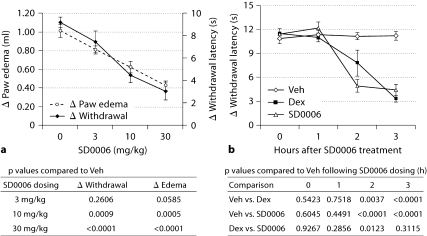
Fig. 6.
In the carrageenan rat paw model, SD0006 reduces pain and swelling prophylactically (a) and therapeutically reduces pain with efficacy equal to steroids (b). a, b The data represent the means ± SEM based on 5 rats per group. Procedures and quantification of responses were as described in Materials and Methods. Veh = Vehicle; Dex = dexamethasone. a Hyperalgesia (pain; determined by change in paw withdrawal latency time) and edema (paw swelling; determined by change in paw volume) doseresponse to pretreatment dosed 2 h before carrageenan injection and measured 3 h after for response. One-way analysis of variance (ANOVA) on the hyperalgesia and edema data from the SD0006 dose-response study was performed separately and then each dose group was compared to the vehicle control using Dunnett‘s t test, and the p values from the pairwise comparisons are shown. The 10 mg/kg and 30 mg/kg SD0006 dosings were statistically highly significant compared to controls for both readouts at the 5% significance level. b Hyperalgesia therapeutic response compared to steroid. Vehicle (0 mg/kg), SD0006 (30 mg/kg), or dexamethasone (3 mg/kg) were administered orally 3 h after carrageenan injection, and pain was measured 1, 2, and 3 h afterwards. A repeated analysis of variance model was utilized on the longitudinal data set of the change in withdrawal latency times considering that measurements from the same animal are correlated. Based on results of the repeated analysis of variance, at 2 and 3 h posttreatment both dexamethasone and SD0006 were strongly significantly different from vehicle control at the 5% test level. Also, at the 2-hour time point, SD0006 was significantly different from dexamethasone. p values of the comparisons at the posttreatment hours are shown.