity in the human at 30°, as is done in the Lachman test. In a preliminary study we found that the AP laxity of the pig knee was greater at 60° (6.4±1.4 mm) as compared to that of 30° (5.5±0.9 mm) or 90° (5.9±1.6mm) using our testing system, as previously shown by Li et al. 21 Thus, 60° flexion was selected because the contribution of the capsule and menisci to joint stiffness would be minimal at this angle.
A limitation of this study is its ex vivo nature. While the procedures resulted in repeatable laxity values after each repair construct, all measurements were taken at the time of repair. It is not known how the repairs may function with time in the healing knee joint. This is an important question that certainly needs to be answered with an animal model before considering the translation of these results into clinical practice. In addition, this study does not account for the effects of cyclic loading. Ex vivo testing under cyclic loads, followed by in vivo testing of the most promising techniques are needed to document maintenance of knee laxity over time and the biomechanical properties of the healing ligament, and the preservation of cartilage health, all of which are important next steps in the development of a suitable ACL repair strategy.
There are several surgical variables that could affect joint laxity during ACL repair. The three that were evaluated in this study were soft-tissue versus bone fixation, the locations of the tibial attachment for bone fixation, and the knee flexion angle at which the sutures are tensioned and tied. Other factors that could also influence AP laxity are the type and size of the suture material, and the knots used to create the repair. Only one suture type was used for this study and standard knots were used for each configuration. The effects that the suture material and the knots may have on AP laxity were not evaluated in this study.
It should also be noted that the AP laxity values were obtained with constrained axial rotation. Laxity values would likely be slightly greater if the tibia were left free to rotate during the test.27 We elected to test the specimen in this manner because of the difficulty in perfectly aligning the longitudinal axis of the tibia with that of the tibial support. If the axis of rotation permitted by the jig were not collinear with the long axis of the tibia, motion constraints would be introduced. With the technique used here, the tibia was free to rotate when the specimen was aligned in the fixture and then locked in the perceived neutral position just prior to loading the system, which avoided these artificial motion constraints. When comparing the five techniques, all conditions were tested in the same manner so this should not adversely affect our findings.
The test order for the repair constructs was not randomized though no consistent biases were evident. It is possible that the AP laxity values might be better when the testing is performed at the same knee angle as the repair was performed giving an advantage to the 60° repair strategy. This would primarily influence the MARSHALL procedure where the sutures were directly secured to the tibial stump. Considering that the AP laxity value for both MARSHALL conditions (30° and 60°) are almost twice that of the tunnel-based condition, this concern is minimized. It should be noted that the joints were flexed and extended between the repair procedure and the AP laxity test. Although the 30° repairs were tested first, they were always more lax than the 60° repairs (which is the opposite of what would be expected if sub-critical damage to the joint capsule or other structures important in joint laxity were occurring during the testing process). Similarly, the group tested last (the ANT-MID tunnel group) had one of the least laxity means, a finding which would not be expected if damage to the joint were accumulating and resulting in bias. This was likely due to the fact that the number of laxity tests and the magnitude of the shear loads applied to each specimen were relatively low and that the testing of each specimen was easily completed within one day. The specimens were inspected between each repair construct to ensure that the femoral anchor was intact and that there was no cutting at the intra-articular tunnel sites.
Prior to initiating the study, we intended to measure the AP laxity of the ACL-deficient knee to make statistical comparisons with the repaired techniques. However, the pig knee proved to be very lax when the ACL was severed, producing AP laxity values that consistently exceeded the displacement limit of our testing system (32 mm). Primary repair using the Marshall technique (with the sutures tied with the knee at 30° of flexion) produced the largest post-repair laxity values. However, even these values were only half of the total displacement limit. Thus, it is reasonable to conclude that all repair techniques reduced the AP laxity values substantially relative to the ACL-deficient joint.
In conclusion, suture repair techniques that traverse the ligament and insert through bone tunnels in the anterior half of the tibial ACL footprint were shown to restore the normal AP laxity of the knee at the time of surgery, particularly if the sutures were tied with the joint flexed at 60°. Further cyclical testing and in vivo evaluation of these repair techniques are warranted to investigate the best suture repair techniques to promote ACL healing after suture repair. Here, we have identified three suture techniques that may provide a temporary stent to facilitate functional healing of the ACL for these future studies.
ACKNOWLEDGEMENTS
The authors would like to acknowledge David Spenciner, David Paller, Ryan Rich, Eduardo Abreu, Megan McElfresh and Matthew Palmer for their assistance with this project, as well as David Zurakowski for his assistance with the statistical analysis. In addition, funding was received from NIH grants AR054099 (MMM) and AR049199 (BCF).
REFERENCES
- 1.Marshall JL, Warren RF, Wickiewicz TL. Primary surgical treatment of anterior cruciate ligament lesions. Am J Sports Med. 1982;10:103–107. doi: 10.1177/036354658201000208. [DOI] [PubMed] [Google Scholar]
- 2.Mrshall JL, Warren RF, Wickiewicz TL, et al. The anterior cruciate ligament: a technique of repair and reconstruction. Clin Orthop. 1979;143:97–106. [PubMed] [Google Scholar]
- 3.Feagin JA, Curl WW. Isolated tear of the anterior cruciate ligament: 5-year follow-up study. Am J Sports Med. 1976;4:95–100. doi: 10.1177/036354657600400301. [DOI] [PubMed] [Google Scholar]
- 4.Murray MM, Spindler KP, Abreu E, et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25:820–830. doi: 10.1002/jor.20282. [DOI] [PubMed] [Google Scholar]
- 5.Murray MM, Spindler KP, Ballard P, et al. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25:1007–1017. doi: 10.1002/jor.20367. [DOI] [PubMed] [Google Scholar]
- 6.Murray MM, Spindler KP, Devin C, et al. Use of a collagen-platelet rich plasma scaffold to stimulate healing of a central defect in the canine ACL. J Orthop Res. 2006;24:820–830. doi: 10.1002/jor.20073. [DOI] [PubMed] [Google Scholar]
- 7.Abramowitch SD, Papageorgiou CD, Withrow JD, et al. The effect of initial graft tension on the biomechanical properties of a healing ACL replacement graft: A study in goats. J Orthop Res. 2003;21:707–715. doi: 10.1016/S0736-0266(02)00265-6. [DOI] [PubMed] [Google Scholar]
- 8.Cummings JF, Grood ES. The progression of anterior translation after anterior cruciate ligament reconstruction in a caprine model. J Orthop Res. 2002;20:1003–1008. doi: 10.1016/S0736-0266(02)00033-5. [DOI] [PubMed] [Google Scholar]
- 9.Papageorgiou CD, Ma CB, Abramowitch SD, et al. A multidisciplinary study of the healing of an intraarticular anterior cruciate ligament graft in a goat model. Am J Sports Med. 2001;29:620–626. doi: 10.1177/03635465010290051501. [DOI] [PubMed] [Google Scholar]
- 10.Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21:176–185. doi: 10.1177/036354659302100203. [DOI] [PubMed] [Google Scholar]
- 11.Amis AA, Jakob RP. Anterior cruciate ligament graft positioning, tensioning, and twisting. Knee Surg Sports Traumatol Arthrosc. 1998;6:S2–S12. doi: 10.1007/s001670050215. [DOI] [PubMed] [Google Scholar]
- 12.Fleming BC, Abate JA, Peura GD, et al. The relationship between graft tensioning and the anterior-posterior laxity in the anterior cruciate ligament reconstructed goat knee. J Orthop Res. 2001;19:841–844. doi: 10.1016/S0736-0266(01)00020-1. [DOI] [PubMed] [Google Scholar]
- 13.Sherman MF, Lieber L, Bonamo JR, et al. The long-term followup of primary anterior cruciate ligament repair. Defining a rationale for augmentation. Am J Sports Medicine. 1991;9:243–255. doi: 10.1177/036354659101900307. [DOI] [PubMed] [Google Scholar]
- 14.Grontvedt T, Engebretsen L, Bredland T. Arthroscopic reconstruction of the anterior cruciate ligament using bone-patellar tendon-bone grafts with and without augmentation. A prospective randomised study. J Bone Joint Surg [Br] 1996;78:817–822. [PubMed] [Google Scholar]
- 15.Stewart NJ, Engebretsen L, Lewis JL, et al. Maintenance of set force in anterior cruciate ligament grafts. J Orthop Res. 1993;11:149–153. doi: 10.1002/jor.1100110117. [DOI] [PubMed] [Google Scholar]
- 16.Kdolsky R, Kwasny O, Schabus R. Synthetic augmented repair of proximal ruptures of the anterior cruciate ligament. Long-term results of 66 patients. Clin Orthop. 1993;295:183–189. [PubMed] [Google Scholar]
- 17.Lew WD, Engebretsen L, Lewis JL, et al. Method for setting total graft force and load sharing in augmented ACL grafts. J Orthop Res. 1990;8:702–711. doi: 10.1002/jor.1100080512. [DOI] [PubMed] [Google Scholar]
- 18.Lewis JL, Poff BC, Smith JJ, et al. Method for establishing and measuring in vivo forces in an anterior cruciate ligament composite graft: Response to differing levels of load sharing in a goat model. J Orthop Res. 1994;12:780–788. doi: 10.1002/jor.1100120605. [DOI] [PubMed] [Google Scholar]
- 19.Markolf KL, Burchfield DM, Shapiro MM, et al. Biomechanical consequences of replacement of the anterior cruciate ligament with a patellar ligament allograft .1. Insertion of the graft and anterior-posterior testing. J Bone Joint Surg [Am] 1996;78:1720–1727. doi: 10.2106/00004623-199611000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Beynnon BD, Howe JG, Pope MH, et al. Anterior cruciate ligament strain, in vivo. Int Orthop. 1992;16:1–12. doi: 10.1007/BF00182976. [DOI] [PubMed] [Google Scholar]
- 21.Li GA, Rudy TW, Allen CR, et al. Effect of combined axial compressive and anterior tibial loads on in situ forces in the anterior cruciate ligament: A Porcine Study. J Orthop Res. 1998;16:122–127. doi: 10.1002/jor.1100160121. [DOI] [PubMed] [Google Scholar]
- 22.Almekinders LC, Pandarinath R, Rahusen FT. Knee stability following anterior cruciate ligament rupture and surgery. The contribution of irreducible tibial subluxation. J Bone Joint Surg [Am] 2004;86:983–987. doi: 10.2106/00004623-200405000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Daniel DM, Malcom LL, Losse GM, et al. Instrumented measurement of anterior laxity of the knee. J Bone Joint Surg [Am] 1985;67:720–726. [PubMed] [Google Scholar]
- 24.Fleming BC, Brattbakk B, Peura GD, et al. Measurement of anterior-posterior knee laxity: A comparison of three techniques. J Orthop Res. 2002;20:421–426. doi: 10.1016/S0736-0266(01)00134-6. [DOI] [PubMed] [Google Scholar]
- 25.Grood ES, Walz-Hasselfeld KA, Holden JP, et al. The correlation between anterior-posterior translation and cross-sectional area of anterior cruciate ligament reconstructions. J Orthop Res. 1992;10:878–885. doi: 10.1002/jor.1100100617. [DOI] [PubMed] [Google Scholar]
- 26.Beynnon BD, Johnson RJ, Tohyama H, et al. The relationship between anterior-posterior knee laxity and the structural properties of the patellar tendon graft. A study in canines. Am J Sports Med. 1994;22:812–820. doi: 10.1177/036354659402200613. [DOI] [PubMed] [Google Scholar]
- 27.Fukubayashi T, Torzilli PA, Sherman MF, et al. An in vitro biomechanical evaluation of anterior-posterior motion of the knee: Tibial displacement, rotation, and torque. J Bone Joint Surg [Am] 1982;64:258–264. [PubMed] [Google Scholar]