Abstract
This paper proposes that reduced allergen exposure is one of the factors underlying the higher risk of IgE-mediated allergic disease in populations with an urbanized, westernized, and affluent lifestyle. This lower allergen exposure results in the failure to induce and maintain immune tolerance to common environmental allergens. The paper summarizes different lines of evidence that may support or contradict this hypothesis and points to areas where more knowledge is needed.
Key Words: Allergy, Allergen, Respiratory hypersensitivity, Tolerance
Immune Tolerance: A Dose-Dependent Phenomenon
Immune responses are directed against most pathogens but normally not against non-pathogenic antigens abundantly present in the environment such as allergens from pollens, house dust mites (HDMs), moulds, and animals. This differential responsiveness is important to avoid hypersensitivity reactions against allergens penetrating mucosal barriers and other non-specific defence lines of the immune system. Allergy can be viewed as a failure of the immune system to develop and maintain specific unresponsiveness (immune tolerance) against allergens. In 1911, Wells and Osborne [1] showed that anaphylactic reactions to ovalbumin (OVA) in guinea pigs could be prevented by prior oral administration of OVA. The concept of tolerance is well established for the oral route of exposure where the events takes place or starts in the gastrointestinal mucosa and gut-associated lymphoid tissue [2,3,4,5]. However, tolerance induced via the inhalation route of respiratory airway mucosa is also well-documented and important to obtain unresponsiveness to inhalant allergens [6, 7]. Holt et al. [8] demonstrated that prolonged as opposed to brief exposure of mice to OVA via the respiratory tract induced unresponsiveness to subsequent OVA inhalation. In mice, this tolerant immune response is characterized by decreased specific IgE and IgG1 response (Th2-associated response) accompanied by increased specific IgG2a response (Th1-associated response) [8, 9]. Thus, data from experimental animal studies have shown that high-dose allergen exposure independent of the route of administration favours immune tolerance, while low-dose allergen exposure favours immune responsiveness [2,10,11,12,13,14,15,16]. Notably, Tsitoura et al. [17] demonstrated that intranasally administered OVA could prevent OVA-induced airway hyperresponsiveness and airway inflammation in mice immunized intraperitoneally with OVA. It may be of potential importance that induction of antigen-specific tolerance may have non-specific effects in terms of increased tolerance to unrelated antigens, also known as bystander suppression possibly through control of regulatory T cell responses [6].
Factors other than the allergen dose are important. Most importantly, there exists an individual genetic susceptibility to allergic disease and this also relates to tolerance. It has been shown in inbred strains of rodents that tolerance to inhalation of both experimental allergen (OVA) [18] and HDM allergens [19] is highly strain-dependent and thus dependent on genetic susceptibility. Thus, the threshold dose of inhaled allergen required for tolerance induction is highly dependent on genetic susceptibility. Furthermore, other environmental factors, e.g. the presence of microbial products/antigens, may influence the outcome of allergen exposure. The complex mechanisms of immune tolerance and gene-environmental interactions are beyond the scope of this paper and have been described in more detail elsewhere [2, 11, 12, 15,20,21,22,23,24,25].
Allergy Is Associated with Urbanized, Westernized, and Affluent Lifestyle
There is strong evidence that the prevalence of aeroallergen sensitization and atopic diseases, such as allergic rhinitis, asthma, and atopic dermatitis, has increased over recent decades [26, 27]. The specific factors responsible for these trends are largely unknown. However, three factors have reasonably consistently been associated with a high prevalence of atopic disease: westernization [28], urbanization [29], and affluence [30]. It is almost a law of nature that populations that undergo changes towards a westernized, urbanized, and affluent lifestyle develop an increasing prevalence of aeroallergen sensitization and atopic diseases. In some populations, these developments have occurred over a surprisingly short period of time. For example, an increase in hay fever was observed in the former East Germany following the unification of the former East and West Germanies possibly resulting in rapid changes towards a western lifestyle [31]. Accordingly, in Greenland, rapid lifestyle changes from a native Inuit lifestyle towards a modern westernized lifestyle were followed by a rapid increase in atopy [32]. The urban-rural difference in prevalence of allergy is of particular interest, since the urban-rural gradient has been observed within populations considered relatively homogeneous with regard to their genetic make-up and other factors of potential importance to allergy development. In Denmark, adults who had migrated from rural areas to the urbanized area of Copenhagen were at increased risk of novel allergy development suggesting that the factors underlying the urban-rural gradient also operate beyond childhood and that tolerance is not always a permanent immunological state in adult life [33]. Hence, it appears that persons migrating from rural to urban areas meet some new risk factors in the urban environment or lose some protective factors in the rural environment decreasing immune tolerance and increasing allergy risk.
Is Urbanization Associated with High or Low Exposure to Allergens?
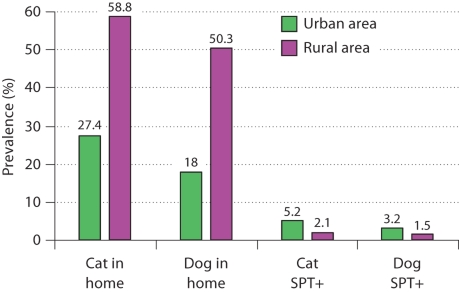
It has often been stated that urban living is associated with high exposure to allergens, e.g. allergens from pets that may more often be kept indoors in urban areas and more often outdoors in rural areas. However, there is surprisingly little evidence to support this notion. In fact, the available evidence seems to suggest that high allergen exposure levels are present in rural environments and it is plausible that the quantitatively most important allergen sources, such as allergens from pollens, moulds, and animals, are more abundantly present in rural compared to urban areas. In Denmark, pets are less frequently kept indoors in urban as compared to rural settings [34]. Thus, a nationwide representative Danish survey found that the prevalence of cats or dogs in the home was 59, 44, 27 and 11% in farm houses, detached houses, non-detached town houses, and apartment houses, respectively [34]. In Denmark, the most urbanized area (the municipality of Copenhagen) has the highest prevalence of self-reported allergy. Nevertheless, in Copenhagen the prevalence of cats or dogs in the home is very low (12%) compared to more rural counties (ranging between 32 and 46%) [34]. In a recent study in Greece it has been found that both cats and dogs were markedly more often kept in the home by a rural population compared to an urban population (fig. 1). In striking contrast, both cat and dog IgE sensitization as reflected by skin prick test reactivity were markedly more prevalent in the urban compared to the rural population (fig. 1) [35]. Although it may be speculated that pets spend more time indoors in urban homes as compared to rural homes, the above observations suggest that pet allergen exposure is frequent and significant in rural homes and somehow this does not confer a high risk of sensitization.
Fig. 1.
Prevalence of cats and dogs in the home and skin prick test reactivity (SPT+) to cat and dog in an urban and rural area in Greece [from [35]].
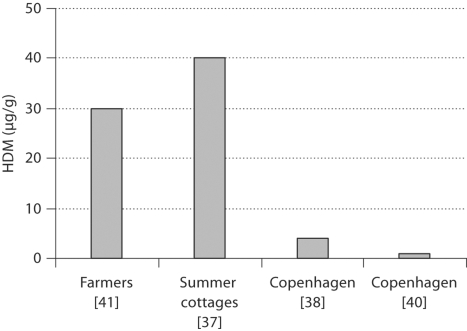
There is also limited data on possible urban-rural differences in HDM exposure. It has been assumed that increased insulation of buildings and increased indoor humidity have resulted in an increase in HDM exposure [36] and that this might particularly be the case in urban areas. However, poorer housing conditions present in developing, rural, and poor populations may result in high concentrations of allergens from HDMs, storage mites, and moulds. Notably, a Danish study revealed levels of HDM allergens in unheated summer cottages that were at least 3-fold higher than levels in ordinary houses and higher than levels usually found in urban dwellings in Denmark [37] (fig. 2). In mattress dust from summer cottages the median concentration was 2,000 HDMs/g corresponding to 40 μg/g of mattress dust. In the urbanized area of Copenhagen, mattress dust samples from a general population sample showed a median level of 4 μg/g of mattress dust [38], which was comparable to levels observed in previous studies in Copenhagen [39, 40] (fig. 2). In comparison, mattress dust from Danish farmers contained high levels of HDMs: median count 148/0.1 g of mattress dust [41], which corresponds to 30 μg/g of mattress dust. A German study revealed that HDM levels were 50-fold higher in the homes of pig farmers as compared to the homes of urban dwellers [42]. Thus, the median concentration of Der p 1 was 53.4 and 1.1 μg/g dust from mattresses of farmers and urban dwellers, respectively. In the PARCIFAL study conducted in five European countries, HDM levels were higher in dust samples from homes of children living on farms as compared to samples from homes of children who lived in the same area, but not on farms [43].
Fig. 2.
Studies on the levels of HDM in mattress dust from Danish homes. Copenhagen is the most urbanized area in Denmark.
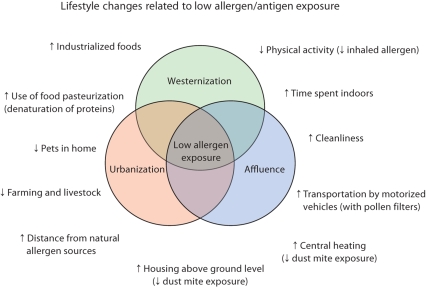
Data from the European Community Respiratory Health Survey (ECRHS) showed that living in a flat, having a bedroom above ground level, and central heating were associated with lower levels of HDM allergens in mattress dust [44]. In many countries, these housing characteristics are more common in urbanized as compared to rural settings. Furthermore, it is conceivable that some of the lifestyle factors characterizing urban, westernized, and affluent lifestyle may be associated with reduced allergen exposure. Thus, city dwellers may spend more time indoors at the work place and during leisure time, and often use the bus, train, or car as means of transport (many of these have pollen filters!) reducing the exposure at least to outdoor allergens. A physically inactive as compared to a physically active lifestyle may decrease the volume of inhaled air manifold, thereby decreasing the cumulated dose of allergen filtrated by the airway mucosa. Industrialized foods undergo procedures such as pasteurization that decrease the antigenic properties of the dietary protein contents decreasing the exposure to dietary antigens. The above-described lifestyle factors are often more common in urbanized, westernized, and affluent populations, and may result in an overall generally lower allergen exposure (fig. 3). However, few studies have specifically addressed these issues.
Fig. 3.
Common characteristics of a westernized, urbanized, and affluent lifestyle that may be associated with low allergen exposure.
In summary, there is limited data to support the idea that increased prevalence of allergy in urbanized areas as compared to rural areas is due to a higher exposure to allergens in the urban areas. On the contrary, the available data seem to support the notion that allergen exposure may be lower in urban as compared to rural settings.
What Can Be Learned from Observational Epidemiology?
It seems beyond doubt that IgE sensitization to an allergen requires exposure to that particular allergen. Not surprisingly, the pattern of sensitization in a particular population reflects the allergens prevailing in the environment. Hence, the prevalence of sensitization to olive pollen is high in Mediterranean regions (e.g. Southern France and Italy), but low in Scandinavia (e.g. Sweden and Norway), while the prevalence of sensitization to birch tree pollen is low in Mediterranean regions, but high in Scandinavia [45]. Nevertheless, at the individual level within each community there is often no clear relationship between exposure to allergens and risk of sensitization. Many epidemiological studies have looked at the relationship of pet keeping to IgE sensitization against pets and allergic disease. It has generally been assumed that pets confer an increased risk of sensitization and atopic disease. However, during the 1990s a number of studies emerged reporting a negative association between pet keeping and the risk of pet allergy. Since then several studies have reproduced this finding, although results have not been consistent. It was suggested that this negative association was due to selective mechanisms, i.e. the ‘healthy pet keeper effect’. Nevertheless, many of these studies have observed that previous pet keeping was associated with a decreased risk of sensitization to between that of current and never pet keeping. This finding seems to be inconsistent with a healthy pet keeper effect.
The relationship between HDM exposure and HDM allergy seems to contradict the idea that high allergen exposure induces tolerance and protection against HDM allergy [46]. In a prospective German birth cohort (the MAS study), there was a dose-response relationship between increased exposure to HDM levels in the home and later development of HDM sensitization, but not to the development of asthma [47]. These German homes, however, were in the lower range of HDM allergen exposure [43]. It might be hypothesized that such low levels of exposure are insufficient to induce and maintain tolerance in genetically susceptible children. In other words, the dose-response relationship between HDM exposure and HDM sensitization may be bell-shaped [43, 48, 49], i.e. intermediate exposure confers an increased risk, while high exposure facilitates tolerance induction, as has been proposed for cat allergen exposure and cat sensitization [50]. Of note is that estimates for the quantity of inhaled cat allergens in homes with a cat are 10- to 100-fold higher than those for the quantity of inhaled HDM allergens [51, 52]. In communities with a high prevalence of cats and high levels of cat allergens in the society in general, a cat in the home may not carry an increased risk of allergic disease, whereas in communities with a low prevalence of cat keeping a cat in the home confers an increased risk [53]. The interpretation of these findings is not straightforward, but might be explained by the proposed bell-shaped relationship between exposure and sensitization [50]. When it comes to exposure to dogs in the home and dog allergens, the balance of evidence supports that exposure decreases the risk of sensitization to dog allergens, and a non-specific protective effect against sensitization to other allergens has even been suggested.
What Can Be Learned from Intervention Studies on Allergen Avoidance?
For many years allergen avoidance, if possible, has been recommended for the treatment of IgE-mediated allergic disease. In some countries, allergen avoidance has also been recommended for primary prevention in high-risk infants. Recommendations include avoidance of pet keeping in early life and measures for reducing HDM exposure (vacuum cleaning, no carpets, washing of bedding at a high temperature, impermeable mattress covers, ventilation, etc.). Nevertheless, the available evidence from HDM avoidance-randomized intervention studies in high-risk infants does not support the recommendation of HDM avoidance for primary prevention of allergic disease. Some of these intervention studies have included interventions other than HDM avoidance making it difficult to estimate the effects attributable to HDM avoidance. However, in three studies it was possible to estimate the independent effects of HDM avoidance [54,55,56]. Significant reductions in levels of HDM allergens were obtained without obtaining any significant beneficial effects on the incidence of health outcomes in the active group as compared to the placebo group [54,55,56]. On top of this, the effects of HDM avoidance measures for treatment of HDM-allergic respiratory disease have been questioned [57,58,59,60]. A Cochrane review of the effects of chemical and physical measures for reducing HDM exposure concluded that these measures cannot be recommended for treatment of asthma [59]. Although a number of methodological limitations should be considered in the interpretation of the data, there has been some concern that narrative reviews on this topic have been shown to be biased, favouring a positive effect of HDM avoidance [61]. To what extent such publication bias plays a role and whether this favours a positive effect of HDM avoidance is difficult to determine.
The generally recommended strategy for treatment of IgE-mediated food allergy is the elimination diet (ED), i.e. strict avoidance of the food items responsible for the induction of IgE-mediated food-allergic reactions. Few studies have compared the effect of ED on the effect of specific oral tolerance induction (SOTI), which is an alternative approach for treating food allergy [62]. The concept of SOTI is to increase tolerance to the allergenic food item by ingestion of increasing doses of the food item [63]. To the best of my knowledge only one randomized study has compared the effect of ED to the effect of SOTI. SOTI was found to be just as effective in inducing tolerance, as defined by double-blind placebo-controlled food challenge, to the allergenic food item as compared to ED (approximately 35% in both the SOTI and the ED group) [64]. Importantly, an additional 30% in the group receiving SOTI was able to integrate the allergenic food item in their diet, although they were not completely tolerant as defined by double-blind, placebo-controlled food challenge. Thus, SOTI may further have the advantage of increasing the threshold dose for allergic reactions and decreasing the risk of severe allergic reactions of inadvertent ingestion of the allergen. The value of SOTI for treatment of food allergy needs to be further evaluated in randomized studies.
The lifetime cumulated incidence of IgE-mediated food allergy seems to be considerably lower compared to the incidence of IgE-mediated allergic respiratory disease. Furthermore, the spontaneous course of food allergy is transient with remission in the majority of cases as opposed to allergic respiratory disease, which tends to be more chronic in nature [65]. A possible explanation for this apparent differential course of these two IgE-mediated diseases was put forward by Holt [66], who noted that the dose of allergens introduced to the immune system is quantitatively manifold higher for food allergens (doses in the range of micrograms to grams) as compared to inhalant allergens (doses in the range of nanograms). Thus, the lower prevalence and transient nature of food allergy might be due to the high allergen dose obtained from foods and the fact that for many food allergens, e.g. cow's milk proteins, complete avoidance of exposure is difficult.
What Can Be Learned from Allergen-Specific Immunotherapy?
Meta-analyses of randomized trials conclude that allergen-specific immunotherapy (SIT) improves symptoms of allergic rhinitis [67] and allergic asthma [68]. A long-term effect after discontinuation of treatment has been shown indicating that SIT potentially can induce long-term tolerance and change the course of disease [69]. The concept of subcutaneously administrated SIT is to induce immune tolerance to specific allergens by injection of high doses of allergens. To increase accessibility and reduce the risk of side effects, novel routes of administrating SIT have been developed, i.e. sublingually administrated SIT in the form of allergen drops or tablets [70, 71]. Although the mechanisms underlying the effects of SIT have not been fully elucidated [22, 72], the experience from SIT tells us that the induction of tolerance in IgE-sensitized allergic patients is not only biologically plausible but a scientific fact. It is of potential interest that SIT might also have non-specific immunological effects in terms of a protective effect against the development of new allergies [73]. This observation might raise the hypothesis that a high level of natural exposure to specific allergens, e.g. allergens from grass pollens, may have non-specific effects in terms of increasing the likelihood of tolerance to other allergens.
In summary, in a clinical setting high exposure to specific allergens by the subcutaneous or intravenous or oral or mucosal routes induces immune tolerance in allergic patients. We have no reason to assume that this phenomenon is not at play when allergen exposure takes place in the form of natural environmental allergens.
Other Explanations for the Allergy Epidemic
It is important to acknowledge that many factors are likely to play a role in the inception of the allergy epidemic, and that no single factor can explain all the variations in the prevalence of allergic disease over time, and the variation between and within populations. The hygiene hypothesis proposes that the rise in allergy is explained by a decrease in exposure to infections and microbes including microbial antigens leading to reduced stimulation of the immune system and reduced immune tolerance. Results of randomized studies of the effects of novel treatments such as probiotics and microbial products may provide evidence for or against this hypothesis. Furthermore, increasing knowledge on the function of genes and genetic variations in metabolism and detoxification enzymatic systems may help researchers in the pursuit of novel risk factors and evidence of causality [74, 75]. However, the hygiene hypothesis does not in its current version take into account reduced exposure to the quantitatively most important environmental airborne antigens, i.e. antigens of common allergens such as pollens, HDMs, animals, and moulds. Lower allergen exposure as a contributing factor to the allergy epidemic may be viewed as an extension of the hygiene hypothesis. Recently, lifestyle factors such as obesity, physical inactivity, decreasing exposure to sunlight (decreased vitamin D status), and diet have attracted increasing interest and should be considered in this context.
Summary and Perspectives
Allergy has reasonably consistently been linked to an urban, western, and affluent lifestyle. The author hypothesizes that a common characteristic of this lifestyle is reduced exposure to the quantitatively most important environmental airborne antigens, i.e. antigens of common allergens such as pollens, HDMs, animals, and moulds. In genetically susceptible individuals low allergen exposure results in a failure to induce and maintain immune tolerance to allergens and increased risk of allergic disease. There is very limited data on levels of allergen exposure according to gradients of urbanization, westernization, and affluence. Studies of these gradients seem warranted. However, several lines of evidence support the proposed hypothesis. First, numerous experimental animal studies have shown that exposure to high allergen doses (introduced by the oral route, respiratory route, or by injection) induces tolerance, whereas exposure to low allergen doses confers an increased risk of IgE sensitization. Second, many, but not all, observational epidemiological studies suggest that exposure to pets decreases the risk of pet allergy. When it comes to HDM exposure, the overall balance of evidence from observational studies suggests that increasing exposure to HDM allergens increases the risk of HDM IgE sensitization. Although this seems to contradict the proposed hypothesis, it might be explained by the possibility that the majority of populations studied were at the very low end of the range of exposure, which may be insufficient to induce and maintain tolerance to HDMs. Third, randomized intervention studies of HDM allergen avoidance (both primary/secondary and tertiary prevention) do not support that avoidance has beneficial effects on sensitization and clinical outcomes. Finally, there is a large body of evidence to support that the induction of immune tolerance in allergic patients can be obtained by allergen SIT underlining the pivotal role of the concept of immune tolerance induction by allergen exposure. New routes of administrating SIT may facilitate the implementation of large-scale intervention studies of the effects of SIT with regard to its ability to induce long-term immune tolerance and its preventive effects on the development of novel allergies and asthma. New target groups for SIT may be considered, e.g. asymptomatic sensitized persons or even persons with low risk of allergy.
It should be emphasized that some of the data presented in this paper are indirect evidence and not specifically designed to address the research questions. Clearly, more data from randomized intervention studies and epidemiological studies of gene-environment interactions are needed. It is going to take time to prove and accept the proposed hypothesis, and it requires a change in the paradigm of allergy treatment. In particular, the concept of allergen avoidance needs to be critically appraised. Importantly, caution should be exercised in this process, since a sudden increase in exposure to allergens in predisposed populations with a currently low allergen exposure may increase morbidity and disease prevalence. The author of this paper acknowledges to the greatest extent possible the complexity of this issue. Indeed, factors other than allergen exposure are needed to explain the increase in allergy and the observed variation in allergy prevalence between and within populations. However, reduced allergen exposure may serve as a unifying explanation for the increase in allergy associated with changes towards westernized, affluent, and urbanized lifestyle. At present, if this ‘pill’ is too hard to swallow, one may suggest that it is left under the tongue – slowly releasing allergens.
References
- 1.Wells Hg, Osborne TB. The biological reactions of the vegetable proteins: anaphylaxis. J Infect Dis. 1911;8:66–124. [Google Scholar]
- 2.Strober W, Kelsall B, Marth T. Oral tolerance. J Clin Immunol. 1998;18:1–30. doi: 10.1023/a:1023222003039. [DOI] [PubMed] [Google Scholar]
- 3.Mayer L, Shao L. Therapeutic potential of oral tolerance. Nat Rev Immunol. 2004;4:407–419. doi: 10.1038/nri1370. [DOI] [PubMed] [Google Scholar]
- 4.Mayer L, Sperber K, Chan L, Child J, Toy L. Oral tolerance to protein antigens. Allergy. 2001;56(suppl 67):12–15. doi: 10.1111/j.1398-9995.2001.00904.x. [DOI] [PubMed] [Google Scholar]
- 5.Mayer L. Oral tolerance: new approaches, new problems. Clin Immunol. 2000;94:1–8. doi: 10.1006/clim.1999.4791. [DOI] [PubMed] [Google Scholar]
- 6.Hoyne GF, Tan K, Corsin-Jimenez M, Wahl K, Stewart M, Howie SE, Lamb JR. Immunological tolerance to inhaled antigen. Am J Respir Crit Care Med. 2000;162:S169–S174. doi: 10.1164/ajrccm.162.supplement_3.15tac6. [DOI] [PubMed] [Google Scholar]
- 7.Lowrey JL, Savage ND, Palliser D, Corsin-Jimenez M, Forsyth LM, Hall G, Lindey S, Stewart GA, Tan KA, Hoyne GF, Lamb JR. Induction of tolerance via the respiratory mucosa. Int Arch Allergy Immunol. 1998;116:93–102. doi: 10.1159/000023931. [DOI] [PubMed] [Google Scholar]
- 8.Holt PG, Batty JE, Turner KJ. Inhibition of specific IgE responses in mice by pre-exposure to inhaled antigen. Immunology. 1981;42:409–417. [PMC free article] [PubMed] [Google Scholar]
- 9.McMenamin C, Pimm C, McKersey M, Holt PG. Regulation of IgE responses to inhaled antigen in mice by antigen-specific gamma delta T cells. Science. 1994;265:1869–1871. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- 10.Sakai K, Yokoyama A, Kohno N, Hiwada K. Effect of different sensitizing doses of antigen in a murine model of atopic asthma. Clin Exp Immunol. 1999;118:9–15. doi: 10.1046/j.1365-2249.1999.01036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolbe L, Heusser C, Kolsch E. Epitope-dependent nonreciprocal regulation of IgE and IgG2a antibody formation. Int Arch Allergy Immunol. 1994;103:214–216. doi: 10.1159/000236630. [DOI] [PubMed] [Google Scholar]
- 12.Saklayen MG, Pesce AJ, Pollak VE, Michael JG. Kinetics of oral tolerance: study of variables affecting tolerance induced by oral administration of antigen. Int Arch Allergy Appl Immunol. 1984;73:5–9. doi: 10.1159/000233428. [DOI] [PubMed] [Google Scholar]
- 13.Clausen SK, Bergqvist M, Poulsen LK, Poulsen OM, Nielsen GD. Development of sensitisation or tolerance following repeated OVA inhalation in BALB/cJ mice. Dose-dependency and modulation by the Al(OH)3 adjuvant. Toxicology. 2003;184:51–68. doi: 10.1016/s0300-483x(02)00583-8. [DOI] [PubMed] [Google Scholar]
- 14.Marcelletti JF, Katz DH. Antigen concentration determines helper T cell subset participation in IgE antibody responses. Cell Immunol. 1992;143:405–419. doi: 10.1016/0008-8749(92)90036-o. [DOI] [PubMed] [Google Scholar]
- 15.Holt PG. Mucosal immunity in relation to the development of oral tolerance/sensitization. Allergy. 1998;53:16–19. doi: 10.1111/j.1398-9995.1998.tb04952.x. [DOI] [PubMed] [Google Scholar]
- 16.Seymour BW, Gershwin LJ, Coffman RL. Aerosol-induced immunoglobulin (Ig)-E unresponsiveness to ovalbumin does not require CD8+ or T cell receptor (TCR)-gamma/delta+ T cells or interferon (IFN)- gamma in a murine model of allergen sensitization. J Exp Med. 1998;187:721–731. doi: 10.1084/jem.187.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsitoura DC, Blumenthal RL, Berry G, DeKruyff RH, Umetsu DT. Mechanisms preventing allergen-induced airways hyperreactivity: role of tolerance and immune deviation. J Allergy Clin Immunol. 2000;106:239–246. doi: 10.1067/mai.2000.108429. [DOI] [PubMed] [Google Scholar]
- 18.Holt PG, Britten D, Sedgwick JD. Suppression of IgE responses by antigen inhalation: studies on the role of genetic and environmental factors. Immunology. 1987;60:97–102. [PMC free article] [PubMed] [Google Scholar]
- 19.Stewart GA, Holt PG. Immunogenicity and tolerogenicity of a major house dust mite allergen, Der p I from Dermatophagoides pteronyssinus, in mice and rats. Int Arch Allergy Appl Immunol. 1987;83:44–51. doi: 10.1159/000234329. [DOI] [PubMed] [Google Scholar]
- 20.Romagnani S. Coming back to a missing immune deviation as the main explanatory mechanism for the hygiene hypothesis. J Allergy Clin Immunol. 2007;119:1511–1513. doi: 10.1016/j.jaci.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Romagnani S. The increased prevalence of allergy and the hygiene hypothesis: missing immune deviation, reduced immune suppression, or both? Immunology. 2004;112:352–363. doi: 10.1111/j.1365-2567.2004.01925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jutel M, Akdis M, Blaser K, Akdis CA. Mechanisms of allergen specific immunotherapy – T-cell tolerance and more. Allergy. 2006;61:796–807. doi: 10.1111/j.1398-9995.2006.01175.x. [DOI] [PubMed] [Google Scholar]
- 23.Akdis CA, Blaser K, Akdis M. Genes of tolerance. Allergy. 2004;59:897–913. doi: 10.1111/j.1398-9995.2004.00587.x. [DOI] [PubMed] [Google Scholar]
- 24.von Bubnoff D, de la Salle H, Wessendorf J, Koch S, Hanau D, Bieber T. Antigen-presenting cells and tolerance induction. Allergy. 2002;57:2–8. doi: 10.1034/j.1398-9995.2002.01150.x. [DOI] [PubMed] [Google Scholar]
- 25.Tournoy KG, Hove C, Grooten J, Moerloose K, Brusselle GG, Joos GF. Animal models of allergen-induced tolerance in asthma: are T-regulatory-1 cells (Tr-1) the solution for T-helper-2 cells (Th-2) in asthma? Clin Exp Allergy. 2006;36:8–20. doi: 10.1111/j.1365-2222.2005.02385.x. [DOI] [PubMed] [Google Scholar]
- 26.Jarvis D, Luczynska C, Chinn S, Potts J, Sunyer J, Janson C, Svanes C, Kunzli N, Leynaert B, Heinrich J, Kerkhof M, Ackermann-Liebrich U, Anto JM, Cerveri I, De Marco R, Gislason T, Neukirch F, Vermeire P, Wjst M, Burney P. Change in prevalence of IgE sensitization and mean total IgE with age and cohort. J Allergy Clin Immunol. 2005;116:675–682. doi: 10.1016/j.jaci.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Linneberg A, Gislum M, Johansen N, Husemoen LL, Jorgensen T. Temporal trends of aeroallergen sensitization over twenty-five years. Clin Exp Allergy. 2007;37:1137–1142. doi: 10.1111/j.1365-2222.2007.02760.x. [DOI] [PubMed] [Google Scholar]
- 28.Douwes J, Pearce N. Asthma and the westernization ‘package’. Int J Epidemiol. 2002;31:1098–1102. doi: 10.1093/ije/31.6.1098. [DOI] [PubMed] [Google Scholar]
- 29.Nicolaou N, Siddique N, Custovic A. Allergic disease in urban and rural populations: increasing prevalence with increasing urbanization. Allergy. 2005;60:1357–1360. doi: 10.1111/j.1398-9995.2005.00961.x. [DOI] [PubMed] [Google Scholar]
- 30.Von Hertzen LC, Haahtela T. Asthma and atopy – the price of affluence? Allergy. 2004;59:124–137. doi: 10.1046/j.1398-9995.2003.00433.x. [DOI] [PubMed] [Google Scholar]
- 31.von Mutius E, Weiland SK, Fritzsch C, Duhme H, Keil U. Increasing prevalence of hay fever and atopy among children in Leipzig, East Germany. Lancet. 1998;351:862–866. doi: 10.1016/S0140-6736(97)10100-3. [DOI] [PubMed] [Google Scholar]
- 32.Krause T, Koch A, Friborg J, Poulsen LK, Kristensen B, Melbye M. Frequency of atopy in the Arctic in 1987 and 1998. Lancet. 2002;360:691–692. doi: 10.1016/s0140-6736(02)09841-0. [DOI] [PubMed] [Google Scholar]
- 33.Linneberg A. Hypothesis: urbanization and the allergy epidemic – a reverse case of immunotherapy? Allergy. 2005;60:538–539. doi: 10.1111/j.1398-9995.2005.00721.x. [DOI] [PubMed] [Google Scholar]
- 34.Keiding L, Gunnarsen L, Rosdahl N, Machon M, M⊘ller R, Valbj⊘rn O. Environmental factors of everyday life in Denmark – with specific focus on housing environment. Copenhagen: National Institute of Public Health; 2003. [Google Scholar]
- 35.Priftis KN, Anthracopoulos MB, Nikolaou-Papanagiotou A, Mantziou V, Paliatsos AG, Tzavelas G, Nicolaidou P, Mantzouranis E. Increased sensitization in urban vs. rural environment – rural protection or an urban living effect? Pediatr Allergy Immunol. 2007;18:209–216. doi: 10.1111/j.1399-3038.2006.00514.x. [DOI] [PubMed] [Google Scholar]
- 36.Korsgaard J, Iversen M. Epidemiology of house dust mite allergy. Allergy. 1991;46(suppl 11):14–18. doi: 10.1111/j.1398-9995.1991.tb00643.x. [DOI] [PubMed] [Google Scholar]
- 37.Korsgaard J, Harving H. House-dust mites and summer cottages. Allergy. 2005;60:1200–1203. doi: 10.1111/j.1398-9995.2005.00840.x. [DOI] [PubMed] [Google Scholar]
- 38.Sidenius KE, Hallas TE, Poulsen LK, Mosbech H. House dust mites and their allergens in Danish mattresses – results from a population based study. Ann Agric Environ Med. 2002;9:33–39. [PubMed] [Google Scholar]
- 39.Mosbech H, Veggerby C, Steensen M, Poulsen LK, Johnsen CR, Heinig JH, Lange P. House dust mite allergens and mite allergy in Copenhagen dwellings. A cross-sectional study (in Danish). Ugeskr Laeger. 1999;161:419–423. [PubMed] [Google Scholar]
- 40.Mosbech H, Jensen A, Heinig JH, Schou C. House dust mite allergens on different types of mattresses. Clin Exp Allergy. 1991;21:351–355. doi: 10.1111/j.1365-2222.1991.tb01667.x. [DOI] [PubMed] [Google Scholar]
- 41.Iversen M, Korsgaard J, Hallas T, Dahl R. Mite allergy and exposure to storage mites and house dust mites in farmers. Clin Exp Allergy. 1990;20:211–229. doi: 10.1111/j.1365-2222.1990.tb02670.x. [DOI] [PubMed] [Google Scholar]
- 42.Radon K, Schottky A, Garz S, Koops F, Szadkowski D, Radon K, Nowak D, Luczynska C. Distribution of dust-mite allergens (Lep d 2, Der p 1, Der f 1, Der 2) in pig-farming environments and sensitization of the respective farmers. Allergy. 2000;55:219–225. doi: 10.1034/j.1398-9995.2000.00461.x. [DOI] [PubMed] [Google Scholar]
- 43.Schram-Bijkerk D, Doekes G, Boeve M, Douwes J, Riedler J, Ublagger E, von Mutius E, Budde J, Pershagen G, van Hage M, Wickman M, Braun-Fahrlander C, Waser M, Brunekreef B. Nonlinear relations between house dust mite allergen levels and mite sensitization in farm and nonfarm children. Allergy. 2006;61:640–647. doi: 10.1111/j.1398-9995.2006.01079.x. [DOI] [PubMed] [Google Scholar]
- 44.Zock JP, Heinrich J, Jarvis D, Verlato G, Norback D, Plana E, Sunyer J, Chinn S, Olivieri M, Soon A, Villani S, Ponzio M, Dahlman-Hoglund A, Svanes C, Luczynska C. Distribution and determinants of house dust mite allergens in Europe: the European Community Respiratory Health Survey II. J Allergy Clin Immunol. 2006;118:682–690. doi: 10.1016/j.jaci.2006.04.060. [DOI] [PubMed] [Google Scholar]
- 45.Bousquet PJ, Chinn S, Janson C, Kogevinas M, Burney P, Jarvis D. Geographical variation in the prevalence of positive skin tests to environmental aeroallergens in the European Community Respiratory Health Survey I. Allergy. 2007;62:301–309. doi: 10.1111/j.1398-9995.2006.01293.x. [DOI] [PubMed] [Google Scholar]
- 46.Nielsen GD, Hansen JS, Lund RM, Bergqvist M, Larsen ST, Clausen SK, Thygesen P, Poulsen OM. IgE-mediated asthma and rhinitis. I. A role of allergen exposure? Pharmacol Toxicol. 2002;90:231–242. doi: 10.1034/j.1600-0773.2002.900502.x. [DOI] [PubMed] [Google Scholar]
- 47.Lau S, Illi S, Sommerfeld C, Niggemann B, Bergmann R, von Mutius E, Wahn U. Early exposure to house-dust mite and cat allergens and development of childhood asthma: a cohort study. Multicentre Allergy Study Group. Lancet. 2000;356:1392–1397. doi: 10.1016/s0140-6736(00)02842-7. [DOI] [PubMed] [Google Scholar]
- 48.Holt PG, Thomas WR. Sensitization to airborne environmental allergens: unresolved issues. Nat Immunol. 2005;6:957–960. doi: 10.1038/ni1005-957. [DOI] [PubMed] [Google Scholar]
- 49.Cullinan P, MacNeill SJ, Harris JM, Moffat S, White C, Mills P, Newman Taylor AJ. Early allergen exposure, skin prick responses, and atopic wheeze at age 5 in English children: a cohort study. Thorax. 2004;59:855–861. doi: 10.1136/thx.2003.019877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Platts-Mills T, Vaughan J, Squillace S, Woodfolk J, Sporik R. Sensitisation, asthma, and a modified Th2 response in children exposed to cat allergen: a population-based cross-sectional study. Lancet. 2001;357:752–756. doi: 10.1016/S0140-6736(00)04168-4. [DOI] [PubMed] [Google Scholar]
- 51.Platts-Mills TA, Erwin E, Heymann P, Woodfolk J. Is the hygiene hypothesis still a viable explanation for the increased prevalence of asthma? Allergy. 2005;60(suppl 79):25–31. doi: 10.1111/j.1398-9995.2005.00854.x. [DOI] [PubMed] [Google Scholar]
- 52.Custis NJ, Woodfolk JA, Vaughan JW, Platts-Mills TA. Quantitative measurement of airborne allergens from dust mites, dogs, and cats using an ion-charging device. Clin Exp Allergy. 2003;33:986–991. doi: 10.1046/j.1365-2222.2003.01706.x. [DOI] [PubMed] [Google Scholar]
- 53.Svanes C, Heinrich J, Jarvis D, Chinn S, Omenaas E, Gulsvik A, Kunzli N, Burney P. Pet-keeping in childhood and adult asthma and hay fever: European Community Respiratory Health Survey. J Allergy Clin Immunol. 2003;112:289–300. doi: 10.1067/mai.2003.1596. [DOI] [PubMed] [Google Scholar]
- 54.Koopman LP, Van Strien RT, Kerkhof M, Wijga A, Smit HA, de Jongste JC, Gerritsen J, Aalberse RC, Brunekreef B, Neijens HJ. Placebo-controlled trial of house dust mite-impermeable mattress covers: effect on symptoms in early childhood. Am J Respir Crit Care Med. 2002;166:307–313. doi: 10.1164/rccm.2106026. [DOI] [PubMed] [Google Scholar]
- 55.Marks GB, Mihrshahi S, Kemp AS, Tovey ER, Webb K, Almqvist C, Ampon RD, Crisafulli D, Belousova EG, Mellis CM, Peat JK, Leeder SR. Prevention of asthma during the first 5 years of life: a randomized controlled trial. J Allergy Clin Immunol. 2006;118:53–61. doi: 10.1016/j.jaci.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 56.Woodcock A, Lowe LA, Murray CS, Simpson BM, Pipis SD, Kissen P, Simpson A, Custovic A. Early life environmental control: effect on symptoms, sensitization, and lung function at age 3 years. Am J Respir Crit Care Med. 2004;170:433–439. doi: 10.1164/rccm.200401-083OC. [DOI] [PubMed] [Google Scholar]
- 57.Woodcock A, Forster L, Matthews E, Martin J, Letley L, Vickers M, Britton J, Strachan D, Howarth P, Altmann D, Frost C, Custovic A. Control of exposure to mite allergen and allergen-impermeable bed covers for adults with asthma. N Engl J Med. 2003;349:225–236. doi: 10.1056/NEJMoa023175. [DOI] [PubMed] [Google Scholar]
- 58.Marinho S, Simpson A, Custovic A. Allergen avoidance in the secondary and tertiary prevention of allergic diseases: does it work? Prim Care Respir J. 2006;15:152–158. doi: 10.1016/j.pcrj.2006.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gotzsche PC, Johansen HK, Schmidt LM, Burr ML. House dust mite control measures for asthma. Cochrane Database Syst Rev. 2004;4 doi: 10.1002/14651858.CD001187.pub2. CD001187. [DOI] [PubMed] [Google Scholar]
- 60.Sheikh A, Hurwitz B. House dust mite avoidance measures for perennial allergic rhinitis: a systematic review of efficacy. Br J Gen Pract. 2003;53:318–322. [PMC free article] [PubMed] [Google Scholar]
- 61.Schmidt LM, Gotzsche PC. Of mites and men: reference bias in narrative review articles: a systematic review. J Fam Pract. 2005;54:334–338. [PubMed] [Google Scholar]
- 62.Patriarca G, Nucera E, Roncallo C, Pollastrini E, Bartolozzi F, De Pasquale T, Buonomo A, Gasbarrini G, Di Campli C, Schiavino D. Oral desensitizing treatment in food allergy: clinical and immunological results. Aliment Pharmacol Ther. 2003;17:459–465. doi: 10.1046/j.1365-2036.2003.01468.x. [DOI] [PubMed] [Google Scholar]
- 63.Niggemann B, Staden U, Rolinck-Werninghaus C, Beyer K. Specific oral tolerance induction in food allergy. Allergy. 2006;61:808–811. doi: 10.1111/j.1398-9995.2006.01066.x. [DOI] [PubMed] [Google Scholar]
- 64.Staden U, Rolinck-Werninghaus C, Brewe F, Wahn U, Niggemann B, Beyer K. Specific oral tolerance induction in food allergy in children: efficacy and clinical patterns of reaction. Allergy. 2007;62:1261–1269. doi: 10.1111/j.1398-9995.2007.01501.x. [DOI] [PubMed] [Google Scholar]
- 65.Bodtger U, Linneberg A. Remission of allergic rhinitis: an 8-year observational study. J Allergy Clin Immunol. 2004;114:1384–1388. doi: 10.1016/j.jaci.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 66.Holt PG. Immunoprophylaxis of atopy: light at the end of the tunnel? Immunol Today. 1994;15:484–489. doi: 10.1016/0167-5699(94)90194-5. [DOI] [PubMed] [Google Scholar]
- 67.Calderon M, Alves B, Jacobson M, Hurwitz B, Sheikh A, Durham S. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007;1 doi: 10.1002/14651858.CD001936.pub2. CD001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abramson MJ, Puy RM, Weiner JM. Allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2000;4 doi: 10.1002/14651858.CD001186. CD001186. [DOI] [PubMed] [Google Scholar]
- 69.Durham SR, Walker SM, Varga EM, Jacobson MR, O'Brien F, Noble W, Till SJ, Hamid QA, Nouri-Aria KT. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–475. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 70.Dahl R, Stender A, Rak S. Specific immunotherapy with SQ standardized grass allergen tablets in asthmatics with rhinoconjunctivitis. Allergy. 2006;61:185–190. doi: 10.1111/j.1398-9995.2005.00949.x. [DOI] [PubMed] [Google Scholar]
- 71.Wilson DR, Torres LI, Durham SR. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev. 2003;2 doi: 10.1002/14651858.CD002893. CD002893. [DOI] [PubMed] [Google Scholar]
- 72.Moingeon P, Batard T, Fadel R, Frati F, Sieber J, Van Overtvelt L. Immune mechanisms of allergen-specific sublingual immunotherapy. Allergy. 2006;61:151–165. doi: 10.1111/j.1398-9995.2006.01002.x. [DOI] [PubMed] [Google Scholar]
- 73.Roches A, Paradis L, Menardo JL, Bouges S, Daures JP, Bousquet J. Immunotherapy with a standardized Dermatophagoides pteronyssinus extract. VI. Specific immunotherapy prevents the onset of new sensitizations in children. J Allergy Clin Immunol. 1997;99:450–453. doi: 10.1016/s0091-6749(97)70069-1. [DOI] [PubMed] [Google Scholar]
- 74.Bieli C, Eder W, Frei R, Braun-Fahrlander C, Klimecki W, Waser M, Riedler J, von Mutius E, Scheynius A, Pershagen G, Doekes G, Lauener R, Martinez FD. A polymorphism in CD14 modifies the effect of farm milk consumption on allergic diseases and CD14 gene expression. J Allergy Clin Immunol. 2007;120:1308–1315. doi: 10.1016/j.jaci.2007.07.034. [DOI] [PubMed] [Google Scholar]
- 75.Simpson A, John SL, Jury F, Niven R, Woodcock A, Ollier WE, Custovic A. Endotoxin exposure, CD14 and allergic disease: an interaction between genes and the environment. Am J Respir Crit Care Med. 2006;174:386–392. doi: 10.1164/rccm.200509-1380OC. [DOI] [PubMed] [Google Scholar]