Abstract
Background/Aims
Alcohol use alters inflammatory cell responses. While alcohol has direct effects on pancreatic acinar cells, activation of inflammatory cells is a major component of the pathology of alcoholic pancreatitis.
Methods
The effects of acute or chronic alcohol exposure were evaluated in human monocytes on the production of TNFα or IL-10 production, pro-inflammatory gene and nuclear factor-κB (NF-κB) activation.
Results
Moderate, acute alcohol consumption or equivalent doses of alcohol in vitro had anti-inflammatory effects on monocyte activation via inhibition of pro-inflammatory genes and NF-κB activation, inhibition of TNFα production and augmentation of the anti-inflammatory cytokine, IL-10. In contrast, acute alcohol treatment augmented NF-κB activation and TNFα production and inhibited IL-10 levels in the presence of complex stimulation with combined TLR2 and TLR4 ligands. Prolonged alcohol exposure also resulted in an increase in NF-κB and TNFα production in response to TLR4 stimulation with LPS.
Conclusion
These results suggest that alcohol can either attenuate or promote inflammatory responses that are critical in pancreatitis. Our results support the hypothesis that both acute alcohol intake in the presence of complex stimuli (such as necrotic cells) and chronic alcohol exposure result in hyper-responsiveness of monocytes to inflammatory signals and may contribute to increased inflammation in pancreatitis.
Key Words: Alcohol, Ethanol, Monocyte, Cytokine, NF-κB
Introduction
Previous studies have demonstrated that chronic alcohol consumption leads to alterations in immune responses including decreased antimicrobial and antiviral immunity. Excessive alcohol consumption is associated with increased incidence of pneumonias, tuberculosis, hepatitis C virus (HCV) infection, increased susceptibility to HIV infection, and acceleration of HCV-induced liver disease [1,2,3]. Specific immune functions identified in these defects of immune responses include reduced antigen-specific T cell activation, antigen-presenting cell defects and reduced elimination of bacterial and viral pathogens [1,4,5,6]. Innate immune responses that are pivotal in recognition of danger signals from pathogens or damaged host tissue are critical in initiation of appropriate immune responses and T cell activation for mounting adaptive immune responses [7]. Cells of the innate immune system such as blood monocytes, tissue macrophages and dendritic cells express pattern recognition receptors including Toll-like receptors (TLRs) that specifically recognize microbial or host-derived danger signals [7, 8]. In response to such signals monocytes can differentiate into macrophages which are a cell type that can produce massive amounts of pro-inflammatory cytokines such as TNFα, IL-1 as well as reactive oxygen species that mediate tissue damage. Pro-inflammatory cytokines are also produced by dendritic cells, however at much lower amounts compared to macrophages. Dendritic cells have a unique capacity to induce the most potent T cell activation in an antigen-specific manner [9]. For example, innate immune cells have a key role not only in pathogen-specific immune responses but also in ‘sterile’ inflammation triggered by host-derived factors from damaged cells and tissues [10]. In addition, Toll-like receptor 2 and 4 have been indicated in recognition of hsp70 while TLR4 recognizes Hsp60 [11, 12].
Of all pancreatitis cases 66% are alcohol-induced and chronic pancreatitis develops in 10% of alcohol addicts after 6–12 years of 80 g daily alcohol intake [13] Unfortunately, there are no controlled studies investigating the rate of nonpancreatic infections or infected pancreatic necrosis in alcohol-induced acute or chronic pancreatitis. However, tissue damage is a major component of alcoholic pancreatitis [14, 15]. Multiple theories have been suggested in explanation of the pathomechanisms of alcohol-induced pancreatitis [3,14,15,16,17]. While typically excessive and prolonged alcohol use is associated with alcoholic pancreatitis, an acute alcohol consumption event or binge drinking can also trigger acute pancreatitis [18]. Ethanol-derived toxic metabolites induce reactive oxygen species (ROS) and oxidative stress induces autodigestion of the acinar cells [17, 19]. This leads to pancreatic necrosis that triggers recruitment and activation of inflammatory cells [16,17,18,19,20]. Local recruitment and activation of inflammatory cells results in production of pro-inflammatory cytokines TNFα, IL-6 and chemokines. It has been proposed that the inflammatory cytokines in turn can activate stellate cells and trigger fibrin deposition and scarring [21].
In addition to the direct effects of alcohol and its metabolites on acinar cells, alcohol-induced regulation of inflammatory cells and inflammatory cytokine production is also likely to contribute to pancreatic pathology. It has been demonstrated that both chronic alcohol consumption and moderate, acute drinking can modulate the function of the innate immune cells, monocytes, macrophages and dendritic cells [1,4,5,6,22,23,24,25,26,27,28,29]. For example, in alcoholic hepatitis increased circulating levels of pro-inflammatory cytokines, particularly TNFα, correlates with clinical outcome and survival. In chronic pancreatitis alcohol ingestion leads to increased circulating levels of TNFα [30]. Kupffer cell activation and increased levels of the nuclear regulatory factor-κB (NF-κB), a transcription factor of pro-inflammatory cytokine genes are present at increased levels in alcoholic liver disease [26,27,28]. In contrast, acute alcohol administration has anti-inflammatory effects both in mice and man and may attenuate inflammatory ctyokine production [22,23,24,25, 31].
In this study, we evaluated whether acute, moderate and chronic alcohol consumption have different effects on inflammatory cell functions. Furthermore, we investigated the effect of acute alcohol ingestion on inflammatory cell activation in combination with a single or multiple pathogen-derived stimuli.
Methods
Human Subjects and Monocytes
Healthy volunteers without history of alcohol consumption (less than 12 drinks/week for males and less than 9 drinks/week for females) were recruited according to the protocol approved by the IRB at University of Massachusetts Medical School. Volunteers consumed 2 ml vodka/kg body weight and blood samples were collected for monocyte isolation before and 24 h after the alcohol intake as previously described [24, 31].
Monocyte Separation and Stimulation
Monocytes from human peripheral blood were isolated by selective adherence from Ficoll-Hypaque purified mononuclear cell preparations as previously described [22]. Cells were stimulated with Escherichia coli-derived LPS (0.1 μg/ml), 25 mM ethanol and the combination of LPS and ethanol together for acute alcohol experiments or for chronic alcohol experiments, exposed to 25 mM ethanol for 5 days followed by LPS. The 25 mM in vitro ethanol concentration approximates a 0.1-g/dl blood alcohol level, which is achieved in vivo after a dose of moderate drink [31]. Cell viability was not affected by ethanol or LPS treatment. Cell supernatants were harvested after 18hour treatment for TNFα or 40hours for IL-10 determinations or for 1 h for NFκB-binding activity. TNFα and IL-10 was evaluated in cell supernatants using ELISA from Endogen Inc. (Cambridge, Mass., USA).
Preparation of Nuclear and Cytoplasmic Extracts
Nuclear and cytoplasmic extracts from cells with or without stimulation at 37°C were performed by the method previously described [31]. Protein content was determined in nuclear extracts by the Bio-Rad Dye Reagent Assay.
Electrophoretic Gel Mobility Shift Assay
A consensus double-stranded nuclear factor-κB (NF-κB) oligonucleotide (5′AGTTGAGGGGACTTTCGC3′) was used for EMSA [29]. End-labeling was accomplished by treatment with T4 polynucleotide kinase in the presence of γ32P-ATP (Dupont-NEN, Boston, Mass., USA). Labeled oligonucleotides were purified on a polyacrylamide copolymer column (Bio-Rad). Five micrograms of nuclear protein was added to a binding reaction mixture containing 50 mM Tris-HCl (pH 7.5), 5 mM MgCl2, 2.5 mM EDTA, 2.5 mM DTT, 250 mM NaCl, 20% glycerol, 20 μg/ml of bovine serum albumin, 2 μg of poly (dI-dC) and 30,000 cpm of γ32P-labeled NF-κB oligonucleotide. Samples were incubated at room temperature for 30 min. All reactions were run on a 5% polyacrylamide gel and the dried gel was exposed to an X-ray film at –80°C overnight. For the cold competition reaction a 20-fold excess of specific unlabeled double-stranded probe was added to the reaction mixture before adding the labeled oligonucleotide.
Superarray Method
Total cellular RNA was isolated after stimulation of monocytes for 1 h with acid-guanidinium-thiocyanate-phenol chloroform method using the Tri-Reagent (Molecular Research, Inc., Cincinnati, Ohio, USA). The NF-κB pathway GEArray kit was obtained from SuperArray Inc., (Bethesda, Md., USA). This kit determines differential expression levels of multiple genes involved in a biological pathway. Briefly, the total RNA from the respective samples was used as a template to generate (γ-32P) cDNA probes using the GEAprimer mix as a reverse transcriptase primer. The cDNA probes, which represent abundance of mRNA population, were then denatured and hybridization was conducted in GEHybridization solution to two nylon membranes spotted with gene-specific cDNA fragments. Membranes were then washed in 2× SSC, 1% SDS twice for 20 min each followed by 0.1× SSC, 0.5% SDS twice for 20 min each. The membranes were then exposed to Kodak X-Omat film at –70°C with intensifying screens (Dupont Cronex Lightening Plus, Wilmington, Del., USA). The relative expression level of each gene was then determined through densitometric scanning. Data are presented as densitometric units corrected for equal RNA loading based on the corresponding β-actin mRNA levels.
Statistical Analysis
All data are expressed as means ± SE. Results between treatment groups were compared by Wilcoxon signed-rank, nonparametric data analysis.
Results
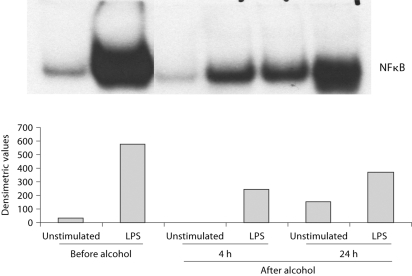
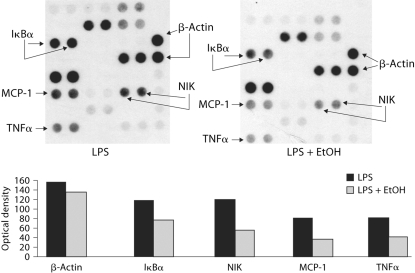
Events of clinically significant acute pancreatitis have been reported even after one occasion of alcohol consumption [18]. Thus, we investigated the effects of acute alcohol intake on the induction of the pro-inflammatory cytokines, TNFα and IL-1β. As reported earlier, production of both TNFα and IL-1β are significantly decreased in response to stimulation with lipopolysaccharide (LPS) in monocytes obtained after alcohol consumption compared to monocytes collected from the same individual before the alcohol intake [31]. These results suggested that acute alcohol intake has anti-inflammatory effects. Production of TNFα and IL-1β is regulated transcriptionally by the NF-κB. We found that consistent with inhibition of TNFα production, acute alcohol intake attenuated LPS-induced NF-κB actvation in monocytes compared to monocytes of the same individual before alcohol intake (fig. 1). The NF-κB response element is common in the promoter region of most genes involved in inflammation. We found that treatment of monocytes in vitro with a physiologically relevant dose of acute alcohol (25 mM) resulted in reduced activation of pro-inflammatory cytokines [22, 24, 31]. Here, RNA samples from alcohol-treated and alcohol-naive monocytes were evaluated in a superarray to assess expression of pro-inflammatory genes. We found that NF-κB responsive pro-inflammatory genes were induced to a lesser extent in the presence of alcohol treatment by LPS compared to non-alcohol-treated cells (fig. 2). The downregulated genes included MCP-1, a chemokine that recruits T cells, TNFα and the NF-κB regulating members of the NF-κB signaling pathway including IκBα and NIK. There was no difference in the expression of β-actin, a housekeeping gene. These results demonstrated that acute alcohol intake can have anti-inflammatory effects in normal monocytes by attenuating pro-inflammatory cell activation by LPS.
Fig. 1.
Moderate alcohol consumption inhibits LPS-induced NFkB activation in human monocytes. Monocytes isolated before (day 1), 4 h or 24 h after (day 2) alcohol intake were stimulated with LPS for 1 h and nuclear extracts were tested in EMSA for binding to a consensus NF- κB sequence. One representative gel density units ± SE of a total of 5 individuals are shown.
Fig. 2.
Acute alcohol attenuates LPS-induced gene expression of the pro-inflammatory pathway. Peripheral blood monocytes stimulated with 1 μg/ml LPS in the presence or absence of 25 m M alcohol for 2 h were subjected to RNA extraction using the Tri-Reagent. Radiolabeled cDNA was prepared and hybridized to the NFκB pathway gene arrays according to the manufacturer's instructions as described in ‘Materials and Methods’. The microarray data is representative of three individual experiments and shows the density units (lower panel) for the expression of IκBκ, NIK, MCP-1 and TNFα gene spotted in duplicate and the positive control β-actin used to normalize the loading of the samples.
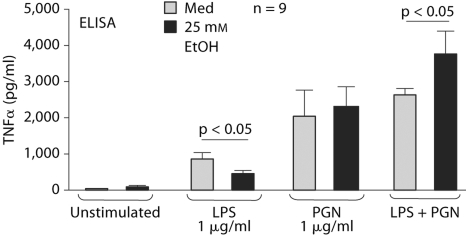
It has been proposed that gut-derived endotoxin plays a central role in Kupffer cell activation and in the induction of the pro-inflammatory cytokine cascade in alcoholic liver disease [32]. At this time it is unclear whether other gut-derived factors may also contribute to alcoholic liver disease. It is conceivable that in pancreatitis, the ongoing acinar cell activation and damage may result in host-derived factors that could activate inflammatory cells [10, 16, 17]. Here, we investigated the effect of acute alcohol treatment on monocyte activation by single stimulation with two prototype bacterial stimuli, LPS and PGN. LPS is a component of Gram-negative bacteria and it activates cells through TLR4 [7]. Peptidoglycan is found in Gram-positive microorganisms and it is a ligand for TLR2 [7]. We found that while acute alcohol treatment inhibited TLR4-induced TNFα production in monocytes, it had no significant effect on TLR2-induced TNFα production. Interestingly, when acute alcohol was combined with TLR4 plus TLR2 stimulation, it had an augmenting effect on TNFα production (fig. 3). At the level of NF-κB, a nuclear regulator of TNFα gene induction, we found the same pattern [33]. Acute alcohol inhibited LPS but not PGN-induced NF-κB but augmented NF-κB DNA binding in the presence of dual TLR2 plus TLR4 stimulation.
Fig. 3.
Opposite effects of acute alcohol on TLR4 and/or TLR2 stimulation on TNFα production in human monocytes. Adherence isolated normal human monocytes were stimulated with purified LPS (1 μg/ml), PGN (1 μg/ml) or their combination in the presence or absence of 25 mM alcohol for 16 h and TNFα was measured in the supernatants by ELISA. The data are represented as mean ± SE (n = 9).
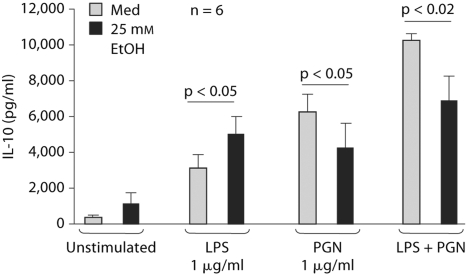
The production of pro-inflammatory cytokines is regulated by anti-inflammatory cytokines such as IL-10 [23]. Our results demonstrated that acute alcohol augmented LPS-induced IL-10 production in monocytes which is consistent with the anti-inflammatory effects of acute alcohol on TLR4-induced monocyte stimulation (fig. 4). In contrast, there was a moderate attenuation of TLR2- or TLR2 plus TLR4-induced IL-10 production by acute alcohol (fig. 4). These results suggested that acute alcohol treatment can inhibit or augment inflammation depending on the co-stimulatory signals.
Fig. 4.
Regulation of the anti-inflammatory cytokine, IL-10, in TLR4- and/or TLR2-stimulated human monocytes. Normal human monocytes were stimulated with purified LPS (1 μg/ml), PGN (1 μg/ml) or their combination in the presence or absence of 25 m M alcohol for 40 h and IL-10 was measured in the supernatants by ELISA. The data are represented as mean ± SE (n = 6).
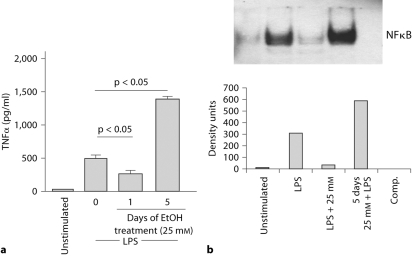
Finally, we were interested to delineate the effects of chronic alcohol treatment on inflammatory cell functions. Human monocytes were cultured in the presence of 25 mM alcohol for 5 days and then stimulated with LPS. Data in figure 5a demonstrate that monocytes exposed to prolonged alcohol treatment produced greater amounts of TNFα in response to LPS compared to alcohol-naive monocytes from the same individual. This increased TNFα production by monocytes exposed to chronic alcohol was in contrast to the attenuation of LPS-induced TNFα production by acute alcohol treatment. Furthermore, chronic alcohol treatment increased LPS-induced NF-κB binding activity (fig. 5b) suggesting increased inflammatory responses after chronic alcohol exposure.
Fig. 5.
Opposite effects of acute and chronic alcohol on monocyte TNFα production and NF- κB binding. Normal human monocytes were stimulated with exposed to 25 mM alcohol for 1 or 5 days followed by LPS (100 ng/ml) in the presence or absence of 25 m M alcohol (a) for 18 h and TNFα was measured in the supernatants by ELISA (data are represented as mean ± SE, n = 10) and (b) 1 h for NF-κB and nuclear extracts were tested in EMSA for binding to a consensus NF-κB sequence. One representative gel density unit ± SE of a total of 6 experiments is shown.
Discussion
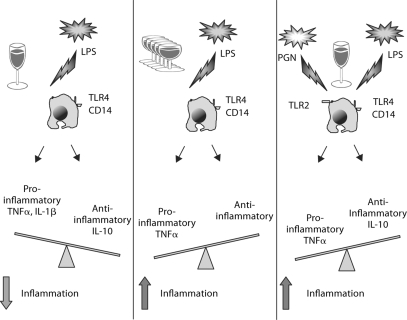
Alcohol-induced etiology represents the most common cause of acute pancreatitis in most countries [14, 15]. While the pathomechanisms of alcohol-induced pancreatitis are yet to be fully understood, there is substantial evidence for both direct acinar cell damage mediated by alcohol and its metabolites as well as inflammatory cell recruitment and activation as pivotal components of alcoholic pancreatitis [14,15,16,17]. In addition, regulation of inflammatory cell responses by alcohol may play a role in the development and progression of pancreatitis. In this study, we investigated the effect of acute alcohol treatment on inflammatory cell function and activation in response to a single or multiple inflammatory stimuli. We found that acute alcohol can differently regulate monocyte TNFα production depending on the complexity of the co-stimulatory signals (fig. 6). In vivo alcohol intake or acute alcohol treatment in vitro had anti-inflammatory effects on TLR4-induced cell activation [22,23,24,25, 31]. In contrast, even acute alcohol could augment inflammation when monocytes were stimulated with both TLR2 and TLR4 ligands, suggesting that alcohol regulates monocytes differently in the presence of more complex pro-inflammatory or danger signals [33]. It is tempting to speculate that some of these mechanisms may play a role in acute pancreatitis related to acute alcohol intake or binge drinking where local tissue damage could produce multiple different ligands for inflammatory cell activation. For example, acinar cell death could be triggered as a result of direct effects of alcohol and these apoptotic and necrotic cells induce pro-inflammatory activation of monocytes [14,15,16,17]. It has been demonstrated that chronic alcohol exposure increases the susceptibility of the pancreas to LPS-induced necrosis [34]. While LPS induces acinar cell apoptosis, alcohol-exposed acinar cells have increased susceptibility to LPS-induced necrosis. It is conceivable that LPS-induced TLR4 stimulation occurs if excessive amounts of acute or binge drinking cause an increase in gut permeability and result in elevations in serum LPS [35]. Elevated levels of serum endotoxin have been reported after chronic alcohol intake both in humans and in animal models [35, 36]. Furthermore, proteins derived from dying cells such as acinar cells are recognized as ‘danger’ signals by inflammatory cells [10]. For example, toll-like receptor 2 (TLR2) is activated by heat shock protein 70 and cellular components of dying cells activate damage-associated pattern recognition receptors [10,11,12]. Thus, under conditions where multiple cellular stimuli occur even acute alcohol could augment pro-inflammatory cell activation. Our results demonstrated that increased TNFα production in the presence of acute alcohol and dual stimulation with TLR2 and TLR4 ligands was mediated by an increase in NF-κB activation and DNA binding [33]. We found that TLR-induced IRAK-1 phosphorylation was attenuated by acute alcohol after TLR4 but it was upregulated after TLR2 plus TLR4 stimulation [33]. In addition, we have recently reported that differential activation of MAP kinases by acute alcohol in the presence of a single or multiple TLR ligands correlates with these effects [33]. Specifically, acute alcohol selectively inhibits Erk phosphorylation in LPS stimulated cells while JNK phosphorylation is increased by acute alcohol in the presence of dual TLR2 plus TLR4 stimulation. Taken together, we conclude that acute alcohol may attenuate or augment TNFα production and pro-inflammatory signaling pathways depending on the presence of co-stimulatory signals from the environment (fig. 6).
Fig. 6.
Regulation of monocyte TNF-α production is dependent on the TLR costimulatory signals.
Studies in acute non-alcoholic experimental pancreatitis attempting to delineate the effect of TLR-4 signaling resulted in conflicting results. While in the report by Pastor et al. the absence of TLR-4 expression had no impact on local or systemic injury in caerulein-induced pancreatitis [36], Saluja et al. [pers. commun.] found ameliorated pancreatitis in TLR4-deficient mice. In another study, administration of anti-TLR-4 blocking antibody led to increased local pancreatic and systemic injury [37]. Interestingly, MD-2, an essential co-receptor of TLR-4, is proteolytically inactivated by trypsin abundantly released in acute pancreatitis, favoring the notion of attenuated pancreatitis in the TLR4-deficient mice.
Inflammation is regulated at multiple levels and under normal conditions monocytes also produce anti-inflammatory cytokines that can keep the initial pro-inflammatory activation under control [39]. Interleukin-10 (IL-10) produced by monocytes and T cells is an anti-inflammatory cytokine that also has immunosuppressive effects. We found that the anti-inflammatory and immunosuppressive potential of acute alcohol is partially linked to increased IL-10 induction in LPS-stimulated monocytes [5, 23, 31]. Conversely, IL-10 production was attenuated by acute alcohol in combination with TLR2 or TLR2 plus TLR4 stimulation [33]. While the mechanisms for this difference in the effects of alcohol on TLR2 and TLR4-induced IL-10 production are yet to be delineated, it is striking that the overall inflammatory cell regulation by alcohol appear to show a coordinated fashion. When acute alcohol inhibited LPS-induced NF-κB and TNFα production, IL-10 was concomitantly induced. In contrast, augmentation of TLR2 plus TLR4-induced TNFα and NF-κB activation by acute alcohol occurred in the presence of reduced monocyte IL-10 production. The role of IL-10 is yet to be explored in alcoholic pancreatitis; however, monocytes recruited to the local tissue damage site are likely to respond in a fashion similar to our in vitro observations.
In alcoholic liver disease, where alcoholic hepatitis is induced by chronic and usually excessive alcohol intake, increased serum levels of pro-inflammatory cytokines correlate with disease outcome [26,27,28,29]. While IL-8, IL-1, IL-6 and TNFα levels are all elevated in alcoholic hepatitis, increased serum TNFα is a predictor of poor survival [28]. Elevations in pro-inflammatory cytokines have also been found in chronic pancreatitis, Circulating levels of IL-6 and MCP-1 were increased by acute alcohol intake in chronic pancreatitis [40]. In in vitro experiments we demonstrated that prolonged alcohol exposure resulted in sensitization of monocytes to LPS-induced TNFα production. We also showed that monocytes exposed to prolonged alcohol treatment showed an increase in NF-κB activation suggesting that this is a likely mechanism involved in increased TNFα induction. Importantly, increased NF-κB activation was associated with pancreatitis induced by hormones (CCK and caerulein) or taurocholate [41, 42] and ethanol potentiated the cholecystokinine-induced NF-κB activation in pancreatic acinar cells [43]. Thus, it appears that NF-κB activation is a major indicator and mediator of cellular activation both in acinar cells and in monocytes and it is likely to play a regulatory role in alcoholic pancreatitis.
The clinical course of chronic alcoholic pancreatitis often evolves into pancreatic fibrosis due to pancreatic stellate cell activation which is similar to the outcome of chronic alcoholic hepatitis leading to liver fibrosis and cirrhosis. The mechanisms of stellate cell activation both in the pancreas and in the liver likely involve direct activation by alcohol and its metabolites and pro-inflammatory mediators. In addition to the pro-inflammatory mediators, transforming factor-β (TGFβ) is a major regulator of collagen production in stellate cells [21, 44]. We have previously reported that alcohol treatment of monocytes results in both induction and augmentation of TGFβ production suggesting an inflammatory cell-mediated mechanisms for stellate cell activation [44]. Thus, it is conceivable that monocyte-derived pro-inflammatory and pro-fibrinogenic cytokines contribute not only to the inflammation but also to fibrosis in chronic alcoholic pancreatitis.
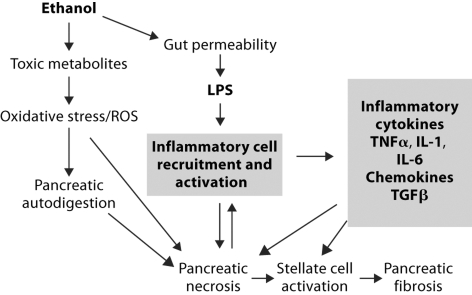
In summary, we propose that alcohol may amplify inflammatory cell activation which is a potential mechanism contributing to alcoholic pancreatitis. In addition to the components of alcohol-induced pancreatitis elegantly summarized by Apte et al. [17], we speculate that inflammatory cell activation can be induced by necrotic acinar cells and LPS from increased gut permeability after alcohol use (fig. 7). These stimuli in combination with acute or chronic alcohol intake can increase inflammatory responses. Inflammatory cell recruitment and activation can induce a vicious cycle in the presence of ongoing alcohol-induced insult to stimulate local production of pro-inflammatory cytokines that may worsen pancreatic necrosis. In addition, inflammatory cell-derived pro-inflammatory cytokines and TGFβ will result in stellate cell activation leading to fibrosis in the pancreas.
Fig. 7.
The role of inflammatory responses in alcohol-induced pancreatitis.
Acknowledgements
This work was supported by NIH grants AA011576 and AA00577. The discussion of the results represents the views of the authors and not the official view of the National Institute on Alcoholism and Alcohol Abuse. The authors are grateful to Donna Catalano, BS for the technical support, to Mrs. Bernadette White, RN for volunteer recruitment, and to Ms. Gail Bird for clerical support.
References
- 1.Cook RT. Alcohol abuse, alcoholism and damage to the immune system: a review. Alcohol Clin Exp Res. 1998;22:1927–1942. [PubMed] [Google Scholar]
- 2.Naveau S, Raynard B, Ratziu V, Abella A, Imbert-Bismut F, Messous D, Beuzen F, Capron F, Thabut D, Munteanu M, Chaput JC, Poynard T. Biomarkers for the prediction of liver fibrosis in patients with chronic alcoholic liver disease. Clin Gastroenterol Hepatol. 2005;3:167–174. doi: 10.1016/s1542-3565(04)00625-1. [DOI] [PubMed] [Google Scholar]
- 3.Jaakola M, Sillanaukee P, Lof K, Koivula T, Nordack I. Amount of alcohol is an important determinant of the severity of acute alcoholic pancreatitis. Surgery. 1994;115:31–38. [PubMed] [Google Scholar]
- 4.Szabo G, Mandrekar P, Dolganiuc A, Catalano D, Kodys K. Reduced alloreactive T-cell activation after alcohol intake is due to impaired monocyte accessory cell function and correlates with elevated IL-10, IL-13, and decreased IFNγ levels. Alcohol Clin Exp Res. 2001;25:1766–1772. [PubMed] [Google Scholar]
- 5.Szabo G, Catalano D, White B, Mandrekar P. Acute alcohol consumption inhibits accessory cell function of monocytes and dendritic cells. Alcohol Clin Exp Res. 2004;28:824–828. doi: 10.1097/01.alc.0000127104.80398.9b. [DOI] [PubMed] [Google Scholar]
- 6.Mandrekar P, Catalano D, Dolganiuc A, Kodys K, Szabo G. Inhibition of myeloid dendritic cell accessory cell function by alcohol correlates with reduced CD80/CD86 expression and decreased IL-12 production. J Immunol. 2004;173:3398–3407. doi: 10.4049/jimmunol.173.5.3398. [DOI] [PubMed] [Google Scholar]
- 7.Takeda K, Akira S. TLRs in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto M, Akira S. TIR domain-containing adaptors regulate TLR signaling pathways. Adv Exp Med Biol. 2005;560:1–9. doi: 10.1007/0-387-24180-9_1. [DOI] [PubMed] [Google Scholar]
- 9.Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001;31:3388–3393. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 10.Wagner H, Bauer S. All is not Toll: new pathways in DNA recognition. J Exp Med. 2006;203:265–268. doi: 10.1084/jem.20052191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- 12.Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the Toll-like receptor-4 complex. J Immunol. 2000;164:558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 13.Dufour MC, Adamson MD. The epidemiology of alcohol-induced pancreatitis. Pancreas. 2003;27:286–290. doi: 10.1097/00006676-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Apte MV, Wilson JS. Alcohol-induced pancreatic injury. Best Pract Res Clin Gastroenterol. 2003;17:593–612. doi: 10.1016/s1521-6918(03)00050-7. [DOI] [PubMed] [Google Scholar]
- 15.Schneider A, Singer MV. Alcoholic pancreatitis. Dig Dis. 2005;23:222–231. doi: 10.1159/000090169. [DOI] [PubMed] [Google Scholar]
- 16.Pandol SJ, Gukovsky I, Satoh A, Lugea A, Gukovskaya AS. Emerging concepts for the mechanism of alcoholic pancreatitis from experimental models. J Gastroenterol. 2003;38:623–628. doi: 10.1007/s00535-003-1134-7. [DOI] [PubMed] [Google Scholar]
- 17.Apte MV, Pirola RC, Wilson JS. Molecular mechanisms of alcoholic pancreatitis. Dig Dis. 2005;23:232–240. doi: 10.1159/000090170. [DOI] [PubMed] [Google Scholar]
- 18.Somogyi L, Martin S, Venkatesan T, Ulrich C. Recurrent pancreatitis: an algorithmic approach to identification and elimination of initiating factors. Gastroenterology. 2001;120:944–952. doi: 10.1053/gast.2001.22333. [DOI] [PubMed] [Google Scholar]
- 19.McKim SE, Uesugi T, Raleigh JA, McClain CJ, Arteel GA. Chronic intragastic alcohol exposure causes hypoxia and oxidative stress in the rat pancreas. Arch Biochem Biophys. 2003;417:34–43. doi: 10.1016/s0003-9861(03)00349-7. [DOI] [PubMed] [Google Scholar]
- 20.Weber CK, Adler G. From acinar cell damage to systemic inflammatory response: current concepts in pancreatitis. Pancreatology. 2001;1:356–362. doi: 10.1159/000055834. [DOI] [PubMed] [Google Scholar]
- 21.Siegmund SV, Brenner DA. Molecular pathogenesis of alcohol-induced hepatic fibrosis. Alcohol Clin Exp Res. 2005;29:102S–109S. doi: 10.1097/01.alc.0000189275.97419.58. [DOI] [PubMed] [Google Scholar]
- 22.Verma BK, Fogarsi M, Szabo G. Down-regulation of TNF activity by acute ethanol treatment in human peripheral blood monocytes. J Clin Immunol. 1993;13:8–23. doi: 10.1007/BF00920631. [DOI] [PubMed] [Google Scholar]
- 23.Mandrekar P, Catalano D, Szabo G. Human monocyte IL-10 production is increased by acute ethanol treatment. Cytokines. 1996;8:567–577. doi: 10.1006/cyto.1996.0076. [DOI] [PubMed] [Google Scholar]
- 24.Szabo G, Chavan S, Mandrekar P, Catalano D. Acute alcohol consumption attenuates IL-8 and MCP-1 induction in response to ex vivo stimulation. J Clin Immunol. 1999;19:67–76. doi: 10.1023/a:1020518703050. [DOI] [PubMed] [Google Scholar]
- 25.Szabo G, Mandrekar P, Catalano D. Inhibition of superantigen-induced T cell proliferation and monocyte IL-1β, TNF-α, and IL-6 production by acute ethanol treatment. J Leukoc Biol. 1995;58:342–350. doi: 10.1002/jlb.58.3.342. [DOI] [PubMed] [Google Scholar]
- 26.McClain CJ, Cohen DA. Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. Hepatology. 1989;9:349–351. doi: 10.1002/hep.1840090302. [DOI] [PubMed] [Google Scholar]
- 27.McClain CJ, Hill DB, Song Z, Deaciuc I, Barve S. Monocyte activation in alcoholic liver disease. Alcohol. 2002;27:53–61. doi: 10.1016/s0741-8329(02)00212-4. [DOI] [PubMed] [Google Scholar]
- 28.Khoruts A, Stahnke L, McClain CJ, Logan G, Allen JI. Circulating tumor necrosis factor, interleukin-1 and interlukin-6 concentrations in chronic alcoholic patients. Hepatology. 1991;13:267–276. [PubMed] [Google Scholar]
- 29.Hill DLB, Marsano LS, McClain CJ. Increased plasma interleukin-8 concentrations in alcoholic hepatitis. Hepatology. 1993;18:576–580. [PubMed] [Google Scholar]
- 30.Pastor CM, Pugin J, Kwak B, Chanson M, Mach F, Hadengue A, Frossard JL. Role of Toll-like receptor 4 on pancreatic and pulmonary injury in a mice model of acute pancreatitis associated with endotoxemia. Crit Care Med. 2004;32:1759–1763. doi: 10.1097/01.ccm.0000133020.47243.8e. [DOI] [PubMed] [Google Scholar]
- 31.Mandrekar P, Catalano D, White B, Szabo G. Moderate alcohol intake in humans attenuates monocyte inflammatory responses: inhibition of NF-3B and induction of IL-10. Alcohol Clin Exp Res. 2006;30:135–139. doi: 10.1111/j.1530-0277.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- 32.Enomoto N, Schemmer P, Ikejima K, Takei Y, Sato N, Brenner DA, Thurman RG. Long-term alcohol exposure changes sensitivity of rat Kupffer cells to lipopolysaccharide. Alcohol Clin Exp Res. 2001;25:1360–1367. [PubMed] [Google Scholar]
- 33.Oak S, Mandrekar P, Szabo G. TLR2 and TLR4-mediated signals determine attenuation or augmentation of inflammation by acute alcohol in monocytes. J Immunol. 2006;176:7628–7635. doi: 10.4049/jimmunol.176.12.7628. [DOI] [PubMed] [Google Scholar]
- 34.Fortunato F, Deng X, Gates LK, McClain CJ, Bimmler D, Graf R, Whitcomb DC. Pancreatic response to endotoxin after chronic alcohol exposure: switch from apoptosis to necrosis? Am J Physiol. 2006;290:232–241. doi: 10.1152/ajpgi.00040.2005. [DOI] [PubMed] [Google Scholar]
- 35.Bode C, Bode JC. Effect of alcohol consumption on the gut. Best Pract Res Clin Gastroenterol. 2003;17:575–592. doi: 10.1016/s1521-6918(03)00034-9. [DOI] [PubMed] [Google Scholar]
- 36.Thurman RG. II Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275:G605–G611. doi: 10.1152/ajpgi.1998.275.4.G605. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Wu L, Wu K, Zhang R, Dong Y. Roles of endotoxin-related signaling molecules in the progression of acute necrotizing pancreatitis in mice. Pancreas. 2005;31:251–257. doi: 10.1097/01.mpa.0000175179.62916.17. [DOI] [PubMed] [Google Scholar]
- 38.Cario E, Golenbock DT, Visintin A, Runzi M, Gerken G, Podolsky DK. Trypsin-sensitive modulation of intestinal epithelial MD-2 as mechanism of lipopolysaccharide tolerance. J Immunol. 2006;1(176):4258–4266. doi: 10.4049/jimmunol.176.7.4258. [DOI] [PubMed] [Google Scholar]
- 39.Wang P, Wu P, Siegel MI, Egan RW, Billah MM. IL-10 inhibits transcription of cytokine genes in human peripheral blood mononuclear cells. J Immunol. 1994;153:811–816. [PubMed] [Google Scholar]
- 40.Pedersen N, Larsen S, Seidelin JB, Nielsen OH. Alcohol modulates circulating levels of Interleukin-6 and monocyte chemoattractant protein-1 in chronic pancreatitis. Scand J Gastroenterol. 2004;3:277–282. doi: 10.1080/00365520310008296. [DOI] [PubMed] [Google Scholar]
- 41.Gukovsky I, Gukovskaya AS, Blinman TA, Zaninovic V, Pandol SJ. Early NF-kappaB activation is associated with hormone-induced pancreatitis. Am J Physiol. 1998;275:G1402–G1414. doi: 10.1152/ajpgi.1998.275.6.G1402. [DOI] [PubMed] [Google Scholar]
- 42.Vacquero E, Gukovsky I, Zaninovic V, Gukovskaya AS, Pandol SJ. Localized pancreatic NF-kappaB activation and inflammatory response in taurocholate-induced pancreatitis. Am J Physiol. 2001;280:G1197–G1208. doi: 10.1152/ajpgi.2001.280.6.G1197. [DOI] [PubMed] [Google Scholar]
- 43.Hosseini S, Gukovskaya A, Cheng J, Gukosky I, Pandol S. Ethanol potentiates cholecystokinin-induced but inhibits thapsigargin-induced NFκB activation in pancreatic acinar cells (abstract) Gastroenterology. 2002;122:A-282. [Google Scholar]
- 44.Deng X, Wang L, Elm MS, Gabazadeh D, Diorio GJ, Eagon PK, Whitcomb DC. Chronic alcohol consumption accelerates fibrosis in response to caerulein-induced pancreatitis in rats. Am J Pathol. 2005;166:93–106. doi: 10.1016/S0002-9440(10)62235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szabo G, Verma BK, Fogarasi M, Catalano D. Induction of transforming growth factor-beta and prostaglandin E2 production by ethanol in human monocytes. J Leuk Biol. 1992;52:602–610. doi: 10.1002/jlb.52.6.602. [DOI] [PubMed] [Google Scholar]