Abstract
Hemophilia A gene therapy using recombinant adenovirus-associated virus (AAV) vectors has been hampered by the size of the factor VIII (FVIII) cDNA. Previously, splitting the FVIII coding sequence into a heavy-chain (HC) fragment and a light-chain (LC) fragment for dual recombinant AAV vector delivery has been successfully explored. However, the main disadvantage of this approach is a “chain imbalance” problem in which LC secretion is ~1–2 logs higher than that of HC, and therefore, the majority of protein synthesized is nonfunctional. To improve HC secretion, we constructed alternate FVIII HCs based on our observation that LC facilitates HC secretion. To our surprise, most of the new HC molecules exhibited enhanced expression over the traditional HC molecule (HC745). The optimized HC mutein, HCHL, including additional acidic-region-3 (ar3) sequences, exhibited three- to fivefold higher activity in both enzyme-linked immunosorbent assay (ELISA) and activated partial thromboplastin time (aPTT) assay in in vitro testing. Further characterization suggested ar3 sequences increased HC secretion, rather than promoting HC synthesis. Intravenous delivery of AAV8-HCHL+AAV8-LC or AAV8-HC745+AAV8-LC achieved phenotypic correction in hemophilia A mice. Mice receiving AAV8-HCHL+AAV8-LC achieved three- to fourfold higher HC expression than AAV8-HC745+AAV8-LC, consistent with the FVIII functional assays. HCHL should be substituted for HC745 in a dual AAV vector strategy due to its enhanced expression.
Introduction
Hemophilia A is an X-linked, recessive bleeding disorder that affects ~1 in 5,000 males. This disease is caused by defects in the gene coding for coagulation Factor VIII (FVIII) and comprises the majority of all hemophilia cases. Clinically, it is characterized by frequent spontaneous joint hemorrhages, easy bruising, and prolonged bleeding. The consequences of bleeding into critical closed spaces, such as the intracranial or the retroperitoneal space, are severe and can be life threatening. Clotting factor concentrate infusions are the only currently effective treatment options for severe hemophilia A patients.1,2 However, recombinant FVIII is very costly and often not available for patients in developing counties.
Gene therapy has shown great promise for curing hemophilia.3,4,5,6,7 For hemophilia B, adenovirus-associated virus (AAV) vectors have been demonstrated to be very efficient in a variety of animal models ranging from mice and dogs to nonhuman primates.8,9,10,11,12 Significant levels of factor IX expression were detected in the first AAV factor IX human clinical trials targeting the liver.4 Robust alternative AAV serotypes are also ready to be tested for hemophilia B in human clinical trials.13,14,15,16 In contrast to hemophilia B, gene therapy for hemophilia A has lagged behind, even though hemophilia A patients far outnumber hemophilia B patients. The major hurdle for developing AAV-FVIII for gene therapy lies in the size of the FVIII gene.17 In contrast to the 1.5 kb factor IX cDNA, the full size of FVIII is 7.0 kb and the B-domain-deleted FVIII (4.37 kb) alone is close to the 4.4 kb packaging capacity of AAV, which leaves little space for regulatory elements such as promoters, poly A sequences, and introns.18,19,20,21
In addition to efforts to deliver FVIII using a single recombinant AAV vector, an alternate strategy has been developed to deliver FVIII using dual AAV vectors.22,23 This is based on the discovery that separate FVIII HC and LC can reassociate in vitro and regenerate coagulation activity.24 In this approach, one AAV vector was used to carry FVIII HC745, which included 1–745 amino acids of mature single-chain FVIII (referred to as HC745). Another AAV vector is used exclusively to express FVIII LC. The combination of AAV-HC745+AAV-LC had been demonstrated to correct the hemophilia A phenotype in animal experiments.22,23,25 However, the main drawback is “chain imbalance” because the levels of LC were typically 10- to 100-fold higher than those of HC.22,23 Thus, the majority of secreted LC was not associated with its counterpart HC and did not contribute to coagulation activity at all.
In this study, we developed alternate FVIII HC molecules that target the “chain imbalance” issue. The optimized FVIII HC mutein, HCHL, was expressed more efficiently than the conventional HC745. AAV-HCHL secreted approximately fivefold more protein than AAV-HC745 and resulted in a similar increase in coagulation activity that corrected the hemophilic phenotype in small animal models, which overcomes a major obstacle of the dual AAV vector system.
Results
Construction of FVIII HC muteins with improved expression
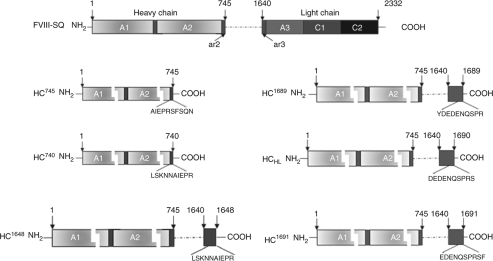
As shown in Figure 1, the only HC molecule (HC745) explored so far for AAV delivery contains the first 745 amino acids of FVIII single-chain polypeptide. The poor efficiency of HC745 in expression as compared to FVIII itself prompted us to investigate alternative HC molecules for AAV delivery. Our previous study demonstrated that FVIII LC can significantly enhance HC secretion.26 Thus, we hypothesized that there exists some elements in the LC that facilitate HC expression or secretion. To avoid the potential complication of creating neoantigens, we decided not to introduce nonnative amino acids. Representative HC muteins are illustrated in Figure 1. They can be divided into two categories. HC1689, HCHL, HC1648, and HC1691 all have additional FVIII residues compared to HC745. On the other hand, HC740 includes only the first 740 amino acids of FVIII protein. All these muteins were constructed by PCR-mediated mutagenesis and cloned into the expression backbone, pAAV-CB-FVIII, which is under the control of a β-actin promoter along with a CMV enhancer flanked by AAV inverted terminal repeats.
Figure 1.
Schematic representation of key elements of FVIII HC muteins. HC745 is the traditional HC molecule. Key amino acids are identified. The break between A1 and A2 domain stands for the region that is not shown. The abbreviations, “ar2” and “ar3” stand for acidic region 2 and 3, respectively. FVIII-SQ is the B-domain-deleted FVIII (Refacto). FVIII, factor VIII; HC, heavy chain.
Acidic-region-3 enhanced HC secretion
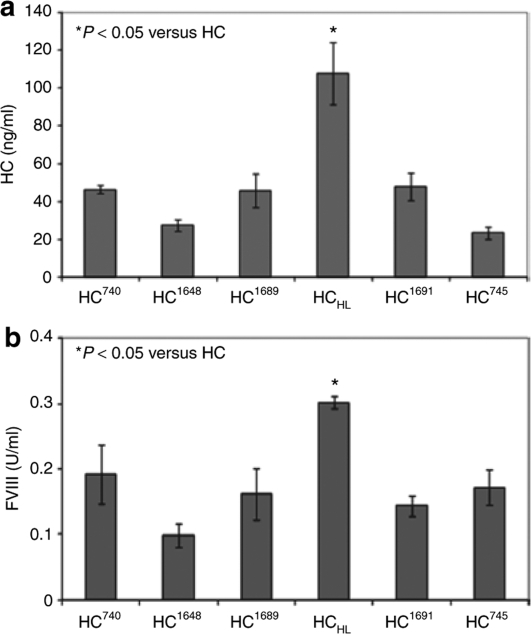
The FVIII HC muteins outlined in Figure 1 were tested for their expression levels in tissue culture cells. Each HC mutein was transfected into 293 cells along with an LC expression plasmid. At 72 hours post-transfection, levels of secreted FVIII HC were evaluated by ELISA (Figure 2a). Surprisingly, HC745 showed the lowest levels of expression. HC1689, HC1691, and HC740 were approximately one- to twofold higher than HC745 in expression. The most significant construct is HCHL, which was about five- to sixfold higher than HC745. The coagulation activities of secreted HC were then measured by aPTT assay. As shown in Figure 2b, the activity of HC745 appeared to be only 50% higher than HC1648 and was comparable to HC740, HC1648, and HC1691. In contrast, aPTT assay showed that HCHL exhibited approximately twofold higher coagulation activity compared to HC745. Therefore, there is a modest discordance between the activities of secreted FVIII in the media and the antigen levels measured by ELISA. Nevertheless, these results suggested that HCHL is more efficiently expressed than HC745 and that the inclusion of the acidic-region-3 (ar3) sequence in HCHL does not interfere with the HC coagulation activity.
Figure 2.
HC muteins with improvement in HC expression. Expression vectors for HC (HC745), HC740, HC1648, HC1689, HCHL, or HC1691 were co-transfected with the LC expression plasmid into 293 cells by calcium phosphate precipitation. FVIII HC secretion and FVIII activity were determined at 72 hours post-transfection. (a) HC antigen in the media was measured by HC-specific ELISA. (b) Functional FVIII coagulation activity converted from activated partial thromboplastin time (aPTT) assay. Standard error is shown in the figure (n = 3). ELISA, enzyme-linked immunosorbent assay; FVIII, factor VIII; HC, heavy chain; LC, light chain.
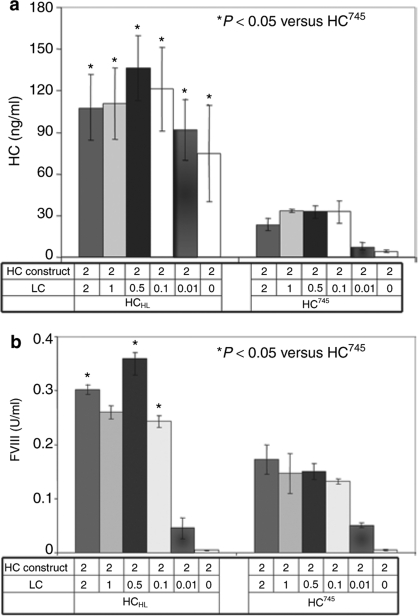
Previously we have demonstrated that LC could facilitate HC745 secretion.26 The effects of LC on HCHL were also analyzed. For transfections in 293 cells, we used a fixed amount of HC plasmid (HCHL or HC745) and varying amounts of LC plasmid ranging from 0 to 2 µg. As shown in Figure 3a, in the absence of FVIII LC, HCHL antigen in the media is much higher than HC745. The HC745 antigen was barely detectable in the absence of LC. The inclusion of even a trivial amount of LC plasmid (0.01 µg or 0.1 µg) had significant effects on HC745 secretion. The enhancement of LC on HC745 antigen expression as measured by ELISA is in the range of 10- to 100-fold. In contrast, although the effect of LC on HCHL expression was noticeable, the enhancement for HCHL expression was only in the range of 1.3- to 2-fold. The coagulation activity of the secreted FVIII in the media (Figure 3b) confirmed the observation from the antigen assay.
Figure 3.
The effects of LC on HC secretion in vitro. HCHL or HC745 were co-transfected into 293 cells along with LC expression plasmid at ratios as indicated by calcium phosphate precipitation in triplicate. Media were harvested 72 hours after transfection. (a) HC antigen was measured by heavy-chain-specific ELISA. (b) Functional FVIII was converted from activated partial thromboplastin time assay. Standard error is shown (n = 3). ELISA, enzyme-linked immunosorbent assay; FVIII, factor VIII; HC, heavy chain; LC, light chain.
FVIII HCHL secreted higher levels of HC antigen
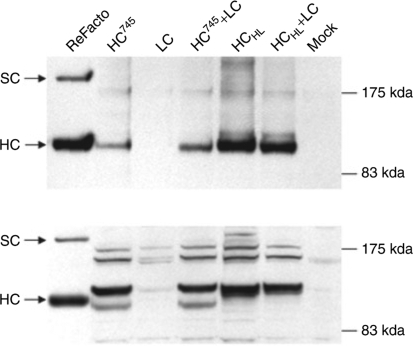
To determine whether the increased HC antigen in the media was due to increased HCHL synthesis in the cells, we compared intracellular levels of HCHL and HC745 by western blot. As presented in Figure 4, the intracellular levels of HCHL (lane HCHL and HCHL+LC) were similar to HC745 (lane HC745 and HC745+LC). Although the intracellular size of HC differed from the HC fragment of commercially available ReFacto, the secreted FVIII HC matched the HC of ReFacto (Figure 4, top panel). Quantitatively, intracellular levels of HC745 and HCHL were also measured by ELISA. As shown in Figure 4, there were no significant differences between HC745 and HCHL with or without LC molecules. The ELISA results, therefore, support the observation from the western blot. These results suggested the HC molecule synthesized in the cells from HCHL was similar to HC745 and BDD-FVIII-SQ (ReFacto). The antigen-level difference in the media did not arise from a different level of intracellular synthesis, but rather from a difference in secretion efficiency.
Figure 4.
Western blot analysis of HC synthesis and secretion. HCHL or HC745 were co-transfected into 293 cells along with LC expression plasmid at a ratio of 4:1. The media and the cells were collected at 72 hours post-transfection. The HC in the media concentrate and cell lysates were prepared for western blot analysis in 4–12% gradient polyacrylamide gel electrophoresis gel. The blot was probed with a mouse antihuman FVIII A2 domain monoclonal antibody (Meridian Life Science, Saco, ME). Top panel, HC or SC secreted in media. Bottom panel, the intracellular HC or SC. Intracellular HCHL lane at HC position is a doublet. FVIII, factor VIII; HC, heavy chain; LC, light chain; SC, single chain.
AAV-delivered FVIII HCHL secreted high levels of HC in vivo
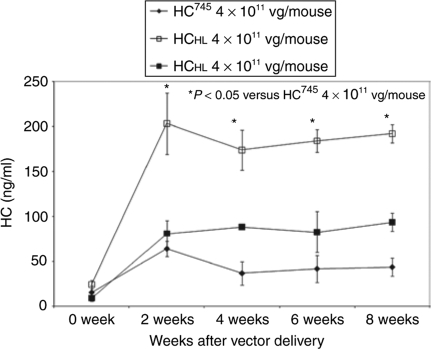
To confirm that HCHL is expressed efficiently in vivo, we generated AAV vectors carrying HCHL and HC745, respectively. We injected 4 × 1011 vg/mouse of AAV-HC745, 1 × 1011 vg/mouse of AAV-HCHL, and 4 × 1011 vg/mouse of AAV-HCHL into the hemophilia A and CD4 double-knockout mice and monitored HC secretion in the mouse plasma after vector infusion. HA mice in CD4 KO background were selected because of the inhibitor concern. The antigen level of HC was measured by ELISA (Figure 5). Consistent with in vitro results, in the absence of LC, plasma HC antigen levels in the mice receiving 4 × 1011 vg/mice AAV-HCHL were in the range of 200 ng/ml, which is about four to five times higher than the group receiving a similar dose of AAV-HC745. Even at a lower dose of 1 × 1011 vg/mouse, AAV-HCHL expression was twice as high as that with 4 × 1011 vg/mouse AAV-HC745. This result confirmed that HCHL is expressed at a higher level in vivo when delivered by AAV vectors.
Figure 5.
Enhanced HC secretion from HCHL mutein in vivo. CD4KOHA mice were injected with 4 × 1011 vg/mouse AAV8-HCHL vector (n = 4, open square), 4 × 1011 vg/mouse AAV8-HC745 vector (n = 4, close diamond), or 1 × 1011 vg/mouse AAV8-HCHL vector (n = 4, open square). The expression of FVIII heavy chain (y-axis) in mouse plasma was measured by FVIII heavy-chain-specific ELISA biweekly (x-axis). Error bars indicate standard error. ELISA, enzyme-linked immunosorbent assay; FVIII, factor VIII; HC, heavy chain.
AAV-delivered FVIII HCHL corrects the hemophilic phenotype in vivo
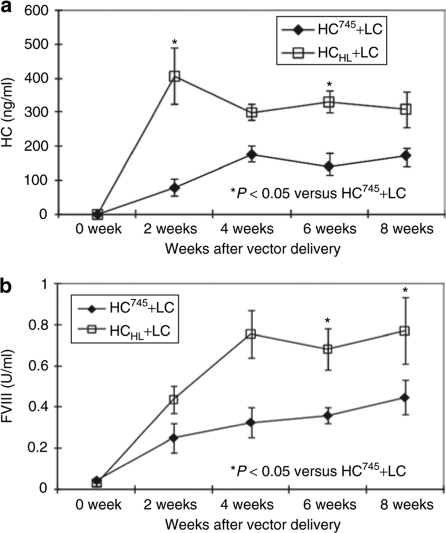
The ultimate goal of engineering HC molecules is to enhance the ability to correct hemophilia. Therefore, we evaluated the performance of AAV-HCHL when delivered along with AAV-LC. At a dose of 5 × 1011 vg/mouse for combined HC and LC vectors, CD4KOHA mice receiving AAV-HCHL+AAV-LC had a stable level of FVIII coagulation activity at 0.8 IU/ml (Figure 6b). This is significantly higher than AAV-HC745+AAV-LC, which is in the range of 0.2–0.4 IU/ml. The antigen level of HC is consistent with the functional activity measured by aPTT assay. As shown in Figure 6a, the HC antigen level in mice receiving AAV-HCHL+AAV-LC was stable at 300–400 ng/ml, which is higher than 100–200 ng/ml for mice receiving AAV-HC745+AAV-LC.
Figure 6.
HCHL improved the phenotypic hemophilia correction in vivo. CD4KOHA mice were injected with 5 × 1011 vg/mouse AAV8-HCHL+AAV8-LC vector (n = 5, open square) or AAV8-HC745+AAV8-LC (n = 5, close diamond) at a ratio of 4:1. (a) The expression of FVIII heavy chain (y-axis) in mouse plasma was measured by FVIII heavy-chain-specific ELISA biweekly (x-axis). (b) Functional FVIII was then assayed and converted from aPTT (y-axis). Error bars indicate standard error. aPTT, activated partial thromboplastin time; FVIII, factor VIII; HC, heavy chain; LC, light chain.
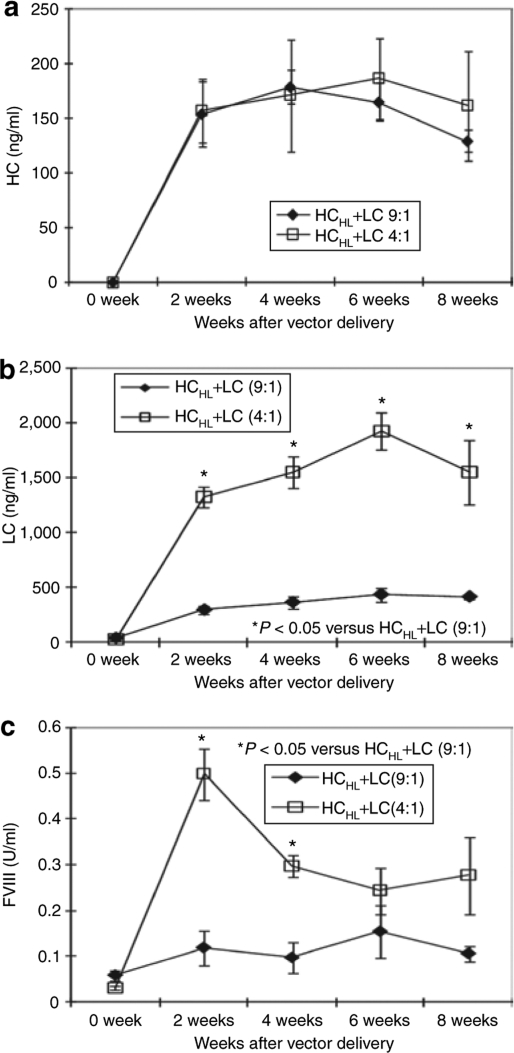
The main problem for HC745 is the chain imbalance issue.22,23,25 To assess whether HCHL molecule could overcome the chain imbalance, we compared the levels of HC and LC when AAV-HCHL+AAV-LC vectors were administered at ratios of 9:1 and 4:1. The antigen levels of HC and LC measured by ELISA are presented in Figure 7. Because the amount of the HCHL vector is fixed, the expression level of HC remained similar at either vector ratio (Figure 7a). HC expression was between 150 and 200 ng/ml. In contrast, LC expression level was reduced from approximately 1,500–2,000 to 300–400 ng/ml when ratio of LC vector reduced from 4:1 to 9:1, respectively (Figure 7b). Therefore, the chain imbalance issue was significantly ameliorated with the improved HCHL molecule.
Figure 7.
Optimizaiton of FVIII heavy-chain and light-chain expression in vivo. CD4KOHA mice received 2.5 × 1011 vg/mouse AAV8-HCHL+AAV8-LC vector (n = 4, open square) at a ratio of 4:1 (open square) or 9:1 (closed diamond). (a) The expression of FVIII heavy chain (y-axis) in mouse plasma was measured by FVIII heavy-chain-specific ELISA biweekly (x-axis). (b) The expression of FVIII light chain (y-axis) in mouse plasma was measured by FVIII light-chain-specific ELISA biweekly (x-axis). (c) Functional FVIII was then assayed and converted from aPTT (y-axis). Error bars indicate standard error. aPTT, activated partial thromboplastin time; ELISA, enzyme-linked immunosorbent assay; FVIII, factor VIII; HC, heavy chain; LC, light chain.
On the other hand, we noted that the coagulation activity (Figure 7c) does not match the level of antigen precisely; at HCHL to LC vector ratio of 9:1, only 0.1 units of FVIII were achieved with 200 ng of HC antigen. In contrast, 0.8 to 0.9 units of functional FVIII activity was achieved when the HCHL and LC ratio was at 4:1 with similar amounts of HC antigen. These results also suggested overexpressed LC is required to maximize the association of HC and LC, which determines the coagulation activity of secreted FVIII HC and LC antigen. We conclude that balanced HC and LC at the antigen level may not guarantee maximal coagulation activity when adopting a dual AAV vector approach.
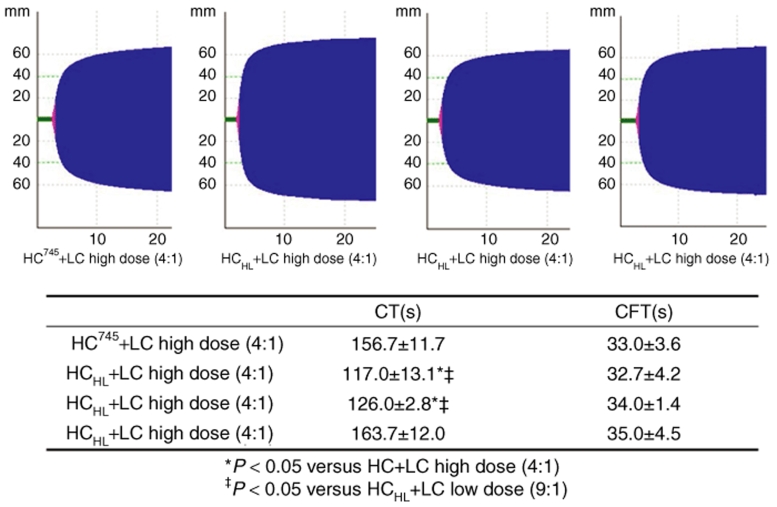
The improved secretion of HCHL was also reflected in the thromboelastographic profile measured by ROTEM assays (Figure 8). ROTEM assays were conducted at 16 weeks postvector delivery. Although there were no significant differences in clot formation time between mice receiving HC745 and HCHL vectors, the CTs, which corresponds to the reaction time in a conventional thromboelastogram, were dramatically different. Mice receiving HCHL vectors with optimized ratios of HC745 and LC had a CT time in the range of 120 seconds, in contrast to mice receiving HC745 vectors at 160 seconds. The CT time was consistent with high levels of FVIII coagulation activity measured by aPTT assays.
Figure 8.
Analysis of coagulation profile of mice receiving dual-chain vectors. The blood of mice receiving dual vector via intravenous injection was evaluated for coagulation profile using ROTEM. HC745+LC high dose (4:1) and HCHL+LC high dose (4:1) are those mice that received 5 × 1011 vg/mouse AAV8-HC745+AAV8-LC or AAV8-HCHL+AAV8-LC vector, respectively. HCHL+LC low dose (4:1) and HCHL+LC low dose (9:1) are mice that received 2.5 × 1011 vg/mouse AAV8-HCHL+AAV8-LC vector at a ratio of 4:1 or 9:1, respectively. Initiatison of coagulation was measured as coagulation times (CTs). The clot formation time (CFT) was defined as the time needed to achieve a clot firmness of 20 mm. Results shown as mean ± SEM.
Discussion
Although AAV is the vector of choice for correcting genetic diseases due to its persistent gene expression with only minimal levels of integration into the host chromosome, the inability to package inserts greater than ~5 kb has been a challenge for delivering FVIII and other large cDNAs.27,28,29 Splitting FVIII into two AAV vectors was a clever approach that takes advantage of the fact that the HC and LC of FVIII could associate to regenerate coagulation activity.22,23,25 FVIII is a protein that secretes inefficiently as compared to other similar proteins such as factor V;17,30 this problem hampered the two-chain approach as well because FVIII HC but not LC was inefficiently secreted.22,23,25 The disparity of HC and LC secretion has led to a chain imbalance issue that decreased the efficacy of AAV-FVIII vector delivery and resulted in the majority of expressed transgene products being nonfunctional. Such chain imbalance may also destabilize the host cells and induce apoptosis.31
It has been suggested that the interactions between cellular chaperones and elements in the FVIII HC reduce FVIII secretion.30,32,33,34 The currently used FVIII HC molecules (HC745) seem to be far less efficient than full-length FVIII in secretion. Thus, it is necessary to improve HC745 secretion. Although it is established that certain modifications in FVIII such as F309S and additional B-domain sequence increases FVIII secretion, simply adding these modifications to the HC did not increase HC secretion.26 On the contrary, the secretion of HC was reduced even further.26 Therefore, improving HC secretion cannot be achieved by simply following what has been done for FVIII.
Our recent studies have demonstrated that LC facilitates HC secretion.26 The HC molecules secrete much more efficiently when LC molecules were coexpressed. One possibility is that elements in the LC may facilitate HC secretion and thus ameliorate this problem. Although many HC molecules we constructed with partial C1 and C2 elements were not functional (data not shown), HC molecules with complete or partial ar3 sequences were capable of complementing LC to restore coagulation activity. Surprisingly, one HC mutein, HCHL, which has an intact ar3 sequence along with the HC coding sequence, secretes better than HC745 in both in vitro and in vivo testing (Figures 1–7). HCHL may thus be a better choice for delivery by AAV vectors. It is worth noting that such enhancements by ar3 would not be predicted by current working models of FVIII structure. The expression of HC appeared to be greatly influenced by the number of amino acids in the HC molecules. How these individual amino acids affect FVIII secretion remains to be elucidated.
The increased HC molecules in the media or blood circulation were not due to increased polypeptide translation in cells. This is evident in the western blot and ELISA data showing similar levels of intracellular FVIII HC-related polypeptides for both HC745 and HCHL expression plasmid transfection (Figure 4). Thus, the secretion of HC appears to be the determining factor. Because the only difference between HC745 and HCHL lies in the ar3, the enhanced performance of HCHL in expression suggested that this sequence may also have a role in facilitating FVIII HC secretion. Therefore, this could be a new function of ar3 in addition to its role in mediating FVIII and von Willebrand factor interaction.35,36
In this study, we successfully demonstrated that HCHL molecules can be used in AAV vectors to complement LC vectors and fully correct hemophilia A in the mouse model. HC expression from AAV-HCHL vector was significantly higher than the traditional AAV-HC745 vectors. An approximate fivefold increase in the HC antigen level is consistent with in vitro results (Figures 2–8). The increase in HC secretion paralleled the increase in coagulation activity in mouse plasma (Figure 7). By adjusting the HCHL to LC vector ratio to 9:1, the overall antigen ratio of HC and LC was close to 1. Thus, the goal of achieving chain balance was achieved. However, the coagulation FVIII activity by aPTT assay was only 0.1 units, which suggested only 10% of either HC or LC (200 ng) associated with the other molecule to contribute to the coagulation activity. We postulate that the inefficient association of HC and LC is the primary cause of this discordance between antigen and activity levels.
The observation that a balanced HC and LC antigen could not maximize the coagulation is unexpected. Because there existed disulfide bonds between HC and LC, we speculated that HC and LC mostly likely associate with each other more efficiently during the secretion pathway. In other words, the disulfide bond between HC and LC only formed efficiently with helps from cellular chaperons in the secretion process. If HC and LC do not follow exactly the same pathway for secretion or not all cells are transduced equally with both vectors, HC and LC would not be associated with each other efficiently. We found simply mixing purified HC and LC does not recover FVIII activity (data not shown), which may explain why HC and LC did not achieve maximum activity when they are expressed separately. This probably is an inherent disadvantage for the dual-chain strategy.
On the other hand, when the HCHL and LC vectors were injected at a ratio of 4:1, the antigen ratio of HC to LC protein was approximately 1:7–10, and nearly 80–90% of HC contributed to coagulation activity. Therefore, to achieve optimal therapeutic effects, it may be necessary to sacrifice balanced HC and LC levels at the antigen level. There is no evidence showing that LC negatively affected HC and LC association. Higher levels of LC protein appeared to be helpful in allowing all HC molecules to be associated with LC molecules. Considering the difficulties in achieving high-level HC expression, it would be logical to have approximately tenfold higher LC protein if excess LC protein at this level does not pose immunological or other concerns. Stated differently, high-level FVIII activity using the two-chain approach may inevitably require excess LC synthesis. The existence of discrepancy between antigen level and coagulation activity in HC740, HC1648, and HC1691 muteins (Figure 2) also suggested that the association of HC and LC is a critical issue for consideration in the strategy of expressing HC and LC from separate AAV vectors. Future studies would be warranted to investigate the mechanism regulating the HC and LC association.
One important feature of the HCHL molecules is that they were synthesized without the introduction of additional non-FVIII amino acids. This is of special importance for hemophilia A patients because inhibitor formation is tenfold higher than in hemophilia B patients even with native FVIII infusion. In HCHL, the only difference from HC745 is inclusion of additional amino acids from ar3 of FVIII. Although ar3 sequence has been reported to affect von Willebrand factor and FVIII LC interaction,37 it is less likely that ar3 directly affected HC stability of HCHL for two reasons. First, ar3 sequence has no direct binding activity to von Willebrand factor.38,39 Second, the secreted HC from HCHL was also similar to HC745 because the additional ar3 region amino acids were removed in posttranslational processing due to the proteolytic site at 1648 (Figure 4). In all, there are few disadvantages in adopting HCHL over traditional HC745 as the HC molecule for AAV vector delivery due to its improvements in secretion. Further studies of HCHL in large animal models will be necessary to evaluate its potential in human gene therapy for hemophilia A.
Materials and Methods
FVIII expression plasmids construction. Human FVIII heavy-chain (HC745) and light-chain (LC) coding sequences were obtained from plasmids described in previous studies.22,23 The plasmid pAAV-HC encodes the human FVIII A1 and A2 domains along with residues 741–745 from the B-domain. The plasmid pAAV-LC contained residues 1563–1648 from the B-domain and the whole A3–C1–C2 sequences. The plasmids pAAV-HCHL, pAAV-HC740, pAAV-HC1689, and pAAV-HC1648, pAAV-HC1691 are constructed by removing corresponding regions from B-domain-deleted FVIII using PCR-mediated site-specific mutagenesis. The detailed FVIII-related sequences for these plasmids were illustrated in Figure 1. Besides flanking inverted terminal repeats, all expressing cassettes were under identical regulatory elements that included a 562-bp human β-actin promoter with a CMV enhancer, a 99-bp modified SV40 intron, and a 232-bp bovine growth hormone polyadenylation signal.
Tissue culture and transfection. HEK293 and HEK293FT cells were purchased from the American Type Culture Collection (Manassas, VA) and cultured in Dulbecco's modified Eagle medium (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (HyClone, Logan, UT), penicillin (100 U/ml), and streptomycin (100µg/ml) at 37 °C in a moisturized environment supplied with 5% CO2. Transfections were carried out using calcium phosphate precipitation as described previously. After transfection, the cells were grown for 12 hours in Dulbecco's modified Eagle medium with 10% fetal bovine serum to minimize cell death. The cells were then maintained in OPTI-MEM (Invitrogen) medium for 72 hours before the cells and medium were collected and the secreted FVIII antigens were analyzed.
AAV vector preparation. Recombinant AAV vectors used in this study were based on AAV serotype 8. They were produced by a triple plasmid co-transfection method as described previously.40,41 Pseudotype AAV8 vector was packaged by using AAV helper plasmid containing AAV2 rep and AAV8 cap genes. In detail, AAV8 helper plasmid, adenovirus function helper plasmid, and AAV-FVIII vector plasmid were co-transfected into HEK293 cells cultured in roller bottles at a ratio of 1:2:1. The transfected cells were harvested 3 days later. AAV vectors were purified by two rounds of cesium chloride gradient ultracentrifuge. After the collected AAV vectors were buffer exchanged extensively against PBS with 5% D-sorbitol, vector purity and genome titer were analyzed by silver staining. The final vectors were stored at −80 °C before administration.
Animal procedures. Exon 16 FVIII knockout (HA KO) mice were obtained from Haig Kazazian.18,42 Immunodeficient CD4 knockout mice (CD4 KO) were purchased from the Jackson Laboratories (Bar Harbor, ME). CD4 and HA double-knockout mice (CD4KOHA) were generated by repeated breeding of HA KO with CD4 KO mice to obtain a pure genetic background. All mice were housed in a specific pathogen-free environment with a normal diet. All surgical procedures involving mice were in accordance with institutional guidelines under approved protocols at the Children's Hospital of Philadelphia.
For tail vein injection, a typical injection volume is 200 µl vector diluted in saline. The mice are initially put under a heat lamp to increase blood flow to the tail vein. Then they are transferred to a holding device that restrains the animal while allowing access to the tail vein. The lateral tail vein is identified on either side and vector is injected using a 30 g needle. After injection, bleedings at the injection was monitored and the mouse was returned to the cage after the bleeding was completely controlled.
For collecting plasma for coagulation analysis, blood was collected by tail clipping using sodium citrate as an anticoagulant at a final concentration of 0.38% (wt/vol). The blood samples were then centrifuged at 4 °C for 10 minutes at 9,000g in a microcentrifuge. The plasmas were then collected and stored in −80 °C prior to FVIII assays. For AAV vector administration, vectors were injected by tail vein intravenous infusion. Mouse plasma postvector administration was harvested as described above.
Quantitative analysis of FVIII antigen and activities. FVIII HC and FVIII LC antigen were determined using chain-specific enzyme-linked immunosorbent assays (ELISAs). For human FVIII HC-specific ELISA, Nunc maxisorp (Nalge Nunc International, Rochester, NY) plates were coated with 2 µg/ml HC-specific monoclonal antibody GMA-012 (Green Mountain Antibodies, Burlington, VT). After the samples and standards (100 µl/well) were incubated at room temperature for 2 hours, they were washed 3× with PBS+0.5% Tween 20. Then a horseradish peroxidase–conjugated ESH5 (American Diagnostica, Greenwich, CT) was added to the plates and incubated for 2 hours at room temperature. After the final wash, the antigen was detected using ABTS substrate (Roche, Germany) and the absorbance was read at 405 nm. The hFVIII LC ELISA was performed similarly with the following modifications: (i) the capture antibody was changed to 2 µg/ml human FVIII LC-specific N55195M (Biodesign International, Saco, ME), and (ii) the detection antibody was changed to horseradish peroxidase–conjugated ESH8 (American Diagnostica, Greenwich, CT). For all ELISAs, the standard used was recombinant B-domain-deleted FVIII, ReFacto (Genetics Institute, Cambridge, MA).
Biologically active FVIII in media and plasma was measured using the activated partial thromboplastin time (aPTT) assay as previously described.18,22,42 ReFacto was used as the standard.
Western blot analysis of FVIII. To analyze intracellular FVIII antigen, cells transfected with FVIII plasmids were lysed with RIPA buffer (50 mmol/l Tris-HCl pH 7.4,150 mmol/l NaCl, 1 mmol/l phenylmethylsulphonyl fluoride, 1 mmol/l EDTA, 1% Triton x-100, 1% sodium dodecyl sulfate). After sonication to break the genomic DNA, equal volumes of 2× sample buffer was added and boiled for 2 minutes. For secreted FVIII, medium were concentrated and desalted by Centricon (Millipore, Bedford, MA) before 2× sample buffer was added. The resulting samples were analyzed by 4–12% gradient sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels and then transferred to nitrocellulose membrane for western blot. After blocking the membrane in PBS/10% skim milk powder/0.3% Tween-20, a mouse anti-hFVIII A2 domain antibody, N77210M (1:1,000 dilution; Biodesign International, Saco, ME), was added, and the membrane was incubated overnight at 4°C. The membrane was washed, incubated with a HPR-conjugated sheep antimouse IgG antibody (1:2,000 dilution; Sigma, St Louis, MI), and developed using an enhanced chemiluminescent substrate (Amersham-Pharmacia Biotech, Piscataway, NJ).
Thrombelastographic measurements. Thrombelastographic measurements were performed by ROTEM (Pentapharm, Munich, Germany) in citrated whole blood using the intrinsically activated tests. In detail, whole blood samples were collected and tested within 30. All tests were performed in Rotem cup and pins prewarmed to 37 °C, and 300 µl of whole blood was dispensed into the sample cups according to the IN-TEM program. The parameters of ROTEM analysis include “coagulation time” (CT), which corresponds to the reaction time in a conventional thrombelastogram, and “clot formation time,” which stands for the CT. All reagents were purchased from Pentapharm. The ROTEM devices were monitored for correct function using quality-control serum (ROTROL; Pentapharm).
Statistical analyses. Two-tailed Student's t-tests and one-way ANOVA with Bonferroni multiple comparison post-test were performed. The differences were considered significant when P was <0.05. The analysis was performed using the SPSS 11.0.
Acknowledgments
H.L., L.C., and W.X. designed and performed the research, analyzed the data; H.L. screened and characterized the HCHL molecule. X.Y. and J.W. performed research. R.S., H.W., and K.A.H. contributed vital new reagents or analytic tools. H.L., K.A.H., and W.X. wrote the article. We also thank Marlene Webber and Junwei Sun for their help in manuscript preparations. This work was supported by the National Institutes of Health (HL080789 and HL084381 to W.X.).
REFERENCES
- Lofqvist T, Nilsson IM, Berntorp E., and , Pettersson H. Haemophilia prophylaxis in young patients—a long-term follow-up. J Intern Med. 1997;241:395–400. doi: 10.1046/j.1365-2796.1997.130135000.x. [DOI] [PubMed] [Google Scholar]
- Kaufman RJ. Advances toward gene therapy for hemophilia at the millennium. Hum Gene Ther. 1999;10:2091–2107. doi: 10.1089/10430349950017095. [DOI] [PubMed] [Google Scholar]
- Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A, et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000;24:257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR, et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- Hasbrouck NC., and , High KA. AAV-mediated gene transfer for the treatment of hemophilia B: problems and prospects. Gene Ther. 2008;15:870–875. doi: 10.1038/gt.2008.71. [DOI] [PubMed] [Google Scholar]
- Ishiwata A, Mimuro J, Kashiwakura Y, Niimura M, Takano K, Ohmori T, et al. Phenotype correction of hemophilia A mice with adeno-associated virus vectors carrying the B domain-deleted canine factor VIII gene. Thromb Res. 2006;118:627–635. doi: 10.1016/j.thromres.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Herzog RW, Hagstrom JN, Kung SH, Tai SJ, Wilson JM, Fisher KJ, et al. Stable gene transfer and expression of human blood coagulation factor IX after intramuscular injection of recombinant adeno-associated virus. Proc Natl Acad Sci USA. 1997;94:5804–5809. doi: 10.1073/pnas.94.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog RW, Yang EY, Couto LB, Hagstrom JN, Elwell D, Fields PA, et al. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector [see comments] Nat Med. 1999;5:56–63. doi: 10.1038/4743. [DOI] [PubMed] [Google Scholar]
- Arruda VR, Schuettrumpf J, Herzog RW, Nichols TC, Robinson N, Lotfi Y, et al. Safety and efficacy of factor IX gene transfer to skeletal muscle in murine and canine hemophilia B models by adeno-associated viral vector serotype 1. Blood. 2004;103:85–92. doi: 10.1182/blood-2003-05-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Sun J, Zhang T, Yin C, Yin F, Van Dyke T, et al. Optimization of self-complementary AAV vectors for liver-directed expression results in sustained correction of hemophilia B at low vector dose. Mol Ther. 2008;16:280–289. doi: 10.1038/sj.mt.6300355. [DOI] [PubMed] [Google Scholar]
- Monahan PE, Samulski RJ, Tazelaar J, Xiao X, Nichols TC, Bellinger DA, et al. Direct intramuscular injection with recombinant AAV vectors results in sustained expression in a dog model of hemophilia. Gene Ther. 1998;5:40–49. doi: 10.1038/sj.gt.3300548. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Gray JT, McIntosh J, Ng CY, Zhou J, Spence Y, et al. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007;109:1414–1421. doi: 10.1182/blood-2006-03-010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH., and , Wilson JM. New recombinant serotypes of AAV vectors. Curr Gene Ther. 2005;5:285–297. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J., and , Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Chirmule N, Berta SC, McCullough B, Gao G., and , Wilson JM. Gene therapy vectors based on adeno-associated virus type 1. J Virol. 1999;73:3994–4003. doi: 10.1128/jvi.73.5.3994-4003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann KG. Biochemistry and physiology of blood coagulation. Thromb Haemost. 1999;82:165–174. [PubMed] [Google Scholar]
- Sarkar R, Xiao W., and , Kazazian HH., Jr A single adeno-associated virus (AAV)-murine factor VIII vector partially corrects the hemophilia A phenotype. J Thromb Haemost. 2003;1:220–226. doi: 10.1046/j.1538-7836.2003.00096.x. [DOI] [PubMed] [Google Scholar]
- Jiang H, Lillicrap D, Patarroyo-White S, Liu T, Qian X, Scallan CD, et al. Multiyear therapeutic benefit of AAV serotypes 2, 6, and 8 delivering factor VIII to hemophilia A mice and dogs. Blood. 2006;108:107–115. doi: 10.1182/blood-2005-12-5115. [DOI] [PubMed] [Google Scholar]
- Chao H., and , Walsh CE. Induction of tolerance to human factor VIII in mice. Blood. 2001;97:3311–3312. doi: 10.1182/blood.v97.10.3311. [DOI] [PubMed] [Google Scholar]
- Chao H, Mao L, Bruce AT., and , Walsh CE. Sustained expression of human factor VIII in mice using a parvovirus-based vector. Blood. 2000;95:1594–1599. [PubMed] [Google Scholar]
- Scallan CD, Liu T, Parker AE, Patarroyo-White SL, Chen H, Jiang H, et al. Phenotypic correction of a mouse model of hemophilia A using AAV2 vectors encoding the heavy and light chains of FVIII. Blood. 2003;102:3919–3926. doi: 10.1182/blood-2003-01-0222. [DOI] [PubMed] [Google Scholar]
- Burton M, Nakai H, Colosi P, Cunningham J, Mitchell R., and , Couto L. Coexpression of factor VIII heavy and light chain adeno-associated viral vectors produces biologically active protein. Proc Natl Acad Sci USA. 1999;96:12725–12730. doi: 10.1073/pnas.96.22.12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay PJ. Reconstitution of human factor VIII from isolated subunits. Arch Biochem Biophys. 1988;262:525–531. doi: 10.1016/0003-9861(88)90404-3. [DOI] [PubMed] [Google Scholar]
- Mah C, Sarkar R, Zolotukhin I, Schleissing M, Xiao X, Kazazian HH, et al. Dual vectors expressing murine factor VIII result in sustained correction of hemophilia A mice. Hum Gene Ther. 2003;14:143–152. doi: 10.1089/104303403321070838. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhu F, Li J, Lu H, Jiang H, Sarkar R, et al. The enhancing effects of the light chain on heavy chain secretion in split delivery of factor VIII gene. Mol Ther. 2007;15:1856–1862. doi: 10.1038/sj.mt.6300268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong JY, Fan PD., and , Frizzell RA. Quantitative analysis of the packaging capacity of recombinant adeno- associated virus. Hum Gene Ther. 1996;7:2101–2112. doi: 10.1089/hum.1996.7.17-2101. [DOI] [PubMed] [Google Scholar]
- Cao L, Liu Y, During MJ., and , Xiao W. High-titer, wild-type free recombinant adeno-associated virus vector production using intron-containing helper plasmids. J Virol. 2000;74:11456–11463. doi: 10.1128/jvi.74.24.11456-11463.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Zhao W, Zhong L, Han Z, Li B, Ma W, et al. Self-complementary recombinant adeno-associated viral vectors: packaging capacity and the role of rep proteins in vector purity. Hum Gene Ther. 2007;18:171–182. doi: 10.1089/hum.2006.088. [DOI] [PubMed] [Google Scholar]
- Kaufman RJ, Pipe SW, Tagliavacca L, Swaroop M., and , Moussalli M.Biosynthesis, assembly and secretion of coagulation factor VIII Blood Coagul Fibrinolysis 19978S3–S14.Suppl 2 [PubMed] [Google Scholar]
- Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT, et al. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124:587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- Pipe SW. Coagulation factors with improved properties for hemophilia gene therapy. Semin Thromb Hemost. 2004;30:227–237. doi: 10.1055/s-2004-825636. [DOI] [PubMed] [Google Scholar]
- Miao HZ, Sirachainan N, Palmer L, Kucab P, Cunningham MA, Kaufman RJ, et al. Bioengineering of coagulation factor VIII for improved secretion. Blood. 2004;103:3412–3419. doi: 10.1182/blood-2003-10-3591. [DOI] [PubMed] [Google Scholar]
- Cunningham MA, Pipe SW, Zhang B, Hauri HP, Ginsburg D., and , Kaufman RJ. LMAN1 is a molecular chaperone for the secretion of coagulation factor VIII. J Thromb Haemost. 2003;1:2360–2367. doi: 10.1046/j.1538-7836.2003.00415.x. [DOI] [PubMed] [Google Scholar]
- Pipe SW., and , Kaufman RJ. Factor VIII C2 domain missense mutations exhibit defective trafficking of biologically functional proteins. J Biol Chem. 1996;271:25671–25676. doi: 10.1074/jbc.271.41.25671. [DOI] [PubMed] [Google Scholar]
- d'Oiron R, Lavergne JM, Lavend'homme R, Benhida A, Bordet JC, Negrier C, et al. Deletion of alanine 2201 in the FVIII C2 domain results in mild hemophilia A by impairing FVIII binding to VWF and phospholipids and destroys a major FVIII antigenic determinant involved in inhibitor development. Blood. 2004;103:155–157. doi: 10.1182/blood-2003-04-1321. [DOI] [PubMed] [Google Scholar]
- Foster PA, Fulcher CA, Houghten RA., and , Zimmerman TS. An immunogenic region within residues Val1670-Glu1684 of the factor VIII light chain induces antibodies which inhibit binding of factor VIII to von Willebrand factor. J Biol Chem. 1988;263:5230–5234. [PubMed] [Google Scholar]
- Saenko EL, Loster K, Josic D., and , Sarafanov AG. Effect of von Willebrand Factor and its proteolytic fragments on kinetics of interaction between the light and heavy chains of human factor VIII. Thromb Res. 1999;96:343–354. doi: 10.1016/s0049-3848(99)00123-1. [DOI] [PubMed] [Google Scholar]
- Saenko EL., and , Scandella D. The acidic region of the factor VIII light chain and the C2 domain together form the high affinity binding site for von willebrand factor. J Biol Chem. 1997;272:18007–18014. doi: 10.1074/jbc.272.29.18007. [DOI] [PubMed] [Google Scholar]
- Hauck B, Xu RR, Xie J, Wu W, Ding Q, Sipler M, et al. Efficient AAV1-AAV2 hybrid vector for gene therapy of hemophilia. Hum Gene Ther. 2006;17:46–54. doi: 10.1089/hum.2006.17.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Xie J, Lu H, Chen L, Hauck B, Samulski RJ, et al. Existence of transient functional double-stranded DNA intermediates during recombinant AAV transduction. Proc Natl Acad Sci USA. 2007;104:13104–13109. doi: 10.1073/pnas.0702778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar R, Tetreault R, Gao G, Wang L, Bell P, Chandler R, et al. Total correction of hemophilia A mice with canine FVIII using an AAV 8 serotype. Blood. 2004;103:1253–1260. doi: 10.1182/blood-2003-08-2954. [DOI] [PubMed] [Google Scholar]