Abstract
Background and Objectives
Leprosy involves both the skin and peripheral nervous system. Leprosy patients display an increased incidence of xerosis and altered sensory thresholds, which persist in previously active skin sites. We assessed here whether alterations in stratum corneum (SC) function persist in cured leprosy, and the relationship of epidermal functional abnormalities to each clinical subtype of leprosy.
Methods
A total of 43 cured leprosy subjects and 29 normal control subjects were enrolled in this study. Basal skin surface pH, SC hydration, permeability barrier function as well as barrier recovery rates were measured over previously involved skin sites with a skin physiology monitor. One-way ANOVA and two-tailed Student's t test were used to determine the significance between 2 groups and 3 or more groups, respectively.
Results
Competent barrier function was observed in all subtypes of cured leprosy subjects. All cured leprosy subjects except those with the borderline tuberculoid type exhibited a significantly lower SC hydration in comparison with normal subjects. Skin surface pH was significantly elevated in all cured leprosy subjects in comparison with normal subjects.
Conclusions
A varied spectrum of alterations in SC function remains in all subjects who have recovered from leprosy, but the spectrum of SC functional abnormalities varies with disease subtype.
Key Words: Barrier function, Leprosy, pH, Stratum corneum hydration
Introduction
Key stratum corneum (SC) functions include maintenance of permeability barrier homeostasis, SC hydration and SC integrity (the converse of desquamation), which all play important roles in cutaneous function. Studies have demonstrated that permeability barrier function regulates (1) epidermal proliferation, (2) epidermal lipid synthesis and epidermal lipid processing, (3) primary cytokine release, the initial stage of cutaneous inflammation, (4) epidermal antimicrobial peptide expression, (5) and epidermal terminal differentiation (cornification), signaled in part by changes in the epidermal calcium gradient [1,2,3,4,5,6,7,8,9,10]. Moreover, SC hydration has been shown to regulate epidermal proliferation, differentiation, inflammation, melanin content, as well as serving as a ‘driver’ of epidermal permeability barrier function [11,12,13,14,15,16]. The acidic pH of SC serves as a type of functional superregulator, influencing epidermal permeability barrier homeostasis, desquamation, skin infections, initiation of inflammation in the skin, antimicrobial defense production and primary cytokine release [17,18,19,20,21,22]. SC functions are also regulated by a variety of local and systemic signaling mechanisms. For example, the epidermal calcium gradient and cytokines are critical regulators of epidermal lamellar body secretion, lipid synthesis and DNA synthesis [23,24,25,26,27]. Not only changes in barrier function, but also a number of environmental stressors, such as increased psychological stress, can alter epidermal structure and function [28,29,30].
Leprosy is caused by Mycobacterium leprae, a bacterium that attacks both the skin and the peripheral nervous system. Both residual hyperpigmentation or hypopigmentation and reduction or loss of cutaneous sensory function (anesthetic lesions) can remain in treated leprosy patients [31,32,33,34]. Moreover, xerosis is a prominent feature of treated leprosy, a feature that can become ichthyosiform in some subjects [35,36,37]. A reduction in epidermal proliferative activity, as measured by thymidine incorporation, has been observed in lepromatous leprosy, and a decreased SC sphingolipid content has been found in leprosy lesions, which further points to potential changes in epidermal function in leprosy [38, 39]. However, epidermal function has not yet been assessed in either active or microbially cured leprosy lesions.
Clinical cure in leprosy means only that subjects are free of the causative pathogen. Yet, in most subjects who have recovered from leprosy, some structural and functional alterations, such as deformities, localized anesthesia and dyspigmentation remain, and even worsen [40,41,42,43]. Even bone lesions have been reported to progress for years after termination of treatment, and lepromatous granulomas can persist in cured leprosy subjects [44, 45]. Clinically, the skin tends to be drier in cured leprosy than in normal subjects. Moreover, cured leprosy subjects may have persistent ulcers [46, 47]. In addition, leprosy patients generally experience increased psychological stress [48], which in turn could compromise epidermal function [29], especially in regions where subjects are restricted to remote rural areas and deprived from social activities. All these problems suggest that persistent alterations in SC function could occur in microbially cured leprosy subjects, which would predispose these patients to ongoing and diverse clinical complications. Accordingly, we measured epidermal functions, including basal transepidermal water loss (TEWL), barrier recovery rates, SC hydration and skin surface pH in cured leprosy patients. We identified multiple alterations in SC function in cured leprosy subjects, which could both complicate rehabilitation.
Subjects and Methods
Experimental Subjects
A total of 29 normal control subjects, aged 66–88 years (10 males, 19 females), and 43 subjects, whose forearms had previously been involved in leprosy and who had recovered from leprosy, aged 49–86 years (31 males, 12 females), were enrolled in this study (table 1). The leprosy cases included multiple lepromatous (LL), borderline lepromatous (BL), borderline (BB), borderline tuberculoid (BT) and tuberculoid (TT) types (table 1), which were determined by both histology and clinical symptoms when patients were first admitted to the leprosy control center located in a remote rural area. Control subjects had no skin disorders nor systemic diseases such as diabetes and renal disease, which could influence epidermal function. In addition, skin care products were not applied to measurement sites for at least 24 h, and no soap wash, which could affect SC pH, hydration and barrier function, for at least 4 h prior to skin measurements. All human research procedures were conducted according to a protocol approved by the local human ethnical research committee (Dalian Skin Disease Hospital, Liaoning, PR China).
Table 1.
Characteristics of the study subjects
| Normal | LL | BL | BB | BT | TT | |
|---|---|---|---|---|---|---|
| Males | 10 | 5 | 10 | 10 | 9 | 7 |
| Females | 19 | 8 | 0 | 0 | 2 | 2 |
| Mean age, years | 76.14±1.2 | 66.74±1.69 | 67.3±2.13 | 65.2±2.38 | 66.58±1.47 | 70.8±2.27 |
Functional Studies
Skin surface pH, TEWL, and SC hydration were measured on the forearm (flexor side, about 10 cm above wrist) with a Courage-Khazaka MPA5 (CK Electronic GmbH, Germany), as described previously [48, 49]. In addition to baseline readings, TEWL was measured at 0 and 3 h after acute barrier disruption by repeated tape-stripping to assess barrier recovery. Before all measurements were taken, subjects rested for 30 min in a temperature-controlled room (22–24°C), with a relative humidity of 45–55%. These studies were performed in early December.
Statistics
A two-tailed Student's t test was used to determine the significance between 2 groups, and a one-way ANOVA was used to determine the significance when 3 or more groups were compared. Data are expressed as means ± SEM.
Results
Competent or Supernormal Barrier Function in Subjects Who Had Recovered from Leprosy
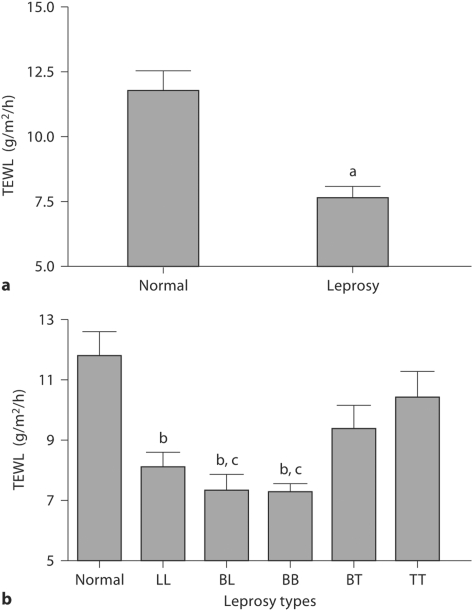
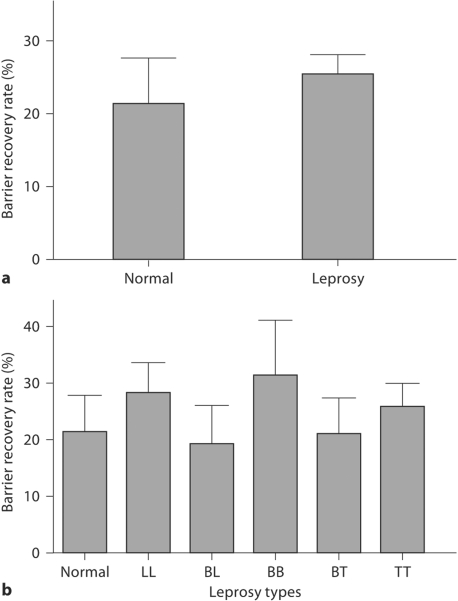
To determine whether epidermal permeability barrier function is abnormal in cured leprosy, we first measured basal TEWL. Basal TEWL was significantly lower in the cured leprosy group as a whole than in normal controls (fig. 1a; p < 0.0001), but basal barrier function did not differ significantly from normal controls in BT and TT cases (fig. 1b). However, the TEWL in BL, BB and LL types was significantly lower than in normal controls (fig. 1b; p < 0.01), and basal TEWL was also significantly lower in BL and BB types than in TT type subjects (fig. 1b; p < 0.05). These results show that basal permeability barrier function varies according to the prior type of leprosy. Although cured leprosy subjects showed competent or superior basal permeability barrier function, we next determined whether barrier recovery kinetics differed among cured leprosy and normal subjects. In contrast to basal barrier function, there were no significant differences in barrier recovery kinetics among cured leprosy and normal subjects (fig. 2).
Fig. 1.
Enhanced permeability barrier in cured leprosy subjects. a p < 0.0001, b p < 0.01 versus normal; c p < 0.05 versus TT type. a TEWL in normal and cured leprosy subjects (numbers of subjects are listed in table 1). b TEWL in subjects with various types of leprosy.
Fig. 2.
No difference in barrier recovery rate between cured leprosy and normal subjects. Barrier disruption was achieved by repeated tape stripping, and the TEWL was measured immediately after tape stripping and 3 h after barrier disruption. a Barrier recovery rate in normal and cured leprosy subjects. b Barrier recovery rate in subjects with various types of leprosy
Reduced SC Hydration in Subjects Who Had Recovered from Leprosy
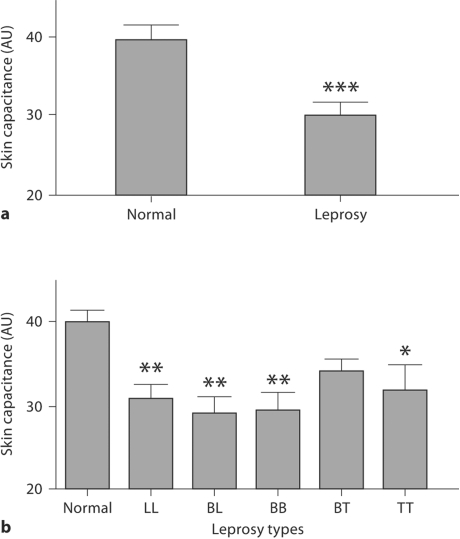
Clinical observations suggest that the skin of cured leprosy subjects is drier than normal. Hence, we next measured skin capacitance, an indicator of SC hydration. In contrast to basal permeability barrier function, which was supernormal in some cured leprosy subjects, SC hydration was significantly subnormal in cured leprosy as a whole compared to normal subjects (fig. 3a; p < 0.0001). Moreover, BL, BB and LL types, which showed the best basal barrier function among the 5 subtypes, displayed the lowest SC hydration (fig. 3b; p < 0.01 vs. normal), but SC hydration did not differ significantly among the 5 leprosy subtypes. Finally, SC hydration in BT type leprosy did not differ significantly from normal subjects (fig. 3b). These results suggest that SC hydration is impaired in most cured leprosy subjects.
Fig. 3.
Decreased SC capacitance (arbitrary units, AU) in cured leprosy subjects. ∗ p < 0.05, ∗∗ p < 0.01, ∗∗∗ p < 0.0001 versus normal subjects. a SC capacitance in normal and cured leprosy subjects. b SC capacitance in subjects with various types of leprosy
Increased Skin Surface pH in Subjects Who Had Recovered from Leprosy
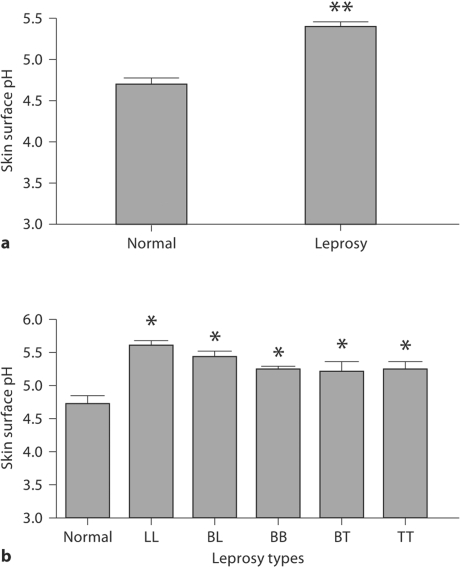
We next assessed skin surface pH in cured leprosy subjects. Skin surface pH was significantly elevated in the cured leprosy group as a whole (fig. 4a) and in all subtypes of leprosy (fig. 4b; p < 0.01). Yet, in contrast to permeability barrier function and SC hydration, there were no significant differences in skin surface pH among the 5 subtypes of cured leprosy.
Fig. 4.
Increased skin surface pH in cured leprosy subjects. ∗ p < 0.01, ∗ ∗ p < 0.0001 versus normal subjects. a Skin surface pH in normal and cured leprosy subjects. b Skin surface pH in subjects with various types of leprosy.
Discussion
The importance of epidermal protective functions is well appreciated [49], and measurements of SC function can be an indicator of the clinical status of skin disease [50], but little is known about SC function in leprosy subjects. Here, we examined epidermal permeability barrier function, skin surface pH and SC hydration in 5 different subtypes of microbially cured leprosy subjects. Our results demonstrated that the SC hydration was significantly lower in cured leprosy subjects although their barrier function was competent. Interestingly, BT and TT cases showed better SC hydration in comparison with LL, BL and BB subjects.
Regarding the mechanism by which SC hydration was reduced, defective sweat gland function is likely to be a key contributor. It has been demonstrated that the sweat gland response to stimulation was altered in active leprosy lesions [51,52,53,54]. In addition, a reduced content of water-soluble, osmotically active components has been found in the SC of active leprosy patients [55]. But whether these alterations persist in microbially cured leprosy subjects is not known. Furthermore, SC lipids, especially sphingolipids, are important regulators for SC hydration [56]. It has been shown that the SC sphingolipid content declines in leprosy patients [39]. Decreased SC hydration could have a significant impact on cutaneous function. It has been demonstrated that lower SC hydration alone can increase epidermal DNA synthesis and amplify epidermal proliferation induced by repeated barrier abrogation [11, 57]. Conversely, topical moisturizers reduce epidermal proliferation and decrease epidermal thickness in both photodamaged skin and psoriasis [12, 58]. Moreover, decreased SC hydration could increase cytokine release [59, 60], which in turn could exacerbate cutaneous inflammation, thereby complicating rehabilitation in leprosy. Thus, increased epidermal proliferative activity and epidermal thickness in leprosy might result from the decreased SC hydration. Hence, improving SC hydration could be a valuable treatment option for patients with microbially cured leprosy.
The present study also showed higher skin surface pH in cured leprosy in comparison with normal subjects. In contrast to SC hydration, skin surface pH did not vary between the leprosy subtypes. However, the basis for the elevation of skin surface pH is unknown. Whether there are alterations in epidermal Na+/H+ exchanger isoform 1 and/or secretory phospholipase A, which both have been shown to regulate SC pH [61,62,63], is not clear. Regardless of causes, if pH increases sufficiently, this change could have dramatic adverse effects on cutaneous structure and function, such as epidermal permeability barrier homeostasis, SC integrity as well as keratinocyte differentiation [10,17,18,19,20,21, 64]. Maintenance of SC pH at optimal levels could be a valuable approach to improve cutaneous function in cured leprosy patients.
In summary, our results demonstrate that there are alterations in SC function in cured leprosy subjects. Treatment strategies that improve SC function should be considered in cured leprosy subjects.
Acknowledgements
This work was supported by National Institutes of Health grants AR 050629, AR 19098 and PO 39448 and the Medical Research Service, Department of Veterans Affairs Medical Center.
References
- 1.Proksch E, Feingold KR, Man MQ, Elias PM. Barrier function regulates epidermal DNA synthesis. J Clin Invest. 1991;87:1668–1673. doi: 10.1172/JCI115183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denda M, Wood LC, Emami S, et al. The epidermal hyperplasia associated with repeated barrier disruption by acetone treatment or tape stripping cannot be attributed to increased water loss. Arch Dermatol Res. 1996;288:230–238. doi: 10.1007/BF02530090. [DOI] [PubMed] [Google Scholar]
- 3.Proksch E, Elias PM, Feingold KR. Regulation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase activity in murine epidermis: modulation of enzyme content and activation state by barrier requirements. J Clin Invest. 1990;85:874–882. doi: 10.1172/JCI114514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holleran WM, Takagi Y, Menon GK, et al. Permeability barrier requirements regulate epidermal beta-glucocerebrosidase. J Lipid Res. 1994;35:905–912. [PubMed] [Google Scholar]
- 5.Wood LC, Jackson SM, Elias PM, et al. Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice. J Clin Invest. 1992;90:482–487. doi: 10.1172/JCI115884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai JC, Feingold KR, Crumrine D, et al. Permeability barrier disruption alters the localization and expression of TNF alpha/protein in the epidermis. Arch Dermatol Res. 1994;286:242–248. doi: 10.1007/BF00387595. [DOI] [PubMed] [Google Scholar]
- 7.Wood LC, Elias PM, Sequeira-Martin SM, et al. Occlusion lowers cytokine mRNA levels in essential fatty acid-deficient and normal mouse epidermis, but not after acute barrier disruption. J Invest Dermatol. 1994;103:834–838. doi: 10.1111/1523-1747.ep12413597. [DOI] [PubMed] [Google Scholar]
- 8.Wood LC, Elias PM, Calhoun C, et al. Barrier disruption stimulates interleukin-1 alpha expression and release from a pre-formed pool in murine epidermis. J Invest Dermatol. 1996;106:397–403. doi: 10.1111/1523-1747.ep12343392. [DOI] [PubMed] [Google Scholar]
- 9.Aberg KM, Man MQ, Gallo RL, et al. Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J Invest Dermatol. 2008;128:917–925. doi: 10.1038/sj.jid.5701099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demerjian M, Hachem JP, Tschachler E, et al. Acute modulations in permeability barrier function regulate epidermal cornification: role of caspase-14 and the protease-activated receptor type 2. Am J Pathol. 2008;172:86–97. doi: 10.2353/ajpath.2008.070161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denda M, Sato J, Tsuchiya T, et al. Low humidity stimulates epidermal DNA synthesis and amplifies the hyperproliferative responses to barrier disruption: implication for seasonal exacerbations of inflammatory dermatoses. J Invest Dermatol. 1998;111:873–878. doi: 10.1046/j.1523-1747.1998.00364.x. [DOI] [PubMed] [Google Scholar]
- 12.Short RW, Chan JL, Choi JM, et al. Effects of moisturization on epidermal homeostasis and differentiation. Clin Exp Dermatol. 2007;32:88–90. doi: 10.1111/j.1365-2230.2006.02297.x. [DOI] [PubMed] [Google Scholar]
- 13.Katagiri C, Sato J, Nomura J, et al. Changes in environmental humidity affect the water-holding property of the stratum corneum and its free amino acid content, and the expression of filaggrin in the epidermis of hairless mice. J Dermatol Sci. 2003;31:29–35. doi: 10.1016/s0923-1811(02)00137-8. [DOI] [PubMed] [Google Scholar]
- 14.Ashida Y, Denda M. Dry environment increases mast cell number and histamine content in dermis in hairless mice. Br J Dermatol. 2003;149:240–247. doi: 10.1046/j.1365-2133.2003.05408.x. [DOI] [PubMed] [Google Scholar]
- 15.De Paepe K, Hachem JP, Vanpee E, et al. beneficial effects of a skin tolerance-tested moisturizing cream on the barrier function in experimentally elicited irritant and allergic contact dermatitis. Contact Dermatitis. 2001;44:337–343. doi: 10.1034/j.1600-0536.2001.044006337.x. [DOI] [PubMed] [Google Scholar]
- 16.Sato J, Denda M, Chang S, et al. Abrupt decreases in environmental humidity induce abnormalities in permeability barrier homeostasis. J Invest Dermatol. 2002;119:900–904. doi: 10.1046/j.1523-1747.2002.00589.x. [DOI] [PubMed] [Google Scholar]
- 17.Hachem JP, Man MQ, Crumrine D, et al. Sustained serine proteases activity by prolonged increase in pH leads to degradation of lipid processing enzymes and profound alterations of barrier function and stratum corneum integrity. J Invest Dermatol. 2005;125:510–520. doi: 10.1111/j.0022-202X.2005.23838.x. [DOI] [PubMed] [Google Scholar]
- 18.Hachem JP, Crumrine D, Fluhr J, et al. pH directly regulates epidermal permeability barrier homeostasis, and stratum corneum integrity/cohesion. J Invest Dermatol. 2003;121:345–353. doi: 10.1046/j.1523-1747.2003.12365.x. [DOI] [PubMed] [Google Scholar]
- 19.Mauro T, Holleran WM, Grayson S, et al. Barrier recovery is impeded at neutral pH, independent of ionic effects: implications for extracellular lipid processing. Arch Dermatol Res. 1998;290:215–222. doi: 10.1007/s004030050293. [DOI] [PubMed] [Google Scholar]
- 20.Runeman B, Faergemann J, Larko O. Experimental Candida albicans lesions in healthy humans: dependence on skin pH. Acta Derm Venereol. 2000;80:421–424. doi: 10.1080/000155500300012819. [DOI] [PubMed] [Google Scholar]
- 21.Selander C, Zargari A, Mollby R, et al. Higher pH level, corresponding to that on skin of patients with atopic eczema, stimulates the release of Malasseziasympodialis allergens. Allergy. 2006;61:1002–1008. doi: 10.1111/j.1398-9995.2006.01108.x. [DOI] [PubMed] [Google Scholar]
- 22.Elias PM. The skin barrier as an innate immune element. Semin Immunopathol. 2007;29:3–14. doi: 10.1007/s00281-007-0060-9. [DOI] [PubMed] [Google Scholar]
- 23.Bikle DD, Chang S, Crumrine D, et al. 25-Hydroxyvitamin D1 alpha-hydroxylase is required for optimal epidermal differentiation and permeability barrier homeostasis. J Invest Dermatol. 2004;122:984–992. doi: 10.1111/j.0022-202X.2004.22424.x. [DOI] [PubMed] [Google Scholar]
- 24.Lee SH, Elias PM, Proksch E, et al. Calcium and potassium are important regulators of barrier homeostasis in murine epidermis. J Clin Invest. 1992;89:530–538. doi: 10.1172/JCI115617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schultz K, Murthy V, Tatro JB, Beasley D. Endogenous interleukin-1 alpha promotes a proliferative and proinflammatory phenotype in human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2007;292:H2927–H2934. doi: 10.1152/ajpheart.00700.2006. [DOI] [PubMed] [Google Scholar]
- 26.Fang Y, Gong SJ, Xu YH, et al. Impaired cutaneous wound healing in granulocyte/macrophage colony-stimulating factor knockout mice. Br J Dermatol. 2007;157:458–465. doi: 10.1111/j.1365-2133.2007.07979.x. [DOI] [PubMed] [Google Scholar]
- 27.Detmar M, Orfanos CE. Tumor necrosis factor-alpha inhibits cell proliferation and induces class II antigens and cell adhesion molecules in cultured normal human keratinocytes in vitro. Arch Dermatol Res. 1990;282:238–245. doi: 10.1007/BF00371643. [DOI] [PubMed] [Google Scholar]
- 28.Aberg KM, Radek AR, Choi EH, et al. Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. J Clin Invest. 2007;117:3339–3349. doi: 10.1172/JCI31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garg A, Chren MM, Sands PL, et al. Psychological stress perturbs epidermal permeability barrier homeostasis: implications for the pathogenesis of stress-associated skin disorders. Arch Dermatol. 2001;137:53–59. doi: 10.1001/archderm.137.1.53. [DOI] [PubMed] [Google Scholar]
- 30.Denda M, Tsychiya T, Elis PM, Feingold KR. Stress alters cutaneous permeability barrier homeostasis. Am J Physiol Regul Integr Comp Physiol. 2000;278:R367–R372. doi: 10.1152/ajpregu.2000.278.2.R367. [DOI] [PubMed] [Google Scholar]
- 31.Daniel E, Premkumar R, Koshy S, et al. Hypopigmented face patches: their distribution and relevance to ocular complications in leprosy. Int J Lepr Other Mycobact Dis. 1999;67:388–391. [PubMed] [Google Scholar]
- 32.Grover S, Singh G, Dash K. Primary hyperpigmented palmar lesion: a rare presentation of borderline tuberculoid leprosy. Indian J Lepr. 1997;69:191–193. [PubMed] [Google Scholar]
- 33.Shereef PH, Thomas M. Hypopigmented macules in leprosy – a histopathological and histochemical study of melanocytes. Indian J Lepr. 1992;64:189–191. [PubMed] [Google Scholar]
- 34.Facer P, Mathur R, Pandya SS, et al. Correlation of quantitative tests of nerve and target organ dysfunction with skin immunohistology in leprosy. Brain. 1998;121:2239–2247. doi: 10.1093/brain/121.12.2239. [DOI] [PubMed] [Google Scholar]
- 35.Ramu G, Iyer GG. Side effects of clofazimine therapy. Lepr India. 1976;48(suppl):722–731. [PubMed] [Google Scholar]
- 36.Okhandiar RP, Sinha E, Sinha RK, Mishra AD. Morphometric study of stratum corneum in leprosy. Indian J Lepr. 1989;61:49–53. [PubMed] [Google Scholar]
- 37.Patki AH, Jadhac Vh, Mehta JM. A study of dermatological conditions in leprosy in-patients. Indian J Lepr. 1989;61:92–95. [PubMed] [Google Scholar]
- 38.Palermo MH, Vugman I, Fleury RN, Zucoloto S. Reduction of epidermal cell proliferation in skin lesions in lepromatous leprosy is greater than in indeterminate and tuberculoid leprosy lesions. Int J Lepr Other Mycobact Dis. 1996;64:37–43. [PubMed] [Google Scholar]
- 39.Lee SJ, Kim DW, Jun JB, et al. Lipid composition of the stratum corneum of the sole in patients with leprosy. Int J Lepr Other Mycobact Dis. 1994;62:574–579. [PubMed] [Google Scholar]
- 40.Banerjee S. Reconstruction of facial deformities in leprosy patients. J Indian Med Assoc. 2004;102:700–701. [PubMed] [Google Scholar]
- 41.MacMoran JW, Brand PW. Bone loss in limbs with decreased or absent sensation: ten year follow-up of the hands in Hansen's disease. Skeletal Radiol. 1987;16:452–459. doi: 10.1007/BF00350539. [DOI] [PubMed] [Google Scholar]
- 42.Enna CD. Skeletal deformities of a denervated hand in Hansen's disease. J Hand Surg. 1979;4:227–233. doi: 10.1016/s0363-5023(79)80157-4. [DOI] [PubMed] [Google Scholar]
- 43.Agarwal US, Handa AK, Mathur D, et al. Hypopigmented lesions in early leprosy – a clinical and histological study. Indian J Lepr. 1990;62:416–421. [PubMed] [Google Scholar]
- 44.Carpintero P, Logrono C, Carrascal A, et al. Progression of bone lesions in cured leprosy patients. Acta Leprol. 1998;11:21–24. [PubMed] [Google Scholar]
- 45.Desikan P, Desikan KV. Persistence of lepromatous granuloma in clinically cured cases of leprosy. Int J Lepr Other Mycobact Dis. 1995;63:417–421. [PubMed] [Google Scholar]
- 46.Ganapati R, Pai VV, Kingsley S. Disability prevention and management in leprosy: a field experience. Indian J Dermatol Venereol Leprol. 2003;69:369–374. [PubMed] [Google Scholar]
- 47.Madhavan K, Vijayakumaran P, Ramachandran L, et al. Sustainable leprosy related disability care within integrated general health services: findings from Salem District, India. Lepr Rev. 2007;78:353–361. [PubMed] [Google Scholar]
- 48.Bahlinger VM, Brantley PJ, Madrigal DR, et al. Psychosocial stress in Hansen's disease: a comparison with other chronic illness patients. Int J Lepr Other Mycobact Dis. 1985;53:251–254. [PubMed] [Google Scholar]
- 49.Elias PM, Choi EH. Interactions among stratum corneum defensive functions. Exp Dermatol. 2005;14:719–726. doi: 10.1111/j.1600-0625.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- 50.Tomita Y, Akiyama M, Shimizu H. Stratum corneum hydration and flexibility are useful parameters to indicate clinical severity of congenital ichthyosis. Exp Dermatol. 2005;14:619–624. doi: 10.1111/j.0906-6705.2005.00341.x. [DOI] [PubMed] [Google Scholar]
- 51.Markendeya N, Srinivas CR. Ninhydrin sweat test in leprosy. Indian J Lepr. 2004;76:299–304. [PubMed] [Google Scholar]
- 52.Markendeya N, Srinivas CR, Shanthakumari S. Ninhydrin sweat test in the early detection of leprosy. Int J Lepr Other Mycobact Dis. 2002;70:125–126. [PubMed] [Google Scholar]
- 53.Rao KS, Balakrishnan S, Oommen PK, et al. Restoration of plantar sweat secretion in the feet of leprosy patients. Indian J Lepr. 1987;59:442–449. [PubMed] [Google Scholar]
- 54.Stenstrom SJ. A study on skin humidity in leprosy patients using a new type of humidity meter. Int J Lepr Other Mycobact Dis. 1984;52:10–18. [PubMed] [Google Scholar]
- 55.Okhandiar RP, Sinha RK, Sinha RK. Study of hydration of stratum corneum in leprosy. Indian J Lepr. 1986;58:395–400. [PubMed] [Google Scholar]
- 56.Coderch L, López O, de la Maza A, Parra JL. Ceramides and skin function. Am J Clin Dermatol. 2003;4:107–129. doi: 10.2165/00128071-200304020-00004. [DOI] [PubMed] [Google Scholar]
- 57.Engelke M, Jensen JM, Ekanayake-Mudiyanselage S, Proksch E. Effects of xerosis and ageing on epidermal proliferation and differentiation. Br J Dermatol. 1997;137:219–225. doi: 10.1046/j.1365-2133.1997.18091892.x. [DOI] [PubMed] [Google Scholar]
- 58.Hagemann I, Proksch E. Topical treatment by urea reduces epidermal hyperproliferation and induces differentiation in psoriasis. Acta Derm Venereol. 1996;76:353–356. doi: 10.2340/0001555576353356. [DOI] [PubMed] [Google Scholar]
- 59.Hosoi J, Hariya T, Denda M, Tsuchiya T. Regulation of the cutaneous allergic reaction by humidity. Contact Dermatitis. 2000;42:81–84. doi: 10.1034/j.1600-0536.2000.042002081.x. [DOI] [PubMed] [Google Scholar]
- 60.Ashida Y, Ogo M, Denda M. Epidermal interleukin-1 alpha generation is amplified at low humidity: implications for the pathogenesis of inflammatory dermatoses. Br J Dermatol. 2001;144:238–243. doi: 10.1046/j.1365-2133.2001.04007.x. [DOI] [PubMed] [Google Scholar]
- 61.Behne MJ, Meyer JW, Hanson KM, et al. NHE1 regulates the stratum corneum permeability barrier homeostasis: Microenvironment acidification assessed with fluorescence lifetime imaging. J Biol Chem. 2002;277:47399–47406. doi: 10.1074/jbc.M204759200. [DOI] [PubMed] [Google Scholar]
- 62.Behne MJ, Barry NP, Hanson KM, et al. Neonatal development of the stratum corneum pH gradient: localization and mechanisms leading to emergence of optimal barrier function. J Invest Dermatol. 2003;120:998–1006. doi: 10.1046/j.1523-1747.2003.12262.x. [DOI] [PubMed] [Google Scholar]
- 63.Fluhr JW, Behne MJ, Brown BE, et al. Stratum corneum acidification in neonatal skin: secretory phospholipase A2 and the sodium/hydrogen antiporter-1 acidify neonatal rat stratum corneum. J Invest Dermatol. 2004;122:320–329. doi: 10.1046/j.0022-202X.2003.00204.x. [DOI] [PubMed] [Google Scholar]
- 64.Thune P, Nilsen T, Hanstad IK, et al. The water barrier function of the skin in relation to the water content of stratum corneum, pH and skin lipids: the effect of alkaline soap and syndet on dry skin in elderly, non-atopic patients. Acta Derm Venereol. 1988;68:277–283. [PubMed] [Google Scholar]