Short abstract
In a randomized phase III trial, sunitinib was associated with significantly superior progression-free survival when compared with interferon alfa as first-line therapy in patients with metastatic renal cell carcinoma. This article investigates whether baseline quality of life and demographic and clinical variables were predictive for progression-free survival.
Abstract
Purpose:
In a randomized phase III trial, sunitinib was associated with significantly superior progression-free survival (PFS) when compared with interferon alfa (IFN-α) as first-line therapy in patients with metastatic renal cell carcinoma. We investigated whether baseline quality-of-life (QOL) and demographic and clinical variables were predictive for PFS.
Methods:
Patients were randomly assigned to receive sunitinib or IFN-α at a ratio of one to one. QOL was measured using the Functional Assessment of Cancer Therapy–General scale (FACT-G), the FACT–Kidney Symptom Index–Disease-Related Symptoms subscale (FKSI-DRS), and the EuroQol (EQ) Group's visual analog scale (EQ-VAS; Rotterdam, the Netherlands). In all scales, higher scores indicate better QOL or fewer symptoms. Controlling for other baseline demographic and clinical variables, Cox proportional hazards models—one for each QOL variable—were used to test if difference in baseline QOL scores predicted PFS.
Results:
The superior treatment effect on PFS of sunitinib versus IFN-α remained robust (hazard ratio [HR], 0.34, 0.33, and 0.33 for each model, respectively; P < .0001 for each model). Higher baseline FACT-G, FKSI-DRS, and EQ-VAS scores were associated with longer PFS (HR, 0.93, 0.89, and 0.91, respectively; P ≤ .001, P ≤ .001, and P = .008, respectively). Presence of liver metastases (HR, 1.59 to 1.71; P = .0009 to .0044) and number of Memorial Sloan-Kettering Cancer Center (MSKCC; New York, NY) risk factors (HR, 1.52 to 1.60; P < .0001 for each) were significant negative predictors of PFS, independent of other variables.
Conclusion:
Sunitinib conferred significantly superior PFS compared with IFN-α, irrespective of baseline QOL or clinical characteristics. Higher baseline QOL correlated with longer PFS, whereas the presence of liver metastases and more MSKCC risk factors at baseline correlated with shorter PFS. This remains an area for future study.
Introduction
Renal cell carcinoma (RCC) is associated with a high incidence of metastatic disease (25% to 30% of patients) and poor prognosis because of its resistance to traditional chemotherapeutic regimens.1 Patients present with a variety of symptoms, including abdominal pain, fever, night sweats, malaise, and weight loss,1 and adverse effects associated with treatment contribute to additional patient disability. Improving quality of life (QOL), therefore, is important to optimize palliative care. Previous research in patients with advanced or metastatic lung,2 breast,3 colorectal,4–6 head and neck,7 bladder,8 and cervical9 cancers has shown an association between QOL and survival. A recent study demonstrated an association between QOL and overall survival (OS) in patients with RCC.10 Whether this relationship exists in patients with metastatic RCC (mRCC) is not yet known.
A number of clinical factors have been investigated in multivariate analyses to predict survival in patients with mRCC. These include hematologic and inflammatory markers, factors related to site of metastasis, performance status, tumor stage, time to treatment, and previous surgery.11–16 These factors have been used to classify patients into risk categories to offer more accurate predictions of treatment outcomes and more tailored treatment strategies and counseling.
In a randomized phase III trial, sunitinib malate (SUTENT; Pfizer, New York, NY), administered as a first-line therapy, was associated with significantly superior progression-free survival (PFS) and overall response rate compared with interferon alfa (IFN-α) in patients with mRCC.17 In the study, patients in the sunitinib group reported significantly better QOL than did patients in the IFN-α group (P < .001).17,18 Here, we report data from an analysis of this study that investigated whether baseline QOL and clinical variables were predictive for PFS.
Methods
Patients and Study Design
In this international, multicenter, randomized phase III trial, male and female patients age 18 years or older were eligible for inclusion if they had mRCC with a component of clear-cell histology. Patients were randomly assigned at a ratio of one to one to receive either sunitinib or IFN-α. Sunitinib was administered at a starting dose of 50 mg orally per day, irrespective of food intake, in a 6-week cycle consisting of 4 weeks on treatment followed by 2 weeks off treatment (schedule 4/2). IFN-α was administered subcutaneously three times per week on nonconsecutive days at a dose of 3 million units (MU) in the first week, 6 MU in the second week, and 9 MU thereafter. Dose modifications were allowed for toxicity management on both treatments. Full details of the study design were reported by Motzer et al.17 This study was approved by the institutional review board or ethics committee at each participating center and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
PFS Assessments
PFS was defined as the time from random assignment to first documentation of objective tumor progression or death as a result of any cause. Tumor imaging was performed at screening, on day 28 of first to fourth cycles and every other cycle thereafter, at the end of treatment, and whenever progression was suspected or confirmation of a response was required. An independent central laboratory reviewed the images to assess responses.
QOL Assessments
Three validated patient self-reported questionnaires were completed at screening, on days 1 and 28 of each cycle, and at the end of treatment or on withdrawal from the study. The first questionnaire, the Functional Assessment of Cancer Therapy–General scale (FACT-G), is a 27-item instrument with scores ranging from 0 (worst cancer-related QOL) to 108 (best cancer-related QOL).19 The second questionnaire used was the Functional Assessment of Cancer Therapy–Kidney Symptom Index–Disease-Related Symptoms subscale (FKSI-DRS), a nine-item scale20 with scores ranging from 0 (most severe symptoms) to 36 (no symptoms). The third instrument, the EuroQol (EQ) Group's visual analog scale (EQ-VAS; Rotterdam, the Netherlands), is a 100-point visual analog scale ranging from 0 (worst imaginable health state) to 100 (best imaginable health state) that forms part of the group's five-domain self-reported questionnaire (EQ-5D).21,22 For the current analysis, only baseline data (first cycle, day 1, administered before the start of study treatment) were used. For all three QOL variables, higher scores indicated better outcomes (ie, better QOL or fewer or less severe symptoms).
Statistical Methods
A Cox proportional hazards model was fitted to the data to test whether the baseline QOL scores predicted PFS.23 Because we found moderate to high correlations between baseline scores for the three QOL variables (r = 0.61-0.69), three separate models were fitted, each distinguished by its baseline QOL variable. Each model included treatment (sunitinib or IFN-α), one baseline QOL variable, and additional covariates concerning baseline demographic and clinical factors (ie, age, sex, baseline Eastern Cooperative Oncology Group performance status [ECOG PS], number of Memorial Sloan-Kettering Cancer Center [MSKCC] risk factors, previous nephrectomy and radiotherapy, number of metastases, and sites of metastases [lung, liver, bone, and lymph nodes]). Each resulting hazard ratio (HR) corresponded to a given variable and was adjusted for the effects of the other variables on PFS. The HR for the QOL variable was the key measure of treatment effect, and was calculated as the hazard of PFS per minimally important difference (MID) in each of the three baseline QOL variables (FACT-G, MID = 5; FKSI-DRS, MID = 2; EQ-VAS, MID = 10). Also obtained were 95% CIs for the HR and P values, with P < .05 set as the criterion for significance. Adjustments for multiple comparisons were not made because these were exploratory analyses.
Results
Patient Baseline Characteristics
In total, 750 patients were randomly assigned (375 patients enrolled in each treatment arm) in this study between August 2004 and October 2005. There were no significant differences between treatment groups in terms of baseline demographic or clinical characteristics or QOL (Table 1). The majority of patients in both groups had at least three disease sites (the lungs were the predominant site), and most had undergone previous nephrectomy. In each group, 14% of patients had previously received radiotherapy. Baseline QOL scores were in the moderate range. At baseline, complete data for the three QOL variables were available for approximately 97% to 98% of patients in the total population, and the modeling analysis was based on these patients with complete data.
Table 1.
Baseline Patient Demographics and Clinical Characteristics
| Characteristic | Sunitinib (n = 375) | IFN-α (n = 375) |
|---|---|---|
| Median age, years | 62 | 59 |
| Sex, % | ||
| Male | 71 | 72 |
| Female | 29 | 28 |
| ECOG PS, %* | ||
| 0 | 62 | 61 |
| 1 | 38 | 38 |
| 2 | 0 | 1 |
| MSKCC risk factors, % | ||
| 0 | 38 | 34 |
| 1-2 | 56 | 59 |
| ≥ 3 | 6 | 7 |
| Previous nephrectomy, % | 91 | 89 |
| Previous radiotherapy, % | 14 | 14 |
| No. of disease sites, % | ||
| 1 | 15 | 19 |
| 2 | 28 | 30 |
| ≥ 3 | 57 | 51 |
| Site of metastasis, % | ||
| Lung | 78 | 79 |
| Liver | 26 | 24 |
| Bone | 30 | 30 |
| Lymph nodes | 58 | 53 |
| QOL scores, mean ± SD | ||
| FACT-G | 82.30 ± 15.2 | 81.25 ± 16.04 |
| FKSI-DRS | 29.74 ± 5.24 | 29.55 ± 5.03 |
| EQ-VAS | 73.80 ± 18.50 | 71.43 ± 19.51 |
Abbreviations: IFN-α, interferon alfa; ECOG PS, Eastern Cooperative Oncology Group performance status; MSKCC, Memorial Sloan-Kettering Cancer Center; QOL, quality of life; SD, standard deviation; FACT-G, Functional Assessment of Cancer Therapy–General scale; FKSI-DRS, Functional Assessment of Cancer Therapy–Kidney Symptom Index–Disease-Related Symptoms subscale; EQ-VAS, EuroQol Group's visual analog scale.
* All patients had an ECOG PS of 0 or 1 at the time eligibility was determined; four patients in the IFN-α group had an ECOG PS of 2 on the day study treatment started.
Predictive Value of Baseline QOL for PFS
As previously reported, median PFS (by independent central review) was 11 months in the sunitinib group (95% CI, 10 to 12) and 5 months in the IFN-α group (95% CI, 4 to 6), corresponding to an HR of 0.42 (95% CI, 0.32 to 0.54; P < .001).17 After controlling for baseline QOL, a numerically greater treatment effect on PFS was seen with sunitinib versus IFN-α, with estimated HRs of 0.34, 0.33, and 0.33 for the three models, respectively (P < .0001 for each model), each containing one baseline QOL variable (FACT-G, FKSI-DRS, or EQ-VAS; Table 2).
Table 2.
Cox Proportional Hazards Models for Effect of Baseline Quality-of-Life and Clinical and Demographic Variables on Progression-Free Survival
| Variable | Model 1 (FACT-G) | Model 2 (FKSI-DRS) | Model 3 (EQ-VAS) | |||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Treatment, sunitinib | 0.34 | 0.26 to 0.45 | 0.33 | 0.25 to 0.43 | 0.33 | 0.25 to 0.44 |
| Baseline QOL variables | ||||||
| FACT-G total score, per 5-point change | 0.93* | 0.89 to 0.97 | ||||
| FKSI-DRS score, per 2-point change | 0.89* | 0.84 to 0.94 | ||||
| EQ-VAS score, per 10-point change | 0.91* | 0.84 to 0.97 | ||||
| Baseline clinical and demographic variables | ||||||
| Age, years | 1.00 | 0.98 to 1.01 | 0.99 | 0.98 to 1.01 | 0.99 | 0.98 to 1.00 |
| Sex, female | 1.30 | 0.98 to 1.71 | 1.29 | 0.97 to 1.71 | 1.37† | 1.04 to 1.82 |
| Baseline ECOG PS, 1 or 2 | 1.08 | 0.80 to 1.46 | 1.01 | 0.75 to 1.36 | 1.07 | 0.79 to 1.45 |
| No. of MSKCC risk factors‡ | 1.60§ | 1.38 to 1.86 | 1.52§ | 1.30 to 1.76 | 1.56§ | 1.34 to 1.83 |
| Previous nephrectomy | 1.11 | 0.70 to 1.76 | 1.05 | 0.66 to 1.68 | 1.07 | 0.67 to 1.72 |
| Previous radiotherapy | 0.78 | 0.50 to 1.21 | 0.80 | 0.52 to 1.24 | 0.79 | 0.50 to 1.25 |
| No. of disease sites | 1.04 | 0.91 to 1.18 | 1.03 | 0.90 to 1.17 | 1.06 | 0.92 to 1.21 |
| Metastases in lung | 1.27 | 0.88 to 1.83 | 1.33 | 0.92 to 1.92 | 1.26 | 0.87 to 1.82 |
| Metastases in liver | 1.71§ | 1.24 to 2.34 | 1.61§ | 1.17 to 2.21 | 1.59§ | 1.16 to 2.18 |
| Metastases in bone | 1.16 | 0.79 to 1.70 | 1.19 | 0.81 to 1.74 | 1.11 | 0.75 to 1.64 |
| Metastases in lymph nodes | 1.25 | 0.93 to 1.69 | 1.27 | 0.94 to 1.72 | 1.27 | 0.94 to 1.72 |
Abbreviations: FACT-G, Functional Assessment of Cancer Therapy–General scale; FKSI-DRS, Functional Assessment of Cancer Therapy–Kidney Symptom Index–Disease-Related Symptoms subscale; EQ-VAS, EuroQol Group's visual analog scale; HR, hazard ratio; QOL, quality of life; ECOG PS, Eastern Cooperative Oncology Group performance status; MSKCC, Memorial Sloan-Kettering Cancer Center.
* P ≤ .001 for HR of FACT-G and FKSI-DRS scores. P = .008 for HR of EQ-VAS score. All other P values exceeded .05 except those noted.
† P = .03.
‡ MSKCC risk factors: low serum hemoglobin, elevated corrected serum calcium, elevated serum lactate dehydrogenase, poor performance status, interval > 1 year between diagnosis and treatment.
§ P < .005.
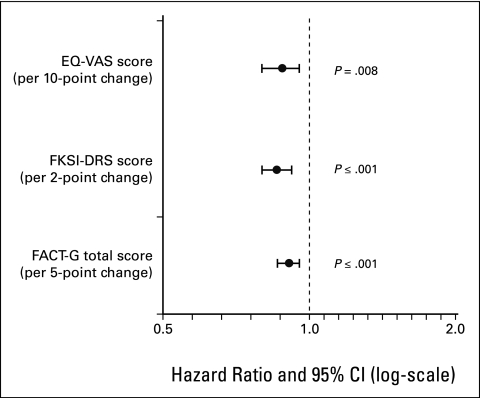
All three baseline QOL variables were individually predictive of PFS (Table 2). Higher scores at baseline of FACT-G (per 5-point change), FKSI-DRS (per 2-point change), and EQ-VAS (per 10-point change) were significantly associated with longer PFS (HR, 0.93, 0.89, and 0.91; P ≤ .001, P ≤ .001, and P = .008, respectively; Fig 1). These data suggest that the rate of tumor progression or death at any given time was approximately 7%, 11%, and 9% lower, respectively, for every higher unit score of MID in the three patient-reported variables.
Figure 1.
Relationship between progression-free survival and baseline quality-of-life variables. EQ-VAS, EuroQol Group's visual analog scale; FKSI-DRS, Functional Assessment of Cancer Therapy–Kidney Symptom Index–Disease-Related Symptoms subscale; FACT-G, Functional Assessment of Cancer Therapy–General scale.
Predictive Value of Other Baseline Variables for PFS
The presence of liver metastases (HR, 1.71 [P = .0009]; HR, 1.61 [P = .0032]; and HR, 1.59 [P = .0044], respectively, for FACT-G, FKSI-DRS, and EQ-VAS) and the number of MSKCC risk factors (HR, 1.60, 1.52, and 1.56, respectively; P < .0001 for each) were both significant negative predictors for PFS, independent of other variables in the models (Table 2). Thus, the presence of liver metastases conferred an estimated 59% to 71% greater risk of tumor progression or death at any given time, and each additional MSKCC risk factor conferred an estimated 52% to 60% greater risk of tumor progression or death at any given time. Female sex had a small but significant negative predictive effect on PFS in the model with baseline EQ-VAS (HR, 1.37; P = .027) but not in the other two models.
Discussion
This analysis of interim data from the phase III randomized trial of 750 patients with mRCC demonstrated the significantly superior effect of sunitinib on PFS, compared with that of IFN-α (HR, 0.42; P < .001), independent of baseline QOL and demographic and clinical variables. To investigate whether there is an association between baseline parameters and PFS, we performed a multivariate regression analysis. Several previous analyses have assessed demographic and clinical predictors of PFS or OS in patients with RCC, but few have evaluated baseline QOL as a predictor.11–13,16
In the current study, we found that patients' baseline self-reported cancer-related QOL, kidney cancer–related symptoms, and overall health status, as measured by three validated scales, were predictive of time to progression or death in patients with mRCC; better baseline QOL was associated with longer PFS. This association was independent of treatment and key demographic and clinical variables. The FKSI scale is a 15-item questionnaire that is similar to the FKSI-DRS but also includes treatment-related symptoms for kidney cancer.20 A recent phase III trial of sorafenib versus placebo as second-line therapy for mRCC showed that total baseline FKSI score was predictive for OS (P < .0001), and 11 of the 15 individual items of the scale were also predictive for OS (P ≤ .001).10 Favorable QOL scores at baseline were associated with subsequent improvement in survival outcomes in patients with RCC.
Our study showed the presence of liver metastases and the number of MSKCC risk factors to be independent negative predictors of PFS in patients with mRCC. To our knowledge, no previous studies have found an association between a particular site of metastasis and shorter survival, but the presence of liver metastases showed borderline significance in terms of predicting OS in two other studies.14,15 This association reflects the implications of a compromised liver in patient functioning, survival, and QOL.
MSKCC risk factors, identified in a multivariate analysis of mRCC patients treated with IFN-α, have been most widely used in large clinical trials and include low serum hemoglobin, elevated corrected serum calcium, elevated serum lactate dehydrogenase, poor Karnofsky performance status, and an interval longer than 1 year between diagnosis and treatment.14 Our results showed the number of MSKCC risk factors to be predictive of shorter PFS in patients treated with either IFN-α or sunitinib. Other multivariate analyses of baseline clinical characteristics have found factors including (but not restricted to) baseline platelet and neutrophil counts, T stage, and number of disease sites to be independent predictors for shorter survival in patients with mRCC.11–13,16 We did not examine all of these factors, but we did examine the number of disease sites, and did not find it predictive for PFS in any of our QOL models.
Interestingly, female sex was predictive of shorter PFS on the EQ-VAS, but not on the other two QOL scales. To our knowledge, no other study has found sex to predict for survival in mRCC, but few have examined the predictive value of demographic characteristics. The reason why female sex was associated with shorter PFS warrants additional investigation.
In summary, patients with mRCC receiving sunitinib showed significantly superior PFS compared with those who received IFN-α, irrespective of baseline QOL or clinical characteristics. Better baseline QOL was associated with longer PFS, whereas the presence of liver metastases and the number of MSKCC risk factors were associated with shorter PFS. This study suggests the importance of good QOL as a prognostic factor for improved clinical outcomes in patients with advanced RCC or mRCC.
Authors' Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Acknowledgment
We thank the patients who participated in this study, as well as their families. Editorial assistance was provided by ACUMED (Tytherington, United Kingdom). The study was funded by Pfizer (New York, NY).
References
- 1.Motzer RJ, Bander NH, Nanus DM: Renal-cell carcinoma. N Engl J Med 335:865-875, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Dharma-Wardene M, Au HJ, Hanson J, et al: Baseline FACT-G score is a predictor of survival for advanced lung cancer. Qual Life Res 13:1209-1216, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Efficace F, Biganzoli L, Piccart M, et al: Baseline health-related quality-of-life data as prognostic factors in a phase III multicentre study of women with metastatic breast cancer. Eur J Cancer 40:1021-1030, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Lis CG, Gupta D, Granick J, et al: Can patient satisfaction with quality of life predict survival in advanced colorectal cancer? Support Care Cancer 14:1104-1110, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Maisey NR, Norman A, Watson M, et al: Baseline quality of life predicts survival in patients with advanced colorectal cancer. Eur J Cancer 38:1351-1357, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Efficace F, Bottomley A, Coens C, et al: Does a patient's self-reported health-related quality of life predict survival beyond key biomedical data in advanced colorectal cancer? Eur J Cancer 42:42-49, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Fang FM, Liu YT, Tang Y, et al: Quality of life as a survival predictor for patients with advanced head and neck carcinoma treated with radiotherapy. Cancer 100:425-432, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Roychowdhury DF, Hayden A, Liepa AM: Health-related quality-of-life parameters as independent prognostic factors in advanced or metastatic bladder cancer. J Clin Oncol 21:673-678, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Monk BJ, Huang HQ, Cella D, et al: Quality of life outcomes from a randomized phase III trial of cisplatin with or without topotecan in advanced carcinoma of the cervix: A Gynecologic Oncology Group Study. J Clin Oncol 23:4617-4625, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Bukowski R, Cella D, Gondek K, et al: Effects of sorafenib on symptoms and quality of life: Results from a large randomized placebo-controlled study in renal cancer. Am J Clin Oncol 30:220-227, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Bensalah K, Leray E, Fergelot P, et al: Prognostic value of thrombocytosis in renal cell carcinoma. J Urol 175:859-863, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Choueiri TK, Garcia JA, Elson P, et al: Clinical factors associated with outcome in patients with metastatic clear-cell renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. Cancer 110:543-550, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Kwak C, Park YH, Jeong CW, et al: Characteristics of metastasis as a prognostic factor for immunotherapy in metastatic renal cell carcinoma. Tumori 93:68-74, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Motzer RJ, Bacik J, Murphy BA, et al: Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 20:289-296, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Motzer RJ, Bacik J, Schwartz LH, et al: Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol 22:454-463, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Suppiah R, Shaheen PE, Elson P, et al: Thrombocytosis as a prognostic factor for survival in patients with metastatic renal cell carcinoma. Cancer 107:1793-1800, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Motzer RJ, Hutson TE, Tomczak P, et al: Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356:115-124, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Cella D, Li JZ, Cappelleri JC, et al: Quality of life in patients with metastatic renal cell carcinoma treated with sunitinib or interferon alfa: Results from a phase III randomized trial. J Clin Oncol 26:3763-3769, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Cella DF, Tulsky DS, Gray G, et al: The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J Clin Oncol 11:570-579, 1993 [DOI] [PubMed] [Google Scholar]
- 20.Cella D, Yount S, Du H, et al: Development and validation of the Functional Assessment of Cancer Therapy-Kidney Symptom Index (FKSI). J Support Oncol 4:191-199, 2006 [PubMed] [Google Scholar]
- 21.de Boer AG, van Lanschot JJ, Stalmeier PF, et al: Is a single-item visual analogue scale as valid, reliable and responsive as multi-item scales in measuring quality of life? Qual Life Res 13:311-320, 2004 [DOI] [PubMed] [Google Scholar]
- 22.EuroQol: A new facility for the measurement of health-related quality of life—The EuroQol Group. Health Policy 16:199-208, 1990 [DOI] [PubMed] [Google Scholar]
- 23.Kleinbaum DG, Klein M: Survival Analysis: A Self-Learning Text, ed 2, New York, NY, Springer, 2005