Abstract
IRF4, a member of the IRF family of transcription factors, is expressed in cells of the immune system where it transduces signals from various receptors to activate or repress gene expression. IRF4 expression is a key regulator of several steps in lymphoid, myeloid and dendritic cell differentiation, including the differentiation of mature B cells into antibody-secreting plasma cells. IRF4 expression is also associated with many lymphoid malignancies, with recent evidence pointing to an essential role in multiple myeloma, a malignancy of plasma cells. Interference with IRF4 expression is lethal to multiple myeloma cells, irrespective of their genetic etiology, making IRF4 an “Achilles’ heel” that may be exploited therapeutically.
Background
The Interferon Regulatory Factor (IRF) family is a broadly expressed set of transcription factors first described as downstream regulators of interferon signaling. Some IRFs also participate in signal transduction through pattern recognition receptors, such as the Toll-like receptors (TLRs). IRF4 (also known as pip, MUM1, LSIRF, NFEM5, and ICSAT) is an IRF family member that is restricted in expression to the immune system, a property it shares with the closely related family member IRF8. IRF4 and IRF8 have evolved as critical mediators of lymphoid, myeloid, and dendritic cell development (1–5).
Unlike other IRF family members, IRF4 is not induced by interferon but rather by diverse mitogenic stimuli, including antigen receptor engagement, lipopolysaccharide, and CD40 signaling (6–8). These stimuli all activate the NF-kB pathway, which leads to IRF4 promoter activation by NF-kB heterodimers (7–10). In addition, IRF4 transcription can be activated by the cytokine IL-4, implicating the transcription factor STAT6 in its activation (7, 8). The abundance of IRF4 varies within the hematopoietic system in a lineage and stage-specific manner (Figure 1). In mature B cells, IRF4 expression is repressed by the Mitf transcription factor (11). Consequently, Mitf-deficient B cells appear to undergo high rates of spontaneous activation and differentiation to plasma cells, ultimately leading to the production of autoantibodies. Germinal center B cells have particularly low levels of IRF4, possibly due the absence of NF-kB in these cells (12). Highest levels of IRF4 are achieved in terminally differentiated plasma cells by an unknown mechanism, although the potential for IRF4 to regulate its own transcription may contribute (13, 14).
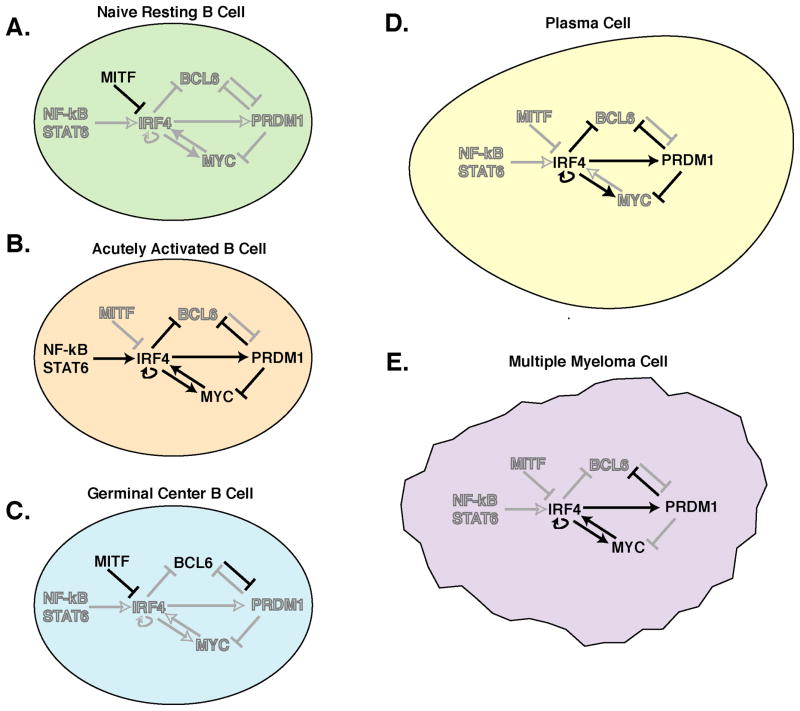
Figure 1. IRF4 regulatory networks in B cell differentiation and malignancy.
IRF4 lies at the center of regulatory networks that function at several stages of B cell differentiation and drive malignancy. A. In resting mature B cells IRF4 levels are low or absent due to MITF repression of the IRF4 gene. B. IRF4 expression is induced by BCR, CD40, and cytokine stimulation via NF-kB and STAT factors in activated B cells, and MITF repression is relieved. IRF4 acutely drives MYC and PRDM1 expression, as well as feeding back to drive its own expression. This results in a burst of cell division (driven by MYC) and subsequent differentiation of a proportion of the cells into short-lived Ig-secretors (driven by PRDM1). C. Germinal center B cells (GC) express IRF8 while IRF4 levels are low due to lack of NF-kB activation as well as repression of IRF4 by MITF. This allows the expression of the key GC regulator, BCL6, which in turn represses PRDM1, the plasma cell master regulator, locking the cells into the GC phenotype. Lack of IRF4 also contributes to the absence of MYC expression in GC B cells. D. As B cells exit the GC, they may differentiate to PCs. IRF4 expression, initiated by activation stimuli, becomes activation-independent via IRF4 auto-induction. IRF4 represses the GC regulator BCL6 and directly induces expression of the PC regulator PRDM1. PRDM1 also directly or indirectly represses BCL6 expression, committing cells to a PC fate. In addition, PRDM1 directly represses MYC, leading to the non-dividing, Ig-secreting phenotype of terminally differentiated PCs. E. In multiple myeloma (MM), the malignant counterpart of normal PCs, IRF4 represses BCL6 and promotes PRDM1 expression as in PCs, but both MYC and IRF4 are over-expressed. For unknown reasons, MYC is not repressed by PRDM1 in MM, leading to a positive feedback loop between IRF4 and MYC as seen in activated B cells. Thus MM fuses the activated B cell and PC IRF4-driven gene expression programs, leading to malignant transformation and cell division. Solid black arrows/lines – active regulation; Gray arrows/lines – inactive regulation; solid letters – active factor; open letters – inactive factor.
For many genes, IRF4 functions as a positive regulator of transcription, using its C-terminal transactivation domain (15). By contrast, IRF8 lacks a transactivation domain and therefore its binding to PU.1/SPIB may interfere with the ability of IRF4 to transactivate genes (15). IRF4 can repress the expression of some interferon-inducible genes by binding to interferon-stimulated response elements (ISREs) in their promoters, displacing the interferon-responsive IRF factors, Irf1 and Irf2 (15, 16). IRF4 can also repress other genes, such as BCL6, through as yet undefined mechanisms (10).
Alone, IRF4 binds DNA weakly due to its C-terminal autoinhibitory domain (15). However, IRF4 can bind with high avidity to the 3′ enhancers of both kappa and lambda immunoglobulin light chains in conjunction with the ETS-family transcription factor PU.1 or the closely related factor SPIB (17–19). Cooperative DNA binding is facilitated by two separate protein-protein interfaces between IRF4 and PU.1; a strong interaction is mediated by a C-terminal regulatory domain of IRF4 and PU.1’s phosphorylated PEST domain. In the absence of PU.1, this IRF4 regulatory domain inhibits DNA binding, but the interaction with PU.1 relieves this autoinhibition (20, 21). A second protein-protein interface exists between the DNA binding domains of IRF4 and PU.1 (15, 22). When binding with a partner such as PU.1 or SPIB, IRF4 regulates gene expression by binding a composite DNA element called ETS/ISRE-consensus element (EICE), which has the consensus sequence GGAANNGAAA that fuses the ETS binding motif (GGAA) with the IRF4 binding motif (AANNGAAA) (2, 15, 16). Although IRF4 can weakly interact with this element alone, cooperative binding with PU.1 increases its avidity by ~5-fold (22). IRF8 can similarly interact with PU.1, by virtue of its structural similarity with IRF4, but other IRF family members cannot (22, 23). Composite EICE elements also mediate cooperative binding of IRF4/IRF8 and PU.1/SPIB on other genes, including TLR4 and CIITA (24, 25).
Additional protein-protein interactions between IRF4 and other regulatory factors modulate its DNA binding properties and/or transactivation potential. The EICE site in the Ig 3′ kappa light chain enhancer is adjacent to a binding site for the E-box protein E47, encoded by the E2A gene (26). Protein-protein interactions between IRF4 and E47 increase the ability of these factors to activate transcription through this element by 50–100-fold (26). Similar transcriptional synergy between IRF4 and E47 operates at the CIITA and CD20 loci (25, 27). In transient transfection studies, IRF4 can also interact and cooperate with STAT6 to induce the STAT6-responsive gene CD23, an effect that can be blocked by physical interaction between IRF4 and either BCL6 or PRDM1 (7, 28). IRF4 can also functionally cooperate with the transcription factor NFATC2 to synergistically regulate the IL4 promoter in T cells (29). Finally, the ability of IRF4 to bind to the promoters of the pro-inflammatory cytokines IL-17 and IL-21 can be blocked by binding of IRF4 to a small GTPase termed IBP (30, 31). Interestingly, mice deficient in IBP develop a virulent autoimmune disease involving excessive IL-17 and IL-21 production, but breeding these mice to an IRF4 knockout strain ameliorates this disease (30).
IRF4 is an essential regulator at multiple steps in B cell differentiation. Both IRF4 and IRF8 are required in a redundant fashion to regulate the pre-B cell transition (32). B cells deficient in both IRF4 and IRF8 are arrested at the large pre-B cell stage in which cells have undergone VDJ recombination at the immunoglobulin heavy chain locus, express cytoplasmic immunoglobulin heavy chain, have elevated expression of the pre-B cell receptor (preBCR) on the cell surface, and are rapidly proliferating. Mechanistically, IRF4 or IRF8 downregulate expression of preBCR components by inducing the Ikaros and Aiolos transcription factors, thereby terminating preBCR-driven proliferation (33). In small pre-B cells, IRF4 activates immunoglobulin light chain gene rearrangement by directly binding to the 3′ kappa and lambda immunoglobulin enhancers (34). Additionally, IRF4 upregulates CXCR4, the receptor for the chemokine SDF-1. The potential consequence is the migration of small pre-B cells towards bone marrow stromal cells that express SDF-1 and away from IL-7-expressing stroma cells that lack SDF-1 (35). Such migration could further augment immunoglobulin light chain gene rearrangement since IL-7 inhibits this process (34). The ability of IRF4 to promote immunoglobulin light chain rearrangement serves an additional important function in central B cell tolerance by “editing” self-reactive B cell receptors (36). Exposure of a self-reactive B cell to its cognate self antigen upregulates IRF4, promoting immunoglobulin light chain rearrangement and replacement, which enables some B cell progeny to escape deletion and populate the peripheral lymphoid organs.
IRF4 plays a critical and non-redundant role in the adaptive immune responses of mature B cells (Figure 1) (6). Without IRF4, mature B cells accumulate in increased numbers in the spleen and lymph node, although there is a quantitative defect in the percentage of IgMhi, IgDlo, CD23lo mature B cells. Most striking is the complete absence of germinal center (GC) formation in response to antigenic challenge. Consequently, IRF4-deficient mice do not produce antigen-specific antibody upon immunization. The failure of IRF4-deficient cells to undergo GC differentiation may be due to a B cell intrinsic deficit since IRF4-deficient B cells proliferate poorly upon BCR-crosslinking or LPS treatment in vitro, although the proliferative response to anti-CD40 stimulation remains intact (6). Cell division in response to LPS is initially normal in IRF4-deficient B cells, but they do not progress through later rounds of division, apparently due to increased cell death (13). The inability of IRF4-deficient B cells to proliferate and survive upon B cell receptor stimulation may be sufficient to prevent GC B cell differentiation, but it is also conceivable that IRF4 may regulate a key gene(s) required to promote GC differentiation.
Serum immunoglobulins of all isotypes are exceedingly low in IRF4-deficient mice (6). This deficit may be attributed in part to the lack of GC formation in these animals but another contributing factor is a severe impairment in antibody secretion (13, 37). In vitro, neither LPS nor anti-CD40 treatment induces IRF4-deficient B cells to secrete antibody. This failure can be traced to a defect in the generation of antibody-secreting B cells and to a decrease in the amount of immunoglobulin secretion on a per cell basis (13, 37). In this regard, it is interesting that a quantitative trait locus in inbred mice for serum IgM levels maps precisely to the IRF4 locus (38).
IRF4 is also required for immunoglobulin class switch recombination (CSR) (13, 37). IRF4-deficient B cells fail to upregulate AID, the critical enzyme that mediates CSR (13, 37). Ectopic provision of AID to IRF4-deficient cells improved CSR but ectopic restoration of IRF4 was more effective (13), suggesting that IRF4 may control CSR in other ways. CSR requires sterile transcription through the immunoglobulin switch regions (39). Although sterile Cγ1 transcription was activated normally in IRF4-deficient B cells (37), it is possible that a relative defect in sterile Cμ transcription may impair CSR in these cells (40).
IRF4-deficient mice completely lack immunoglobulin-secreting plasma cells (PCs) (6). Three lines of evidence demonstrate that this phenotype reflects a B cell-intrinsic requirement for IRF4 during terminal differentiation of B cells to plasma cells. First, purified IRF4-deficient B cells are incapable of forming plasma cells under a variety of differentiation conditions in vitro (13, 37). Second, conditional ablation of the IRF4 locus in B cells blocks plasmacytic differentiation in vivo (37). Third, ectopic expression of IRF4 promotes plasmacytic differentiation (13). Although IRF4 is expressed at varying levels throughout B cell development, its expression peaks in plasma cells (15). Most GC B cells lack IRF4 expression and instead express IRF8 (41, 42). The few GC B cells that do express IRF4 have lost expression of BCL6 and Ki67 and express the master regulator of plasma cell differentiation PRDM1, encoded by PRDM1. These characteristics suggest that these IRF4+ GC cells are preparing to leave the GC and differentiate into plasma cells (43). Upon ectopic expression of IRF4 in mature B cells in vitro, only those cells with the highest IRF4 expression differentiate into plasma cells, suggesting a model in which graded expression of IRF4 during B cell activation and plasmacytic differentiation modulates its biological effect (Figure 1) (13).
Outside the B cell lineage, IRF4 is essential for several stages of T cell and myeloid differentiation. IRF4 is emerging as a critical regulator of T-helper cell differentiation, playing a required role in both Th2 and Th17 development by controlling cytokine expression and apoptosis (29, 44–47). Strikingly, the defective Th17 differentiation in IRF4-deficient animals renders them insensitive to experimental autoimmune encephalomyelitis (45). In addition, both IRF4 and IRF8 control the differentiation of various dendritic cell populations. IRF4 expression is solely required for CD4-positive dendritic cell development whereas IRF8 is solely required for CD8a-positive dendritic cell differentiation. Both factors function in a redundant fashion to promote CD4, CD8-negative dendritic cell and plasmacytoid dendritic cell differentiation (48). IRF4 expression in myeloid lineage interferes with toll-like receptor (TLR) signaling by competing with Irf5 for binding to the Myd88 protein, the key signaling adaptor that transmits signals from these receptors (49). Accordingly, IRF4-deficient mice are hypersensitive to CpG-induced shock, which is mediated by TLR9 signaling.
Finally, it is important to emphasize that IRF4-deficient mice have no apparent phenotypes outside of the lymphoid and myeloid lineages, in keeping with the restricted expression of IRF4 in these cell types. Therefore, potential therapies aimed at IRF4 would have restricted and potentially manageable on-target toxicities within the hematopoietic system (see below).
Clinical-Translational Advances
A recurring theme in the pathogenesis of B cell malignancies is the ability of transcription factors to serve as oncogenes when they are deregulated by chromosomal translocation, amplification, or mutation. Indeed, chromosomal abnormalities involving the transcription factors MYC and BCL6 are among the most common oncogenic events in multiple myeloma and diffuse large B cell lymphoma, respectively. In rare cases of multiple myeloma, the IRF4 gene is brought under the control of immunoglobulin heavy chain regulatory regions by a chromosomal translocation, providing genetic evidence that IRF4 can function as an oncogene (50, 51). Recently, recurrent translocations involving the IRF4 locus were also identified in T cell lymphomas including peripheral T cell lymphoma (PTCL), unspecified (6% of cases) and cutaneous anaplastic large cell lymphoma (57% of cases)(52). Although the exact nature of these translocations breakpoints was not determined, these observations may indicate an important role for IRF4 in the pathogenesis of certain T cell lymphomas. While IRF4 translocations establish its oncogenic potential, cancer cells can be dependent upon IRF4 even if the IRF4 locus is not genetically altered (see below).
The expression of IRF4 in human malignancies mirrors its expression in lymphoid activation and differentiation. Two oncogenic viruses, HTLV-I and EBV, activate the NF-kB pathway and consequently elevate IRF4 expression. HTLV-I, the etiologic agent in adult T cell leukemia (ATL), encodes an NF-kB transactivator, tax, which upregulates IRF4 (53). In some ATL cases, the T cell cytokine IL-15 has been implicated as a growth factor (54). Since IRF4 can transactivate the gene encoding the IL-15 receptor α chain, it is possible that IRF4 contributes to a growth-promoting autocrine loop in these cases (55). The EBV-encoded protein LMP1 activates the NF-kB pathway, thereby upregulating IRF4 (56). EBV infection transforms mature human B cells into lymphoblastoid cell lines. Knockdown of IRF4 in EBV+ lymphoblastoid cells decreases cell division and increases apoptosis. EBV has also been implicated in many lymphomas, including primary central nervous system lymphoma, Hodgkin’s lymphoma and rare nodal DLBCLs. In primary central nervous system lymphoma, expression of IRF4 correlates with expression of LMP1 (56).
Among malignancies of mature B cells, IRF4 is characteristically expressed at high levels in the activated B cell-like (57) subtype of diffuse large B cell lymphoma (DLBCL), which is the least curable subtype by current therapy (58, 59). DLBCL subtypes differ from one another by the expression of thousands of genes owing to their derivation from distinct stages of B cell differentiation (60). The ABC DLBCL subtype appears to be generated from post-germinal center B cells that are blocked during the process of plasmacytic derivation. A hallmark of the ABC DLBCL subtype is constitutive activation of the NF-kB pathway, a process mediated by the signaling adapter CARD11 (61, 62). IRF4 expression is driven by the constitutive NF-kB activity in this DLBCL subtype (63). By contrast, IRF4 is expressed at low levels in the germinal center B cell-like (GCB) DLBCL subtype, which is derived from normal germinal center B cells that have conspicuously low activity of the NF-kB pathway (12, 64).
Although the functional role of IRF4 in ABC DLBCL remains to be fully elucidated, it is intriguing that the IRF4 interacting factor SPIB has emerged as oncogene in this lymphoma subtype (65, 66). SPIB is deregulated by recurrent amplifications in roughly one quarter of ABC DLBCL cases and by a translocation in an ABC DLBCL cell line, but these events occur only rarely in GCB DLBCLs. Moreover, SPIB knockdown was lethal to ABC DLBCL cell lines but not GCB DLBCL cell lines. These results raise the intriguing possibility that IRF4 may cooperate with SPIB to promote the malignant phenotype of ABC DLBCL. However, the role of IRF4 in ABC DLBCL is complicated by the fact that it can promote plasmacytic differentiation of normal B cells by binding and transactivating the gene encoding PRDM1 (Figure 1) (13). Interestingly, many ABC DLBCLs have inactivated PRDM1, either by deletion, translocation or mutation, thereby rendering it insensitive the action of IRF4 (67, 68). Another way to prevent plasmacytic differentiation is to increase expression of BCL6, a direct repressor of PRDM1 (14, 69, 70). IRF4 binds to a region in the BCL6 first intron and represses its expression (10). Consequently, some DLBCLs accumulate somatic mutations in the IRF4 binding region of BCL6, thereby causing high expression of BCL6 to be maintained in the face of IRF4 expression. Thus, ABC DLBCLs sustain a variety of genetic lesions that may allow IRF4 action as an oncogene while restraining its ability to promote plasmacytic differentiation.
In chronic lymphocytic leukemia (CLL), there is a solid yet enigmatic association between polymorphisms in the IRF4 locus and the development of the disease. In a genome-wide association study, single nucleotide polymorphisms (SNPs) in the 3′ UTR region of IRF4 exhibited the strongest statistical association with CLL susceptibility, and this association was observed in several independent patient cohorts (71). The functional effect of the IRF4 alleles on IRF4 expression or function is unknown. Interestingly, the IRF4 SNP was associated more strongly with the subtype of CLL that has mutated immunoglobulin genes, presumably indicating an origin from post-germinal center memory B cells. Given the important role of IRF4 in plasmacytic differentiation, it is conceivable that the IRF4 SNPs influence CLL risk by impairing the differentiation of memory B cells into plasma cells. Alternatively, given the requirement for IRF4 in B cell activation (6), the IRF4 SNPs may control an aspect of the B cell activation program that contributes to aberrant B cell receptor signaling in CLL.
The role of IRF4 in promoting malignancy has been most clearly shown in multiple myeloma (MM), a malignancy of plasma cells. Multiple myeloma presents at a peak age of 65–70 and accounts for approximately 15% of all hematological malignancies, second only to non-Hodgkin’s lymphoma. Despite vigorous clinical research efforts directed at discovering curative therapies, MM patients still have a median survival time of just three years. Current therapy includes the use of alkylating agents, glucocorticoids, proteosome inhibitors, farnesylation inhibitors, and thalidomide (and its analogs) (72). An effective therapy for patients under sixty-five years of age combines high-dose cytotoxic agents with autologous bone marrow transplantation to reconstitute the patient’s hematologic system (73). Treatment regimens are further limited in older patients who cannot tolerate intensive therapy, and a variety of single-drug and drug cocktail regimens are employed in this population. Recently, the combination of dexamethasone and the thalidomide analog lenolidomide appears promising in relapsed and refractory MM, but still the responses do not appear curative (74).
The developmental and molecular biology of MM contribute to this difficulty in treatment. MM cells, like their normal plasma cell counterparts, reside in the bone marrow, which provides a microenvironment that sustains their proliferation and survival and protects them from the cytotoxic effect of therapy (75). In addition, MM is initiated and maintained by a wide spectrum of genetic anomalies, including translocations of oncogenes to the immunoglobulin loci (D-type cyclins, c-Maf, MafB, c-myc, MMSET, FGFR3), as well as mutations of oncogenes (N-ras, K-ras) and tumor suppressors (p53, p18) (76). In addition, translocations between the immunoglobulin heavy chain locus and the IRF4 locus have been characterized in a few MM cell lines and rare MM patient samples (50, 77). This genetic heterogeneity is reflected in the gene expression patterns of MM patient samples, which have been used to classify MM into 7 molecular subtypes (78).
Recently, we have designed and employed an RNA interference genetic screen to identify new therapeutic targets in cancer (61). This so-called “Achilles heel” screen identifies genes that are critical for tumor proliferation and/or survival. Applying this Achilles heel screen in MM revealed IRF4 to be an essential gene in this malignancy (14). Knockdown of IRF4 by RNA interference induces a rapid and profound non-apoptotic cell death in all MM cell lines tested, regardless of the suite of genetic anomalies inherent to each line. The genetic repertoire controlled by IRF4 was defined using gene expression profiling following IRF4 knockdown together with genome-wide chromatin immunoprecipitation analysis of IRF4 binding sites (14). These efforts revealed that IRF4 controls an aberrant gene expression program in MM that fuses the gene expression programs of activated B cells and normal plasma cells. Several key metabolic pathways lie downstream of IRF4 in MM cells, including lipid and cholesterol biosynthesis, glucose metabolism, general transcriptional regulation and cell cycle progression. The toxicity of IRF4 knockdown in MM appears to be ‘death by a thousand cuts’ since numerous IRF4 target genes play crucial roles in the proliferation and survival of MM cells (Figure 2).
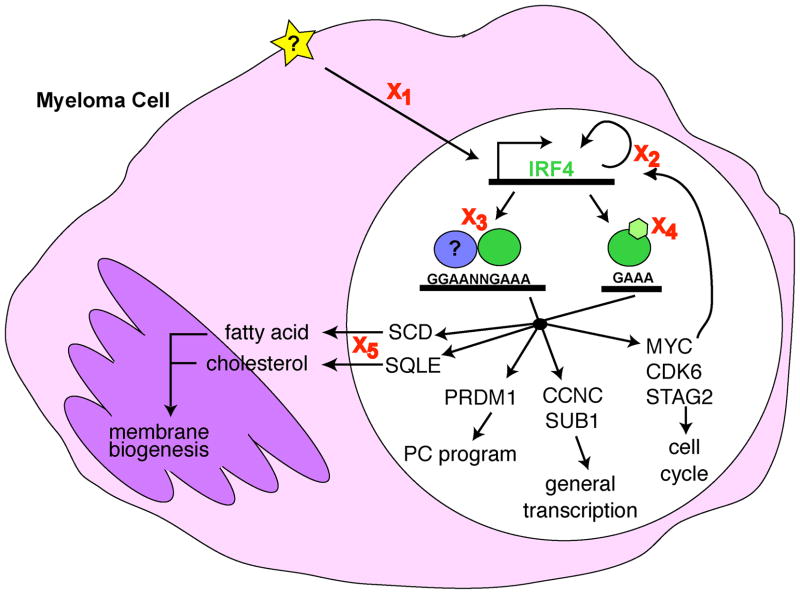
Figure 2. Disrupting IRF4 Function in Myeloma.
Myeloma cells are addicted to IRF4 expression such that even a modest decrease in IRF4 levels leads to cell death. IRF4 (green) controls an aberrant gene expression program in MM cells that fuses and expands the gene expression programs of activated B cells and PCs and directly controls the expression genes critical for cell cycle control, transcriptional regulation, plasma cell differentiation, and membrane biogenesis. There are several points at which IRF4 activity might be interrupted in a therapeutically advantageous manner (X). X1, target the signals activating (star) IRF4 expression, such as NF-kB. X2, target the known regulators of IRF4 expression in MM, MYC and IRF4 itself. X3, target the ability of IRF4 to interact with a binding partner (blue), thereby preventing IRF4 binding to target DNA sequences. X4, target putative post-translational modifications of IRF4 that may alter its ability to bind DNA or activate transcription. X5, target critical pathways downstream of IRF4. For example, the isoprenoid/cholesterol biosynthesis pathway can be targeted by drugs such as statins or farnesylation inhibitors. Any reduction in IRF4 activity will result in cell ‘death by a thousand cuts’ due to metabolic collapse following the downregulation of several key pathways.
A particularly noteworthy IRF4 target gene in MM is MYC (Figures 1 & 2) (14). IRF4 binds directly to the MYC promoter region in MM cells and transactivates its expression. Conversely, MYC transactivates IRF4 by binding to an evolutionarily conserved intronic region, creating a positive autoregulatory feedback loop. The expression of MYC in the malignant cells of MM is decidedly abnormal since normal plasma cells do not express MYC due to repression by PRDM1 (Figure 1). MYC is frequently deregulated by translocation or amplification in MM (79), events that presumably upregulate both MYC and IRF4. Indeed, the expression of IRF4 and MYC are positively correlated in primary bone marrow samples from patients with MM (14). It is important to emphasize that most MMs do not have genetic lesions in the IRF4 locus but are nonetheless completely dependent upon the aberrant genetic program controlled by IRF4. IRF4 dependency in MM is thus a prime example of a newly appreciated phenomenon termed ‘non-oncogene addiction’ in which cancer cells develop an exaggerated reliance upon a normal cellular protein as a result of their genetic and biological abnormalities (80).
Although transcription factors have been considered intractable therapeutic targets, recent successful targeting of p53 (81) and BCL6 (82) using small molecule inhibitors provides hope that IRF4 can be attacked as an Achilles heel of multiple myeloma and other IRF4-dependent malignancies. There are several aspects of IRF4 biology that may be exploited for therapeutic purposes (Figure 2). First, therapies that target the transcription of IRF4 could be envisioned. In malignancies in which IRF4 is dependent upon NF-kB signaling – ABC DLBCL, ATL and EBV-associated processes – inhibitors of IkB kinase β would be effective. Although NF-kB is constitutively active many cases of MM (83, 84), IRF4 expression is not dependent upon this pathway since MM cell lines without NF-kB activation nonetheless have high IRF4 expression. Rather, the expression of IRF4 in MM reflects the constitutive high expression of IRF4 that is also observed in normal plasma cells (13, 14, 37). The mechanisms responsible for IRF4 expression in normal and malignant plasma cells are largely unknown, but interestingly IRF4 binds to its own promoter region, creating the potential for positive autoregulation (Figures 1 & 2). Such autoregulation might create a therapeutic opportunity since any maneuver that would downregulate IRF4 expression or activity would secondarily downregulate IRF4 transcription, thereby potentiating the effect. In addition, MYC and IRF4 form a positive autoregulatory loop (Figures 1 & 2), implying that any therapy targeting IRF4 transcription would have the added benefit of decreasing MYC transcription. Of interest in this regard is a recent study showing that the protein kinase Cβ inhibitor, enzastaurin, has anti-proliferative and pro-apoptotic activity in MM and coordinately downregulates expression of both IRF4 and MYC (85).
A second therapeutic opportunity may be provided by the fact that IRF4 binds DNA cooperatively with several DNA-binding factors, notably the ETS-family proteins PU.1 and SPIB (Figure 2). The IRF4-PU.1 interaction requires phosphorylation of PU.1 on a single serine in its PEST domain (17), and this serine is conserved in SPIB. Casein kinase II can phosphorylate this site in vitro, but the responsible kinase in vivo has not been identified. It is conceivable that kinase inhibitors that block PU.1 and possibly SPIB phosphorylation might be exploited for the treatment of B cell malignancies that express these ETS factors together with IRF4, such as ABC DLBCL. Since PU.1 and SPIB are not expressed in MM, it is currently unclear whether IRF4 requires other cooperating transcription factors to regulate gene expression in MM and plasma cells.
A third potential strategy to inhibit IRF4 might be to alter its post-translational modifications (Figure 2). IRF4 appears to undergo a regulated conformational change by interacting with a member of the immunophilin family, FKBP52 (86). FKBP52 is a peptidyl-prolyl isomerase that associates with a central proline-rich domain of IRF4. FKBP52 alters IRF4 protein structure, as evidenced by altered proteolytic cleavage, and inhibits the ability of IRF4 to bind DNA and transactivate reporter constructs. Drugs that inhibit FKBP52, such as ascomycin, increase IRF4 function (86). One would predict that cellular mechanisms must exist that release IRF4 from the inhibitory effects of FKBP52; these might provide therapeutic opportunities. A second post-translational modification to consider is phosphorylation. The IRF4 homologue IRF8 is tyrosine phosphorylated in cells that overexpress this factor, although the responsible kinase has not been identified (87).
Finally, it is important to envision potential side effects of a therapy aimed at IRF4. The phenotypes of IRF4-deficient mice are strictly limited to the immune system, including defects the differentiation of plasma cells and certain dendritic cell subsets as well as in lymphocyte activation. Notably, mice lacking one allele of IRF4 are phenotypically normal (6) yet a ~50% knockdown of IRF4 mRNA and protein was sufficient to kill myeloma cell lines (14). Thus, a therapeutic window could exist in which IRF4-directed therapy would kill IRF4-addicted malignant cells while sparing normal cells.
Acknowledgments
The authors declare no competing financial interests. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. P.R. is a HHMI-NIH Research Scholar.
References
- 1.Ozato K, Tailor P, Kubota T. The interferon regulatory factor family in host defense: mechanism of action. J Biol Chem. 2007;282:20065–9. doi: 10.1074/jbc.R700003200. [DOI] [PubMed] [Google Scholar]
- 2.Pernis AB. The role of IRF-4 in B and T cell activation and differentiation. J Interferon Cytokine Res. 2002;22:111–20. doi: 10.1089/107999002753452728. [DOI] [PubMed] [Google Scholar]
- 3.Lu R. Interferon regulatory factor 4 and 8 in B-cell development. Trends Immunol. 2008;29:487–92. doi: 10.1016/j.it.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanno Y, Levi BZ, Tamura T, Ozato K. Immune cell-specific amplification of interferon signaling by the IRF-4/8-PU.1 complex. J Interferon Cytokine Res. 2005;25:770–9. doi: 10.1089/jir.2005.25.770. [DOI] [PubMed] [Google Scholar]
- 5.Tamura T, Yanai H, Savitsky D, Taniguchi T. The IRF family transcription factors in immunity and oncogenesis. Annu Rev Immunol. 2008;26:535–84. doi: 10.1146/annurev.immunol.26.021607.090400. [DOI] [PubMed] [Google Scholar]
- 6.Mittrucker HW, Matsuyama T, Grossman A, et al. Requirement for the transcription factor LSIRF/IRF4 for mature B and T lymphocyte function. Science. 1997;275:540–3. doi: 10.1126/science.275.5299.540. [DOI] [PubMed] [Google Scholar]
- 7.Gupta S, Jiang M, Anthony A, Pernis AB. Lineage-specific modulation of interleukin 4 signaling by interferon regulatory factor 4. J Exp Med. 1999;190:1837–48. doi: 10.1084/jem.190.12.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grumont RJ, Gerondakis S. Rel induces interferon regulatory factor 4 (IRF-4) expression in lymphocytes: modulation of interferon-regulated gene expression by rel/nuclear factor kappaB. J Exp Med. 2000;191:1281–92. doi: 10.1084/jem.191.8.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaffer AL, Wright G, Yang L, et al. A library of gene expression signatures to illuminate normal and pathological lymphoid biology. Immunol Rev. 2006;210:67–85. doi: 10.1111/j.0105-2896.2006.00373.x. [DOI] [PubMed] [Google Scholar]
- 10.Saito M, Gao J, Basso K, et al. A signaling pathway mediating downregulation of BCL6 in germinal center B cells is blocked by BCL6 gene alterations in B cell lymphoma. Cancer Cell. 2007;12:280–92. doi: 10.1016/j.ccr.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Lin L, Gerth AJ, Peng SL. Active inhibition of plasma cell development in resting B cells by microphthalmia-associated transcription factor. J Exp Med. 2004;200:115–22. doi: 10.1084/jem.20040612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaffer AL, Rosenwald A, Hurt EM, et al. Signatures of the immune response. Immunity. 2001;15:375–85. doi: 10.1016/s1074-7613(01)00194-7. [DOI] [PubMed] [Google Scholar]
- 13.Sciammas R, Shaffer AL, Schatz JH, Zhao H, Staudt LM, Singh H. Graded expression of interferon regulatory factor-4 coordinates isotype switching with plasma cell differentiation. Immunity. 2006;25:225–36. doi: 10.1016/j.immuni.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Shaffer AL, Emre NC, Lamy L, et al. IRF4 addiction in multiple myeloma. Nature. 2008;454:226–31. doi: 10.1038/nature07064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brass AL, Kehrli E, Eisenbeis CF, Storb U, Singh H. Pip, a lymphoid-restricted IRF, contains a regulatory domain that is important for autoinhibition and ternary complex formation with the Ets factor PU.1. Genes Dev. 1996;10:2335–47. doi: 10.1101/gad.10.18.2335. [DOI] [PubMed] [Google Scholar]
- 16.Yamagata T, Nishida J, Tanaka S, et al. A novel interferon regulatory factor family transcription factor, ICSAT/Pip/LSIRF, that negatively regulates the activity of interferon-regulated genes. Mol Cell Biol. 1996;16:1283–94. doi: 10.1128/mcb.16.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pongubala JM, Nagulapalli S, Klemsz MJ, McKercher SR, Maki RA, Atchison ML. PU.1 recruits a second nuclear factor to a site important for immunoglobulin kappa 3′ enhancer activity. Mol Cell Biol. 1992;12:368–78. doi: 10.1128/mcb.12.1.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisenbeis CF, Singh H, Storb U. Pip, a novel IRF family member, is a lymphoid-specific, PU.1-dependent transcriptional activator. Genes Dev. 1995;9:1377–87. doi: 10.1101/gad.9.11.1377. [DOI] [PubMed] [Google Scholar]
- 19.Su GH, Ip HS, Cobb BS, Lu MM, Chen HM, Simon MC. The Ets protein Spi-B is expressed exclusively in B cells and T cells during development. J Exp Med. 1996;184:203–14. doi: 10.1084/jem.184.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brass AL, Zhu AQ, Singh H. Assembly requirements of PU.1-Pip (IRF-4) activator complexes: inhibiting function in vivo using fused dimers. Embo J. 1999;18:977–91. doi: 10.1093/emboj/18.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pongubala JM, Van Beveren C, Nagulapalli S, et al. Effect of PU.1 phosphorylation on interaction with NF-EM5 and transcriptional activation. Science. 1993;259:1622–5. doi: 10.1126/science.8456286. [DOI] [PubMed] [Google Scholar]
- 22.Escalante CR, Brass AL, Pongubala JM, et al. Crystal structure of PU.1/IRF-4/DNA ternary complex. Mol Cell. 2002;10:1097–105. doi: 10.1016/s1097-2765(02)00703-7. [DOI] [PubMed] [Google Scholar]
- 23.Marecki S, Atchison ML, Fenton MJ. Differential expression and distinct functions of IFN regulatory factor 4 and IFN consensus sequence binding protein in macrophages. J Immunol. 1999;163:2713–22. [PubMed] [Google Scholar]
- 24.Rehli M, Poltorak A, Schwarzfischer L, Krause SW, Andreesen R, Beutler B. PU.1 and interferon consensus sequence-binding protein regulate the myeloid expression of the human Toll-like receptor 4 gene. J Biol Chem. 2000;275:9773–81. doi: 10.1074/jbc.275.13.9773. [DOI] [PubMed] [Google Scholar]
- 25.van der Stoep N, Quinten E, Marcondes Rezende M, van den Elsen PJ. E47, IRF-4, and PU.1 synergize to induce B-cell-specific activation of the class II transactivator promoter III (CIITA-PIII) Blood. 2004;104:2849–57. doi: 10.1182/blood-2004-03-0790. [DOI] [PubMed] [Google Scholar]
- 26.Nagulapalli S, Atchison ML. Transcription factor Pip can enhance DNA binding by E47, leading to transcriptional synergy involving multiple protein domains. Mol Cell Biol. 1998;18:4639–50. doi: 10.1128/mcb.18.8.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Himmelmann A, Riva A, Wilson GL, Lucas BP, Thevenin C, Kehrl JH. PU.1/Pip and basic helix loop helix zipper transcription factors interact with binding sites in the CD20 promoter to help confer lineage- and stage-specific expression of CD20 in B lymphocytes. Blood. 1997;90:3984–95. [PubMed] [Google Scholar]
- 28.Gupta S, Anthony A, Pernis AB. Stage-specific modulation of IFN-regulatory factor 4 function by Kruppel-type zinc finger proteins. J Immunol. 2001;166:6104–11. doi: 10.4049/jimmunol.166.10.6104. [DOI] [PubMed] [Google Scholar]
- 29.Rengarajan J, Mowen KA, McBride KD, Smith ED, Singh H, Glimcher LH. Interferon regulatory factor 4 (IRF4) interacts with NFATc2 to modulate interleukin 4 gene expression. J Exp Med. 2002;195:1003–12. doi: 10.1084/jem.20011128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Q, Yang W, Gupta S, et al. IRF-4-binding protein inhibits interleukin-17 and interleukin-21 production by controlling the activity of IRF-4 transcription factor. Immunity. 2008;29:899–911. doi: 10.1016/j.immuni.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fanzo JC, Yang W, Jang SY, et al. Loss of IRF-4-binding protein leads to the spontaneous development of systemic autoimmunity. J Clin Invest. 2006;116:703–14. doi: 10.1172/JCI24096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu R, Medina KL, Lancki DW, Singh H. IRF-4,8 orchestrate the pre-B-to-B transition in lymphocyte development. Genes Dev. 2003;17:1703–8. doi: 10.1101/gad.1104803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma S, Pathak S, Trinh L, Lu R. Interferon regulatory factors 4 and 8 induce the expression of Ikaros and Aiolos to down-regulate pre-B-cell receptor and promote cell-cycle withdrawal in pre-B-cell development. Blood. 2008;111:1396–403. doi: 10.1182/blood-2007-08-110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson K, Hashimshony T, Sawai CM, et al. Regulation of immunoglobulin light-chain recombination by the transcription factor IRF-4 and the attenuation of interleukin-7 signaling. Immunity. 2008;28:335–45. doi: 10.1016/j.immuni.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 35.Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20:707–18. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Pathak S, Ma S, Trinh L, Lu R. A role for interferon regulatory factor 4 in receptor editing. Mol Cell Biol. 2008;28:2815–24. doi: 10.1128/MCB.01946-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein U, Casola S, Cattoretti G, et al. Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nat Immunol. 2006;7:773–82. doi: 10.1038/ni1357. [DOI] [PubMed] [Google Scholar]
- 38.Corte-Real J, Rodo J, Almeida P, et al. Irf4 is a positional and functional candidate gene for the control of serum IgM levels in the mouse. Genes Immun. 2008 doi: 10.1038/gene.2008.73. [DOI] [PubMed] [Google Scholar]
- 39.Chaudhuri J, Alt FW. Class-switch recombination: interplay of transcription, DNA deamination and DNA repair. Nat Rev Immunol. 2004;4:541–52. doi: 10.1038/nri1395. [DOI] [PubMed] [Google Scholar]
- 40.Nagulapalli S, Goheer A, Pitt L, McIntosh LP, Atchison ML. Mechanism of e47-Pip interaction on DNA resulting in transcriptional synergy and activation of immunoglobulin germ line sterile transcripts. Mol Cell Biol. 2002;22:7337–50. doi: 10.1128/MCB.22.20.7337-7350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cattoretti G, Shaknovich R, Smith PM, Jack HM, Murty VV, Alobeid B. Stages of germinal center transit are defined by B cell transcription factor coexpression and relative abundance. J Immunol. 2006;177:6930–9. doi: 10.4049/jimmunol.177.10.6930. [DOI] [PubMed] [Google Scholar]
- 42.Lee CH, Melchers M, Wang H, et al. Regulation of the germinal center gene program by interferon (IFN) regulatory factor 8/IFN consensus sequence-binding protein. J Exp Med. 2006;203:63–72. doi: 10.1084/jem.20051450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Falini B, Fizzotti M, Pucciarini A, et al. A monoclonal antibody (MUM1p) detects expression of the MUM1/IRF4 protein in a subset of germinal center B cells, plasma cells, and activated T cells. Blood. 2000;95:2084–92. [PubMed] [Google Scholar]
- 44.Lohoff M, Mittrucker HW, Brustle A, et al. Enhanced TCR-induced apoptosis in interferon regulatory factor 4-deficient CD4(+) Th cells. J Exp Med. 2004;200:247–53. doi: 10.1084/jem.20040182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brustle A, Heink S, Huber M, et al. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007;8:958–66. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 46.Honma K, Kimura D, Tominaga N, Miyakoda M, Matsuyama T, Yui K. Interferon regulatory factor 4 differentially regulates the production of Th2 cytokines in naive vs. effector/memory CD4+ T cells. Proc Natl Acad Sci U S A. 2008;105:15890–5. doi: 10.1073/pnas.0803171105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu CM, Jang SY, Fanzo JC, Pernis AB. Modulation of T cell cytokine production by interferon regulatory factor-4. J Biol Chem. 2002;277:49238–46. doi: 10.1074/jbc.M205895200. [DOI] [PubMed] [Google Scholar]
- 48.Tamura T, Tailor P, Yamaoka K, et al. IFN regulatory factor-4 and -8 govern dendritic cell subset development and their functional diversity. J Immunol. 2005;174:2573–81. doi: 10.4049/jimmunol.174.5.2573. [DOI] [PubMed] [Google Scholar]
- 49.Negishi H, Ohba Y, Yanai H, et al. Negative regulation of Toll-like-receptor signaling by IRF-4. Proc Natl Acad Sci U S A. 2005;102:15989–94. doi: 10.1073/pnas.0508327102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iida S, Rao PH, Butler M, et al. Deregulation of MUM1/IRF4 by chromosomal translocation in multiple myeloma. Nat Genet. 1997;17:226–30. doi: 10.1038/ng1097-226. [DOI] [PubMed] [Google Scholar]
- 51.Tsuboi K, Iida S, Inagaki H, et al. MUM1/IRF4 expression as a frequent event in mature lymphoid malignancies. Leukemia. 2000;14:449–56. doi: 10.1038/sj.leu.2401696. [DOI] [PubMed] [Google Scholar]
- 52.Feldman AL, Law M, Remstein ED, et al. Recurrent translocations involving the IRF4 oncogene locus in peripheral T-cell lymphomas. Leukemia. 2008 doi: 10.1038/leu.2008.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharma S, Mamane Y, Grandvaux N, et al. Activation and regulation of interferon regulatory factor 4 in HTLV type 1-infected T lymphocytes. AIDS Res Hum Retroviruses. 2000;16:1613–22. doi: 10.1089/08892220050193047. [DOI] [PubMed] [Google Scholar]
- 54.Kukita T, Arima N, Matsushita K, et al. Autocrine and/or paracrine growth of adult T-cell leukaemia tumour cells by interleukin 15. Br J Haematol. 2002;119:467–74. doi: 10.1046/j.1365-2141.2002.03813.x. [DOI] [PubMed] [Google Scholar]
- 55.Mariner JM, Mamane Y, Hiscott J, Waldmann TA, Azimi N. IFN regulatory factor 4 participates in the human T cell lymphotropic virus type I-mediated activation of the IL-15 receptor alpha promoter. J Immunol. 2002;168:5667–74. doi: 10.4049/jimmunol.168.11.5667. [DOI] [PubMed] [Google Scholar]
- 56.Xu D, Zhao L, Del Valle L, Miklossy J, Zhang L. Interferon regulatory factor 4 is involved in Epstein-Barr virus-mediated transformation of human B lymphocytes. J Virol. 2008;82:6251–8. doi: 10.1128/JVI.00163-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Majumder S, Zhou LZ, Chaturvedi P, Babcock G, Aras S, Ransohoff RM. p48/STAT-1alpha-containing complexes play a predominant role in induction of IFN-gamma-inducible protein, 10 kDa (IP-10) by IFN-gamma alone or in synergy with TNF-alpha. J Immunol. 1998;161:4736–44. [PubMed] [Google Scholar]
- 58.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–11. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 59.Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937–47. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 60.Staudt LM, Dave S. The biology of human lymphoid malignancies revealed by gene expression profiling. Adv Immunol. 2005;87:163–208. doi: 10.1016/S0065-2776(05)87005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ngo VN, Davis RE, Lamy L, et al. A loss-of-function RNA interference screen for molecular targets in cancer. Nature. 2006;441:106–10. doi: 10.1038/nature04687. [DOI] [PubMed] [Google Scholar]
- 62.Lenz G, Davis RE, Ngo VN, et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science. 2008;319:1676–9. doi: 10.1126/science.1153629. [DOI] [PubMed] [Google Scholar]
- 63.Lam LT, Davis RE, Wright G, et al. Small Molecule Inhibitors of IkB-Kinase are Selectively Toxic for Subgroups of Diffuse Large B Cell Lymphoma Defined by Gene Expression Profiling. Clin Cancer Res. 2005;11:28–40. [PubMed] [Google Scholar]
- 64.Basso K, Klein U, Niu H, et al. Tracking CD40 signaling during germinal center development. Blood. 2004;104:4088–96. doi: 10.1182/blood-2003-12-4291. [DOI] [PubMed] [Google Scholar]
- 65.Lenz G, Nagel I, Siebert R, et al. Aberrant immunoglobulin class switch recombination and switch translocations in activated B cell-like diffuse large B cell lymphoma. J Exp Med. 2007;204:633–43. doi: 10.1084/jem.20062041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lenz G, Wright GW, Emre NC, et al. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci U S A. 2008;105:13520–5. doi: 10.1073/pnas.0804295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pasqualucci L, Compagno M, Houldsworth J, et al. Inactivation of the PRDM1/BLIMP1 gene in diffuse large B cell lymphoma. J Exp Med. 2006;203:311–7. doi: 10.1084/jem.20052204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tam W, Gomez M, Chadburn A, Lee JW, Chan WC, Knowles DM. Mutational analysis of PRDM1 indicates a tumor-suppressor role in diffuse large B-cell lymphomas. Blood. 2006;107:4090–100. doi: 10.1182/blood-2005-09-3778. [DOI] [PubMed] [Google Scholar]
- 69.Shaffer AL, Yu X, He Y, Boldrick J, Chan EP, Staudt LM. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity. 2000;13:199–212. doi: 10.1016/s1074-7613(00)00020-0. [DOI] [PubMed] [Google Scholar]
- 70.Tunyaplin C, Shaffer AL, Angelin-Duclos CD, Yu X, Staudt LM, Calame KL. Direct repression of prdm1 by Bcl-6 inhibits plasmacytic differentiation. J Immunol. 2004;173:1158–65. doi: 10.4049/jimmunol.173.2.1158. [DOI] [PubMed] [Google Scholar]
- 71.Di Bernardo MC, Crowther-Swanepoel D, Broderick P, et al. A genome-wide association study identifies six susceptibility loci for chronic lymphocytic leukemia. Nat Genet. 2008;40:1204–10. doi: 10.1038/ng.219. [DOI] [PubMed] [Google Scholar]
- 72.San-Miguel J, Harousseau JL, Joshua D, Anderson KC. Individualizing treatment of patients with myeloma in the era of novel agents. J Clin Oncol. 2008;26:2761–6. doi: 10.1200/JCO.2007.15.2546. [DOI] [PubMed] [Google Scholar]
- 73.Barlogie B, Jagannath S, Vesole DH, et al. Superiority of tandem autologous transplantation over standard therapy for previously untreated multiple myeloma. Blood. 1997;89:789–93. [PubMed] [Google Scholar]
- 74.Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123–32. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 75.Podar K, Chauhan D, Anderson KC. Bone marrow microenvironment and the identification of new targets for myeloma therapy. Leukemia. 2008 doi: 10.1038/leu.2008.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bergsagel PL, Kuehl WM. Molecular pathogenesis and a consequent classification of multiple myeloma. J Clin Oncol. 2005;23:6333–8. doi: 10.1200/JCO.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 77.Yoshida S, Nakazawa N, Iida S, et al. Detection of MUM1/IRF4-IgH fusion in multiple myeloma. Leukemia. 1999;13:1812–6. doi: 10.1038/sj.leu.2401563. [DOI] [PubMed] [Google Scholar]
- 78.Zhan F, Huang Y, Colla S, et al. The molecular classification of multiple myeloma. Blood. 2006;108:2020–8. doi: 10.1182/blood-2005-11-013458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dib A, Gabrea A, Glebov OK, Bergsagel PL, Kuehl WM. Characterization of MYC translocations in multiple myeloma cell lines. J Natl Cancer Inst Monogr. 2008:25–31. doi: 10.1093/jncimonographs/lgn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Solimini NL, Luo J, Elledge SJ. Non-oncogene addiction and the stress phenotype of cancer cells. Cell. 2007;130:986–8. doi: 10.1016/j.cell.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 81.Vassilev LT, Vu BT, Graves B, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–8. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 82.Polo JM, Dell’Oso T, Ranuncolo SM, et al. Specific peptide interference reveals BCL6 transcriptional and oncogenic mechanisms in B-cell lymphoma cells. Nat Med. 2004;10:1329–35. doi: 10.1038/nm1134. [DOI] [PubMed] [Google Scholar]
- 83.Annunziata CM, Davis RE, Demchenko Y, et al. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 2007;12:115–30. doi: 10.1016/j.ccr.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Keats JJ, Fonseca R, Chesi M, et al. Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell. 2007;12:131–44. doi: 10.1016/j.ccr.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Verdelli D, Nobili L, Todoerti K, et al. Molecular targeting of the PKC-beta inhibitor enzastaurin ( LY317615) in multiple myeloma involves a coordinated downregulation of MYC and IRF4 expression. Hematol Oncol. 2008 doi: 10.1002/hon.875. [DOI] [PubMed] [Google Scholar]
- 86.Mamane Y, Sharma S, Petropoulos L, Lin R, Hiscott J. Posttranslational regulation of IRF-4 activity by the immunophilin FKBP52. Immunity. 2000;12:129–40. doi: 10.1016/s1074-7613(00)80166-1. [DOI] [PubMed] [Google Scholar]
- 87.Sharf R, Meraro D, Azriel A, et al. Phosphorylation events modulate the ability of interferon consensus sequence binding protein to interact with interferon regulatory factors and to bind DNA. J Biol Chem. 1997;272:9785–92. doi: 10.1074/jbc.272.15.9785. [DOI] [PubMed] [Google Scholar]