Abstract
Background/Aims
Calcium and dairy proteins have been postulated to explain why the intake of dairy products correlates inversely with body mass index in several populations. We have shown that a high-calcium diet with whey protein attenuates weight gain and now we describe the effects of this diet on adipose tissue gene expression.
Methods
Nine-week-old C57Bl/6J mice were divided into two groups (n = 10/group). The control diet was a standard high-fat diet (60% of energy) low in calcium (0.4%). The whey protein diet was a high-calcium (1.8%), high-fat diet with whey protein. After the 21-week treatment, adipose tissue transcript profiling (2 mice/group) was performed using Affymetrix Mouse Genome 430 2.0.
Results
The high-calcium diet with whey protein altered the expression of 129 genes (± 1.2 fold). Quantitative RT-PCR analysis confirmed the significant up-regulation of Adrb3 (p = 0.002) and leptin (p = 0.0019) in the high-calcium whey group. Insulin and adipocytokine signaling pathways were enriched among the up-regulated genes and the fatty acid metabolism pathway among the down-regulated genes.
Conclusions
High-calcium diet with whey protein significantly modifies adipose tissue gene expression. These preliminary findings reveal that targets of a high-calcium diet with whey protein include genes for Adrb3 and leptin, and help to explain how the intake of dairy products might attenuate obesity.
Key Words: Dietary calcium, Diet-induced obesity, Gene expression, Whey protein
Introduction
The inverse association between dairy calcium intake and body mass index has been found in several cross-sectional and longitudinal studies [1,2,3,4,5]. High-calcium diet has also been demonstrated to inhibit weight gain in both rats and mice [6,7,8]. Increased calcium intake has also been established to effectively accelerate weight loss both in rodents [7, 9] and humans [10, 11]. However, not all the calcium interventions have been successful in modulating body weight [12,13,14].
The mechanism by which dietary calcium affects body weight is still controversial. Calcium intake has been suggested to modify adipocyte metabolism via 1,25(OH)2-D3-vitamin, which increases the adipocyte intracellular calcium content [15]. This active form of vitamin D has been shown to promote lipogenesis in adipocytes by increasing the expression and activity of fatty acid synthase (FAS) [16]. The increased intracellular calcium on the other hand has been shown to exert antilipolytic effects through activation of phosphodiesterase [17]. In addition 1,25(OH)2-D3-vitamin has been shown to act on its nuclear receptors and inhibit uncoupling protein 2 (UCP2) expression in adipocytes and regulate thermogenesis and UCP2 overexpression-induced apoptosis [18, 19].
In a recent human study, modification of serum 1,25(OH)2-D3-vitamin concentration did not lead to significant changes in the expression of essential genes related to fatty acid metabolism [20]. However, a 1-week intervention with high-calcium dairy diet has been shown to reduce fat tissue FAS expression in comparison with a low-calcium diet [21]. Another mechanism, which has been suggested to explain the effects of calcium on body weight, is the capacity of calcium to form insoluble soaps with fatty acids and thereby reduce the absorption of fat [8, 22, 23]. However, it is controversial whether the fat-binding capacity of calcium is large enough to explain the effects seen in the intervention studies.
The intervention studies which have successfully demonstrated the anti-obesity effect of calcium have repeatedly shown that the effect of calcium from dairy sources is superior to the effect of supplemental calcium [6, 9, 24]. So far, the mechanisms explaining this difference are not understood. It has been suggested that dairy products contain bioactive peptides, which might modulate adipose tissue metabolism, energy expenditure or satiety signals [15]. Dairy products are known to contain bioactive peptides, e.g. with ACE-inhibitory properties, opioid-like activities and mineral-binding and antithrombotic properties [25]. However, it is presently unclear how these or other dairy-derived peptides affect adipose tissue metabolism.
We have previously shown that a high-calcium, high-fat diet containing whey protein isolate (WPI) inhibits body weight and fat tissue gain in C57Bl/6J mice fed a high-fat diet, a widely used model of diet-induced obesity [23]. However, the knowledge on the mechanisms of action of the whey protein diet on fat tissue metabolism is still sparse. Whey protein has been shown to affect satiety at least acutely, but in this animal model WPI did not decrease the cumulative energy intake during the 21-week treatment period. A high-calcium diet with WPI increased fecal fat excretion, which may partly explain the inhibitory effect on weight gain.
In this paper, we clarify the effect of a high-calcium diet with whey protein on fat tissue metabolism using microarray technology. We show that a high-calcium diet with whey protein significantly regulates adipose tissue gene expression, including leptin and adrenergic receptor expression, in C57Bl/6J mice fed a high-fat diet.
Methods
Animals and Diets
The animals and treatments have been described in detail in our publication describing the effects of high-calcium diets on weight gain [23]. In brief, 8- to 9-week-old male C57Bl/6J mice were purchased from Harlan (Horst, The Netherlands). After a 1-week acclimatization period, the body-weight-matched mice (25.6 ± 0.1 g) were divided into two groups (n = 10/group) receiving modified high-fat diets (60% of energy from fat). The high-calcium whey group received a high-fat diet (D05031104M; Research Diets, New Brunswick, N.J., USA) with 1.8% CaCO3 and all protein (18% of energy) from WPI (Alacen™ 895; NZMP, Auckland, New Zealand). The control group received a high-fat diet (D05031101M; Research Diets) with 0.4% CaCO3 and all protein (18% of energy) from casein isolate (Alacid 714; New Zealand Milk Products, Santa Rosa, Calif., USA). At the end of the 21-week treatment period, the body weight (44.1 ± 1.1 g) and body fat content (41.6 ± 0.6%, measured by DEXA, Lunar PIXImus, GE Healthcare, Chalfont St. Giles, UK) were significantly lower (p < 0.05) in the high-calcium whey group than in the control group (48.1 ± 0.8 g and 44.9 ± 0.8%).
At the end of the treatment period, the animals were rendered unconscious with CO2/O2 (95%/5%; AGA, Riihimäki, Finland) and decapitated, and the epididymal fat pads were dissected. The distal end of the fat pad was fixed in 10% formalin and embedded in paraffin with routine techniques. The rest of the epididymal fat pads were snap-frozen in liquid nitrogen and stored at −80°C until analyzed.
Immunohistochemical Staining for F4/80 and Determination of the Adipocyte Cross-Sectional Area
Sections (5 μm) of paraffin-embedded adipose tissue samples were cut with a microtome and mounted on charged glass slides, deparaffinized in xylene and stainedfor F4/80 expression according to the indirect peroxidase-conjugated streptavidin procedure with an anti-F4/80 monoclonal antibody [F4/80 antibody (CI: A3-1) ab6640; Abcam, Cambridge, UK]. For each individual mouse adipose depot, three different high-power fields were analyzed. The total number ofnuclei and the number of nuclei of F4/80-expressing cells were counted for each field. The fraction of F4/80-expressing cells for each sample was calculated as the sum of the number of nuclei of F4/80-expressing cells divided by the total number of nuclei in sections of a sample. The adipocyte cross-sectional area was determined for each adipocyte in three fields per sample using Leica QWin Standard software (Leica Microsystems Imaging Solutions, Cambridge, UK).
Extraction of RNA and Microarray Procedure
Total RNA from the epididymal fat pads of 2 control mice and 2 high-calcium, whey-protein-fed mice were collected with TRIzol (Invitrogen, Carlsbad, Calif., USA), purified with the RNeasy Mini Kit (Qiagen) and measured at 260 and 280 nm. RNA quality was analyzed with a Bioanalyzer. RNA (5 μg) was reverse transcribed to cDNA and tagged with biotin with one-cycle target labeling and control reagents (Affymetrix) and hybridized according to the standard protocol using four Mouse Genome 430 2.0 arrays (Affymetrix) in total, representing over 30,000 mouse transcripts. GeneChip Scanner 3000 (Affymetrix) was used for scanning. The complete data set is available from the NCBI Gene Expression Omnibus database and gene expression profiling data comply with the MIAME standard (minimum information about a microarray experiment; accession No. GSE9280).
The data were pre-processed with the robust multichip algorithm [26], normalized per chip to the median and analyzed with Genespring 7.2. (Agilent, Santa Clara, Calif., USA). The 10,235 genes detected to be present in the data from all four microarrays were passed to further analysis. Differentially expressed probe sets were selected based on filtering by the fold change (±1.2-fold) between the control group and the high-calcium whey group, resulting in 1,067 up-regulated and 1,075 down-regulated identifiers. The probe sets passing the initial filtering were further inspected using parametric statistical analysis not assuming equal variances (Welch-type t test) with p < 0.05 as a threshold for significance. The lists of the obtained up- and down-regulated probe sets were inspected for the enriched Gene Ontology (GO) terms and the pathways of the Kyoto Encyclopedia of Genes and Genomes (KEGG) among the genes using the ‘DAVID 2006’ program [27]. Furthermore, the genes were clustered based on the GO terms in order to detect possible subgroups of co-expressed genes with certain functions using the ‘TAFFEL’ [28]. The predicted TF binding sites for the regulated genes were downloaded from the cisRED database [29]. The transcription factors were listed for 40 out of the 64 up-regulated genes.
Epididymal Adipose Tissue Gene Expression Analysis by Quantitative Real-Time PCR Assay
The increased expression of leptin and β3-adrenergic receptor (Adrb3) in the adipose tissue of mice fed a high-calcium diet with whey protein was independently verified by quantitative real-time PCR (qRT-PCR). Total RNA from the epididymal fat pads was collected with TRIzol (Invitrogen, Carlsbad, Calif., USA), treated with DNAse 1 (deoxyribonuclease 1, Sigma, St. Louis, Mo., USA) and reverse transcribed to cDNA by incubation for 50 min at 45°C with the presence of reverse transcription enzyme (ImProm-II™ Reverse Transcription System, Promega). cDNA (1 μl) was subjected to qRT-PCR (Lightcycler; Roche Diagnostics, Neuilly-sur-Seine, France) for detection of leptin, Adrb3 and 18S mRNAs. 18S served as housekeeping gene. The samples were amplified using FastStart DNA Master SYBR Green 1 (Roche Diagnostics) in the presence of 0.5 μM of the following primers: leptin forward AGACCGGGAAAGAGTG and reverse GCCATAGTGCAAGGTT; Adrb3 forward ACCAACGTGTTCGTGACT and reverse CAGCTAGGTAGCGGTCCA, and 18S forward ACATCCAAGGAAGGCAGCAG and reverse TTTTCGTCACTACCTCCCCG. The PCR amplifications consisted of a 10-min incubation at 95°C, following 43 cycles of 15 s at 95°C, annealing for 5 s at 59°C and 10 s at 72°C for leptin; a 10-min incubation at 95°C following 37 cycles of 15 s at 95°C, annealing for 5 s at 58°C and 10 s at 72°C for Adrb3; a 10-min incubation at 95°C following 26 cycles of 15 s at 95°C, annealing for 5 s at 66°C and 10 s at 72°C for 18S. The quantities of leptin, Adrb3 and 18S PCR products were quantified with an external standard curve amplified from purified PCR product.
Results
Changes in Adipose Tissue Gene Expression
A high-calcium diet with whey protein altered the expression of 129 Affymetrix probe sets corresponding to the same number of genes (>1.2-fold change in the expression). The amount of up- and down-regulated genes in the whey group in comparison with the control group was almost equal (64 up-regulated and 65down-regulated). The 45 up-regulated genes associated with GO terms of the biological process category are presented in table 1. The 48 down-regulated genes associated with biological process category GO terms are shown in table 2.
Table 1.
The effect of dietary calcium and dairy proteins on the adipose tissue gene expression profile in diet-induced obesity: up-regulated genes in the high-calcium whey group in comparison with controls
| Gene ID | Gene title | Gene symbol | GO biological process description | Fold change vs. control |
|---|---|---|---|---|
| 66153 | F-box only protein 36 | Fbxo36 | Ubiquitin cycle | 2.24 |
| 11556 | Adrenergic receptor, β3 | Adrb3 | Diet-induced thermogenesis/negative regulation of body size | 2.02 |
| 207304 | HECT domain containing 1 | Hectd1 | Protein modification/ubiquitin cycle | 1.57 |
| 18555 | PCTAIRE-motif protein kinase 1 | Pctk1 | Protein amino acid phosphorylation | 1.51 |
| 110198 | Aldo-keto reductase family 7, member A5 (aflatoxin aldehyde reductase) | Akr7a5 | Carbohydrate metabolism/aldehyde metabolism | 1.49 |
| 19082 | Protein kinase, AMP-activated, γ1 noncatalytic subunit | Prkag1 | Fatty acid biosynthesis/response to stress/lipid biosynthesis | 1.49 |
| 13854 | Epsin 1 | Epn1 | Endocytosis | 1.48 |
| 20624 | Elongation factor Tu GTP binding domain containing 2 | Eftud2 | Nuclear mRNA splicing, via spliceosome/mRNA processing/ protein biosynthesis | 1.47 |
| 319322 | Splicing factor 3b, subunit 2 | Sf3b2 | mRNA processing | 1.45 |
| 52563 | CDC23 (cell division cycle 23, yeast, homolog) | Cdc23 | Ubiquitin cycle/cell cycle/mitosis/cell division | 1.41 |
| 70549 | Talin 2 | Tln2 | Cell adhesion | 1.36 |
| 171567 | Non-metastatic cells 7, protein expressed | Nme7 | GTP biosynthesis/UTP biosynthesis/CTP biosynthesis/ nucleotide metabolism | 1.35 |
| 214585 | RIKEN cDNA 6030465E24 gene | 6030465E24Rik | Aromatic compound metabolism | 1.34 |
| 54151 | Cysteine and histidine rich 1 | Cyhr1 | Ubiquitin cycle | 1.34 |
| 67819 | Der1-like domain family, member 1 | Derl1 | ER-associated protein catabolism/retrograde protein transport, ER to cytosol | 1.33 |
| 17758 | Microtubule-associated protein 4 | Mtap4 | Microtubule-based process/negative regulation of microtubule depolymerization | 1.33 |
| 14252 | Flotillin 2 | Flot2 | Cell adhesion | 1.32 |
| 13424 | Dynein cytoplasmic 1 heavy chain 1 | Dync1h1 | Proteolysis/microtubule-based movement | 1.32 |
| 27967 | Calcium homeostasis endoplasmic reticulum protein | Cherp | Calcium ion homeostasis/negative regulation of cell proliferation/RNA processing | 1.31 |
| 18035 | Nuclear factor of K light chain gene enhancer in B-cells inhibitor, α | Nfkbia | Protein import into nucleus, translocation/regulation of cell proliferation/negative regulation of Notch signaling pathway | 1.30 |
| 67474 | Synaptosomal-associated protein | Snap29 | Intracellular protein transport | 1.30 |
| 19165 | Presenilin 2 | Psen2 | Cell fate specification/Notch signaling pathway/positive regulation of apoptosis/proteolysis during protein maturation/amyloid precursor protein catabolism | 1.29 |
| 53413 | Exocyst complex component 7 | Exoc7 | Protein transport/exocytosis | 1.29 |
| 16598 | Kruppel-like factor 2 (lung) | Klf2 | Positive regulation of transcription, DNA dependent | 1.29 |
| 56032 | Tumor suppressor candidate 4 | Tusc4 | Negative regulation of progression through cell cycle | 1.28 |
| 11651 | Thymoma viral proto-oncogene 1 | Akt1 | Carbohydrate metabolism/insulin signaling pathway/ regulation of protein biosynthesis/transport/inflammatory response/protein ubiquitination/protein catabolism/negative regulation of apoptosis/regulation of survival gene product activity | 1.28 |
| 235344 | SNF1-like kinase 2 | Snf1lk2 | Regulation of insulin receptor signaling pathway/protein kinase cascade | 1.28 |
| 18016 | Neurofibromatosis 2 | Nf2 | Negative regulation of protein kinase activity/regulation of cell proliferation/intercellular junction assembly and maintenance/negative regulation of progression through cell cycle | 1.28 |
| 106068 | Solute carrier family 45, member 4 | Slc45a4 | Phosphoenolpyruvate-dependent sugar phosphotransferase system | 1.28 |
| 107723 | Solute carrier family 12, member 6 | Slc12a6 | Ion transport/amino acid transport/regulation of progression | 1.27 |
| through cell cycle/regulation of cell volume | ||||
| 15461 | Harvey rat sarcoma virus oncogene 1 | Hras1 | Endocytosis/small GTPase mediated signal transduction/ cell aging/cell proliferation/protein biosynthesis | 1.26 |
| 17913 | Myosin IC | Myo1c | Transport/cytoskeleton organization and biogenesis | 1.26 |
| 22031 | TNF receptor-associated factor 3 | Traf3 | Signal transduction/regulation of apoptosis | 1.26 |
| 20364 | Selenoprotein W, muscle 1 | Sepw1 | Cell redox homeostasis | 1.26 |
| 22793 | Zyxin | Zyx | Cell adhesion | 1.26 |
| 207304 | HECT domain containing 1 | Hectd1 | Protein modification/ubiquitin cycle | 1.25 |
| 109689 | Arrestin, β1 | Arrb1 | Signal transduction/regulation of G-protein-coupled receptor protein signaling pathway | 1.25 |
| 19274 | Protein tyrosine phosphatase, receptor type, M | Ptprm | Protein amino acid dephosphorylation/transmembrane receptor protein tyrosine phosphatase signaling pathway | 1.25 |
| 18813 | Proliferation-associated 2G4 | Pa2g4 | Regulation of transcription, DNA dependent/ rRNA processing/regulation of protein biosynthesis | 1.24 |
| 69226 | Sorting nexing 24 | Snx24 | Transport/intracellular signaling cascade | 1.22 |
| 69051 | Pyrroline-5-carboxylate reductase family, member 2 | Pycr2 | Electron transport/amino acid biosynthesis | 1.21 |
| 252875 | cDNA sequence BC020002 | BC020002 | Transport | 1.21 |
| 53625 | UDP-GlcNAc:βGal β-1,3-N- acetylglucosaminyltransferase 2 | B3gnt2 | Protein amino acid glycosylation | 1.20 |
| 116748 | U7 snRNP-specific Sm-like protein LSM10 | Lsm10 | Nuclear mRNA splicing, via spliceosome/histone mRNA 3′-end processing | 1.20 |
| 21769 | Zinc finger, AN1-type domain 3 | Zfand3 | Microtubule-based movement/protein polymerization | 1.20 |
Table 2.
The list of genes which were down-regulated (>1.2 fold) in the adipose tissue of the high-calcium whey group in comparison with controls (only the genes associated with GO terms of the biological process category are listed)
| Gene ID | Gene title | Gene symbol | GO biological process description | Fold change vs. control |
|---|---|---|---|---|
| 226139 | COX15 homolog, cytochrome C oxidase assembly protein (yeast) | Cox15 | Protein complex assembly | 0.56 |
| 20210 | Serum amyloid A3 | Saa3 | Acute-phase response | 0.59 |
| 12894 | Carnitine palmitoyltransferase 1a, liver | Cpt1a | Lipid/fatty acid metabolism | 0.61 |
| 229211 | Acyl-coenzyme A dehydrogenase family, member 9 | Acad9 | Electron transport | 0.65 |
| 668101 | Similar to SIRP _1 isoform 2 | LOC668101 | Intracellular signaling cascade/positive regulation of phagocytosis | 0.65 |
| 54607 | Suppressor of cytokine signaling 6 | Socs6 | Regulation of cell growth/cell glucose homeostasis/ intracellular signaling cascade/negative regulation of signal transduction | 0.65 |
| 13167 | Diazepam binding inhibitor | Dbi | Transport | 0.66 |
| 15950 | Interferon activated gene 203 | Ifi203 | Immune response/regulation of transcription from RNA polymerase II promoter/regulation of transcription, DNA dependent | 0.66 |
| 55932 | Guanylate nucleotide binding protein 4 | Gbp4 | Immune response | 0.67 |
| 17329 | Chemokine (C-X-C motif) ligand 9 | Cxcl9 | Inflammatory response/immune response | 0.67 |
| 11770 | Fatty acid binding protein 4, adipocyte | Fabp4 | Cytokine production/negative regulation of protein kinase activity/transport/negative regulation of transcription/ cholesterol homeostasis/positive regulation of inflammatory response | 0.68 |
| 22359 | Very low density lipoprotein receptor | Vldlr | Lipid metabolism/lipid transport/endocytosis/steroid metabolism/cholesterol metabolism | 0.69 |
| 14081 | Acyl-CoA synthetase long-chain family member 1 | Acsl1 | Lipid metabolism/fatty acid metabolism | 0.69 |
| 108682 | Glutamic pyruvate transaminase (alanine aminotransferase) 2 | Gpt2 | Biosynthesis | 0.70 |
| 17449 | Malate dehydrogenase 1, NAD (soluble) | Mdh1 | Glycolysis/tricarboxylic acid cycle/malate metabolism | 0.70 |
| 22710 | Zinc finger protein 52 | Zfp52 | Regulation of transcription, DNA dependent | 0.71 |
| 11800 | Apoptosis inhibitor 5 | Api5 | Transport/anti-apoptosis | 0.71 |
| 19211 | Phosphatase and tensin homolog | Pten | Protein amino acid dephosphorylation/regulation of apoptosis, cell migration and of the progression through cell cycle/negative regulation of protein kinase B signaling cascade/ regulation of cyclin-dependent protein kinase activity/negative regulation of cell proliferation/regulation of protein stability/ negative regulation of focal adhesion formation | 0.71 |
| 320267 | Far upstream element (FUSE) binding protein 3 | Fubp3 | Positive regulation of transcription from RNA polymerase II promoter | 0.72 |
| 26358 | Aldehyde dehydrogenase family 1, subfamily A7 | Aldh1a7 | Metabolism | 0.72 |
| 11740 | Solute carrier family 25 (mitochondrial carrier, adenine nucleotide translocator), member 5 | Slc25a5 | Transport, mitochondrial transport | 0.74 |
| 14062 | Coagulation factor II (thrombin) receptor | F2r | Signal transduction/G-protein-coupled receptor protein signaling pathway/blood coagulation | 0.75 |
| 19744 | RAS-homolog enriched in brain | Rheb | Small GTPase mediated signal transduction/protein transport | 0.76 |
| 28006 | DNA segment, Chr 6, Wayne State University 116, expressed | D6Wsu116e | Phosphate metabolism | 0.77 |
| 14359 | Fragile X mental retardation gene 1, autosomal homolog | Fxr1h | Muscle development | 0.77 |
| 27362 | DnaJ (Hsp40) homolog, subfamily B, member 9 | Dnajb9 | Protein folding | 0.77 |
| 16403 | Integrin _6 | Itga6 | Cell adhesion/integrin-mediated signaling pathway | 0.78 |
| 76338 | RAB2B, member RAS oncogene family | Rab2b | ER to Golgi vesicle-mediated transport/small GTPase mediated signal transduction/protein transport | 0.78 |
| 14645 | Glutamate-ammonia ligase (glutamine synthetase) | Glul | Glutamine biosynthesis/nitrogen compound metabolism | 0.78 |
| 22359 | Very low density lipoprotein receptor | Vldlr | Lipid metabolism, transport, endocytosis, steroid metabolism, cholesterol metabolism | 0.79 |
| 19038 | Peptidylprolyl isomerase C | Ppic | Protein folding | 0.79 |
| 18970 | Polymerase (DNA directed), β | Polb | DNA replication/base excision repair, gap-filling/ anti-apoptosis/response to DNA damage stimulus/cell death | 0.79 |
| 19248 | Protein tyrosine phosphatase, non-receptor type 12 | Ptpn12 | Protein amino acid dephosphorylation | 0.80 |
| 84092 | Ubiquitin-specific peptidase 8 | Usp8 | DNA topological change/ubiquitin-dependent protein catabolism/Ras protein signal transduction | 0.80 |
| 57279 | Solute carrier family 25 (mitochondrial carnitine/acylcarnitine translocase), member 20 | Slc25a20 | Transport | 0.80 |
| 26413 | Mitogen-activated protein kinase 1 | Mapk1 | MAPKKK cascade/protein amino acid phosphorylation/ response to DNA damage stimulus/cell cycle/signal transduction/organ morphogenesis/cytosine metabolism | 0.81 |
| 69125 | CCR4-NOT transcription complex, subunit 8 | Cnot8 | Regulation of transcription, DNA dependent | 0.81 |
| 72065 | RAP2C, member of RAS oncogene family | Rap2c | Small GTPase mediated signal transduction/protein transport | 0.82 |
| 67030 | Fanconi anemia, complementation group L | Fancl | DNA repair/ubiquitin cycle/gametogenesis/regulation of cell proliferation | 0.82 |
| 319625 | Galactose mutarotase | Galm | Galactose metabolism | 0.82 |
| 14105 | FUS interacting protein (serine-arginine rich) 1 | Fusip1 | Regulation of nuclear mRNA splicing, via spliceosome/ mRNA export from nucleus | 0.82 |
| 72183 | Sorting nexin 6 | Snx6 | Intracellular protein transport/intracellular signaling cascade | 0.82 |
| 14130 | Fc receptor, IgG, low affinity IIb | Fcgr2b | Negative regulation of type I hypersensitivity/defense response/immune response/cell surface receptor linked signal transduction/humoral defense mechanism/negative regulation of B cell proliferation/antigen presentation, exogenous antigen via MHC class II/mast cell activation/positive regulation of phagocytosis/negative regulation of immune response | 0.83 |
| 13136 | CD55 antigen | Cd55 | Immune response/complement activation, classical pathway/ innate immune response | 0.83 |
| 56428 | Mitochondrial carrier homolog 2 (C. elegans) | Mtch2 | Transport | 0.83 |
| 67974 | RIKEN cDNA 5730405I09 gene | 5730405I09Rik | Regulation of progression through cell cycle | 0.83 |
| 71881 | RIKEN cDNA 2310001A20 gene | 2310001A20Rik | Biosynthesis | 0.83 |
| 67204 | Eukaryotic translation initiation factor 2, subunit 2 (β) | Eif2s2 | Protein biosynthesis/translational initiation | 0.83 |
The most highly enriched biological pathways among the altered genes were the insulin and adipocytokine signaling pathway and the fatty acid metabolism pathway. The complete list of the enriched categories for the up- and down-regulated genes is presented in online supplement table 1 (www.karger.com/doi/10.1159/000151238). The data related to the predicted and conserved transcription factor binding sites among the up-regulated genes are also presented as supplementary material (www.karger.com/doi/10.1159/000151238).
Insulin Signaling Pathway
The biggest number of up-regulated genes was enriched in the KEGG insulin signaling pathway, which contained five reporters with over 1.2-fold changes and fourteen genes with a smaller or non-significant difference in the expression. The significantly up-regulated reporters in the insulin signaling pathway corresponded to the genes encoding Flot2 (flotillin 2), Exoc7 (exocyst complex component 7), Prkag1 (AMP-activated protein kinase), Akt1 (serine/threonine protein kinase) and Ras (Harvey rat sarcoma virus oncogene). On the other hand, Rheb (RAS homolog) was significantly down-regulated and there was a downward trend in the expression of Pik3r1 (phosphatidylinositol 3-kinase, PI3-kinase) and Ppp1r3c (protein phosphatase 1) genes, but the difference between the groups was not statistically significant.
Adipocytokine Signaling Pathway
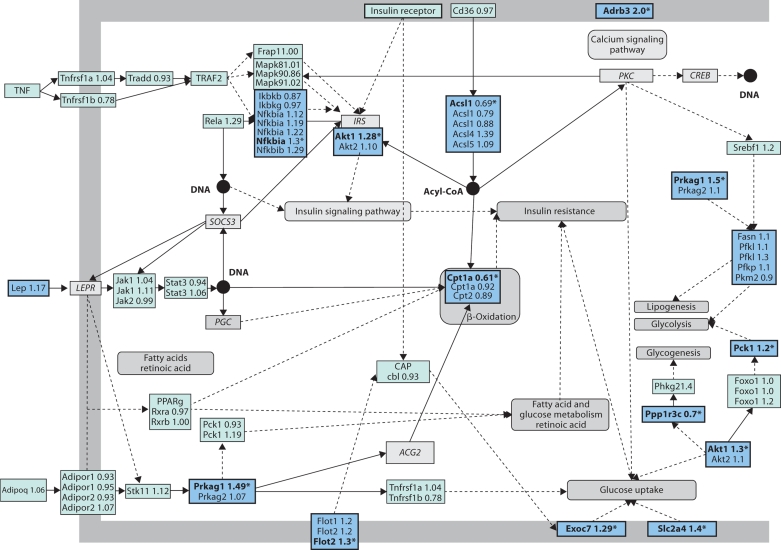
The second biggest number of up-regulated genes was found in the adipocytokine signaling pathway. This pathway contained three significantly up-regulated genes (Prkag1, Akt1 and Nfkbia). In addition, we inspected the microarray data for other genes associated with the adipocytokine KEGG pathway and found trends towards up-regulation in the high-calcium whey group in the expression of leptin (1.17-fold, p = 0.30) and several other genes presented in figure 1. On the other hand, Cpt1a (carnitine palmitoyltransferase 1) was significantly down-regulated together with Acsl1 (long-chain acyl-CoA synthetase, family member 1), as listed in online supplement table 2 (www.karger.com/doi/ 10.1159/00151238). Several key genes in both insulin and adipocytokine signaling pathways are presented in figure 1.
Fig. 1.
Microarray data for the genes in the pathway coined from the central genes in the KEGG pathways ‘insulin signaling pathway’ and ‘adipocytokine signaling pathway’. The figure is modified from the pathway presented in KEGG [51] . Genes with no microarray data are shown in italics. Abstractions are presented with rounded grey shapes. ∗Genes with a ±1.2-fold change in between groups.
Fatty Acid Metabolism Pathway
The expression of Cpt1a, Acsl1 and Acad9, genes related to fatty acid metabolism, were strongly and significantly decreased (p = 0.01, p = 0.01 and p = 0.049, respectively).
Identification and Verification of Target Genes
Based on the expression data and the pathways associated with altered genes, we identified two interesting up-regulated genes in the microarray data that may transmit alterations in metabolism in the fat tissue. The putative targets were β3-adrenergic receptor and leptin, which could be related to the inhibition of fat tissue gain in the high-calcium whey group.
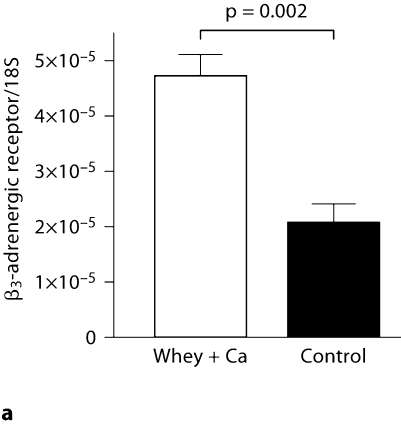
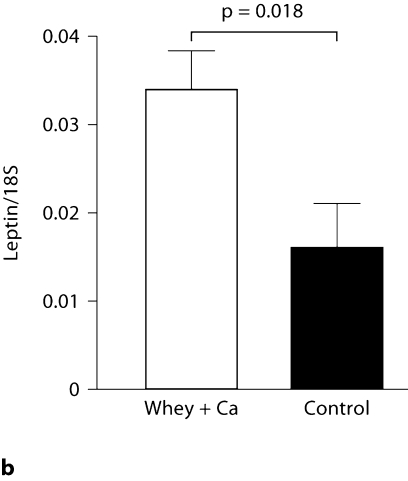
The mRNA abundances of these genes were confirmed by qRT-PCR. According to the microarray data, the expression of Adrb3 was significantly up-regulated (p = 0.03), whereas the 1.17-fold increase in the leptin expression did not reach statistical significance (p = 0.33). In accordance with the microarray data, qRT-PCR analysis confirmed the 2.3-fold up-regulation in the expression of Adrb3 in the high-calcium whey group (p = 0.0002; fig. 2a). Also, the leptin mRNA expression was 2.1 times greater in the high-calcium whey group than in the control group (p = 0.02), confirming the upward trend found in the microarray data (fig. 2b).
Fig. 2.
Effect of a high-calcium diet with whey protein on Adrb3 (a) and leptin mRNA expression (b) in the epididymal adipose tissue of C57Bl/6J mice (n = 10/group) fed a high-fat diet. Values are presented as means ± SEM.
Macrophage Infiltration and Adipocyte Size
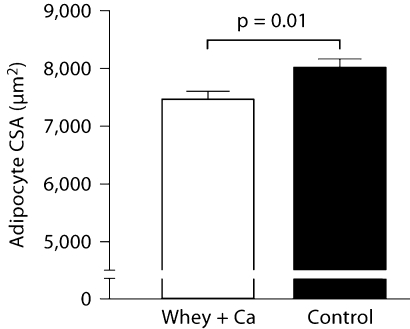
To identify and quantitate macrophages within adipose tissue, we immunohistochemically stained sections for the F4/80 antigen. There was no difference between the groups in the amount of F4/80-expressing cells in the adipose tissue (31.7 ± 3.7% in the high-calcium whey group and 28.2 ± 4.3% in the control group, p = 0.55). The mean adipocyte cross-sectional area was significantly smaller in the high-calcium whey group than in the control group (7,458 ± 147 vs. 8,012 ± 156 μm2, p = 0.01; fig. 3).
Fig. 3.
Effect of a high-calcium diet with whey protein on the adipocyte cross-sectional area (CSA) in the epididymal adipose tissue of C57Bl/6J mice (n = 10/group) fed a high-fat diet. Values are presented as means ± SEM.
Discussion
In this paper, we explored the effects of a whey-protein-containing, high-calcium diet on adipose tissue gene expression. The microarray analysis of two representative samples per group revealed significant changes in the expression of 129 genes, with a similar amount of up- and down-regulated genes in the high-calcium whey group in comparison with the controls. Based on the microarray and qRT-PCR results, adipose tissue of mice fed a high-calcium diet with whey protein was found to have significantly up-regulated expression of Adrb3 and leptin. Furthermore, in line with the alterations in these two genes, there was enrichment of up-regulated genes in the insulin and adipocytokine signaling pathways and enrichment of down-regulated genes in the fatty acid metabolism pathway. These results are in line with the physiological outcome, and thus increase confidence in the data and suggest that the discovered alterations in the transcriptome may be largely valid.
The β3-subtype of adrenergic receptor is known to play an important role in energy homeostasis through its effect on lipolysis and thermogenesis, and there has been a lot of interest in developing selective β3-adrenergic receptor agonists as anti-obesity drugs [30]. Interestingly, high-fat feeding has been demonstrated to suppress the expression of Adrb3 in the adipose tissue of C57Bl/6J mice [31, 32] as well as other mouse models of obesity [33]. However, in this study, a high-calcium diet with whey protein was able to restore the expression of Adrb3 in the adipose tissue of C57Bl/6J mice fed a high-fat diet at a significantly higher level than in obese controls, and thus to prevent the detrimental effect of a high-fat diet on the expression of this receptor.
Interestingly, we found significantly higher leptin expression in the whey group, which had significantly less body fat than the obese control group. In fact, there was no difference in leptin expression between the group fed a low-fat diet and the obese control group (data not shown). In line with our finding, leptin expression has been shown to be disturbed in C57Bl/6J mice fed a high-fat-diet [34, 35]. In comparison with the obesity-resistant A/J-mice, C57Bl/6J mice fed a high-fat diet had significantly less leptin expression in relation to fat mass. Consequently, it can be argued that the high-calcium diet with whey protein changed the expression of leptin in the direction of an obesity-resistant mouse strain. Twelve-week leptin supplementation has been shown to slow, but not totally prevent, diet-induced obesity in C57Bl/6J mice, and leptin supplementation has been demonstrated to have more effect on energy expenditure than energy intake in these mice [36]. The precise signals mediating the regulation of leptin expression and secretion are unclear, but insulin is known to play an important role [37]. Leptin secretion from the adipocytes is stimulated by insulin and stimulation of the β3-adrenergic receptor is known to inhibit insulin-stimulated leptin secretion [38]. Leptin expression is also involved in the adipocytokine signaling pathway, which according to the microarray data had the second biggest cluster of significantly up-regulated genes.
Both leptin and adrenergic signaling are relevant to the sensitivity of insulin signaling, a pathway which, according to the microarray analysis, was enriched with the largest number of up-regulated genes. Insulin signaling in the adipocytes occurs via the interplay of the insulin receptor and its substrates like IRS-1 and PI3-kinase, whose activation leads to translocation of GLUT4-containing vesicles and subsequent increase in glucose uptake [39, 40]. The obesity-induced impairment in adipose tissue insulin signaling has been shown to be related to a decrease in GLUT-4 expression [41]. Impaired IRS-1 signaling to PI3-kinase has also been observed [42].
Whey protein intake has been linked to insulin metabolism previously, but we show for the first time the effect of whey protein on the level of adipose tissue gene expression. Whey protein is known to have a greater postprandial insulinotrophic effect than casein [43, 44]. The insulinotrophic effect of whey protein is likely to be mediated through rapid amino acid absorption, a substantial amount of certain insulinotrophic amino acids (leucine, isoleucine, valine, lysine and threonine) and the inhibition of dipeptidyl peptidase IV in the intestine, which leads to an increased concentration of incretin hormones [45, 46]. It is also of note that an increase in adipocyte size results in increased insulin resistance, at least in vitro [47, 48]. Thus, smaller adipocyte size in the high-calcium whey group could also partly explain the clustering of up-regulated genes in the insulin signaling pathway.
The microarray data indicated that the expression of genes related to fatty acid metabolism, Cpt1a, ACS and Acad9, were all strongly and significantly decreased. Cpt1a is considered to be one of the key enzymes regulating free fatty acid oxidation, and its function in liver and muscle has been widely studied [49, 50]. However, understanding of the role and regulation of adipose tissue Cpt1a expression in C57Bl/6 mice fed a high-fat diet is still sparse. The role of ACS and Acad9 gene expression in adipose tissue or obesity has not been intensively investigated. ACS is involved in facilitating long-chain fatty acid transport across the plasma membrane, and the exact role of Acad9 has thus far not been reported. Hence, the importance of these preliminary findings remains to be elucidated.
Taken together, we have shown for the first time that whey protein together with calcium supplementation not only inhibits the accumulation of fat during a high-fat diet, but also significantly modulates the gene expression of visceral adipose tissue. Whey protein and calcium feeding showed a protective effect against a high-fat diet-induced decline in Adrb3 expression and corrected leptin expression in the direction normally seen in an obesity-resistant mouse strain, i.e. changes which are likely to contribute to the inhibition of weight gain. Significant up-regulation of leptin and Adrb3 expression is also connected with the insulin-signaling pathway, which according to the microarray data was enriched with up-regulated genes. As the microarray analysis was performed from two replicates per experimental group, the findings related to significantly regulated pathways can be considered preliminary. Hence, the influence of a high-calcium diet with whey protein on insulin and adipocytokine signaling and fatty acid metabolism pathways warrants further studies.
Acknowledgements
The present study was supported by the Foundation for Nutrition Research, Academy of Finland, Sigrid Juselius Foundation and Valio Ltd, Helsinki, Finland. We are grateful to Erik Vahtola (MSc), Ms. Sari Laakkonen, Mrs. Anneli von Behr and Mr. Berndt Köhler for expert technical assistance.
References
- 1.Jacqmain M, Doucet E, Despres JP, Bouchard C, Tremblay A. Calcium intake, body composition, and lipoprotein-lipid concentrations in adults. Am J Clin Nutr. 2003;77:1448–1452. doi: 10.1093/ajcn/77.6.1448. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez AJ, White E, Kristal A, Littman AJ. Calcium intake and 10-year weight change in middle-aged adults. J Am Diet Assoc. 2006;106:1066–1073. doi: 10.1016/j.jada.2006.04.024. quiz 1082. [DOI] [PubMed] [Google Scholar]
- 3.Loos RJ, Rankinen T, Leon AS, Skinner JS, Wilmore JH, Rao DC, Bouchard C. Calcium intake is associated with adiposity in black and white men and white women of the HERITAGE family study. J Nutr. 2004;134:1772–1778. doi: 10.1093/jn/134.7.1772. [DOI] [PubMed] [Google Scholar]
- 4.Skinner JD, Bounds W, Carruth BR, Ziegler P. Longitudinal calcium intake is negatively related to children's body fat indexes. J Am Diet Assoc. 2003;103:1626–1631. doi: 10.1016/j.jada.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Davies KM, Heaney RP, Recker RR, Lappe JM, Barger-Lux MJ, Rafferty K, Hinders S. Calcium intake and body weight. J Clin Endocrinol Metab. 2000;85:4635–4638. doi: 10.1210/jcem.85.12.7063. [DOI] [PubMed] [Google Scholar]
- 6.Zemel MB, Shi H, Greer B, Dirienzo D, Zemel PC. Regulation of adiposity by dietary calcium. FASEB J. 2000;14:1132–1138. [PubMed] [Google Scholar]
- 7.Parra P, Bruni G, Palou A, Serra F. Dietary calcium attenuation of body fat gain during high-fat feeding in mice. J Nutr Biochem. 2008;19:109–117. doi: 10.1016/j.jnutbio.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Papakonstantinou E, Flatt WP, Huth PJ, Harris RB. High dietary calcium reduces body fat content, digestibility of fat, and serum vitamin D in rats. Obes Res. 2003;11:387–394. doi: 10.1038/oby.2003.52. [DOI] [PubMed] [Google Scholar]
- 9.Shi H, Dirienzo D, Zemel MB. Effects of dietary calcium on adipocyte lipid metabolism and body weight regulation in energy-restricted aP2-agouti transgenic mice. FASEB J. 2001;15:291–293. doi: 10.1096/fj.00-0584fje. [DOI] [PubMed] [Google Scholar]
- 10.Zemel MB, Richards J, Milstead A, Campbell P. Effects of calcium and dairy on body composition and weight loss in African-American adults. Obes Res. 2005;13:1218–1225. doi: 10.1038/oby.2005.144. [DOI] [PubMed] [Google Scholar]
- 11.Zemel MB, Richards J, Mathis S, Milstead A, Gebhardt L, Silva E. Dairy augmentation of total and central fat loss in obese subjects. Int J Obes (Lond) 2005;29:391–397. doi: 10.1038/sj.ijo.0802880. [DOI] [PubMed] [Google Scholar]
- 12.Thompson WG, Rostad Holdman N, Janzow DJ, Slezak JM, Morris KL, Zemel MB. Effect of energy-reduced diets high in dairy products and fiber on weight loss in obese adults. Obes Res. 2005;13:1344–1353. doi: 10.1038/oby.2005.163. [DOI] [PubMed] [Google Scholar]
- 13.Harvey-Berino J, Gold BC, Lauber R, Starinski A. The impact of calcium and dairy product consumption on weight loss. Obes Res. 2005;13:1720–1726. doi: 10.1038/oby.2005.210. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Q, Tordoff MG. No effect of dietary calcium on body weight of lean and obese mice and rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R669–R677. doi: 10.1152/ajpregu.00655.2003. [DOI] [PubMed] [Google Scholar]
- 15.Zemel MB. The role of dairy foods in weight management. J Am Coll Nutr. 2005;24:537S–546S. doi: 10.1080/07315724.2005.10719502. [DOI] [PubMed] [Google Scholar]
- 16.Shi H, Norman AW, Okamura WH, Sen A, Zemel MB. 1α,25-dihydroxyvitamin D3 modulates human adipocyte metabolism via nongenomic action. FASEB J. 2001;15:2751–2753. doi: 10.1096/fj.01-0584fje. [DOI] [PubMed] [Google Scholar]
- 17.Xue B, Greenberg AG, Kraemer FB, Zemel MB. Mechanism of intracellular calcium ([Ca2+]i) inhibition of lipolysis in human adipocytes. FASEB J. 2001;15:2527–2529. doi: 10.1096/fj.01-0278fje. [DOI] [PubMed] [Google Scholar]
- 18.Sun X, Zemel MB. Role of uncoupling protein 2 (UCP2) expression and 1α, 25-dihydroxyvitamin D3 in modulating adipocyte apoptosis. FASEB J. 2004;18:1430–1432. doi: 10.1096/fj.04-1971fje. [DOI] [PubMed] [Google Scholar]
- 19.Shi H, Norman AW, Okamura WH, Sen A, Zemel MB. 1α,25-dihydroxyvitamin D3 inhibits uncoupling protein 2 expression in human adipocytes. FASEB J. 2002;16:1808–1810. doi: 10.1096/fj.02-0255fje. [DOI] [PubMed] [Google Scholar]
- 20.Boon N, Hul GB, Sicard A, Kole E, Van Den Berg ER, Viguerie N, Langin D, Saris WH. The effects of increasing serum calcitriol on energy and fat metabolism and gene expression. Obesity (Silver Spring) 2006;14:1739–1746. doi: 10.1038/oby.2006.200. [DOI] [PubMed] [Google Scholar]
- 21.Boon N, Hul GB, Stegen JH, Sluijsmans WE, Valle C, Langin D, Viguerie N, Saris WH. An intervention study of the effects of calcium intake on faecal fat excretion, energy metabolism and adipose tissue mRNA expression of lipid-metabolism related proteins. Int J Obes (Lond) 2007;31:1704–1712. doi: 10.1038/sj.ijo.0803660. [DOI] [PubMed] [Google Scholar]
- 22.Jacobsen R, Lorenzen JK, Toubro S, Krog-Mikkelsen I, Astrup A. Effect of short-term high dietary calcium intake on 24-h energy expenditure, fat oxidation, and fecal fat excretion. Int J Obes (Lond) 2005;29:292–301. doi: 10.1038/sj.ijo.0802785. [DOI] [PubMed] [Google Scholar]
- 23.Pilvi TK, Korpela R, Huttunen M, Vapaatalo H, Mervaala EM. High-calcium diet with whey protein attenuates body-weight gain in high-fat-fed C57Bl/6J mice. Br J Nutr. 2007;98:900–907. doi: 10.1017/S0007114507764760. [DOI] [PubMed] [Google Scholar]
- 24.Sun X, Zemel MB. Calcium and dairy products inhibit weight and fat regain during ad libitum consumption following energy restriction in Ap2-agouti transgenic mice. J Nutr. 2004;134:3054–3060. doi: 10.1093/jn/134.11.3054. [DOI] [PubMed] [Google Scholar]
- 25.Jauhiainen T, Korpela R. Milk peptides and blood pressure. J Nutr. 2007;137:825S–829S. doi: 10.1093/jn/137.3.825S. [DOI] [PubMed] [Google Scholar]
- 26.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 27.Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- 28.Kurki M, Paananen J, Storvik M, Jääskeläinen JE, Wong G. TAFFEL program for discovery of gene modules, submitted
- 29.Robertson G, Bilenky M, Lin K, He A, Yuen W, Dagpinar M, Varhol R, Teague K, Griffith OL, et al. cisRED: a database system for genome-scale computational discovery of regulatory elements. Nucleic Acids Res. 2006;34:D68–D73. doi: 10.1093/nar/gkj075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sawa M, Harada H. Recent developments in the design of orally bioavailable β3-adrenergic receptor agonists. Curr Med Chem. 2006;13:25–37. [PubMed] [Google Scholar]
- 31.Moraes RC, Blondet A, Birkenkamp-Demtroeder K, Tirard J, Orntoft TF, Gertler A, Durand P, Naville D, Begeot M. Study of the alteration of gene expression in adipose tissue of diet-induced obese mice by microarray and reverse transcription-polymerase chain reaction analyses. Endocrinology. 2003;144:4773–4782. doi: 10.1210/en.2003-0456. [DOI] [PubMed] [Google Scholar]
- 32.Collins S, Daniel KW, Petro AE, Surwit RS. Strain-specific response to beta 3-adrenergic receptor agonist treatment of diet-induced obesity in mice. Endocrinology. 1997;138:405–413. doi: 10.1210/endo.138.1.4829. [DOI] [PubMed] [Google Scholar]
- 33.Collins S, Daniel KW, Rohlfs EM. Depressed expression of adipocyte beta-adrenergic receptors is a common feature of congenital and diet-induced obesity in rodents. Int J Obes Relat Metab Disord. 1999;23:669–677. doi: 10.1038/sj.ijo.0800894. [DOI] [PubMed] [Google Scholar]
- 34.Watson PM, Commins SP, Beiler RJ, Hatcher HC, Gettys TW. Differential regulation of leptin expression and function in A/J vs. C57BL/6J mice during diet-induced obesity. Am J Physiol Endocrinol Metab. 2000;279:E356–E365. doi: 10.1152/ajpendo.2000.279.2.E356. [DOI] [PubMed] [Google Scholar]
- 35.Surwit RS, Petro AE, Parekh P, Collins S. Low plasma leptin in response to dietary fat in diabetes- and obesity-prone mice. Diabetes. 1997;46:1516–1520. doi: 10.2337/diab.46.9.1516. [DOI] [PubMed] [Google Scholar]
- 36.Surwit RS, Edwards CL, Murthy S, Petro AE. Transient effects of long-term leptin supplementation in the prevention of diet-induced obesity in mice. Diabetes. 2000;49:1203–1208. doi: 10.2337/diabetes.49.7.1203. [DOI] [PubMed] [Google Scholar]
- 37.Ahima RS. Adipose tissue as an endocrine organ. Obesity (Silver Spring) 2006;14(suppl 5):242S–249S. doi: 10.1038/oby.2006.317. [DOI] [PubMed] [Google Scholar]
- 38.Cammisotto PG, Bukowiecki LJ. Mechanisms of leptin secretion from white adipocytes. Am J Physiol Cell Physiol. 2002;283:C244–C250. doi: 10.1152/ajpcell.00033.2002. [DOI] [PubMed] [Google Scholar]
- 39.Cheatham B, Kahn CR. Insulin action and the insulin signalling network. Endocr Rev. 1995;16:117–142. doi: 10.1210/edrv-16-2-117. [DOI] [PubMed] [Google Scholar]
- 40.White MF, Kahn CR. The insulin signalling system. J Biol Chem. 1994;269:1–4. [PubMed] [Google Scholar]
- 41.Pedersen O, Kahn CR, Flier JS, Kahn BB. High fat feeding causes insulin resistance and a marked decrease in the expression of glucose transporters (Glut 4) in fat cells of rats. Endocrinology. 1991;129:771–777. doi: 10.1210/endo-129-2-771. [DOI] [PubMed] [Google Scholar]
- 42.Björnholm M, He AR, Attersand A, Lake S, Liu SC, Lienhard GE, Taylor S, Arner P, Zierath JR. Absence of functional insulin receptor substrate-3 (IRS-3) gene in humans. Diabetologia. 2002;45:1697–1702. doi: 10.1007/s00125-002-0945-z. [DOI] [PubMed] [Google Scholar]
- 43.Tessari P, Kiwanuka E, Cristini M, Zaramella M, Enslen M, Zurlo C, Garcia-Rodenas C. Slow versus fast proteins in the stimulation of beta-cell response and the activation of the entero-insular axis in type 2 diabetes. Diabetes Metab Res Rev. 2007;23:378–385. doi: 10.1002/dmrr.698. [DOI] [PubMed] [Google Scholar]
- 44.Nilsson M, Stenberg M, Frid AH, Holst JJ, Bjorck IM. Glycemia and insulinemia in healthy subjects after lactose-equivalent meals of milk and other food proteins: the role of plasma amino acids and incretins. Am J Clin Nutr. 2004;80:1246–1253. doi: 10.1093/ajcn/80.5.1246. [DOI] [PubMed] [Google Scholar]
- 45.Nilsson M, Holst JJ, Bjorck IM. Metabolic effects of amino acid mixtures and whey protein in healthy subjects: Studies using glucose-equivalent drinks. Am J Clin Nutr. 2007;85:996–1004. doi: 10.1093/ajcn/85.4.996. [DOI] [PubMed] [Google Scholar]
- 46.Gunnarsson PT, Winzell MS, Deacon CF, Larsen MO, Jelic K, Carr RD, Ahren B. Glucose-induced incretin hormone release and inactivation are differently modulated by oral fat and protein in mice. Endocrinology. 2006;147:3173–3180. doi: 10.1210/en.2005-1442. [DOI] [PubMed] [Google Scholar]
- 47.Molina JM, Ciaraldi TP, Brady D, Olefsky JM. Decreased activation rate of insulin-stimulated glucose transport in adipocytes from obese subjects. Diabetes. 1989;38:991–995. doi: 10.2337/diab.38.8.991. [DOI] [PubMed] [Google Scholar]
- 48.Olefsky JM. Mechanisms of decreased insulin responsiveness of large adipocytes. Endocrinology. 1977;100:1169–1177. doi: 10.1210/endo-100-4-1169. [DOI] [PubMed] [Google Scholar]
- 49.Foster DW. The role of the carnitine system in human metabolism. Ann NY Acad Sci. 2004;1033:1–16. doi: 10.1196/annals.1320.001. [DOI] [PubMed] [Google Scholar]
- 50.Kerner J, Hoppel C. Fatty acid import into mitochondria. Biochim Biophys Acta. 2000;1486:1–17. doi: 10.1016/s1388-1981(00)00044-5. [DOI] [PubMed] [Google Scholar]
- 51.Kanehisa M, Goto S, Hattori M, Aoki-Kinoshita KF, Itoh M, Kawashima S, Katayama T, Araki M, Hirakawa M. From genomics to chemical genomics: new developments in KEGG. Nucleic Acids Res. 2006;34:D354–D357. doi: 10.1093/nar/gkj102. [DOI] [PMC free article] [PubMed] [Google Scholar]