Abstract
Recombineering is an efficient method of in vivo genetic engineering applicable to chromosomal as well as episomal replicons in E. coli. This method circumvents the need for most standard in vitro cloning techniques. Recombineering allows construction of DNA molecules with precise junctions without constraints being imposed by restriction enzyme site location. Bacteriophage homologous recombination proteins catalyze these recombineering reactions using double- and single-strand linear DNA substrates, so-called targeting constructs, introduced by electroporation. Gene knockouts, deletions and point mutations are readily made, gene tags can be inserted, and regions of bacterial artificial chromosomes (BACs) or the E. coli genome can be subcloned by gene retrieval using recombineering. Most of these constructs can be made within about a week's time.
INTRODUCTION
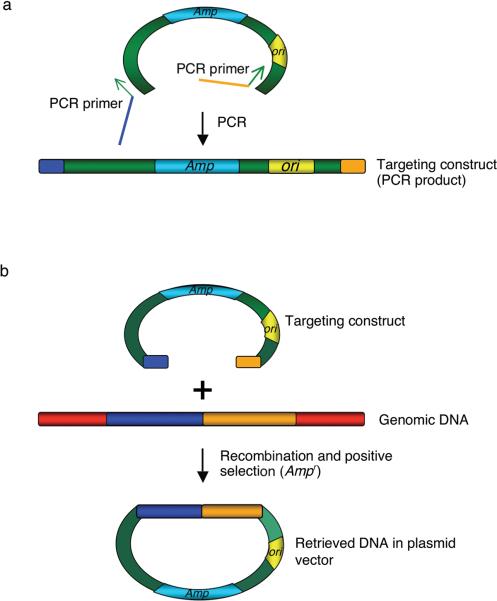
Recombineering is an in vivo method of genetic engineering used primarily in Escherichia coli that utilizes short 50 base homologies 1–5. Because recombineering is based on homologous recombination it allows insertion, deletion or alteration of any sequence precisely and is not dependent on the location of restriction sites (Figure 1). Linear DNAs, either double-stranded (ds), usually in the form of PCR products 3, 6–8 or single-stranded (ss) synthetic oligonucleotides 2, 9 are introduced by electroporation and provide the homologous substrates (i.e. targeting constructs) to create genetic changes. Recombineering is catalyzed by bacteriophage-encoded homologous recombination functions, such as the coliphage λ Red system 3 and the RecET system from the Rac prophage 4, 10.
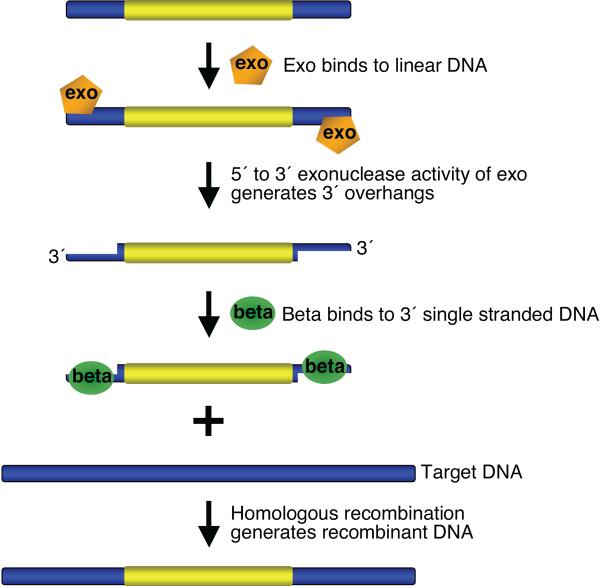
Figure 1. Overview of bacteriophage l recombination system used for recombineering.
Exo has a 5′ to 3′ dsDNA exonuclease activity, which can generate 3′ overhangs on linear DNA. Beta binds the single stranded DNA (3′ overhangs), promotes ss-annealing and generates recombinant DNA. An additional protein, Gam (not shown here), which prevents RecBCD nuclease from degrading double-strand linear DNA fragments, is also required for dsDNA recombineering.
This protocol will emphasize modification of Bacterial Artificial Chromosomes (BACs) and multi-copy plasmids but the procedures described are generally applicable to other replicons. A basic knowledge of molecular and microbiological techniques is required to execute the recombineering protocols described here. These basic techniques are described in detail by Ausubel et al. 11. Recombineering protocols for manipulation of the bacterial and phage chromosomes are described elsewhere 12, 13. Recombineering can also be used to modify episomal DNAs such as the low copy plasmid derivatives of P1 and F that carry artificial chromosomes and are called PAC (P1 artificial chromosome) 14 and BAC 15, 16, respectively. While multi-copy plasmids may be the ideal choice of vector when the insert size is relatively small (up to 50kb), PACs, which accommodate inserts of 50–100kb and BACs, which allow inserts of >100 kb are used for cloning large genomic fragments. A BAC is the vector of choice for cloning and manipulating large DNA fragments. BACs may contain genomic segments that include all of the extragenic cis-regulatory elements (promoter, terminator, and enhancers) of a gene of interest. BACs are therefore ideal for generating transgenic mice because the insert size may allow expression of the cloned gene under the control of its own regulatory elements, mimicking the endogenous expression pattern. In addition, because of the ease of obtaining desirable BAC clones coupled with simple DNA purification protocols, BACs are widely used in gene mapping and functional studies, analysis of regulatory elements, and expression of a transgene under the control of a heterologous promoter 17. They are also used for genetic analysis of mutations that have been identified in human diseases 18, 19. BACs modified by recombineering in E. coli, these modified constructs can then be used to generate knock-out and knock-in mouse models using ES cell technology 20, 21.
The recombineering protocols described here use the bacteriophage λ Red system that includes the phage recombination genes gam, bet and exo. The gam gene function, Gam, prevents an E. coli nuclease, RecBCD, from degrading linear DNA fragments 22, 23, thus allowing preservation of transformed linear DNA in vivo. The bet gene product, Beta, is a ssDNA binding protein that promotes annealing of two complementary DNA molecules 24–26, and the exo gene product, Exo, has a 5′ to 3′dsDNA exonuclease activity 27, 28. Working together these latter two proteins insert linear DNA at the desired target, creating genetic recombinants (Figure 1) 29, 30. For dsDNA, Red Exo is thought to degrade from both 5' ends, exposing ssDNA that is bound by Red Beta. Use of the phage λ Red system for in vivo genetic engineering was pioneered by Murphy et al. 6, 7, who demonstrated dsDNA recombination with the Red functions expressed from the lac promoter, both on a multi-copy plasmid and as an insertion on the E. coli chromosome. Murphy et al. used linear substrate DNA targeting homologies that were greater than 1 kb. Zhang et al. 4 demonstrated that the phage RecET system catalyzes recombination using targeting homologies of only 40–60 bp. This short length requirement allowed the homologies to be incorporated into PCR primers, substantially advancing the technology. The λ Red system was also shown to act on short homologies 3. Ellis et al. 2 demonstrated that the λ Beta protein promotes efficient in vivo recombination with ssDNA, provided as 70-mer ss-oligos. Models for how recombineering occurs 31 propose that the single-strand regions of the incoming linear DNA bound by the Beta protein are annealed to complementary single-strand gaps arising at the replication fork during DNA replication. Consistent with this model, an oligo able to anneal to the discontinuously replicated lagging strand gives a higher recombination frequency than its complementary “leading strand” oligo 2.
When engineering DNA in vivo, it is important to provide a brief high-level pulse of the phage recombination proteins. Limited expression minimizes the toxic effects of the Gam protein32 and minimizes undesirable genome rearrangements between repetitive DNA sequences. Although the phage recombination functions have been produced from multi-copy plasmids under control of the IPTG-inducible lac promoter 6, 7, the lac promoter has a high basal level and requires the lacIQ repressor gene in cis for tight regulation. A better choice is the arabinose-inducible pBAD promoter 33, 34. In Ara+ strains, the pBAD promoter can be tightly repressed by addition of glucose to the growth medium; however, in ara mutants glucose-mediated repression is less effective, even with the araC gene on the plasmid 35. When the pBAD plasmids are used for recombineering 34, glucose is not added during cell growth and arabinose is usually added at least a generation before the cells are made competent for electroporation. This procedure, while convenient for induction, does not yield the tightest possible repression 35. In contrast, expression from the λ prophage system is based on the endogenous lambda regulatory system, which is the natural method for expression of these recombination functions. The most recent studies on λ 36, 37 show that its repression system is uniquely strong: the λ repressor binds cooperatively at the three operator sites present at both the pL and pR promoters, and these two sets of repressor-bound operators interact with each other by protein-protein-mediated looping between pL and pR to generate a handcuff of 12 repressor proteins. To ensure this same tight repression during recombineering, both sets of operators are present on all of the prophage constructs, whether they are in the bacterial chromosome or on low copy plasmids, and a temperature sensitive repressor is expressed from the cI857 gene. At low temperatures (30°C to 34°C) the repressor is active and the recombination genes are not expressed. When the temperature of the bacterial culture is shifted to 42°C for 15 minutes, the repressor is rapidly inactivated, and the recombination genes are expressed at high levels from the powerful λpL promoter. Repressor is renatured and tight repression restored by lowering the temperature after 15 minutes. The short induction time minimizes adventitious recombination and cellular stress 32, 38. An advantage of the natural phage system is that the λ repressor is autoregulated, and thus, better controlled than when recombination genes on multicopy plasmids are expressed from heterologous promoters, which are likely to be leaky, causing unwanted expression and side effects. Red recombination does not require the E. coli RecA function 3 and when recombineering is performed in a strain with a recA mutation, all extraneous homologous recombination is prevented.
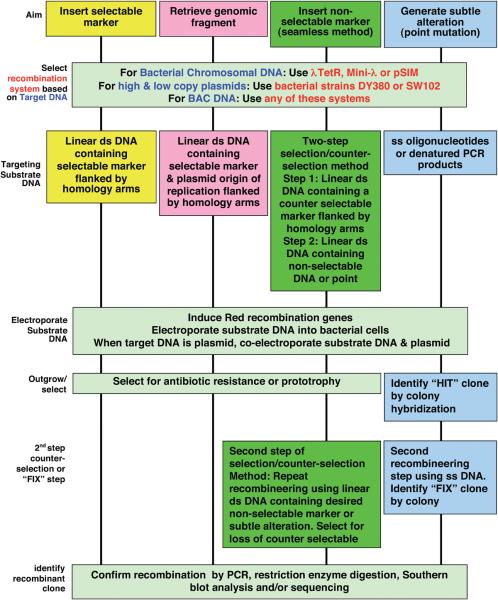
Because of its high efficiency and short homology requirements, recombineering can be used for a wide range of applications (Figure 2). Recombineering can be used to insert selectable or non-selectable markers in plasmids, bacterial chromosomal DNA or BACs. It can be used to generate gene-targeting constructs to be used for making knock-out or knock-in alleles in embryonic stem cells. This technology is also suitable for generating transgenic reporter constructs using BACs to express lacZ, fusion tags (e.g. GFP, tandem affinity, FLAG, HIS etc.), site-specific recombinases (e.g. Cre, Flp), selectable markers (e.g. neomycin resistance gene) or any cDNA under the control of a tissue specific promoter or to make fusion proteins. It can also be used to generate subtle alterations in BACs or bacterial chromosomal DNA without the use of any selectable marker or site-specific recombination system.
Figure 2. A flow-chart of recombineering procedures.
Schematic representation of various steps involved in recombineering. An appropriate system should be selected based on the choice of target DNA. The type of substrate DNA depends upon the choice of method used. The outgrowth procedures and methods to identify the recombinant clone are based on the use a selectable marker, selection/counter-selection method or lack of any selectable marker in the substrate DNA.
In spite of being a very tractable and versatile technology, recombineering has limitations. For example, since very short regions of homology are sufficient for the recombination, manipulating regions containing repetitive sequences can be problematic. Since most recombineering-based methods utilize PCR amplified products as substrate DNA, the recombinant products may occasionally acquire mutations. However, confirming the integrity of the recombinant target DNA by sequencing helps to discard such products. Another limitation of recombineering is that the sequence of the target region must be known. However, because the entire genome of many organisms has been sequenced, this is not a limitation for most commonly used organisms.
EXPERIMENTAL DESIGN
Each recombineering experiment involves the following six steps, which are illustrated in the general flow-chart (Figure 2).
Generation of the appropriate linear targeting substrate DNA
Provision of the λ Red recombination genes
Induction of the λ recombination genes
Preparation of electrocompetent cells and electroporation of the linear targeting substrate DNA
Outgrowth following electroporation
Identification and confirmation of the recombinant clones.
Substrate DNA design and generation
Depending upon the application, different DNA substrates are required (Fig. 2). The creation of appropriate double-stranded and single-stranded linear targeting substrates for specific applications is described below and their construction is detailed in step 3 of the Procedure.
Double-stranded DNA recombination
Linear dsDNA substrates for recombineering consist of a region to be inserted flanked by two homology arms. The inserted region may be either a selectable marker or a non-selectable DNA that is used to replace a counter-selectable marker.
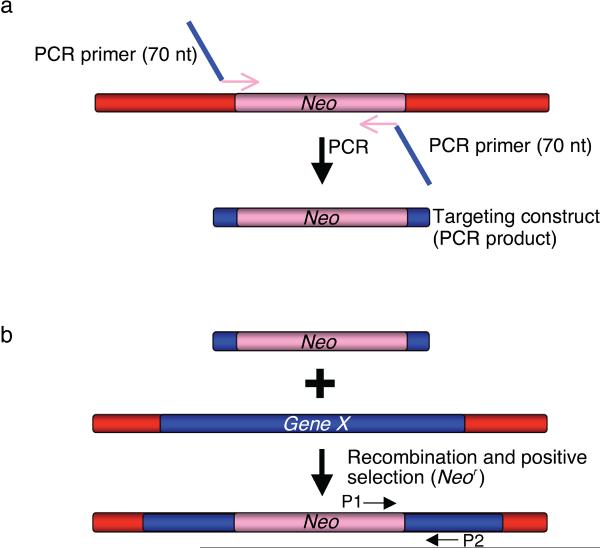
Recombineering can be used to insert a selectable marker (drug resistance gene or a prototrophic marker) into the bacterial chromosome or any episomal DNA. This is done by using linear DNA containing the desired selectable marker flanked by 50 bases of homology to the target site (Figures 1 and 3). Such substrates can be made with the polymerase chain reaction (PCR) by amplifying the selectable markers using a pair of chimeric primers, each about 70 bases in length. Each primer will have 50 bases at the 5' end corresponding to the region to be targeted to provide homology and approximately 20 bases at the 3′ end to prime amplification of the selectable marker as shown in Figure 3. Although flanking 50 base homologies are more than sufficient for recombination, longer homologies of 150–200 bases have been used to further improve the targeting efficiency, however, this substantially increases the time and effort required 6, 21. To use longer homologies, each homology arm is amplified independently by PCR and directionally cloned into a plasmid so as to flank the selectable marker already present in that plasmid. High fidelity Taq DNA polymerase with proof reading ability (such as Invitrogen High Fidelity Platinum Taq or Roche Expand High Fidelity) is used for generating dsDNA PCR products for recombineering substrates; for confirming constructs, standard Taq polymerase can be used. Try not to use supercoiled DNA as template for PCR amplification, since residual intact plasmid will give a high background of transformants, making it difficult to identify actual recombinants. The TKC strain (Table 1) contains the three genes for antibiotic resistance to tetracycline, kanamycin, and chloramphenicol, as well as their regulatory elements. This strain can be used to amplify any of these markers with the colony PCR technique using the primer pairs specified in Table 3 (see Option A in step 3 of the Procedure, Figure 3a). Plasmid templates with selectable markers flanked by loxP and FRT sites are available through the NCI recombineering website: http://recombineering.ncifcrf.gov/ (see Option B in step 3 of the Procedure, Figure 3a). Drug cassettes with dual promoters that allow the use of the same selectable marker (e.g neomycin, hygromycin and blasticidin) in both bacterial and mammalian cells are also available.
Figure 3. Insertion of a selectable marker by recombineering.
(a) Targeting construct can be generated by PCR to introduce the region of homology (in blue) and a selectable marker (e.g. Neo, Kanamycin/Neomycin resistance gene). The PCR primers used to generate the targeting construct are 70-mer oligonucleotides with 50 nucleotides corresponding to the target site (e.g. Gene X, in blue) sequence to introduce the homology arm and 20 bases from the ends of the selectable marker (Neo, in pink). (b) The targeting construct is electroporated into the bacterial cells that are induced to express the phage recombination genes. Recombinant clones are selected as kanamycin resistant colonies and confirmed with PCR using test primers P1 and P2.
Table 1.
Bacterial strains used in Recombineering
| Strain | Primary Use | Relevant Genotype | Reference | Available from |
|---|---|---|---|---|
| For Introduction of BACs | ||||
| DH10B | Parent strain | mcrA Δ(mrr-hsdRMS-mcrBC) φ80dlacZΔM15 ΔlacX74 recA1 endA1 araD139 Δ(ara, leu)7697 galU pglΔ8 rpsL nupG γ(cI857indl) Δ{(cro-bioA)<>tetRA} (TetR) gal490 | 54 | Invitrogen |
| DY380 | BAC recombineering with dsDNA | DH10B γ(cI857indl) Δ{(cro-bioA)<>tetRA} (TetR) gal490 | 44 | Court lab court@ncifcrf.gov |
| EL250 | BAC recombineering: for removing selectable markers flanked by frt sites | DY380 (cro-bioA) <> araC-PBADFlpe | 44 | http://recombineering.ncifcrf.gov/ |
| EL350 | BAC recombineering: for removing selectable markers flanked by loxP sites | DY380 (cro-bioA) <> araC-PBAD Cre | 44 | http://recombineering.ncifcrf.gov/ |
| SW102 | BAC recombineering: galK counter-selection | DY380 ΔgalK | 40 | http://recombineering.ncifcrf.gov/ |
| SW105 | BAC recombineering: galK counter-selection | EL250 ΔgalK | 40 | http://recombineering.ncifcrf.gov/ |
| SW106 | BAC recombineering: galK counter-selection | EL350 ΔgalK | 40 | http://recombineering.ncifcrf.gov/ |
| For Plasmid Propagation and Recombineering | ||||
| DH5α | Plasmid isolation | φ80dlacZΔM15, Δ(lacZYA-argF)U169, deoR, recA1, endA1, hsdR17(rk−, mk+), phoA, supE44, λ-, thi-1, gyrA96, relA1 | Hanahan 1985 | Invitrogen |
| HME70 | Plasmid recombineering: MMR-deficient strain for high efficiency oligo recombination | W3110 Δ(argF-lac)U169 galKtyr145UAG mutS<>cat Δ(srlA-recA)∷Tn10 {λcI857 Δ(cro-bioA)} | 50 | Court lab court@ncifcrf.gov |
| HME71 | Plasmid recombineering | W3110 Δ(argF-lac)U169 galKtyr145UAG Δ(srlA-recA)∷Tn10 {λcI857 Δ(cro-bioA)} | 50 | Court lab court@ncifcrf.gov |
| Drug cassette amplification | ||||
| TKC | Template for tetracycline, kanamycin and chloramphenicol | tetA, cat, kan | Court lab court@ncifcrf.gov | |
| LE392 | For Propagation of λ Tet phage | e14- glnV44 supF58 (lacY1 or ΔlacZY) galK2 galT22 metB1 trpR55 hsdR514(rK− mK+) | NIH Strain Collection | Court lab court@ncifcrf.gov |
| DH10B Containing BACs | DH10B containing BAC libraries | BACPAC Resources Center (BPRC): http://bacpac.chori.org | ||
Table 3.
PCR primers and possible source of template for drug cassette amplification*
| Gene Cassette | Source | Primer Sequence | Annealing Temperature (°C) | Size (kb) |
|---|---|---|---|---|
| ampicillin (amp) | pBluescript SK(+) (Stratagene) | 5' CATTCAAATATGTATCCGCTC 3' 5' AGAGTTGGTAGCTCTTGATC 3' | 53 | 1.2 |
| chloramphenicol (cat) | pPCR-Script Cam (Stratagene) | 5' TGTGACGGAAGATCACTTCG 3' 5' ACCAGCAATAGACATAAGCG 3' | 53 | 0.86 |
| kanamycin (kan) | Tn5 | 5' TATGGACAGCAAGCGAACCG 3' 5' TCAGAAGAACTCGTCAAGAAG 3' | 55 | 0.95 |
| tetracycline (tetA) | Tn10 | 5' TCCTAATTTTTGTTGACACTCTA 3' 5' CTCTTGGGTTATCAAGAGGG 3' | 55 | 1.34 |
When amplified with these primer pairs, all drug cassettes will contain a promoter and all but kan will have a transcriptional terminator.
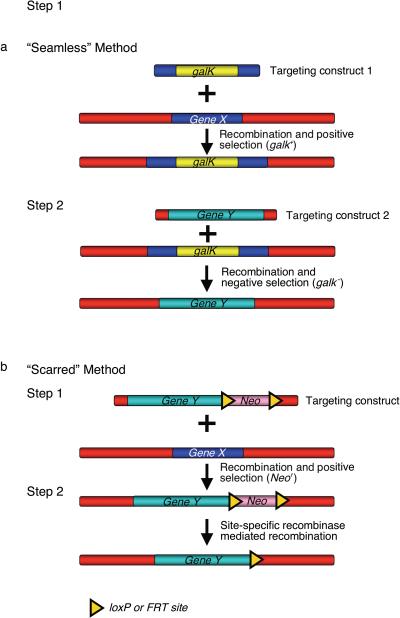
A non-selectable DNA fragment (e.g a reporter gene like lacZ, a transgene like cre, flp or GFP, or a HIS-tag) can also be efficiently inserted into a genomic region using recombineering. There are two approaches to achieve this. The first approach is a two-step selection/counter-selection method that allows insertion of the desired DNA without leaving other undesirable modifications, in a “seamless” event (Figure 4a). Generally, a counter-selectable cassette is first inserted at the site to be changed; this cassette is then replaced with the desired alteration in a second recombineering reaction. In all such procedures, each step can be selected and the final construct will not have a drug marker or other genetic scar. A number of counter-selections are available: these include the sacB 39 gene linked to a drug marker such as cat or kan, the galK gene 40, rpsL 41, thyA 42, and tolC 43. In many situations like when using galK, the same gene can be selected both for and against (Figure 4a). The galK counter-selection has been optimized for use with BACs and utilizes strains that have been deleted for the galK gene at its normal chromosome location; such strains include SW102, SW105 and SW106. A detailed protocol for the galK counter-selection has been published 40. For convenience, the strains and plasmids used in the gaK selection are listed in Tables 1 and 2. In the second approach, a selectable marker is linked to a non-selectable DNA fragment and the two are inserted together (Figure 4b). If subsequent removal of the selectable marker is desired, it can be flanked with loxP or FRT sites, thus allowing excision by expression of the Cre or Flp proteins 44. Template drug cassettes with flanking loxP and FRT sites that can be used for PCR amplification are also available from the National Cancer Institute recombineering website. Since a single loxP or FRT site remains after the genetic manipulation, we refer to this as the “scarred” method (Figure 4b).
Figure 4. Insertion of a non-selectable DNA fragment by recombineering.
(a) “Seamless” method to insert non-selectable DNA fragment makes use of selectable markers that can be used for positive as well as negative selection (e.g. galK). In this two-step method, first the selectable galK marker is targeted to the site (Gene X) where the non-selectable DNA fragment (Gene Y) is to be inserted. In the second step, a targeting construct containing the non-selectable DNA fragment flanked by the same 50 bp of homology to the target site is electroporated into Gal+ bacterial cells containing the recombinant DNA from step 1. Clones in which the Gene Y DNA fragment is correctly targeted are counter-selected for loss of the galK gene. (b) The scarred method: this method targets both the selected (Neo) and non-selected (Gene Y) DNAs jointly. In step 1, the non-selectable DNA fragment (Gene Y) is introduced along with a selectable marker, Neo, which is flanked by loxP or FRT sites. Recombinants are selected for the presence of Neo. In step 2, Neo is deleted by site-specific recombinase mediated recombination (Cre for loxP sites and Flp for FRT sites). Unlike the “seamless” method, a single loxP or FRT site is retained after recombination.
Table 2.
Mini-λs and Plasmids
| Mini-λ or Plasmid | Relevant Genotype | Antibiotics Concentration (μg/ml of media) | Source or Reference |
|---|---|---|---|
| Primary Use: Express Red Functions for recombineering | |||
| mini-λ Kan (kanamycin) | contained in DH10B | 30 | 31 |
| mini-λ Tet (tetracycline) | contained in DH10B | 12.5 | 31 |
| mini-λ Cat (chloramphenicol) | contained in DH10B | 12.5 | 31 |
| mini-λ Amp (ampilcillin) | contained in DH10B | 30 | 31 |
| pSIM5 | pSC101ts gam exo bet chloramphenicol resistance (CmR) | 12.5 | 45 |
| pSIM6 | pSC101ts gam exo bet ampicillin resistance (AmpR) | 100 | 45 |
| pSIM7 | pBBR1 gam exo bet chloramphenicol resistance (CmR) | 12.5 | 45 |
| pSIM8 | pBBR1 gam exo bet ampicillin resistance (AmpR) | 100 | 45 |
| pSIM9 | pRK2 gam exo bet chloramphenicol resistance (CmR) | 12.5 | 45 |
| pSIM17 | pSC101ts gam exo bet blasticidin resistance (BsdR) | 50 | 45 |
| pSIM18 | pSC101ts gam exo bet hygromycin resistance (HygR) | 50 | 45 |
| pSIM19 | pSC101ts gam exo bet spectinomycin resistance (SpecR) | 100 | 45 |
| Primary use: Amplify galK for Counter-selection | |||
| pgalK | pBluescript SK- pEm7-galK ampicillin resistance (AmpR) | 100 | 40 |
Recombineering is now routinely used as a subcloning technique. While classical methods of subcloning using restriction enzymes and DNA ligase allow manipulation of small DNA fragments, recombineering allows precise retrieval of even a large DNA segment from a genomic fragment in a BAC or from the bacterial chromosome 21, 44. Recombineering allows the junctions of the subcloned fragment to be exactly determined, based on need rather than on chance location of restriction sites. For rapid subcloning by recombineering, the small retrieval vectors are generated with PCR using a pair of primers, each with 50 base homologies at the 5' ends corresponding to the region to be retrieved and ~20 bases of plasmid sequence for PCR amplification (Figure 5). As mentioned above, 150–200bp homology arms can be cloned to improve retrieval efficiency if necessary 21. A plasmid template must be used when amplifying the linear retrieval vector backbone, usually consisting of an origin of DNA replication and a selectable marker (Option C in step 3 of the Procedure, Figure 5a). When the linear retrieval vector contains a selectable marker, however, a potential side reaction of end joining can occur, generating a circular plasmid carrying only the selectable marker 5. This is avoided by using a retrieval vector that contains only the origin of replication; in this approach, the selectable marker is first inserted adjacent to the target DNA to be retrieved. See Datta et al. 45 for details. The linear targeting construct recombines with the target DNA and retrieves the desired fragment by gap repair. This results in the generation of a circular plasmid (Figure 5b). When gap repair is used for retrieving DNA, it is not necessary to know the DNA sequence of the entire piece to be retrieved, only that of the flanking region used as targeting homology. When the length of the fragment to be retrieved is less than 15kb, a high copy plasmid (e.g. pBluescript) can be used, but a lower copy plasmid (e.g. pBR322) should be used for larger fragments 44. In a variation of this method known as “in vivo cloning” 5 a linear DNA to be incorporated onto the plasmid, often generated by PCR, is co-electroporated with the linear plasmid. DNA can also be transferred by retrieval from one BAC to another as has been described 46, 47
Figure 5. Subcloning DNA fragments from genomic DNA.
(a) To subclone or retrieve a genomic DNA fragment, generate a targeting construct by PCR. Each primer consists of 50 bases from the end of the genomic DNA that needs to be subcloned (blue and orange) and 20 bases from the plasmid sequence (in green) flanking the region between the origin of replication (ori) and an antibiotic resistance marker (Amp, ampicillin resistance gene). A linear plasmid DNA is used as template for PCR. (b) The linear targeting construct (PCR product) is electroporated into the bacterial cells that are induced to express the phage recombination genes. Recombination between the targeting construct and the genomic DNA results in the formation of a circular plasmid by gap repair. The circular plasmid contains the desired DNA fragment.
Single-strand DNA recombination
Recombineering can also be performed using synthetic oligonucleotides (see Procedure step 2) or short denatured PCR products 2, 8, 9, 48. These oligos (ss-oligos) or ssDNAs can be used to create single base changes, insert or substitute short DNA sequences and generate deletions. Under optimal conditions, creation of small changes with recombineering using ssDNA is highly efficient and often completely eliminates the requirement for any selection. Two different variables contribute to creation of these optimal conditions. One variable is use of a “lagging-strand” oligo, i.e. a single strand corresponding in sequence to the DNA chain that is replicated discontinuously 2, 9. Which of the two complementary oligos corresponds to the lagging-strand oligo will depend on the direction of DNA replication through the region of the chromosome or episome to be modified, and often it is easier to just try both strands; one should recombine with a 20- to 30-fold higher efficiency than the other. The other important variable includes avoidance of the E. coli methyl-directed mismatch repair (MMR) system: preventing MMR increases the effective recombination frequency about 100-fold 9, 49. Since strains mutant for the MMR system acquire adventitious mutations, procedures that avoid the use of MMR mutants have been devised. The simplest of these tricks is to use an oligo that creates a C-C mispair when annealed to the target DNA, since the MMR system does not recognize and remove this particular mismatch. This method has limited utility, however, and other more generally useful two-step procedures are illustrated below.
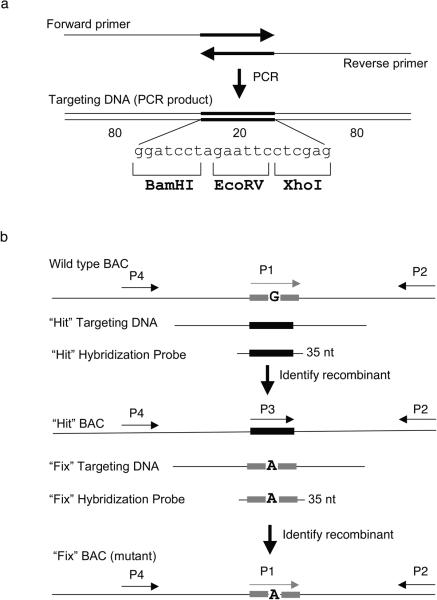
A “hit and fix” two-step recombineering approach 48, in combination with screening by colony hybridization, can be used to generate and find subtle changes in BAC DNA (Figure 6). This approach is particularly useful for modifying DNA regions with repetitive motifs, which can be difficult to screen by PCR. This method utilizes short (~180bp) dsDNA fragments generated by PCR that can be denatured to provide ss-oligonucleotide substrates for recombineering. In the first “hit” step, a stretch of 20 nucleotides is changed, including the nucleotide(s) to be mutated in the final construct. The altered sequence is designed to contain recognition sites for a restriction enzyme. In the second “fix” step, the bases that were modified in the first step are restored to the original sequence, with the exception of the desired mutation(s). Since a run of nucleotides is changed at each step, the recombinant BACs can be detected by colony hybridization using a primer specific for the altered bases. Colony hybridization allows thousands of individual colonies to be screened at once. The presence or absence of the restriction site provides an additional tool to rapidly confirm both the “hit” and “fix” steps. The central 20 bp region can be the same for all “hit” PCR products, allowing one 20 base universal probe to be used to identify the inserts at the “hit” step. See Optional Box 1 for generation of “hit and fix” substrates and step 19 D of the Procedure for identifying recombinants by the “hit and fix” method.
Figure 6. Two-step “hit & fix” method to generate subtle mutations using single stranded short PCR product or oligonucleotides as targeting vector.
(a) The single-stranded oligonucleotides containing 160 bases of homology and 20 unique bases are generated by using two 100-mer oligonucleotides in a PCR reaction. The two 100-mer oligonucleotides have 20 complementary bases (in this case the 20 bp contains restriction sites BamHI, EcoRV, XhoI) at the 3′ end. The 180 bp PCR product can be denatured to obtain single-stranded oligonucleotides that can be used as targeting construct. (b) Schematic representation of the two steps involved in “hit and fix” method to generate subtle alterations (e.g. G to A) without the use of a selectable marker. In step 1, a 180-mer single-strand oligonucleotide is used to replace 20 nucleotides (gray box) around the target site with 20 heterologous nucleotides (black box). Recombinants can be identified by colony hybridization using an end-labeled 35-mer oligonuclotide that can specifically anneal only to the recombinant DNA. A primer set specific for the heterologous “hit” sequence (P3 and P2) can be used to confirm the presence of recombinant clones by PCR. A second primer set (P1 and P2) can be used as a control to amplify only the non-recombinant DNA. Generation of a correct recombinant clone can be confirmed by digesting with BamHI, EcoRV or XhoI the PCR product (~300–500 bp) of primers P2 and P4. In step 2, the 20 nucleotides are restored to the original sequence, except for the desired mutation. Such clones can be identified by colony hybridization using a 35-mer oligonucleotide as probe and further confirmed by PCR amplification using primers P1 and P2, by testing for loss of the restriction sites inserted in step 1, by digesting the PCR product of primers P2 and P4, and by sequencing.
Oligonucleotides can also be used to precisely delete unwanted regions of DNA by designing a 70-mer lagging strand oligo that spans the portion to be deleted, with 35 bases of homology at either side. The efficiency of deleting large segments is lower than that of engineering small single base changes and a selection or screen will be needed to identify recombinants 2, 8, 9, 50. As an example, a counter-selection can be used in combination with an oligo to generate deletions when needed. Alternatively, colony hybridization, using a 20 base probe spanning the deletion with 10 bases on each side, can be used to identify recombinants.
Modifying multi-copy plasmids
Recombineering is useful for making point mutations and small changes on plasmids 3, 5, 50. For inserting or deleting larger pieces of DNA without a selection, classical cloning methods may sometimes be easier, but the precision of making junctions is usually lost. The plasmid to be engineered can be introduced into the Red-expressing cells by co-electroporation with the linear DNA after the recombination functions are induced; alternatively it may be resident in the recombineering host. Co-electroporation of the plasmid with the modifying DNA, as described in step 16 of the Procedure, is often preferable, since it helps control the number of plasmids present at the time of recombineering and may minimize opportunities for plasmid multimer formation. Recombineering will occur only in cells receiving the plasmid, so when introducing the plasmid by co-electroporation with the linear substrate, enough plasmid molecules must be added to obtain a high transformation frequency, but not so high as to introduce large numbers of plasmid molecules per cell. This is generally about 10ng of plasmid DNA per electroporation, but the appropriate amount of plasmid DNA to add may have to be determined empirically by generation of a transformation efficiency curve, since the transformation efficiency of plasmids may differ (see Optional Box 4 in Procedure). Ideally, each cell should receive about one plasmid molecule. If the plasmid is very large and has a low copy number, co-electroporation may not be practical; in this case a resident plasmid should be used for modification.
The intracellular copy number of commonly used plasmids such as pUC derivatives may exceed 500 copies per cell 51. Such a high copy number means that most copies will not be altered during a recombineering reaction, and cells that contain recombinant plasmid molecules will also contain many unmodified parental molecules. To create pure clonal populations of the recombinant it is necessary to separate the recombinant class from the unmodified class; this is done by isolating the plasmid DNA after recombineering and re-transforming it at a low concentration of less than one molecule per cell. If plasmid DNA has been deleted during the recombineering, often the plasmid mixture can be digested with a restriction enzyme that cuts only the parental plasmid species but not the desired recombinant, thus enriching for the recombinant class.
When making changes on plasmids, it is important to begin the procedure with a pure monomer plasmid species. By the same token, it is also important to use a host defective in RecA-mediated homologous recombination to prevent plasmid multimer formation by host recombination pathways. However, it has been observed 3, 50 that when plasmids are modified with recombineering, some larger multimeric circles of these plasmids arise. Since these multimers form during and are dependent upon the recombineering reaction they cannot be totally eliminated. There is anecdotal evidence that other replicons including BACs may also form larger-order multimers during recombineering. Ways to deal with these multimers are suggested in the Troubleshooting section.
Provision of the λ Red recombination genes
There are several ways to provide the prophage system that will express the phage recombination proteins in the bacterial cells where recombineering will be performed (Figure 6). All variations allow highly efficient recombination, and the choice of which to use will depend on the target DNA (see Figure 2). When high or low copy plasmids have to be manipulated, bacterial strains containing the prophage recombination system on the chromosome (e.g DY380 and its derivatives like SW102) should be used. When the bacterial chromosome or BAC DNA will be modified, mobile recombineering systems like the pSIM vectors, mini-λ or the replication-defective λ phage (λTetR) can be introduced into the bacterial cells to be engineered. BAC DNA can also be transformed into bacterial strains containing the repressed recombination system. In all cases it is advisable to check the integrity of the BAC DNA by restriction analysis before proceeding with the recombineering procedure. The individual options are discussed in more detail below.
Bacterial strains with defective prophage; DY380 and derivatives
A number of bacterial strains have been generated that harbor a defective λ prophage that is stably situated in the bacterial chromosome (see Procedure step 4A, Figure 7b and Table 1). Such host strains are ideal for manipulation of plasmid DNA, which can be introduced into these bacterial strains. Another major advantage of using the strains containing the integrated λ prophage is that a drug selection need not be applied to maintain the recombineering system. Strains routinely used for BACs include DY380 44, SW102 and their derivatives 40. To use these strains, the BAC or plasmid to be modified must be introduced into the recombineering strain by transformation (Procedure Optional Box 3). A mini-prep method described below (Procedure Optional Box 2) works well for rapid extraction of BAC DNA and produces DNA that is suitable for restriction digestion, PCR analysis and sequencing. For targeting both BACs and any multicopy plasmids, it is important to use recA mutant strains like these.
Figure 7. Schematic representation of λ phage constructs used for recombineering.
(a) The λ prophage with some of its genes is shown integrated in the bacterial chromosome. It is adjacent to the biotin genes, bioAB. Bacterial DNA is shown as a blue bar; phage DNA is a red bar; and a region containing a deletion and/or a substitution is a white bar. The complete λ prophage is flanked by its attachments site att, where integration occurred. The int and xis genes located in the pL operon encode functions to integrate and excise the phage DNA into and out of the bacterial chromosome. Two operons with their promoters pL and pR are indicated. Transcription of the promoters is controlled by the λ CI857 repressor. This repressor is temperature sensitive in that it is active and represses the promoter at 30–32°C but is inactive at 42°C allowing transcription. The N functions as an anti-transcription terminator and prevents RNA polymerase termination of pL transcripts at terminators tL1, tL2, and tL3. The red genes exo, bet, and gam encoding the homologous recombination functions are also in the pL operon. The kil gene is adjacent to gam and when expressed for over 1 hr kills the bacterial cell 32. The replication genes O and P are in the pR operon. Cro functions as a partial repressor of the pL and pR operons when CI is inactive at 42°C. The lysis and structural genes, SRA-J, are shown located beyond their regulator Q. The λ TetR phage used for recombineering is identical to the above complete prophage with the exception of five changes. The replication gene P has an amber mutation, the cro gene also has an amber mutation, there is an added mutation ind1 in the cI857 repressor gene, and the tetA gene encoding tetracycline resistance replaces rex. (b) The defective prophages in DY380, SW102, and the HME strains have the lytic pR operon deleted from cro through the bioA genes as shown in brackets. The right att site is also deleted preventing any excision of this prophage. In DY380 and SW102 cro through bioA is replaced by the tetracycline resistance cassette, tetRA. (c) The mini-λ phage DNA is shown with the lytic genes cro through J deleted. In different constructs of the mini λ, the cro-J region is replaced with various drug resistant cassettes. Mini-λ has both att sites plus int and xis, which allows for its integration and excision. (d) The λ segment on the pSIM plasmids is shown. The plasmid backbone is not shown. The genes from cro to att are removed as well as genes beyond tL3 including int and xis. The red genes are connected directly to pL by a deletion that removes kil through N. The drug resistant marker characteristic of each SIM plasmid replaces the rex gene adjacent to cI857. The basic features are conserved in all of these Red expression constructs. Red is left under the native phage controlling elements for optimal expression and regulation. The pL promoter drives gene expression and is controlled by the temperature sensitive but renaturable λ CI857 repressor. In all, the cro gene is inactive to maximize pL expression, and the replication genes are absent or inactive to prevent lethal effects on the cell.
Mobile recombineering systems; mini-λ and pSIM plasmids
There are mobile recombineering systems available that can be introduced into any bacterial strain to allow manipulation of the BAC or chromosomal DNA. Such mobile elements include “mini-λ” 52 and the plasmids collectively called the pSIM vectors 45 (see Procedure steps 4B and 4C, respectively). Both mini-λ and the pSIM plasmids provide the same endogenous phage controlling elements and Red recombination functions present in the above-mentioned defective prophage strains (see Figure 7c and d), however these must be introduced into the BAC-containing strain. Mini-λ consists of a defective non-replicating, circular phage DNA, which, once introduced into the strain, integrates into the bacterial chromosome using site-specific recombination 52. The integrants are selected by the presence of the antibiotic resistance gene carried by the mini-λ. Once integrated, mini-λ is stable, replicates as part of the host chromosome, and does not require drug selection for maintenance. The mini-λ includes the pL operon from which are expressed the int and the xis genes that catalyze the site-specific integration/excision events, as well as the exo, bet and gam recombination genes for recombineering (Figure 7c). In addition to being easily introduced into any bacterial cell, the mini-λ can be readily excised to cure the cells of the phage DNA. The excised mini-λ DNA circle can also be purified from the induced bacterial cells using a standard plasmid purification protocol. The pSIM vectors 45 consist of the elements of the prophage necessary for recombineering (see Figure 7d) carried on a number of different plasmid origins. Especially useful is a pSC101 plasmid derivative with temperature-sensitive DNA replication; this plasmid has a low copy number and the ts replicon allows loss of the plasmid after recombineering is completed. These plasmids are available with a variety of selectable antibiotic resistance markers (Table 2). Unlike the prophage and the mini-λ, they require drug selection for stable maintenance.
λ phage carrying tetracycline resistance gene; λTetR
A further means of expressing the Red system is from a λ phage carrying a tetracycline resistance gene 20 (see Procedure step 4D). This phage system combines the advantages of stability and mobility. The genotype of this phage is λ cI857 ind1 rexAB<>tetAR croTYR26am PGLN59am, and it is referred to here as λTetR (see Figure 7a). The λTetR is easy to prepare as a high titer phage lysate; using this lysate, lysogens are readily created by infection of the desired host and TetR selection. The efficiency of lysogeny is greater than that of plasmid transformation, and once introduced into the BAC-containing strain, the prophage is very stable and drug selection is no longer required.
Induction of the λ recombination genes
The Red system is induced by incubating the mid-log bacterial culture in a 42°C water bath shaking at 200 rpm for 15 minutes as detailed in the Procedure steps 6–8. It is important to note that air shakers are not satisfactory for this purpose because the heat transfer is less efficient with an air shaker. Immediately after the heat pulse the cells should be placed in an ice-water slurry for quick-chilling. Failure to rapidly chill the cells may result in premature decay of the recombination activity. Once the cells are induced for the Red functions, it is best to complete the procedure promptly.
Preparation of electrocomptent cells and electroporation of the linear targeting substrate DNA
(see Procedure steps 9–18). The chilled cells are gently washed twice with cold sterile water to remove salts that will cause problems during electroporation. The washed cells are suspended in a small volume of water and mixed with the linear DNA substrate, then aliquoted into chilled electroporation cuvettes. LB is added immediately after electroporation to maximize recovery of viable cells.
Outgrowth following electroporation
The cells are fragile after electroporation and require a minimal 30-minute recovery period in LB before plating. An outgrowth period may also be needed to allow expression of cloned genes such as those encoding antibiotic resistance. The details of the outgrowth procedure will vary according to the type of recombineering reaction performed and how the recombinants will be identified. The various types of outgrowth are described in step 19 A–D of the Procedure.
Identification and confirmation of the recombinant clones
Drug resistant recombinants are first selected using the appropriate antibiotic resistance and then analyzed with PCR to verify that the insertion has gone to the proper location and that the cells are not diploid for the locus. In certain cases, such as for multi-copy plasmids, restriction analysis may be used to confirm the recombinant clones. Southern blots and sequencing may be used to analyze BAC or genomic recombinants. When a point mutation or other subtle change is made, DNA sequencing must be used to confirm correctness of recombinants. Recombinant identification is described in steps 19 A–D of the Procedure.
MATERIALS
REAGENTS
One of the following is required to express the Red functions (available from the Court lab; see Experimental Design for further details):
Bacterial strain containing defective λ prophage (see Table 1)
Mini-λ DNA (see Table 2)
pSIM plasmid (see Table 2)
λTetR phage with titer of at least 109 plaque forming units/ml
Ampicillin (A0166, Sigma)
Kanamycin (K0254, Sigma)
Chloramphenicol (C7795, Sigma)
Tetracycline (T7660, Sigma)
Hygromycin (H3274, Sigma)
Sterile distilled H2O, chilled on ice
Minimal salts solution such as M9
Plasmid DNA isolation reagents (12163, QIAGEN maxiprep kit; 27104, QIAGEN miniprep kit)
PCR purification kit (28104, QIAGEN)
Gel extraction kit to purify DNA from agarose gels (28604, QIAGEN)
High fidelity Taq polymerase with proof reading ability (11304–029, Invitrogen)
Standard Taq polymerase (11342–020, Invitrogen)
dNTP mixture, 10mM each, PCR grade, (10297–018, Invitrogen)
Agarose (SeaKem LE, ISC Bioexpress)
Restriction enzymes (New England Biolabs)
Glycerol (BP229-1, Fisher Scientific)
Chimeric primers for PCR amplification of recombineering substrates, 25 pmol/μl in H2O (Integrated DNA Technologies)
One of the following sources of pure linear DNA for recombineering (see Experimental Design for further details), suspended in sterile H2O:
purified PCR product with 50 bp of homology to the target, ~100 ng/μl
70-mer ss-oligo (salt-free but otherwise unpurified) with desired changes(s) at center and with ~35 bases flanking homology, 0.5 pmol/μl (Integrated DNA Technologies)
plasmid backbone with flanking homology to DNA to be retrieved, ~100 ng/μl
EQUIPMENT
Constant temperature bacterial incubator set at 30–34°C (2005 Low temp Incubator, VWR)
Two shaking H2O baths (200 rpm) set at 30–32°C and at 42°C (G-76, New Brunswick Scientific)
Spectrophotometer and cuvettes (DU 530, Beckman-Coulter)
Electroporator (Genepulser II with Pulse Controller II, Bio-Rad)
Electroporation cuvettes with 0.1 cm gap (870582, BioRad), labeled and prechilled
Floor model low speed centrifuge at 4°C (Avanti, J-25I, Beckman)
Refrigerated microcentrifuge at 4°C (Micromax, RF, IEC)
Thermal cycler and accessories for PCR (MyCycler, BioRad)
Agarose gel electrophoresis apparatus (Bio-Rad)
Sterile 125 and 250 ml Erlenmeyer flasks, preferably baffled (2540–00125 and 2540–00250 respectively, Bellco)
Sterile 35–50 ml centrifuge tubes (03530, Thermo Scientific)
1.5 ml microfuge tubes (022363204, Eppendorf)
0.2 ml flat cap PCR tubes (TFI0201, Bio-Rad)
Insulated ice buckets (35754–100, VWR)
Sterile glass culture tubes (16×150mm) for overnight growth of bacterial cultures (89000–506, VWR)
Stainless steel closures for culture tubes (60825–882, VWR)
Pipetters of various volumes (Gilson) with aerosol-resistant sterile tips
Petri plates, 100×15mm (25834–302, VWR)
Computerized DNA analysis program (Gene Construction Kit, Textco Biosoftware; Vector NTI, Invitrogen)
REAGENT SETUP
M9 medium
3.0 g KH2PO4
12.8 g Na2HPO4•7H2O
1.0 g NH4Cl
0.5 g NaCl
1 liter distilled H2O
LB (Luria Broth)
10 g Bacto-tryptone (Difco)
5 g yeast extract (Difco)
5 g NaCl
1 liter H2O
pH 7.2
Both M9 and LB should be autoclaved for sterility and stored at room temperature.
LB and petri plates (100×15mm) containing 1.5% Difco agar, with antibiotics as needed (see Table 4)
Table 4.
Antibiotics and concentrations (μg/ml) used in media for selection:
| Antibiotic | Single copy* | Multi-copy plasmids |
|---|---|---|
| Ampicillin | 30 | 100 |
| Kanamycin | 30 | 50 |
| Chloramphenicol | 10 | 20 |
| Tetracycline | 12.5 | 25 |
| Hygromycin | not used in single copy | 50 |
| Spectinomycin | 100 | 100 |
| Blastocidin | 50 | 50 |
Use these concentrations for drug markers on BACs or the E. coli chromosome and for single copy plasmids.
PROCEDURE
Design and generation of linear DNA substrate for recombineering
1 Before beginning recombineering, precisely design your desired DNA sequence in silico. This is greatly facilitated by use of computerized DNA analysis programs such as Gene Construction Kit (Textco Software; http://www.textco.com/) or Vector NTI (Invitrogen). CRITICAL STEP: Design the desired molecule on the computer before ordering primers!
2 From the in silico design of the new DNA molecule, generate the sequence(s) of required primers or70-mer ss-oligos (see Experimental Design) and order or synthesize them as salt-free and otherwise unpurified. CRITICAL STEP Be sure to include the 50 bases of flanking homology to the 5' ends of your primers (see Experimental Design). CRITICAL STEP ss oligos can be directly used as substrates for recombineering after appropriate dilution.
3 To generate dsDNA substrates for recombineering by PCR, different templates can be utilized as follows: option A for bacterial DNA (Figure 3A), option B for plasmid DNA (Figure 3A), option C for plasmid origin amplification (Figure 5A):
A. PCR-amplification of dsDNA substrate for recombineering from bacterial DNA TIMING 3–4 h
i) Antibiotic resistance genes containing tetracycline, kanamycin, and chloramphenicol cassettes can be amplified from the TKC strain using “colony PCR” and primer pairs listed in Table 3. To perform colony PCR, prepare a PCR reaction without allowing any volume for the template DNA; for a typical 50μl PCR reaction using the high fidelity Platinum Taq kit from Invitrogen, mix the following in a 0.2 ml PCR tube using sterile technique (for multiple PCRs prepare a master mix and dispense aliquots as appropriate):
5μl 10X PCR Buffer
2μl 50 mM MgSO4,
1μl 10mM dNTP mixture
1μl each primer (25 pmol/μl in H2O)
0.5μl Platinum Taq high fidelity
39.5μl sterile distilled H2O
ii) Lightly touch a fresh colony of the E. coli strain with a sterile inoculating loop and mix cells into the PCR reaction.
iii) Use the following PCR program suitable for amplifying any of the drug cassettes referred to in Table 3 (using optimal conditions for Invitrogen Platinum Taq high fidelity):
Initial denaturation: 94°C 5 min
Subsequent denaturation: 94°C 30 sec
Anneal primers: 53°C 30 sec
Extension: 68°C 1.5 min
Repeat the latter three steps 29 times.
Final extension: 68°C 10 min
Hold at 4°C.
iv) Visualize the PCR reaction on an agarose gel as described elsewhere Ausubel et al. 11.
v) Remove salt and clean up the PCR product by ethanol precipitation as described elsewhere (Ausubel et al. 11) or by using a commercially available kit and following the manufacturer's instructions.
vi) Suspend product in small volume of H2O so as to have a concentration of ~100 ng/μl.
B. PCR-amplification of dsDNA substrate for recombineering from plasmid DNA TIMING 3–4 h
i) Plasmid templates must be linearized prior to PCR amplification. Digest plasmid DNA with a restriction enzyme at sites present in the vector sequence but absent in the sequence to be amplified. Ideally the DNA replication origin should be destroyed.
ii) Run an aliquot of the DNA on an agarose gel to verify complete digestion. (Be aware that super-coiled plasmid migrates at ~70% of the size of a full-length linear species).
iii) If necessary, cut the band containing the linearized DNA from the gel and extract the DNA.
iv) Resuspend the linear plasmid DNA in TE (pH 8.0), check the concentration and dilute to 0.5–1.0 ng/μl. A purified fragment containing just the drug cassette can be stored and reused as template.
v) Amplify the drug cassette with a pair of chimeric primers, using standard PCR conditions and a Taq DNA Polymerase with proof reading ability.
vi) Examine the PCR product by agarose gel electrophoresis.
vii) Gel-purify the desired band to eliminate contamination of supercoiled plasmid.
viii) If linear plasmid DNA was used as template and the product was not gel purified, digest the PCR reaction with the modification-dependent restriction enzyme DpnI, which will cut the plasmid DNA but not the unmodified PCR product. DpnI digestion may not totally eliminate plasmid background, however. CRITICAL STEP Do not expose the PCR product to direct ultraviolet light, which may damage it and result in abnormal recombination frequencies.
ix) Clean up the PCR product as described in step 3. A. v.
CRITICAL STEP Do not use supercoiled DNA as template for PCR amplification. To avoid having to gel purify the DNA or treat with DpnI, use the TKC strain as a template when possible.
C. Generation of dsDNA substrate for recombineering by PCR amplification of plasmid origin for gene retrieval TIMING 3–4 h
i) Digest the plasmid with one or more restriction enzyme(s) that do not cut within the region to be amplified.
ii) Amplify the origin and selectable marker from the linear plasmid by PCR using the primers (reaction conditions will need to be established empirically). Use the least amount of linear plasmid possible for the PCR template, to minimize residual uncut plasmid.
iii) Digest the completed PCR reaction with DpnI to further remove the template plasmid.
iv) Purify the PCR product to remove salt before proceeding. The amplified product will be a linear plasmid with flanking homology to either side of the region to be rescued from the chromosome. It is possible to avoid the potential side reaction of plasmid end-joining by using an alternative approach: amplify a retrieval vector containing only the origin of replication, after first inserting a selectable marker different than the one on the starting plasmid next to the target DNA to be retrieved.
? TROUBLESHOOTING
See OPTIONAL BOX 1 for Generation of “hit” and “Fix” PCR products
Provision of the λ Red recombination genes
4 Provide the λ recombination genes from either the defective prophage present in DY380 and its derivatives (option A), or from the mini-λ (option B), a pSIM plasmid (option C), or the replication-defective λTet phage (option D). It is critical to realize that in all cases, the Red genes are under control of a temperature-sensitive repressor, and strains containing them should always be propagated at low temperature (30–32°C) except during induction of the Red system. Once the recombineering strains are created they can be frozen and stored indefinitely for future use at −70°C as a glycerol stock prepared by mixing 1 ml of a freshly grown overnight culture with 1 ml 50% glycerol (dilute glycerol in water and autoclave).
A. Provision of the λ Red recombination genes from DY380 and derivatives
i) DY380 and derivatives already contain the Red functions and so require no further preparation before using for recombineering.
See OPTIONAL BOX 2 for Small-scale miniprep for BAC DNA preparation See OPTIONAL BOX 3 for Electroporation of BAC DNA into bacterial strain of choice
B. Provision of the λ Red recombination genes from Mini-λ TIMING 2d
i. To prepare the mini-λ DNA, inoculate a single colony of E.coli with an integrated mini-λ prophage into 125 ml of LB containing appropriate antibiotics in a one-liter flask and culture overnight at 30–32°C.
ii) The next day, incubate the culture at 42°C for 15 minutes to induce excision of the mini-λ DNA circles.
iii) Chill the culture on ice for 15 minutes and extract the mini-λ DNA using a standard plasmid midi-prep purification kit (e.g. Qiagen) as described previously52.
iv. Introduce the mini-λ into the desired E.coli strain that will be used for recombineering by mixing 1 μl of mini-λ DNA (25–35 ng) with 25 μl of electro-competent bacterial cells and introduce the DNA by electroporation. Also do a control electroporation of cells alone without added DNA.
v) Grow the transformed cells at 32°C for one hour in LB medium and plate dilutions on an LB agar plate containing the appropriate antibiotic to select the mini-λ. Incubate overnight at 32°C (see Table 4, use antibiotic concentration indicated for single copy plasmids).
vi) Pick isolated colonies for recombineering and test for the presence of the prophage by confirming that the strain is temperature sensitive for colony formation on antibiotic plates at 42°C.
C. Provision of the λ Red recombination genes from pSIM plasmids TIMING 1 d
i) Introduce the pSIM plasmid DNA into the bacterial strain where recombineering will be performed by selecting for the appropriate antibiotic resistance of the plasmid and plating the cells at 30–32°C (see Table 4, use antibiotic concentration indicated for multi-copy plasmids).
ii) Purify a single colony 30–32°C and grow an overnight culture from it with the appropriate antibiotic using methods described elsewhere 11.
D. Provision of the λ Red recombination genes from Replication-defective λTet phage TIMING 1 d
i) Grow a 1 ml culture of the appropriate bacterial strain that will be used for recombineering overnight in LB, adding chloramphenicol (10μg/ml) if a BAC is present.
ii) The next day, pellet the cells by centrifuging at 10,000 × g for 1 min in a microfuge and suspend them in 0.1 ml 10mM MgSO4.
iii) Add 1μl high titer phage stock (at least 109/ml) to the cells and incubate at 32°C for 20 min.
iv) Add 1 ml LB and incubate 1 hr at 30–32°C, then plate a series of 10-fold dilutions on LB-Tet plates (Tetracycline concentration: 12.5μg/ml) and incubate at 30–32°C overnight.
v) Purify Tetracycline resistant colonies by streaking the bacteria and picking isolated colonies on LB-Tet plates.
? TROUBLESHOOTING
5 Inoculate the bacterial strain containing the Red functions from a single colony into 3 to 5 ml LB medium. Incubate the culture at 32°C overnight with aeration. CRITICAL STEP Maintain antibiotic selection for the BAC (chloramphenicol 10μg/ml), and if a pSIM plasmid is used, also include the antibiotic for the pSIM (see Table 4, use antibiotic concentration indicated for multi-copy plasmids).
Induction of the λ recombination genes TIMING 3 h
6 Add 0.5 ml of the overnight culture to 35 ml of LB medium. Supplement with appropriate antibiotic to maintain plasmids, if needed, in a 250-ml baffled Erlenmeyer flask. Use antibiotic concentration indicated in Table 4.
CRITICAL STEP Dilute the overnight at least 70-fold.
7 Place the flask in the 32°C H2O bath and grow cells with shaking for about 2 hr or until the cells reach an OD600 of 0.4–0.6. CRITICAL STEP The growth time may vary with different strains. The cells are ready when the OD600 is 0.4–0.6. It is important not to over-grow the cells, since stationary phase cells are not optimal for recombineering.
8 Transfer half the culture to a 125 ml Erlenmeyer flask and place that flask to shake in a 42°C H2O bath; keep the other flask at 32°C. Shake for 15 min at 200–220 rpm. The culture at 42°C is induced for recombination functions and the 32°C culture is the uninduced control.
? TROUBLESHOOTING
Preparation of electrocompetent cells and electroporation of the linear targeting substrate DNA TIMING 2 h
9 Immediately after the 15 min induction, rapidly chill both cultures in an ice-water slurry; swirl the flasks gently. Leave on ice for 5–10 min. Label and chill two 35–50 ml centrifuge tubes for each set of induced and uninduced cells.
10 Transfer both the induced and uninduced cultures to the centrifuge tubes and centrifuge 7 min at 4600 × g (6700 rpm in a Sorvall SA-600 rotor) at 4°C. Using sterile technique, aspirate or pour off supernatant.
11 Add 1 ml ice-cold sterile distilled H2O to the cell pellet and gently suspend cells with a large pipet tip (do not vortex). Add another 30 ml of ice-cold distilled H2O to each tube, seal, and gently invert to mix, again without vortexing. Centrifuge tubes again as in Step 10.
12 Promptly decant the 30-ml supernatant very carefully from the soft pellet in each tube and gently suspend each cell pellet in 1 ml ice-cold distilled H2O. CRITICAL STEP Remove tubes from the centrifuge promptly at end of spin. The pellet is very soft and care should be taken not to dislodge it or lose the cells, especially when processing multiple tubes. If necessary, leave a little supernatant in the tube.
? TROUBLESHOOTING
13 Transfer the suspended cells to pre-chilled microcentrifuge tubes. Centrifuge for 30 sec at 10,000 × g in refrigerated microcentrifuge at 4°C. Carefully aspirate supernatant and suspend cells in 1ml ice-cold H2O, centrifuge again and aspirate supernatant, being extremely careful with the pellet.
14 Suspend the cell pellet in 200μl sterile cold distilled H2O and keep on ice until used. CRITICAL STEP For highest efficiency, use freshly processed cells.
15 To introduce the linear DNA recombineering substrate (from Step 3) into the electrocompetent cells (from Step 14) by electroporation, chill the desired number of labeled 0.1-cm electroporation cuvettes on ice. Turn on the electroporator and set to 1.80 kV, 25μF capacitance and 200 ohm resistance.
? TROUBLESHOOTING
16 In labeled microcentrifuge tubes on ice, mix 1 μl DNA from Step 3 (~100ng of salt-free PCR fragment or 0.5 pmol (~100ng) ss-oligo) with 50 μl of electrocompetent induced or uninduced cells from Step 14. When modifying a multi-copy plasmid introduced by co-electroporation, also add 1 μl of the multi-copy plasmid DNA (~20ng/μl) at this point. Include the following controls: a. Induced cells without DNA. If colonies are present on this control plate, either the selection is not working properly or the cells have a high reversion frequency for the property selected. b. Uninduced cells plus DNA. This is a control to estimate the number of background colonies due to some contaminating factor in the DNA such as intact plasmid template from a PCR reaction. CRITICAL STEP Never leave the DNA-cell mixes on ice for more than ~10 minutes.
? TROUBLESHOOTING
17 Transfer the cell-DNA mixes to chilled electroporation cuvettes as quickly as possible and introduce the DNA into the cells by electroporation. CRITICAL STEP Make sure that the time constant is greater than 5 msec for optimal results. Lower time constants generally indicate impurities or salts in the cells or the DNA.
? TROUBLESHOOTING
18 Immediately after electroporation, add 1 ml LB medium to the cuvette. Do this before proceeding to the next electroporation. After the electroporations are completed, transfer the electroporation mixes in LB to sterile culture tubes and incubate with shaking at 32°C for a minimum of 30 min. CRITICAL STEP Failure to allow 30 min outgrowth gives poor cell viability.
Outgrowth following electroporation, identification and confirmation of the recombinant clones TIMING 1 d- 2 wk
19 The outgrowth and screening procedures will vary according to the type of recombineering reaction: use option A to select recombinant clones by antibiotic resistance or prototrophy, option B to select for recombinant clones containing a genomic fragment subcloned by recombineering, option C to select for modified multi-copy plasmids, and option D to screen hit and fix recombinants by colony hybridization.
A. Outgrowth and selection of recombinant clones by antibiotic resistance selection or prototrophy TIMING 2 d
i. Continue outgrowth in 1ml of LB without any antibiotics for at least 2 hours at 30–32°C to allow expression of the antibiotic resistance or metabolic gene to be selected.
ii. Following the outgrowth, make six 1:10 serial dilutions of the experimental cultures in a buffered medium lacking a carbon source such as M9 salts.
iii. To select recombinants, spread 0.1 ml of the undiluted culture and the 10−1 and 10−2dilutions on plates selective for the recombinant at 30–32°C. Also assay total viable cells by plating 0.1 ml of the 10−4, 10−5, and 10−6 dilutions on LB; determining this viable cell count will allow calculation of a recombinant frequency. If the number of viable cells is too low (less than 107/ml) recombinants may not be found. For the control cultures, both the uninduced (32°C) and the induced (42°C) to which no DNA was added, plate 0.1 ml of the undiluted culture on a single selective plate. CRITICAL STEP Do not add chloramphenicol for BAC maintenance while outgrowing or selecting for the recombinant on plates.
iv. Incubate plates until colonies appear (generally 22–24 hr for rich plates at 32°C). Generally no or only a few colonies are obtained on the control plates, indicating that most of the colonies on the experimental induced plus DNA plates represent true recombinant clones.
v. Once potential recombinant clones are identified, confirm the construct by PCR analysis, restriction digestion analysis and sequencing where necessary. For confirming insertion of a dsDNA such as a drug cassette, use two pairs of primers, each pair having one primer outside the targeted flanking region and one primer in the inserted DNA, and amplify the two junctions. Another PCR reaction, using the two outside flanking primers, should also be performed to confirm the absence of the gene to be removed, thus ruling out the possibility of a duplication event 53.
? TROUBLESHOOTING
B. Outgrowth and selection of recombinant colonies containing genomic fragments retrieved onto a plasmid by subcloning using recombineering: TIMING 2 d
i. Following electroporation, add the 1ml cell mixture to 9 ml LB medium and grow the culture overnight non-selectively at 32°C.
ii. The next day, isolate plasmid DNA using a standard miniprep protocol. It is not necessary to visualize this DNA by agarose gel electrophoresis.
iii. Introduce the plasmid DNA into a high-efficiency cloning strain by transformation, selecting for the drug resistance of the plasmid or the retrieved selectable marker. Use a low concentration of DNA to minimize uptake of multiple plasmids into the same cell.
iv. Purify drug resistant clones.
v. Grow 5 ml overnight cultures from ~20 isolates with antibiotic selection.
vi. Isolate plasmid DNA and examine by restriction analysis.
? TROUBLESHOOTING
C. Modifying multi-copy plasmids: TIMING 3 d
i. Outgrow the electroporated cultures in at least 1ml LB with aeration for at least 2 h at 30–3°C before applying selection.
ii. After outgrowth, add 9 ml LB and drug for plasmid selection and grow overnight at 30–32°C with aeration.
iii. Isolate plasmid DNA using a standard mini-prep procedure.
iv. When possible, eliminate parental plasmid by cutting with a restriction enzyme that does not cut the recombinant molecule.
v. Introduce plasmid DNA by transformation into a recA strain of E.coli (e.g DH10B) at a low DNA concentration (less than one plasmid per cell). Generally the amount of DNA should be around 0.1ng.
See OPTIONAL BOX 4 for determination of plasmid DNA transformation efficiency.
vi. Select or screen for desired phenotype. Possible methods of screening include restriction digestion, identification of plasmids with altered size as assayed by migration on agarose gels, sequencing, and PCR analysis. CRITICAL STEP Some of the recombinant plasmid molecules will have undergone multimer formation. Avoid these. If monomer recombinants are not apparent a recombinant multimer can be digested with a restriction enzyme having a unique site in the monomer plasmid and the resulting monomer DNA ligated at a low DNA concentration.
vii. Confirm the desired change by sequencing where necessary.
? TROUBLESHOOTING
D. Screening “hit” and “fix” recombinants with colony hybridization TIMING 2 wk
i. After 30 min of outgrowth at 32°C, dilute the cell suspension 10−2 and 10−3 in LB. Spread 200 μl of each diluted suspension onto a 150×10 mm (large) agar plate containing appropriate antibiotic for BACs selection. Try to achieve uniform distribution of colonies throughout the plate. Incubate plates for 18–22 hours at 32°C. Approximately 1–5×103 colonies are expected.
ii. Identify recombinant clones by colony hybridization using a γ-32P-end labeled (or a non-radioactively labeled) oligonucleotide (35-mer) containing the 20 nt heterologous sequence as described in detail by Ausubel et al. 11
iii. Using a bacteriological loop, pick bacterial colonies giving a positive signal and suspend them in 1 ml LB media. Screen at least 3 to 5 positive areas for each BAC modification. Because the recombinant colonies also contain parental cells and because many colonies are close or touching each other on the original plate, a second screening step is required.
iv. Make 10−2, 10−3, and 10−4 dilutions of a bacterial suspension for each colony picked.
v. Plate 50 μl of each dilution on a 15×100mm agar plate containing the appropriate antibiotic for BAC selection. Incubate the plates at 32°C for 18–22 hours.
vi. Select plates with 20–50 clearly isolated colonies and repeat colony hybridization.
vii. Pick two well-isolated positive colonies from each plate for further analysis. Confirm the purity of the clones by colony PCR. Set up two PCR reactions for each clone using primers (20 nt) that will specifically amplify either the recombinant (“hit” clone) or the non-recombinant DNA (Figure 6). Test a total of ten individual colonies corresponding to the 3 to 5 positive areas on the original plate for each BAC modification. CRITICAL STEP: Only one of the two reactions should be positive if the colony is pure. Occasionally, some colonies will amplify PCR products with both sets of primers, implying that at least one modified and one unmodified copy of the BAC is present. These colonies may be due to BAC multimer formation and should be discarded.
viii. Once correctly targeted pure “hit” colonies have been identified, repeat the preceding steps ii through vii used for the “fix” step, beginning with the newly created “hit” strain. CRITICAL STEP When using either the mini-λ or the pSIM plasmids for recombineering, streak the correct “hit” clones on the appropriate antibiotic plate to determine whether they have retained the λ Red system. If necessary, re-introduce the Red system before proceeding.
ix. Confirm the presence of correct mutation after the “fix” step by sequencing.
x. Digest both the original and modified BACs with a few commonly used restriction enzymes (e.g. BamHI, EcoRI, EcoRV, HindIII, PstI etc.). Confirm the integrity of the new BAC by comparing restriction digestion pattern with the original BAC.
? TROUBLESHOOTING
See OPTIONAL BOX 5 for testing colony purity by restriction digestion
See OPTIONAL BOX 6 for detection of selectable marker flanked by loxP or FRT sites
OPTIONAL BOX 1.
Generation of “hit” and “fix” PCR products
1. Synthesize a linear dsDNA cassette with PCR using two 100-mer oligonucleotide primers that overlap by 20 nucleotides (nt) at their 3'-ends, designing the primers so that the resulting DNA contains 80 nt homology arms flanking a 20 nt heterologous region. For each change to be created, the “fix” DNA should contain the same homologies as the corresponding “hit” DNA. Only the central region of the two PCR products will vary. The exact sequence of the “hit” 20 nt central region is not critical as long as it is different from the wild type sequence(s) and contains a restriction site not present in the flanking homology, however, we recommend using our universal 20 base sequence: 5′-GGATCCTAGAATTCCTCGAG-3′.
2. For the “fix” DNA, replace the central 20 bp sequence with the endogenous sequence but include the desired mutational change(s).
3. Verify the size of the PCR products (180 bp) by agarose gel electrophoresis and purify them with a PCR clean-up kit.
OPTIONAL BOX 3.
Electroporation of BAC DNA into bacterial strain of choice TIMING 1 d
1. Pick an isolated colony from an LB plate and grow overnight in 3–5 ml of LB at 32°C.
2. Next morning add 0.5ml of the culture to 25 ml of LB in a 250 ml flask and grow at 32°C to an OD600 of 0.50–0.60. Transfer the culture to a 50 ml Oak Ridge tube and spin at 7000 rpm in pre-chilled rotor for 10 minutes at 4°C.
3. Wash the cell pellet with 20 ml of ice-cold H2O once and then resuspend in 1 ml of H2O and transfer to a chilled 1.5 ml tube. Spin at 14,000 rpm for 20–30 seconds at 4°C.
4. Wash the cells two more times with 1 ml of ice cold H2O. Resuspend cell pellet in H2O in a final volume of 100μl and keep on ice.
5. Mix 100ng of BAC DNA with 50 μl of electro-competent cells and chill on ice for 5 minutes then transfer into a 0.1 cm cuvette. Introduce the BAC DNA into the cells by electroporation (1.8 kV, 25 μF capacitance and 200 ohm resistance). Also do a control electroporation where no DNA is added. After electroporation immediately add 1 ml LB and transfer the cells to a standard sterile culture tube. Incubate cells at 32°C for 1 hour. Spin down cell for 20–30 seconds in a microcentrifuge. Resuspend the pellets in 200 μl of LB. Plate each aliquot of cells on a single LB plate containing chloramphenicol (12.5μg/ml) and incubate for 20–22 hours at 32°C. Only the BAC DNA electroporation should have colonies.
6. Pick 5–10 individual colonies and grow in 3 ml of LB containing 12.5 μg/ml chloramphenicol. Extract BAC DNA and perform restriction digestion with common enzymes like EcoRI, BamHI, PstI, HindIII, EcoRV. Run the sample on 0.8% agarose gel. Compare the restriction pattern with that of the original BAC DNA.
OPTIONAL BOX 2.
Small-scale miniprep for BAC DNA preparation TIMING 1.5 h
1. Inoculate a single colony of DH10B cells containing the BAC into 3 ml of LB containing chloramphenicol (12.5 μg/ml) and grow overnight at 32°C (because of the presence of the Red system) in a shaking incubator at 200–250 rpm.
2. Next day, pellet 1.5 ml of culture by centrifugation in a 1.5 ml tube at 10,000 × g for 1 minute.
3. Add 100 μl of chilled alkaline lysis solution I (25mM Tris-HCl, pH 8.0, 50mM glucose, 10mM EDTA, pH 8.0, 2.5 mg/ml of Lysozyme (Roche Molecular Biochemicals) and RNase A, 100 μg/ml). Resuspend by pipetting the cells up and down using the pipette tip. Place on ice for 5 minutes.
4. Add 200 μl of freshly prepared alkaline lysis solution II (0.2N NaOH and 1% Sarcosyl). Gently invert the tubes until the lysate is clear. Place on ice for 5 minutes.
5. Add 150 μl of alkaline lysis solution III (5M Potassium acetate, pH 4.8) and mix gently until a white precipitate appears. Place on ice for 5 minutes. Centrifuge the tubes at 10,000 × g for 5 minutes and then transfer the supernatant to a clean 1.5 ml tube.
6. Add 2 volumes of 95% ethanol to the supernatant. Mix and place on ice for 30 minutes. Centrifuge at 10,000 × g for 10 minutes. Discard supernatant and wash the pellet with 70% ethanol. Air Dry the pellet and resuspend in 30 – 50μl of 1 X TE (10 mM Tris-HCl, pH 8.0; 1.0 mM EDTA).
OPTIONAL BOX 4.
Determination of plasmid DNA transformation efficiency TIMING 1 d
1. Make electrocompetent cells as described in the protocol, adding a different amount of DNA to each of several electroporations (suggested range is 0.1, 1, 10 and 100 ng).
2. Outgrow the electroporation mixes for 2 hrs and plate 10-fold serial dilutions on petri plates, selecting for the plasmid drug resistance. Use a range of dilutions to obtain countable numbers of colonies for each transformation.
3. The next day, count colonies and generate a semi-log graph of the number of transformants (ordinate) vs. the amount of DNA added (abscissa).
4. From the graph, determine the minimum amount of DNA necessary to achieve maximal transformation efficiency (i.e. without adding excess DNA), and use this amount of DNA for each recombineering reaction.
? TROUBLESHOOTING
OPTIONAL BOX 5.
Testing colony purity by restriction digestion
For this approach the region including the modification site is amplified using PCR primers hybridizing outside the region of homology. The purified PCR product should be digested with a restriction enzyme recognizing the “hit” cassette (Figure 6). Complete digestion of the PCR product indicates that the recombinant BAC is correct and is a pure culture.
OPTIONAL BOX 6.
Deletion of selectable marker flanked by loxP or FRT sites
1. Grow an isolated colony of the recombinant strain containing the correct insertion of the transgene with its selectable marker flanked by loxP or FRT sites at 32°C overnight in 3 ml of LB containing appropriate antibiotics.
2. Dilute 1 ml of the culture into 50 ml of LB containing antibiotics in a 250 ml flask and grow at 32°C.
3. Once the culture reaches an OD600 of 0.3, add 500μl of 10% filter sterilized Arabinose to a final concentration of 0.1%. This induces the flp gene in SW105 or the cre gene in SW106.
4. Grow the culture at 32°C until it reaches an OD600 of 0.5. Plate 100 μl of 10−2, 10−3, 10−4 dilutions on LB plates containing chloramphenicol (12.5 μg/ml) to maintain the BAC and grow overnight at 32°C.
5. Screen for clones that have lost the selectable marker by site-specific recombination, these can be identified by antibiotic sensitivity. Streak ~50–100 colonies in duplicate on LB plates and LB containing the drug. Colonies that grow on LB plates but not in the presence of the drug are the desired clones that have recombined and retain only a single loxP or FRT site.
6. Confirm the loss of the selectable marker by PCR and sequencing.
TIMING
Steps 1–3, design and generation of linear dsDNA substrates for recombineering by PCR: 3–4 h
Steps 4–5, introduction of the λ Red recombination system: 1–2 d
Steps 6–8, induction of the λ Red genes: 3 h
Steps 9–14 preparation of electrocompetent cells: 1 h
Steps 15–18 Electroporation of the linear DNA: <1 h
Step 19: Outgrowth, identification and confirmation of the recombinant clones: 1 d- 2 wk
TROUBLESHOOTING
Troubleshooting advice can be found in Table 5.
TABLE 5.
Troubleshooting guide for Recombineering (also see http://recombineering.ncifcrf.gov/ FAQs)
| Step | Problem | Possible cause(s) | Possible solution(s) |
|---|---|---|---|
| 19 A–D | No recombinants obtained | Problem with bacterial strain Step 4 | Never place recombineering strains at 37°C. |
| For strains containing defective prophage and λTet, verify that strain is temperature sensitive by plating dilutions at 32 and 42°C. | |||
| For strains containing mini-λ and pSIMs, verify drug resistance. | |||
| Do control recombineering reaction (i.e. drug cassette insertion) to check strain. | |||
| Cell loss during washing for electroporation Step 12 | Titer viable cells as well as plating for recombinants - if viables are less than 107/ml, recombinants may not be obtained. Be careful not to lose cells during H2O wash steps. | ||
| Problem with induction procedure Step 8 | Using shaking H2O baths for both 32°C growth and 42°C induction, do not use air shaker. Confirm temperatures of H2O baths. Do not exceed 15 min 42°C induction. Be sure to quick-chill flasks in ice bath after induction. If scaling up procedure maintain 1/10 media/flask ratio. | ||
| Problem with linear DNA targeting construct Step 3 | Remake PCR product or obtain new oligos | ||
| Problem with electrocompetent cells Steps 15–17 | Test cells by transforming supercoiled plasmid. Use correct electroporation conditions. Be sure time constant for electroporation is ~5msec. Add LB immediately after electroporation. |
||
| Drug concentration too high see Table 4 | Be sure to use antibiotic concentrations for single copy molecules when appropriate. | ||
| 19 A | No recombinants obtained with drug selection | Inadequate outgrowth time | Allow greater than 2 hr outgrowth if needed. Increase LB volume so more cell doublings occur. |
| 19 A | No recombinants or transformants with double drug selection | Double drug selection issues | Try to use only one drug selection at a time rather than combining drugs. Do not maintain selection for BAC or pSIM plasmid when selecting drug-resistant recombinants. |
| 19 A–C | Colonies in uninduced control reactions | Incomplete digestion of plasmid template used for PCR so intact plasmid is transformed Step 3 | Digest plasmid before using as template, digest PCR product with DpnI following amplification. |
| Leaky selection | Alter selection e.g. increase drug concentration. | ||
| Contamination | Use sterile technique. Test solutions for source of contamination and discard if necessary. | ||
| 19 A–D | Difficulty obtaining pure clones: BAC, PAC or E. coli chromosome | Incomplete colony purification | Re-streak for single colonies and retest. |
| Difficulty obtaining pure clones: BAC or PAC | Possible multimer formation | Screen more candidates. | |
| 19 B | No DNA retrieved, only empty vector | Vector is re-circularizing by microhomologies. | Either redesign the primers or place a selectable marker next to the DNA to be retrieved and retrieve with plasmid ori only. |
| 19 C | Difficulty obtaining pure clones: multicopy plasmids | Contamination with parental plasmid | Transform DNA prep at low DNA concentration (less than one molecule per cell), select or screen for recombinant. |
| No recombinants | Poor plasmid transformation efficiency | Determine plasmid transformation efficiency as described in optional procedure | |
| Hetero-allelic multimer | Either screen other candidates or digest hetero-allelic multimer with a unique cutter and re-ligate at a low DNA concentration. | ||
| Multimers with plasmid recombineering | Inherent nature of recombineering | Screen other isolates. Use restriction digestion to convert to monomer, re-transform and screen for recombinant plasmids. |
|
| Use of RecA+ host | Use only a recA mutant host for plasmid modification. | ||
| Plasmid was a multimer initially. | Start with a pure monomer plasmid species. |
ANTICIPATED RESULTS
Recombineering is an efficient and relatively straightforward method of in vivo genetic engineering that is rapidly becoming routine for modification of the bacterial genome, plasmids, PACs and BACs. Because recombineering allows DNA to be precisely inserted, altered, or deleted it is very useful for generation of various genomic constructs for functional studies. Linear DNA, both PCR products and synthetic oligonucleotides (oligos) can be used as substrates to create null mutations in genes as well as more subtle genetic changes. Generally, recombinants are obtained at a frequency of 10−4–10−5 when dsDNA is used to make insertions. For optimized recombineering with ss-oligos or largely homologous denatured PCR fragments the frequency of recombinants is in the range of 0.1–10%. The frequency of cloning by gene retrieval is lower, in the range of 10−5–10−6. Insertion of reporter genes at the 5′ or 3′ ends of genes cloned in BACs can be easily achieved. There are a number of critical steps in the recombineering procedure; each step should be optimized. Deviations from the protocol may result in sub-optimal results; the overall effect of not optimizing each step can be a reduction in recombination efficiency and even failure to generate recombinants.
ACKNOWLEDGEMENTS
We thank Nina Costantino and James Sawitzke for helpful comments. This research was supported by the Intramural Research Program of NIH, National Cancer Institute, Center for Cancer Research. This project has been funded in whole or in part with federal funds from the National Cancer Institutes of Health, under contract N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Footnotes
COMPETING INTERESTS STATEMENT The authors declare that they have no competing financial interests.
REFERENCES
- 1.Copeland NG, Jenkins NA, Court DL. Recombineering: a powerful new tool for mouse functional genomics. Nat. Rev. Genet. 2001;2:769–779. doi: 10.1038/35093556. [DOI] [PubMed] [Google Scholar]
- 2.Ellis HM, Yu D, DiTizio T, Court DL. High efficiency mutagenesis, repair, and engineering of chromosomal DNA using single-stranded oligonucleotides. Proc. Natl. Acad. Sci. U. S. A. 2001;98:6742–6746. doi: 10.1073/pnas.121164898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu D, et al. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 2000;97:5978–5983. doi: 10.1073/pnas.100127597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Buchholz F, Muyrers JP, Stewart AF. A new logic for DNA engineering using recombination in Escherichia coli. Nat. Genet. 1998;20:123–128. doi: 10.1038/2417. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Muyrers JP, Testa G, Stewart AF. DNA cloning by homologous recombination in Escherichia coli. Nat. Biotechnol. 2000;18:1314–1317. doi: 10.1038/82449. [DOI] [PubMed] [Google Scholar]
- 6.Murphy KC. Use of bacteriophage λ recombination functions to promote gene replacement in Escherichia coli. J. Bacteriol. 1998;180:2063–2071. doi: 10.1128/jb.180.8.2063-2071.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy KC, Campellone KG, Poteete AR. PCR-mediated gene replacement in Escherichia coli. Gene. 2000;246:321–330. doi: 10.1016/s0378-1119(00)00071-8. [DOI] [PubMed] [Google Scholar]
- 8.Swaminathan S, et al. Rapid engineering of bacterial artificial chromosomes using oligonucleotides. Genesis. 2001;29:14–21. doi: 10.1002/1526-968x(200101)29:1<14::aid-gene1001>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 9.Costantino N, Court DL. Enhanced levels of ⌊ Red-mediated recombinants in mismatch repair mutants. Proc Natl Acad Sci U.S.A. 2003;100:15748–15753. doi: 10.1073/pnas.2434959100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Datta S, Costantino N, Zhou X, Court DL. Identification and analysis of recombineering functions from Gram-negative and Gram-positive bacteria and their phages. Proc. Natl. Acad. Sci. U. S. A. 2008;105:1626–1631. doi: 10.1073/pnas.0709089105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ausubel FM, et al., editors. Current Protocols in Molecular Biology. Chapters 1 6. Vol. 1. John Wiley & Sons, Inc.; Hoboken, N.J., United States of America: 2008. [Google Scholar]
- 12.Sawitzke JA, et al. Recombineering: in vivo genetic engineering in E. coli, S. enterica, and beyond. Methods Enzymol. 2007;421:171–199. doi: 10.1016/S0076-6879(06)21015-2. [DOI] [PubMed] [Google Scholar]
- 13.Thomason LC, et al. In: Current Protocols in Molecular Biology. Chapter 1. Ausubel FM, et al., editors. Vol. 1. John Wiley & Sons, Inc.; Hoboken, N.J.: 2007. pp. 1–24. Unit 16. [Google Scholar]
- 14.Sternberg NL. Cloning high molecular weight DNA fragments by the bacteriophage P1 system. Trends Genet. 1992;8:11–16. doi: 10.1016/0168-9525(92)90018-y. [DOI] [PubMed] [Google Scholar]
- 15.Shizuya H, et al. Cloning and stable maintenance of 300-kilobase-pair fragments of human DNA in Escherichia coli using an F-factor-based vector. Proc. Natl. Acad. Sci. U. S. A. 1992;89:8794–8797. doi: 10.1073/pnas.89.18.8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muyrers JP, et al. In: Methods in Molecular Biology: Bacterial Artificial Chromosomes Volume.2: Functional Studies. Zhao S, Stodolsky M, editors. Vol. 256. Humana Press; Totowa, N.J.: 2004. pp. 107–121. [Google Scholar]
- 17.Heaney JD, Rettew AN, Bronson SK. Tissue-specific expression of a BAC transgene targeted to the Hprt locus in mouse embryonic stem cells. Genomics. 2004;83:1072–1082. doi: 10.1016/j.ygeno.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Kuznetsov S, et al. RAD51C deficiency in mice results in early prophase I arrest in males and sister chromatid separation at metaphase II in females. J. Cell Biol. 2007;176:581–592. doi: 10.1083/jcb.200608130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y, Swaminathan S, Martin BK, Sharan SK. Aberrant splicing induced by missense mutations in BRCA1: clues from a humanized mouse model. Hum. Mol. Genet. 2003;12:2121–2131. doi: 10.1093/hmg/ddg222. [DOI] [PubMed] [Google Scholar]
- 20.Chan W, et al. A recombineering based approach for high-throughput conditional knockout targeting vector construction. Nucleic Acids Res. 2007;35:e64. doi: 10.1093/nar/gkm163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 2003;13:476–484. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karu AE, Sakaki Y, Echols H, Linn S. The gamma protein specified by bacteriophage λ. Structure and inhibitory activity for the RecBC enzyme of Escherichia coli. J. Biol. Chem. 1975;250:7377–7387. [PubMed] [Google Scholar]
- 23.Murphy KC. λ Gam protein inhibits the helicase and chi-stimulated recombination activities of Escherichia coli RecBCD enzyme. J. Bacteriol. 1991;173:5808–5821. doi: 10.1128/jb.173.18.5808-5821.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karakousis G, et al. The β protein of phage ⌊ binds preferentially to an intermediate in DNA renaturation. J. Mol. Biol. 1998;276:721–731. doi: 10.1006/jmbi.1997.1573. [DOI] [PubMed] [Google Scholar]
- 25.Kmiec E, Holloman WK. ® protein of bacteriophage ⌊ promotes renaturation of DNA. J. Biol. Chem. 1981;256:12636–12639. [PubMed] [Google Scholar]
- 26.Muniyappa K, Mythili E. Phage λ β protein, a component of general recombination, is associated with host ribosomal S1 protein. Biochem. Mol. Biol. Int. 1993;31:1–11. [PubMed] [Google Scholar]
- 27.Cassuto E, Radding CM. Mechanism for the action of λ exonuclease in genetic recombination. Nature New Biol. 1971;229:13–16. doi: 10.1038/newbio229013a0. [DOI] [PubMed] [Google Scholar]
- 28.Little JW. An exonuclease induced by bacteriophage ⌊. II. Nature of the enzymatic reaction. J. Biol. Chem. 1967;242:679–686. [PubMed] [Google Scholar]
- 29.Carter DM, Radding CM. The role of exonuclease and β protein of phage λ in genetic recombination. II. Substrate specificity and the mode of action of lambda exonuclease. J. Biol. Chem. 1971;246:2502–2512. [PubMed] [Google Scholar]
- 30.Cassuto E, Lash T, Sriprakash KS, Radding CM. Role of exonuclease and β protein of phage λ in genetic recombination. V. Recombination of λ DNA in vitro. Proc. Natl. Acad. Sci. U. S. A. 1971;68:1639–1643. doi: 10.1073/pnas.68.7.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Court DL, Sawitzke JA, Thomason LC. Genetic engineering using homologous recombination. Annu. Rev. Genet. 2002;36:361–388. doi: 10.1146/annurev.genet.36.061102.093104. [DOI] [PubMed] [Google Scholar]
- 32.Sergueev K, Yu D, Austin S, Court D. Cell toxicity caused by products of the pL operon of bacteriophage lambda. Gene. 2001;272:227–235. doi: 10.1016/s0378-1119(01)00535-2. [DOI] [PubMed] [Google Scholar]
- 33.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, et al. An improved recombineering approach by adding RecA to λ Red recombination. Mol. Biotechnol. 2006;32:43–53. doi: 10.1385/mb:32:1:043. [DOI] [PubMed] [Google Scholar]
- 35.Guzman LM, Belin D, Carson MJ, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose pBAD promoter. J. Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Court DL, Oppenheim AB, Adhya SL. A new look at bacteriophage λ genetic networks. J. Bacteriol. 2007;189:298–304. doi: 10.1128/JB.01215-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dodd IB, Shearwin KE, Egan JB. Revisited gene regulation in bacteriophage lambda. Curr. Opin. Genet. Dev. 2005;15:145–152. doi: 10.1016/j.gde.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Murphy KC, Campellone KG. Lambda Red-mediated recombinogenic engineering of enterohemorrhagic and enteropathogenic E. coli. BMC Mol Biol. 2003;4:11. doi: 10.1186/1471-2199-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gay P, Le Coq D, Steinmetz M, Berkelman T, Kado CI. Positive selection procedure for entrapment of insertion sequence elements in gram-negative bacteria. J. Bacteriol. 1985;164:918–921. doi: 10.1128/jb.164.2.918-921.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warming S, Costantino N, Court DL, Jenkins NA, Copeland NG. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 2005;33:e36. doi: 10.1093/nar/gni035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rivero-Muller A, Lajic S, Huhtaniemi I. Assisted large fragment insertion by Red/ET-recombination (ALFIRE)--an alternative and enhanced method for large fragment recombineering. Nucleic Acids Res. 2007;35:e78. doi: 10.1093/nar/gkm250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong QN, et al. Efficient and seamless DNA recombineering using a thymidylate synthase A selection system in Escherichia coli. Nucleic Acids Res. 2005;33:e59. doi: 10.1093/nar/gni059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeVito JA. Recombineering with tolC as a selectable/counter-selectable marker: remodeling the rRNA operons of Escherichia coli. Nucleic Acids Res. 2008;36:e4. doi: 10.1093/nar/gkm1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee EC, et al. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics. 2001;73:56–65. doi: 10.1006/geno.2000.6451. [DOI] [PubMed] [Google Scholar]
- 45.Datta S, Costantino N, Court DL. A set of recombineering plasmids for gram-negative bacteria. Gene. 2006;379:109–115. doi: 10.1016/j.gene.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 46.Kotzamanis G, Huxley C. Recombining overlapping BACs into a single larger BAC. BMC Biotechnol. 2004;4:1. doi: 10.1186/1472-6750-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Venken KJ, He Y, Hoskins RA, Bellen HJ. P[acman]: a BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science. 2006;314:1747–1751. doi: 10.1126/science.1134426. [DOI] [PubMed] [Google Scholar]
- 48.Yang Y, Sharan SK. A simple two-step, `hit and fix' method to generate subtle mutations in BACs using short denatured PCR fragments. Nucleic Acids Res. 2003;31:e80. doi: 10.1093/nar/gng080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li XT, et al. Identification of factors influencing strand bias in oligonucleotide-mediated recombination in Escherichia coli. Nucleic Acids Res. 2003;31:6674–6687. doi: 10.1093/nar/gkg844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomason LC, Costantino N, Shaw DV, Court DL. Multicopy plasmid modification with phage lambda Red recombineering. Plasmid. 2007;58:148–158. doi: 10.1016/j.plasmid.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. Third Edition Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 2001. [Google Scholar]
- 52.Court DL, et al. Mini-λ: a tractable system for chromosome and BAC engineering. Gene. 2003;315:63–69. doi: 10.1016/s0378-1119(03)00728-5. [DOI] [PubMed] [Google Scholar]
- 53.Bubunenko M, Baker T, Court DL. Essentiality of ribosomal and transcription antitermination proteins analyzed by systematic gene replacement in Escherichia coli. J. Bacteriol. 2007;189:2844–2853. doi: 10.1128/JB.01713-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Durfee T, et al. The complete genome sequence of Escherichia coli DH10B: Insights into the biology of a laboratory workhorse. J. Bacteriol. 2008;190:2597–2606. doi: 10.1128/JB.01695-07. [DOI] [PMC free article] [PubMed] [Google Scholar]