Abstract
Panniculitis is a recognized but unusual complication of a severe deficiency of α1-antitrypsin (AAT), with fewer than 100 cases described to date. Like the pathogenesis of emphysema in severe PiZZ deficiency of AAT, panniculitis has been hypothesized to be an inflammatory process, possibly related to Z AAT polymer formation and to an unopposed anti-inflammatory screen in the context of deficient serum levels of AAT. The current report presents a 31-year-old woman with PiZZ AAT deficiency-associated panniculitis. Our case extends current knowledge of AAT-associated panniculitis in 2 ways: (1) we demonstrate Z-type AAT polymers in the skin, which supports the inflammatory pathogenesis of panniculitis and the potential pro-inflammatory role of polymers; (2) we show that a high dose and long-term use of intravenous augmentation therapy (90 mg/kg body weight once weekly during 3 years) can ameliorate the frequency and severity of panniculitis associated with AAT deficiency.
Key Words: α1-Antitrypsin, Polymers, Panniculitis, Inflammation, Augmentation therapy
Introduction
α1-Antitrypsin (AAT), also referred to as α1-proteinase inhibitor, is one of the most abundant serine protease inhibitors in human plasma and in other biological fluids, including saliva, tears, milk, semen, urine and bile [1]. AAT is a glycoprotein mainly produced in liver parenchymal cells and, to a lesser extent, synthesized by blood monocytes, macrophages, pulmonary alveolar cells, intestinal epithelial cells, and cornea [2, 3]. The normal daily rate of synthesis is approximately 34 mg/kg body weight, leading to a plasma concentration ranging from 0.9 to 1.75 mg/ml, with a half-life of 3–5 days.
More than 100 different alleles of AAT have been identified to date, of which at least 20 affect either the amount and/or the function of the AAT molecule in vivo [4]. To classify allele expression, a protein inhibitor (Pi) system has been developed to describe diverse genotypes, based on the migration of the AAT in an electric field (isoelectric focusing). Normal variants of AAT are named M, while other variants are termed A–L and N–Z, depending on whether they run faster (A–L) or slower (N–Z) than the M band in an isoelectric field [5]. The intermediate and severe AAT deficiency phenotypesmostly result from combinations of S, Z and null alleles. Recent reports suggest that there are as many as 116 million carriers of deficiency alleles (PiMZ and PiMS) and 3.4 million individuals with deficient allele combinations (PiSZ, PiSS and PiZZ) worldwide [6].
α1-Antitrypsin genes are inherited as co-dominant alleles (as products of both genes can be found in the circulation) [7]. Individuals with plasma AAT values below 11 μM (50–80 mg/dl, depending on the assay) are considered to be AAT deficient. In this context, individuals heterozygous for the Z allele (i.e. PiMZ heterozygotes) have serum AAT levels that are 30–40% of normal, whereas individuals homozygous for the Z allele (PiZZ) have serum levels that are only 10–15% of the normal levels found in PiMM individuals [8]. The cause of deficient serum levels in PiZZ individuals (Glu342Lys) is accumulation of Z polymers within the hepatocyte (in a process called loop-sheet polymerization), precluding AAT secretion into the blood [9, 10]. The retained AAT polymers within the endoplasmic reticulum of hepatocytes can cause liver damage with a variable clinical presentation, from neonatal hepatitis to liver cirrhosis and hepatocellular carcinoma in adults. The lack of circulating protein will predispose the carrier to chronic obstructive pulmonary disease through unopposed elastolysis of the lung by proteolytic enzymes (e.g. neutrophil elastase) [11]. Other clear disease associations with AAT deficiency include panniculitis, antineutrophil cytoplasmic antibody vasculitis (e.g. Wegener's granulomatosis) and bronchiectasis [12].
Panniculitis associated with AAT deficiency was first described in 1972 by Warter et al. [13]. Since then, panniculitis has been recognized as a rare complication of AAT deficiency, with an estimated prevalence of approximately 1 per 1,000 [14]. Although individuals with severe AAT deficiency (PiZZ) account for most (i.e. 62–70%) cases of AAT deficiency-associated panniculitis, other AAT deficiency types have also been described (i.e. PiMZ, PiMS, PiSS, PiSNull and PiSZ). Moreover, men and women have been shown to be affected equally, and the age of onset varies widely, i.e. from childhood to the seventh decade of life [15, 16]. Clinically, the lesions of AAT deficiency-associated panniculitis consist of subcutaneous nodules due to neutrophilic inflammation, mostly located on the lower extremities and less commonly on the arms, trunk and face. Early lesions may resemble infectious cellulites, and later may ulcerate with exudation of oily material.
Various therapies for AAT deficiency-associated panniculitis have been reported, including anti-inflammatory drugs, antibiotics, chemotherapeutic agents and plasma exchange [17, 18]. All these treatments confer, at most, modest benefit and do not address the fundamental pathogenesis of α-1 antitrypsin deficiency-related panniculitis, which is likely unopposed elastolytic burden. Reasoning that panniculitis is, in fact, caused by unopposed proteolysis in the skin, several authors have reported successful treatment of AAT deficiency-associated panniculitis with intravenous augmentation therapy infusions (i.e. pooled, purified human plasma α1-antitrypsin) [18,19,20,21]. With available results suggesting that biochemical normalization of AAT levels by augmentation therapy reduces the frequency lung infections, the few available reports of augmentation therapy in panniculitis suggest that symptoms and signs of panniculitis have been shortened, at least in conventional doses (60 mg/kg body weight once weekly), for short durations (weeks) [22,23,24].
The current report extends available experience with treating AAT deficiency-associated panniculitis through describing a woman with panniculitis due to PiZZ AAT deficiency which improved in temporal association with augmentation therapy; resolution appeared to require 90 mg/kg body weight of augmentation therapy, and panniculitis recurred with initial cessation, prompting long-term use to control the panniculitis. Also, a novel finding in this report is that polymers of Z-type AAT were found in biopsies of our patient's skin. Because polymers were found in both lesions and nonlesional skin, their pathogenetic role is, of course, not established from this report.
Case Report
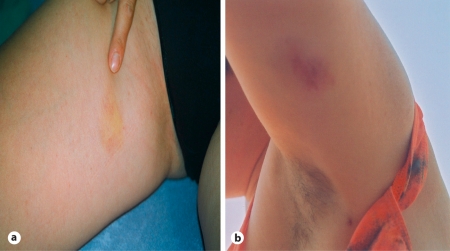
A previously healthy 31-year-old woman had a 2-year history of recurrent skin nodules. The nodules were red and tender, had not ulcerated but, at least in 1 instance, appeared ready to burst and characteristically persisted for 3–4 weeks. The nodules were present mostly on the thighs and calves, abdomen, buttocks and forearms (fig. 1a, b). Histological examination of a skin biopsy obtained on April 19, 2002 (Group Pathology Practice Dr. Hinkeldey, Prof. Kriegsmann and Dr. Otto, Trier, Germany), showed the presence of necrotic lesions in the subcutaneous fatty tissue with moderately pronounced infiltrates of neutrophilic polymorphonuclear granulocytes and occasional lymphocytes, plasma cells and histiocytes. Neither epithelioid cell granulomata nor vasculitis was observed (fig. 2). Histopathological features were suggestive of panniculitis.
Fig. 1.
Proximal thigh (a) and left upper arm (b) before augmentation therapy was begun.
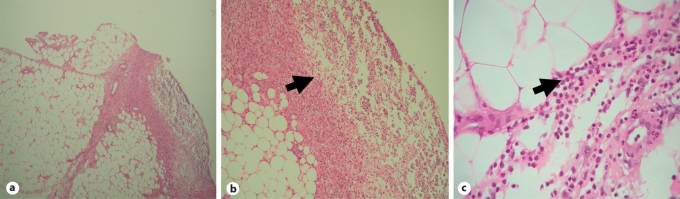
Fig. 2.
Acute lobular and septal panniculitis in skin punch biopsy. The arrows indicate inflammatory infiltrate and focal lobular fat cell degeneration. HE. ×40 (a), ×100 (b) and ×400 (c).
The patient's course had been characterized by episodes of intermittent cutaneous swelling with spontaneous resolution after several weeks and subsequent recurrence elsewhere on the skin. Episodes occurred every fourth to fifth month, and lasted roughly 3 weeks at a time. Trauma was not a triggering factor for these cutaneous flares, and the patient neither experienced fever nor leukocytosis during flares. Initial treatment with ibuprofen over 2 weeks had not been helpful and no further treatment (such as oral dapsone) was offered. A serum electrophoresis showed dysproteinemia with a low α1-globulin fraction. The relative α1-globulin concentration was 0.7% (normal range: 1.4–4.0%) and the absolute concentration was 0.06 g/dl (normal range: 0.19–0.3 g/dl). The serum level of AAT measured by nephelometry was greatly reduced at 22.1 mg/dl (normal range: 90–200 mg/dl), measured in the laboratory of Prof. Seelig and colleagues, Karlsruhe, Germany. Blood samples from this patient were used to perform polymerase chain reaction (PCR) with corresponding primers specific for PiZZ mutation in exon V. PCR genotyping revealed the PiZZ AAT genotype. Testing of first-degree relatives showed that our patient was the only homozygote (PiZZ) in her family; her mother, father and brother were asymptomatic heterozygotes (PiMZ; table 1) and there was no family history of early death due to pulmonary emphysema or liver disease, and no family history of panniculitis. Also, our patient's chest X-ray, pulmonary function and liver function tests were normal.
Table 1.
Serum AAT concentrations in the patient's family members
| Patient's family members | Serum AAT1 mg/dl | AAT Pi geno- type |
|---|---|---|
| Mother | 90.70 | MZ |
| Father | 68.90 | MZ |
| Brother | 87.80 | MZ |
| Son | 100 | MZ |
| Daughter | 83.5 | MZ |
| Husband | 123 | MM |
Normal range: 90–200 mg/dl.
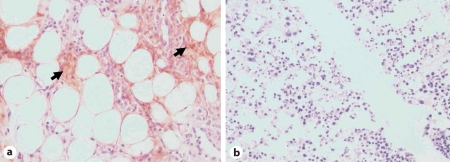
Staining of her skin biopsy with a murine monoclonal antibody ATZ11 (1:100) that was produced in our laboratory and that specifically reacts with polymerized AAT [25] was performed. The staining was positive for AAT polymers in our patient's skin biopsy in contrast to the results of staining a skin biopsy from normal (PiMM) controls (fig. 3), which showed no polymers in the skin. Specimens from our patient showed polymers of Z-type AAT both in areas of panniculitis and in normal-appearing skin.
Fig. 3.
Immunostaining of skin biopsy from the reported PiZZ AAT patient (a) and from a control PiMM AAT individual (b). a Specific staining of subcutaneous fatty tissue with mouse monoclonal ATZ11 antibody (1:100) specific to polymeric form of AAT can be seen in our PiZZ patient. Original magnification ×400. The arrows indicate Z AAT polymers (brown). b No positive staining with ATZ11 antibody (1:100) can be detected in skin biopsy from the control PiMM individual. Original magnification ×100.
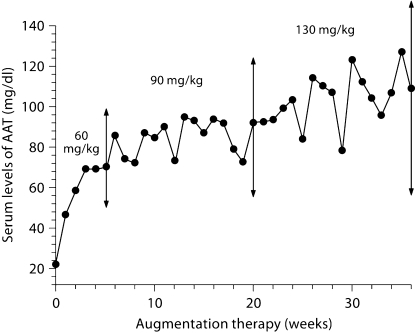
In the context of the aforementioned favorable reports in which augmentation therapy was associated with dramatic and prompt resolution of panniculitis in AAT deficiency, a trial of augmentation therapy was offered. Augmentation therapy with pooled human plasma AAT (Prolastin®) was initiated at a dose of 60 mg/kg body weight (3 g in this patient) once weekly. Within 14 days of initiating augmentation therapy, i.e. after the second dose, the patient reported fewer skin nodules and decreased nodule-associated pain. Nevertheless, while continuing augmentation therapy at this conventional dose, she continued to experience bouts of nodule formation at intervals of weeks to months, albeit with reduced severity and pain. In an attempt to better control her panniculitis, the dose of augmentation therapy was empirically increased to 130 mg/kg body weight once weekly; within a week, the remaining nodules disappeared completely. On this higher dose regimen, the patient's trough serum level of AAT rose to a maximum of 127 mg/dl (fig. 4). With subsequent reduction of the dose to a maintenance regimen of 90 mg/kg body weight, once weekly, and follow-ups from 25 August 2005, the patient developed only occasional, slightly indurated nodules that persisted for no longer than a week, prompting the continuation of augmentation therapy for a total of 3 years to date. No recurrence of panniculitis has been observed over this 3-year period.
Fig. 4.
AAT serum levels prior to next infusion during 36 weeks of augmentation therapy administered to the patient during 2004–2005. The augmentation therapy was started on 17 April 2004 at a dose of 60 mg/kg once weekly. The dose of augmentation therapy was increased to 90 mg/kg between weeks 6 and 20 and went up to 130 mg/kg body weight between weeks 20 and 36. On this dose regimen, the patient's trough serum level of AAT rose to a maximum of 127 mg/dl. Subsequently, the dose was reduced to a maintenance regimen of 90 mg/kg body weight once weekly. No recurrence of panniculitis has been observed over augmentation therapy for a total of 3 years to date.
Discussion
To date, panniculitis has been described in fewer than 100 AAT-deficient individuals [23]. Classically, the panniculitis associated with AAT deficiency is chronic, relapsing and widely disseminated. The inflammation, which can persist for months, can progress from an acute phase to a chronic phase, which is characterized by the development of focal lesions, proliferation of fat-filled histiocytes and giant cell formation. Another distinctive feature of AAT deficiency-associated panniculitis is the absence of a zone of erythema around the sometimes very large skin defects, which can extend down to the level of muscle. Infiltrates range from perivascular round cell infiltrates to masses of neutrophils causing necrosis and replacement of fat lobules without vasculitis [18]. Early on, in a process called ‘splaying’ of collagen, neutrophils infiltrate the tissue within the collagen bundles throughout the reticular dermis. Strands of neutrophils and to some extent also phagocytes project into the necrotic fat lobules. The destruction of fat lobules occurs in direct proximity to normal fat tissue while microscopic examination reveals no primary fat cell changes (fig. 2). Overall, the neutrophilic inflammation, loss of elastin and the absence of vasculitis that were observed in our patient are characteristic features of AAT deficiency-associated panniculitis.
In summarizing possible pathogenetic mechanisms of panniculitis in AAT deficiency, Smith et al. [26] have proposed several mechanisms: (1) insufficient inhibition of membrane-bound serine proteases; (2) increased elastin degradation promoted by the large amounts of available fatty acids; (3) insufficient inhibition of complement activation; (4) neutrophil accumulation at sites of inflammation that may result in the release of serine proteases with subsequent damage to surrounding connective tissue structures; (5) oxidation of the active site of the AAT molecule by myeloperoxidase which reduces antiprotease activity. Keeping in mind that skin biopsies from our patient with AAT deficiency-associated panniculitis showed, for the first time, the co-accumulation of neutrophils and Z AAT polymers (fig. 3), we propose that AAT polymers may represent another possible pathogenetic mechanism of panniculitis in AAT deficiency. Indeed, polymers (which develop as a result of instability of the Z molecule caused by the single amino acid substitution of lysine for glutamic acid at position 342) have been implicated in various manifestations of PiZZ AAT deficiency, including the liver disease [27, 28] and inflammation within the lung, where polymers have also been observed [29, 30]. Indeed, polymers have been shown to be pro-inflammatory, as polymeric AAT has been shown to colocalize with neutrophils in the alveoli of individuals with PiZZ AAT deficiency-related emphysema and polymers have been shown to be chemotactic in vitro [31] and when instilled into murine lungs [32].
Though the precise pathogenesis of panniculitis in AAT deficiency is still unclear, the consistent neutrophilic inflammation and the response to intravenous augmentation therapy strongly support unopposed elastolysis as a key mechanism, just as in the pathogenesis of AAT deficiency-associated emphysema. The pro-inflammatory effects of polymeric AAT, in addition to the impaired inhibitory activity of the Z molecule, may contribute directly to the neutrophil recruitment and development of inflammation in individuals with PiZZ AAT deficiency-associated panniculitis. While demonstrating disappearance of polymers in the skin during remission of the panniculitis might have further supported this pathogenetic mechanism, ethical concerns precluded our performing repeated skin biopsies during the augmentation therapy and associated remission of our patient's symptoms. Thus, while our finding that Z-type AAT polymers colocalize with neutrophils in AAT-associated panniculitis advances suspicion that AAT polymers abet the inflammatory process in panniculitis, demonstrating this proposed pathogenetic mechanism will require further investigation, including serial biopsies showing that polymers become more scarce as the panniculitis remits.
Available reports describe various treatments of panniculitis in AAT deficiency, including anti-inflammatory drugs, antibiotics, chemotherapeutic agents and plasma exchange. As summarized in table 2, treatments to date have had varying levels of effectiveness. Many clinicians regard dapsone as the initial treatment of choice, based on modest efficacy and reasonable expense. At the same time, though described in only 2 reports to date [19, 33], intravenous augmentation therapy has consistently been associated with prompt resolution of the signs and symptoms of AAT deficiency-associated panniculitis. Reports to date have described conventional doses of 60 mg/kg body weight once weekly, which conferred only incomplete benefit in our patient.
Table 2.
Treatment of panniculitis associated with AAT deficiency
| Specific treatment | Response to the treatment, % | n1 |
|---|---|---|
| Doxycycline/minocycline | 87.5 | 8 |
| Cloxacillin/nafcillin | 100 | 3 |
| Dapsone | 90.0 | 23 |
| Corticosteroids | 63.2 | 19 |
| Nonsteroidal anti-inflammatory drugs | 100 | 1 |
| Cyclophosphamide | 50 | 2 |
| Lugol's solution | 0 | 1 |
| Plaquenil/chloroquine | 33.3 | 3 |
| Intravenous augmentation therapy | 100 | 3 |
| Plasma exchange | 100 | 1 |
| Liver transplantation | 100 | 1 |
| Nitrogen mustard | 0 | 1 |
Based on Stoller et al. [19] and other reported cases.
Our report extends the available reports of treating AAT deficiency-associated panniculitis with intravenous augmentation therapy by using higher than conventional doses for longer than usual durations. The observation in our patient that augmentation therapy at a weekly dose of 130 mg/kg was more effective than 60 mg/kg weekly supports the notion of an inflammatory pathogenesis of panniculitis, and suggests that doses described to date to have efficacy in slowing the rate of decline of lung function may be inadequate to ablate inflammation in the skin. Our use of weekly ‘maintenance’ augmentation therapy at a higher than usual dose (90 mg/kg body weight weekly) for several years represents another novel treatment approach to panniculitis, as treatment of panniculitis with augmentation therapy to date has been only episodic (e.g. for several weeks). While we recognize that proof of the efficacy of this approach must await controlled trials and that use of augmentation therapy for panniculitis is both off-label and expensive (estimated at EUR 380 per gram), we also acknowledge that the rarity of AAT-associated panniculitis makes such controlled trials unlikely. Until then, we offer this experience as a guide to clinicians caring for patients with panniculitis associated with AAT deficiency.
In summary, experience with our patient emphasizes not only the importance of testing individuals with panniculitis for AAT deficiency (analysis of serum AAT level and genotype) in the hope of identifying at-risk family members, but also shows that intravenous augmentation therapy can be an effective and direct approach to AAT deficiency-associated panniculitis. In addition, this experience suggests that high doses of augmentation therapy may confer added benefit and that maintenance dosing may ameliorate the frequency and severity of panniculitis associated with AAT deficiency. Recognizing that augmentation therapy has not been established as a first-line therapy of panniculitis and is expensive, this case lends support to the role of augmentation therapy in AAT-associated panniculitis, and invites further study to clarify currently unanswered questions. Finally, our observation that AAT polymers are present in both lesional and nonlesional skin of AAT deficiency-associated panniculitis cases supports, but does not prove, that polymers may play a role in this distinctive form of panniculitis (depending on their size and biochemical characteristics). Whether polymers influence inflammatory reactions in AAT deficiency-associated panniculitis and, in particular, whether augmentation therapy can modulate polymer formation and biological effects are subjects worthy of further investigation.
Acknowledgment
Funding sources. Craffords Foundation (S.J.).
Footnotes
J.K.S. has served as a consultant to Talecris Biotherapeutics and CSL Behring, and has been supported in lectures delivered by Talecris Biotherapeutics, Grifols, Baxter Healthcare and CSL Behring.
References
- 1.Potempa J, Korzus E, Travis J. The serpin superfamily of proteinase inhibitors: structure, function, and regulation. J Biol Chem. 1994;269:15957–15960. [PubMed] [Google Scholar]
- 2.Hutchison DC. Natural history of alpha-1-protease inhibitor deficiency. Am J Med. 1988;84:3–12. doi: 10.1016/0002-9343(88)90153-2. [DOI] [PubMed] [Google Scholar]
- 3.Janciauskiene S. Conformational properties of serine proteinase inhibitors (serpins) confer multiple pathophysiological roles. Biochim Biophys Acta. 2001;1535:221–235. doi: 10.1016/s0925-4439(01)00025-4. [DOI] [PubMed] [Google Scholar]
- 4.Kalsheker N, Morley S, Morgan K. Gene regulation of the serine proteinase inhibitors alpha1-antitrypsin and alpha1-antichymotrypsin. Biochem Soc Trans. 2002;30:93–98. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 5.Carrell RW, Jeppsson JO, Laurell CB, Brennan SO, Owen MC, Vaughan L, Boswell DR. Structure and variation of human alpha 1-antitrypsin. Nature. 1982;298:329–334. doi: 10.1038/298329a0. [DOI] [PubMed] [Google Scholar]
- 6.de Serres FJ. Worldwide racial and ethnic distribution of alpha1-antitrypsin deficiency: summary of an analysis of published genetic epidemiologic surveys. Chest. 2002;122:1818–1829. doi: 10.1378/chest.122.5.1818. [DOI] [PubMed] [Google Scholar]
- 7.Kalsheker NA. Molecular pathology of alpha 1-antitrypsin deficiency and its significance to clinical medicine. QJM. 1994;87:653–658. [PubMed] [Google Scholar]
- 8.Abboud RT, Ford GT, Chapman KR. Emphysema in alpha1-antitrypsin deficiency: does replacement therapy affect outcome? Treat Respir Med. 2005;4:1–8. doi: 10.2165/00151829-200504010-00001. [DOI] [PubMed] [Google Scholar]
- 9.Yu MH, Lee KN, Kim J. The Z type variation of human alpha 1-antitrypsin causes a protein folding defect. Nat Struct Biol. 1995;2:363–367. doi: 10.1038/nsb0595-363. [DOI] [PubMed] [Google Scholar]
- 10.Lomas DA, Evans DL, Finch JT, Carrell R. The mechanism of Z alpha 1-antitrypsin accumulation in the liver. Nature. 1992;357:605–607. doi: 10.1038/357605a0. [DOI] [PubMed] [Google Scholar]
- 11.Crystal RG. Alpha 1-antitrypsin deficiency, emphysema, and liver disease: genetic basis and strategies for therapy. J Clin Invest. 1990;85:1343–1352. doi: 10.1172/JCI114578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janciauskiene S, Stevens T, Blanco I. New insights into the protective effects of alpha-1-antitrypsin in chronic obstructive pulmonary disease. Curr Respir Med Rev. 2007;3:147–158. [Google Scholar]
- 13.Warter J, Storck D, Grosshans E, Metais P, Kuntz JL, Klumpp T. Weber-Christian syndrome associated with an alpha-1-antitrypsin deficiency: familial investigation (in French) Ann Med Interne (Paris) 1972;123:877–882. [PubMed] [Google Scholar]
- 14.McElvaney NG, Stoller JK, Buist AS, Prakash UB, Brantly ML, Schluchter MD, Crystal RD. Baseline characteristics of enrollees in the National Heart, Lung and Blood Institute Registry of alpha 1-antitrypsin deficiency. Chest. 1997;111:394–403. doi: 10.1378/chest.111.2.394. [DOI] [PubMed] [Google Scholar]
- 15.Gaillard MC, Bothwell J, Dreyer L. A case of systemic nodular panniculitis associated with M1 (Val213) Z phenotype of alpha-1-protease inhibitor. Int J Dermatol. 1997;36:278–280. doi: 10.1111/j.1365-4362.1997.tb03043.x. [DOI] [PubMed] [Google Scholar]
- 16.Pinto AR, Maciel LS, Carneiro F, Resende C, Chaves FC, Freitas AF. Systemic nodular panniculitis in a patient with alpha-1-antitrypsin deficiency (PiSS phenotype) Clin Exp Dermatol. 1993;18:154–155. doi: 10.1111/j.1365-2230.1993.tb01000.x. [DOI] [PubMed] [Google Scholar]
- 17.Irvine C, Neild V, Stephens C, Black M. Alpha-1-antitrypsin deficiency panniculitis. J R Soc Med. 1990;83:743–744. doi: 10.1177/014107689008301120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ortiz PG, Skov BG, Benfeldt E. Alpha1-antitrypsin deficiency-associated panniculitis: case report and review of treatment options. J Eur Acad Dermatol Venereol. 2005;19:487–490. doi: 10.1111/j.1468-3083.2005.01194.x. [DOI] [PubMed] [Google Scholar]
- 19.Stoller JK, Piliang M. Panniculitis in alpha-1 antitrypsin deficiency: a review. Clin Pulm Med. 2008;15:113–117. [Google Scholar]
- 20.Wewers MD, Casolaro MA, Sellers SE, Swayze SC, McPhaul KM, Wittes JT, Crystal RG. Replacement therapy for alpha 1-antitrypsin deficiency associated with emphysema. N Engl J Med. 1987;316:1055–1062. doi: 10.1056/NEJM198704233161704. [DOI] [PubMed] [Google Scholar]
- 21.Knight KR, Burdon JG, Cook L, Brenton S, Ayad M, Janus ED. The proteinase-antiproteinase theory of emphysema: a speculative analysis of recent advances into the pathogenesis of emphysema. Respirology. 1997;2:91–95. doi: 10.1111/j.1440-1843.1997.tb00060.x. [DOI] [PubMed] [Google Scholar]
- 22.Soy D, de la Roza C, Lara B, Esquinas C, Torres A, Miravitlles M. alpha-1-Antitrypsin deficiency: optimal therapeutic regimen based on population pharmacokinetics. Thorax. 2006;61:1059–1064. doi: 10.1136/thx.2005.057943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lieberman J. Augmentation therapy reduces frequency of lung infections in antitrypsin deficiency: a new hypothesis with supporting data. Chest. 2000;118:1480–1485. doi: 10.1378/chest.118.5.1480. [DOI] [PubMed] [Google Scholar]
- 24.Stoller JK, Aboussouan LS. α1-Antitrypsin deficiency. 5: intravenous augmentation therapy: current understanding. Thorax. 2004;59:708–712. doi: 10.1136/thx.2003.006544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janciauskiene S, Eriksson S, Callea F, Mallya M, Zhou A, Seyama K, Hata S, Lomas DA. Differential detection of PAS-positive inclusions formed by the Z, Siiyama, and Mmalton variants of alpha1-antitrypsin. Hepatology. 2004;40:1203–1210. doi: 10.1002/hep.20451. [DOI] [PubMed] [Google Scholar]
- 26.Smith KC, Pittelkow MR, Su WP. Panniculitis associated with severe alpha1-antitrypsin deficiency: treatment and review of the literature. Arch Dermatol. 1987;123:1655–1661. [PubMed] [Google Scholar]
- 27.Lomas DA, Mahadeva R. Alpha1-antitrypsin polymerization and the serpinopathies: pathobiology and prospects for therapy. J Clin Invest. 2002;110:1585–1590. doi: 10.1172/JCI16782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ranes J, Stoller JK. A review of alpha-1 antitrypsin deficiency. Semin Respir Crit Care Med. 2005;26:154–166. doi: 10.1055/s-2005-869536. [DOI] [PubMed] [Google Scholar]
- 29.Parmar JS, Mahadeva R, Reed BJ, Farahi N, Cadwallader KA, Keogan MT, Bilton D, Chilvers ER, Lomas DA. Polymers of alpha(1)-antitrypsin are chemotactic for human neutrophils: a new paradigm for the pathogenesis of emphysema. Am J Respir Cell Mol Biol. 2002;26:723–730. doi: 10.1165/ajrcmb.26.6.4739. [DOI] [PubMed] [Google Scholar]
- 30.Elliott PR, Bilton D, Lomas DA. Lung polymers in Z alpha1-antitrypsin deficiency-related emphysema. Am J Respir Cell Mol Biol. 1998;18:670–674. doi: 10.1165/ajrcmb.18.5.3065. [DOI] [PubMed] [Google Scholar]
- 31.Mulgrew AT, Taggart CC, Lawless MW, Greene CM, Brantly ML, O'Neill SJ, McElvaney NG. Z alpha1-antitrypsin polymerizes in the lung and acts as a neutrophil chemoattractant. Chest. 2004;125:1952–1957. doi: 10.1378/chest.125.5.1952. [DOI] [PubMed] [Google Scholar]
- 32.Mahadeva R, Atkinson C, Li Z, Stewart S, Janciauskiene S, Kelley DG, Parmar J, Pitman R, Shapiro SD, Lomas DA. Polymers of Z alpha1-antitrypsin co-localize with neutrophils in emphysematous alveoli and are chemotactic in vivo. Am J Pathol. 2005;166:377–386. doi: 10.1016/s0002-9440(10)62261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chowdhury MM, Williams EJ, Morris JS, Ferguson BJ, McGregor AD, Hedges AR, Stamatakis JD, Pope FM. Severe panniculitis caused by homozygous ZZ alpha1-antitrypsin deficiency treated successfully with human purified enzyme (prolastin) Br J Dermatol. 2002;147:1258–1261. doi: 10.1046/j.1365-2133.2002.05095.x. [DOI] [PubMed] [Google Scholar]