Abstract
The ability of ozone to kill micro-organisms associated with non-cavitated occlusal caries was investigated. The occlusal surfaces were treated with ozone (n = 53) or air (n = 49) for 40 s, and the underlying infected dentine was exposed. There was no significant difference between the number of bacteria recovered from the ozone-treated and the control sites (p > 0.1). Treatment of the exposed dentine with ozone resulted in a just significant (p = 0.044) reduction in bacterial counts. Ozone treatment of non-cavitated occlusal lesions for 40 s failed to significantly reduce the numbers of viable bacteria in infected dentine beneath the demineralized enamel.
Key Words: Diagnodent, Microflora, Occlusal caries, Ozone
Introduction
Despite the recent promising reports regarding the decline in dental caries, the disease is still widely prevalent in many countries. Recently, the total number of claims in England and Wales for dental interventions in the financial year 2002/2003 was 34 million [Brazzelli et al., 2006]. Nowadays, pharmaceutical approaches to treat dental caries are desirable. The development of such approaches raises the possibility of caries treatment without drilling: an approach apparently much welcomed by patients. A novel concept for the treatment of dental caries using ozone gas, as a potent microbicide, has recently been introduced [Baysan et al., 2000; Baysan and Lynch, 2004, 2006]. Previous data had suggested that ozone delivered as a gas on root carious lesions [Baysan et al., 2004] or as ozonated water to bacteria in vitro [Baysan et al., 2000] was extremely bactericidal. Such effects would be expected if the gaseous ozone became dissolved in water since ozonated water is well established as an antimicrobial in many industrial situations [Kim et al., 1999]. Previous reports of the clinical use of ozone gas to treat carious lesions, including non-cavitated occlusal carious lesions, were critically evaluated in two recent independent reviews [Rickard et al., 2004; Brazzelli et al, 2006]. However, the treatment of non-cavitated occlusal caries with ozone gas has not been extensively investigated and the reports of the effects of ozone gas exposure on non-cavitated occlusal lesions are not consistent. Exposure of occlusal carious lesions to ozone gas had no significant effect on caries status for the overall study population [Huth et al., 2005], but in the high current caries risk group there was a significant reduction in the clinical status of these lesions. In none of the clinical studies on non-cavitated lesions have the effects of ozone gas on the microflora underlying the enamel been investigated, although this is the purpose of the inclusion of ozone gas in this treatment procedure. Therefore, in this study, we determined the antimicrobial effect of the application of ozone gas on the microflora of infected dentine associated with non-cavitated occlusal carious lesions and subsequently determined the effect of ozone gas on the viability of bacteria in the exposed infected dentine associated with these lesions.
Materials and Methods
Selection of Teeth for Inclusion in the Study
Freshly extracted molar teeth were used in this study within 15 min of extraction; 1 tooth was used from each patient. All patients gave verbal consent for their teeth to be used in this study, and the collection of teeth for such studies has been approved by the local independent Ethics Committee (04/Q0704/57). The occlusal surfaces of the teeth were cleaned using a sterile polishing brush and prophylaxis paste with water as a lubricant. Each tooth was dried using dry sterile cotton wool rolls and a dental 3-in-1 air syringe. Subsequently, laser fluorescence (LF; Diagnodent, Kavo, Biberach, Germany) was used to identify teeth with non-cavitated occlusal lesions. To be included in the study, the occlusal surface of the tooth had to have an LF score of between 11 and 30 and an Ekstrand score [Ekstrand et al., 1997] of 3, equivalent to a white-spot lesion visible before or after air drying. Ekstrand score 3 has localized enamel breakdown in opaque or discoloured enamel and/or greyish discoloration from the underlying dentine in clinical examination, and histopathological observation reveals demineralization involving the middle third of the dentine. Of 360 surgically extracted teeth examined, 104 met the inclusion criteria and were included in the study.
Ozone Treatment of Non-Cavitated Occlusal Lesions
The 104 teeth were randomly allocated using a computer-generated random number table (http://www.random.org/) to either an ozone treatment group or to a no-treatment group. The teeth in the ozone treatment group were exposed to ozone gas from the Healozone delivery system (Kavo) for 40 s according to the manufacturer's instructions at room temperature. Immediately following ozone exposure, the enamel was removed from above the dentine using a sterile Jet 330 bur. The consistency, colour and wetness of the dentine were recorded [Kidd et al., 1993]. Subsequently, the dentine was sampled using a standardized procedure with a sterile round steel No. 3 bur, and the sample was placed into 1 ml of Fastidious Anaerobe Broth (Lab M, Bury, UK) and immediately transported to the laboratory for processing. This standardized sampling method yields a high level of agreement between the numbers of bacteria recovered in duplicate samples from the same lesion [Kidd et al., 1993; Paddick et al., 2005]. The teeth allocated to the control group were treated in the same manner as in the test group. The same clinical indices were recorded, and dentine samples were taken without the application of ozone.
To assess the effect of ozone exposure on the viability of bacteria in the dentine exposed following either the ozone or control treatments, the dentine of 52 teeth was exposed to ozone for 40 s, and a second dentine sample was taken from another site on the surface of the dentine, with the same clinical appearance as the site from which the first sample was taken, using a sterile round steel No. 3 bur. This allowed for the determination of the direct effects of ozone on the viability of bacteria in infected dentine.
In all cases the samples were numerically coded in the clinic, and the microbiology laboratory did not know the treatment received by any sample until after the numbers of bacteria persample had been determined.
Microbiological Analysis of Dentine Samples
The standardized dentine samples were dispersed by vortexing with sterile 3.5- to 4.5-mm-diameter glass beads (BDH, Lutterworth, UK) for 30 s, decimally diluted in Fastidious Anaerobe Broth and 100-μl volumes of each dilution were plated in duplicate onto Fastidious Anaerobe Agar (Lab M) supplemented with 5% (vol/vol) horse blood. The plates were incubated anaerobically for 7 days at 37°C, and the number of colonies was counted on the most appropriate dilutions.
Data Analysis
The numbers of bacteria in each dentine sample were calculated from the mean of the duplicate Fastidious Anaerobe Agar plate colony counts and these were expressed as log10(colony forming units + 1) persample. The means (±SD) were calculated for microbiological data and LF scores. The data were analysed using unpaired and paired t tests to compare these data sets, for both independent and dependent data while the distributions of categorical data were compared using χ2 tests.
Results
Relationships between Clinical Variables
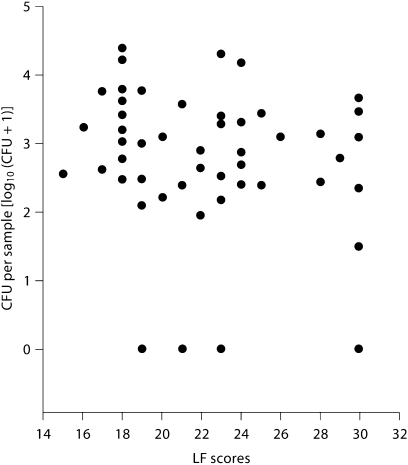
The LF scores for the occlusal surfaces of the 104 teeth included in the final data set ranged from 11 to 30 (mean 21.5 ± 4.9). Data from 2 teeth were lost because of contamination. The exposed dentine in all of the teeth was recorded as dry (wetness) and of leathery consistency, while the colour varied, with 23.5% being recorded as light brown, 64.7% as mid-brown and 11.8% as dark brown, indicating that in every case the dentine had undergone a degree of demineralization. The majority of the dentine samples (91.2%) yielded bacteria but there was no significant relationship between LF score and level of infection (r = −0.121; fig. 1).
Fig. 1.
The relationship between LF scores and level of infection (as colony-forming units per sample expressed as log 10 [CFU + 1] of the underlying dentine for non-cavitated occlusal carious lesions).
Effect of Ozone on Dentine Microflora Associated with Non-Cavitated Occlusal Lesions
The ozone-treated teeth and the control teeth were not significantly different with respect to most clinical criteria, although the proportion of teeth recorded with dentine of mid-brown colour was significantly greater in the ozone-treated group (table 1). The mean LF scores were not significantly different and the distribution of teeth according to the consistency and wetness of the dentine was not significantly different. However, the dentine associated with the teeth in the control group was significantly more likely (χ2 = 5.60; p < 0.01) to be recorded as mid-brown than was the dentine associated with the ozone-treated teeth. There was no significant difference between the numbers of bacteria per sample recovered from dentine of the ozone-treated and non-treated groups (table 2).
Table 1.
Comparison of the clinical status of the non-cavitated occlusal sites of the ozone-gas-treated and control lesions
| Ozone-gas-treated lesions (n = 54) | Control lesions (n = 48) | p value | |
|---|---|---|---|
| LF (moment) | 20.7±5.1 | 20.3±4.5 | 0.665 |
| LF (peak) | 20.3±4.5 | 21.9±5.1 | 0.440 |
| Proportion wet | 0 | 0 | 1.0 |
| Proportion medium consistency | 100 | 100 | 1.0 |
| Proportion mid-brown colour | 75.5 | 53.0 | 0.018 |
Table 2.
Mean 8 SE of the number of bacteria per sample [as log10(CFU + 1)] of dentine associated with non-cavitated occlusal carious lesions and of exposed dentine before and after ozone gas application for a period of 40 s
| Non-cavitated occlusal lesions log10(CFU + 1) | Exposed dentine log10(CFU + 1) | |
|---|---|---|
| Control | 2.95±1.47 | 3.07±0.18 |
| Ozone gas treated | 2.81±1.39 | 2.79±0.14 |
Effect of Ozone on Dentine Microflora of Exposed Dentine
The exposed dentine of 52 of the teeth was treated with ozone for 40 s following the initial treatments. The mean numbers of bacteria in the samples taken before and after exposure of the dentine to ozone gas were significantly different (p = 0.049; table 2). The microbial counts from the dentine samples taken before and after exposure of the dentine to ozone gas were significantly correlated (r = 0.657; p < 0.001). The mean log10 reduction in bacterial counts following exposure of the infecting bacteria to ozone was 0.28 ± 0.98. In 31 (59.6%) cases the bacterial count after exposure to ozone was less than the initial count but the distribution of cases between those having a reduced number of bacteria in the sample and those with no change or more bacteria in the sample was not significantly different (p = 0.32).
Discussion
The teeth selected in this study satisfied the criteria that they had an LF score of between 11 and 30 and an Ekstrand score of 3. These selection criteria were chosen to maximize the likelihood that the dentine underlying the occlusal sites would be infected with bacteria. This was found to be so in the majority of cases, although there was no significant correlation between the level of infection and the LF score as has been reported previously [Astvaldsdottir et al., 2004; Iwami et al., 2004]. In the present study, this was due to the limited range of clinical presentation as no sound sites were included. The present data, derived using freshly extracted teeth, provided support for the use of LF to reliably detect infected dentine associated with non-cavitated occlusal lesions [Lussi et al., 1999]. However, Bader and Shugars [2004] suggested following an analysis of all published reports on the use of LF that it could be used as a supplementary method for occlusal caries detection in conjunction with clinical and radiographic assessments, since this tool demonstrates high sensitivity but low specificity, meaning that many sound sites may be incorrectly diagnosed as carious.
Ozone gas failed to reduce the number of viable micro-organisms in dentine associated with non-cavitated occlusal lesions. This finding contradicts a previous study which suggested that the bactericidal effect was the major cause for the caries-inhibitory effect of ozone on occlusal lesions [Brazzelli et al., 2006], although no microbiological investigations had been included in that particular study.
In the present study, only total bacterial numbers were determined, but these data show that the normal microbiota infecting dentine associated with non-cavitated occlusal lesions, including mutans streptococci, lactobacilli and Actinomyces, were not killed by exposure to ozone gas delivered according to the manufacturers’ instructions. Clearly the applied ozone gas failed to access the microbiota in the underlying dentine, but even when the bacteria in the dentine, were directly exposed to ozone, the reduction in numbers of bacteria per sample did not approach the very large reductions reported previously [Baysan et al., 2000; Baysan and Lynch, 2004]. The present data indicate that gaseous ozone, applied to a biofilm, penetrating tissue, is not effective in killing the bacteria. This discrepancy requires explanation. It may be speculated that there was diffusion-with-reaction; the interaction between ozone gas and the micro-organism/dentine matrix was so strong as to reduce the effective diffusion gradient of ozone within the tissue. However, the solubility of ozone gas in water is very low and it is likely that the concentration of ozone in the aqueous phase was too low to exert an antimicrobial effect. These chemical interactions will significantly moderate the antimicrobial activity of the applied ozone gas. There are other issues arising from a consideration of the previous studies. A significant reduction in microbial counts was reported [Baysan et al., 2000] but this was achieved by the application of ozonated water, not ozone gas. In a study of the effects of the application of ozone gas to root caries lesions, Baysan and Lynch [2004] reported that the greatest reductions in bacterial numbers were in the smallest lesions (1 mm2) while in larger lesions the effects were much smaller or non-existent. In Baysan and Lynch [2004], half of the lesion was sampled before treatment to determine the baseline numbers of bacteria and the other half sampled after treatment to estimate the number remaining after ozone treatment; the difference was attributed to the effect of exposure to ozone gas. It seems likely that this method of sampling was more biased than the standardized sampling method used here, and that sampling reproducibility was influenced by the lesion size with larger lesions more reliably sampled than the smaller lesions from which the first sample (baseline) was consistently larger than the posttreatment sample.
It can also be speculated that the small but significant reduction in total numbers of viable micro-organisms within exposed dentine may be mediated by the oxidant effect of ozone, which kills micro-organisms via a mechanism involving the rupture of bacterial cell membranes [Kim et al., 1999] but modulated by the mechanisms described previously.
It is important to consider the conclusions of the recent Cochrane Review and the report from the National Institute for Health and Clinical Excellence, which highlighted the fact that large, well-conducted randomized controlled trials (duration of at least 2 years) conforming to the CONSORT guidelines [http://www.consort-statement.org/stardstatement.htm] are required by independent research teams to assess the effectiveness and cost-effectiveness of ozone gas delivery systems for the management of both occlusal and root caries [Rickard et al., 2004; Brazzelli et al., 2006].
The data derived from this study indicate that the application of ozone gas to non-cavitated carious lesions does not significantly reduce the number of viable bacteria in the underlying infected dentine. While this study does not prove that gaseous ozone application is not effective at treating caries (occlusal or root), it does provide strong evidence that the mechanism by which ozone application might be effective is not mediated by direct killing of bacteria in infected dentine.
Acknowledgements
This study was supported in part by the Wellcome Trust (grant No. 076381). We also acknowledge Kavo Dental, Germany (Mr. Nick Hartgroves) for lending the ozone delivery system used in this study.
References
- 1.Astvaldsdottir A, Holbrook WP, Tranaeus S. Consistency of Diagnodent instruments for clinical assessment of fissure caries. Acta Odontol Scand. 2004;62:193–198. doi: 10.1080/00016350410001612. [DOI] [PubMed] [Google Scholar]
- 2.Bader JD, Shugars DA. A systematic review of the performance of a laser fluorescence device for detecting caries. J Am Dent Assoc. 2004;135:1413–1426. doi: 10.14219/jada.archive.2004.0051. [DOI] [PubMed] [Google Scholar]
- 3.Baysan A, Lynch E. Effect of ozone on the microbial flora and clinical severity of primary root caries. Am J Dent. 2004;17:56–61. [PubMed] [Google Scholar]
- 4.Baysan A, Lynch E. The use of ozone in dentistry and medicine. 2. Ozone and root caries. Prim Dent Care. 2006;13:37–41. doi: 10.1308/135576106775193897. [DOI] [PubMed] [Google Scholar]
- 5.Baysan A, Whiley R, Lynch E. Anti-microbial effects of a novel ozone generating device on micro-organisms associated with primary root carious lesions in vitro. Caries Res. 2000;34:498–501. doi: 10.1159/000016630. [DOI] [PubMed] [Google Scholar]
- 6.Brazzelli M, McKenzie L, Fielding S, Fraser C, Clarkson J, Kilonzo M, et al. Systematic review of the effectiveness and cost-effectiveness of Healozone® for the treatment of occlusal pit/fissure caries and root caries. Health Technol Assess. 2006;10:iii–iv. doi: 10.3310/hta10160. ix–80. [DOI] [PubMed] [Google Scholar]
- 7.Ekstrand KR, Ricketts DN, Kidd EA. Reproducibility and accuracy of three methods for assessment of demineralization depth of the occlusal surface: an in vitro examination. Caries Res. 1997;31:224–231. doi: 10.1159/000262404. [DOI] [PubMed] [Google Scholar]
- 8.Huth KC, Paschos E, Brand K, Hickel R. Effect of ozone on non-cavitated fissure carious lesions in permanent molars: a controlled prospective clinical study. Am J Dent. 2005;18:223–228. [PubMed] [Google Scholar]
- 9.Iwami Y, Shimzu A, Narimatsu M, Hayashi M, Takeshige F, Ebisu S. Relationship between bacterial infection and evaluation using a laser fluorescence device, Diagnodent. Eur J Oral Sci. 2004;112:419–423. doi: 10.1111/j.1600-0722.2004.00161.x. [DOI] [PubMed] [Google Scholar]
- 10.Kidd EAM, Joyston-Bechal S, Beighton D. Microbiological validation of assessment of caries activity during cavity preparation. Caries Res. 1993;27:402–408. doi: 10.1159/000261571. [DOI] [PubMed] [Google Scholar]
- 11.Kim JG, Yousef AE, Dave S. Application of ozone for enhancing the microbiological safety and quality of foods: a review. J Food Prot. 1999;62:1071–1087. doi: 10.4315/0362-028x-62.9.1071. [DOI] [PubMed] [Google Scholar]
- 12.Lussi A, Imwinkelried S, Pitts N, Longbottom C, Reich E. Performance and reproducibility of a laser fluorescence system for detection of occlusal caries in vitro. Caries Res. 1999;33:261–266. doi: 10.1159/000016527. [DOI] [PubMed] [Google Scholar]
- 13.Paddick JS, Brailsford SR, Kidd EA, Beighton D. Phenotypic and genotypic selection of microbiota surviving under dental restorations. Appl Environ Microbiol. 2005;71:2467–2472. doi: 10.1128/AEM.71.5.2467-2472.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rickard GD, Richardson R, Johnson T, McColl D, Hooper L. Ozone therapy for the treatment of dental caries. Cochrane Database Syst Rev. 2004;3 doi: 10.1002/14651858.CD004153.pub2. CD004153. [DOI] [PubMed] [Google Scholar]