The structures of α- and β-diversonolic esters (1 and 2, Figure 1)[1] define the key structural motifs of a growing family of natural products, some members of which exhibit striking antibiotic activities. Among them are diversonol (3, Figure 1),[2] the first member of the monomeric series within the class, and the rugulotrosins,[3] secalonic acids[4] and hirtusneanoside,[5] all of which are dimeric in nature.
Figure 1.
Structures of α- and β-diversonolic esters (1, 2, 4 and 5) and diversonol (3).
In a program directed towards the total synthesis of these molecules, we focused initially on the development of new synthetic technologies for the synthesis of the basic molecular framework of the class, and, therefore, turned our attention to α- and β-diversonolic esters (1 and 2) and diversonol (3), the simplest members of the family. In this communication we report the development of an expedient synthetic strategy for the construction of the diversonolic architecture, its application to concise total syntheses of all three structures 1–3, and the revision of the originally proposed structures for α- and β-diversonolic esters 1 and 2, to 4 and 5, respectively, through total synthesis.
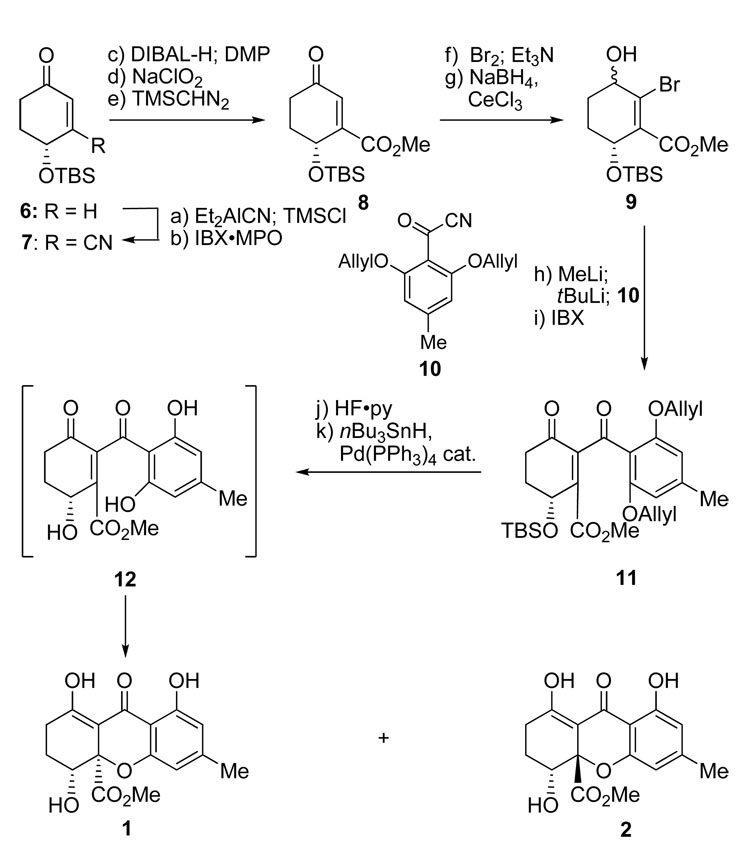
Scheme 1 summarizes the devised synthesis of the originally assigned structures of α- and β-diversonolic esters 1 and 2. Thus, reaction of racemic enone 6 with Et2AlCN followed by trapping with TMSCl in the presence of pyridine furnished the corresponding nitrile TMS enol ether, which was transformed without isolation to nitrile enone 7 through the action of IBX•MPO,[6] in 62 % overall yield. Conversion of the latter to its ester enone counterpart (8) required DIBAL-H reduction (hydroxy aldehyde), DMP oxidation (ketoaldehyde, 83 % yield, two steps), Pinnick oxidation, and treatment with TMSCHN2 (90 % yield, two steps). Ester enone 8 was then converted to bromo hydroxy ester 9 (ca. 1.3:1 dr) through sequential reaction with bromine and Et3N (bromo enone, 94 % yield), followed by reduction with NaBH4-CeCl3 (91 % yield). Deprotonation of bromo hydroxy ester 9 with MeLi (1.1 equiv) followed by sequential treatment with tBuLi and acyl cyanide 10 afforded the expected alcohol (ca. 1.3:1 dr), which was oxidized with IBX to give diketone 11 in 41 % overall yield. Finally, desilylation of 11 (HF•py), followed by deallylation (nBu3SnH, Pd(PPh3)4 cat.), furnished structures 1 and 2 (1:2 ca. 2:1, chromatographically separated), presumably through the intermediacy of 12. The spectroscopic data (1H and 13C NMR) of these compounds, however, did not match those reported[1] for the natural products α- and β-diversonolic esters. The skeletal connectivity of compounds 1 and 2 was established by HMBC studies and their relative stereochemistry was assigned by comparison of NMR data with similar compounds.[7] The structure of 2 (m.p. 183–184 °C, EtOAc:hexanes 1:1) was verified by X-ray crystallographic analysis (see ORTEP drawing, Figure 2).[8]
Scheme 1.
Synthesis of the proposed structures of α- and β-diversonolic esters (1 and 2). Reagents and conditions: a) Et2AlCN (1.0 M in toluene, 1.2 equiv), toluene, 23 °C, 30 min; then pyridine (3.0 equiv), TMSCl (1.8 equiv), 0→23 °C, 1 h; b) IBX (1.2 equiv), MPO (1.2 equiv), DMSO, 23 °C, 1 h, 62 % over two steps; c) DIBAL-H (1.0 M in CH2Cl2, 2.5 equiv), toluene, −78→ −40 °C, 30 min; then DMP (1.2 equiv), CH2Cl2, 23 °C, 45 min, 83 %; d) NaClO2 (3.0 equiv), 2-methyl-2-butene (10 equiv), NaH2PO4 (5.0 equiv), tBuOH/H2O (1:1), 23 °C, 1 h; e) TMSCHN2 (2.0 M in ether, 2.0 equiv), MeOH, 0 °C, 20 min, 90 % over two steps; f) Br2 (1.05 equiv), CH2Cl2, 0 °C, 5 min; then Et3N (1.5 equiv), 0 °C, 5 min, 94 %; g) CeCl3•7H2O (1.2 equiv), NaBH4 (2.0 equiv), MeOH, 0 °C, 30 min, 91 %, ca. 1.3:1 dr; h) MeLi (1.6 M in ether, 1.1 equiv), ether, −78 °C, 15 min; then tBuLi (1.7 M in pentane, 2.2 equiv), −78 °C, 15 min; then 10 (1.5 equiv), −78→ −40 °C, 40 min; i) IBX (2.0 equiv), DMSO, 23 °C, 1 h, 41 % over two steps; j) HF•py/THF (1:5), 23 °C, 12 h, 89 %; k) nBu3SnH (2.2 equiv), AcOH (2.2 equiv), Pd(PPh3)4 (0.05 equiv), benzene, 23 °C, 1 h, 60 %, 1:2 ca. 2:1 dr. DIBAL-H =diisobutylaluminum hydride, DMP=Dess–Martin periodinane, IBX=o-iodoxybenzoic acid, MPO=4-methoxypyridine-N-oxide, py=pyridine, TMS=trimethylsilyl.
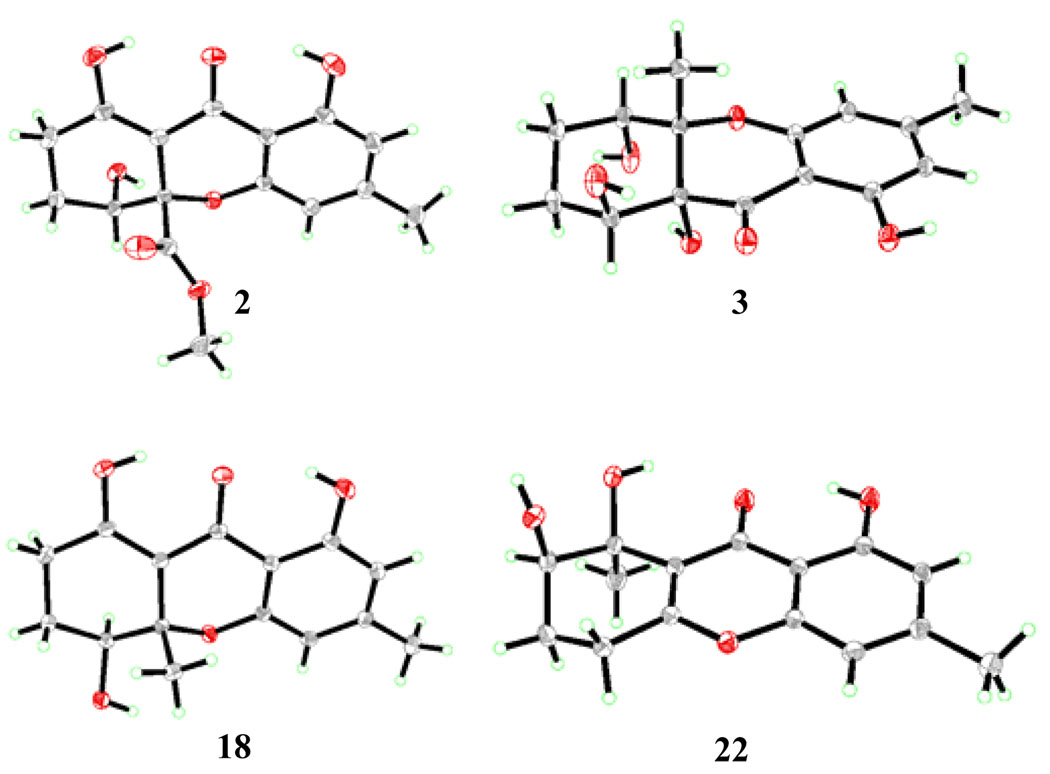
Figure 2.
ORTEP drawings of compound 2, 3, 18 and 22 derived from X-ray crystallographic analysis (non-hydrogen atoms are shown as 30 % ellipsoids).
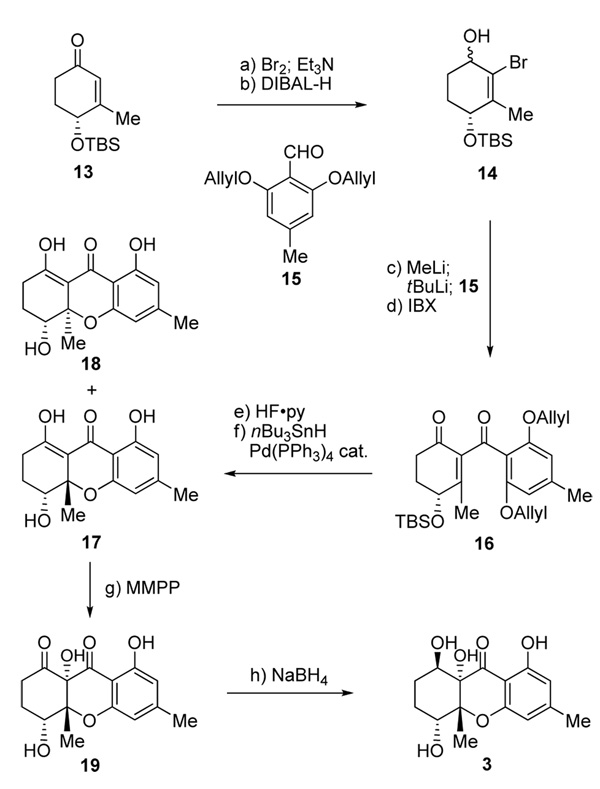
At this stage, and not yet knowing the true structures of α- and β-diversonolic esters, we turned our attention to the synthesis of diversonol (3) through application of the developed synthetic strategy. Thus, substituting the nitrile with a methyl group in the starting enone (now 13, Scheme 2) and following a similar route as before, but proceeding through intermediates 14–19 (17:18 ca. 2:1), we arrived at diversonol (3) in eight steps as summarized in Scheme 2. The spectral data (1H and 13C NMR) of synthetic diversonol matched those reported for the natural substance[2] and those of synthetic diversonol kindly provided by Professor S. Bräse, whose group was the first to synthesize this natural product.[9] The structures of synthetic compounds 18 (m.p. 154–155 °C, EtOAc:hexanes 1:1) and 3 were also confirmed by crystallographic analysis (ORTEP drawing, Figure 2).[8]
Scheme 2.
Total synthesis of diversonol (3). Reagents and conditions: a) Br2 (1.05 equiv), CH2Cl2, 0 °C, 5 min; then Et3N (1.5 equiv), 0 °C, 5 min, 90 %; b) DIBAL-H (1.0 M in hexanes, 1.5 equiv), THF, −78→ −40 °C, 30 min, 95 %, ca. 1:1 dr; c) MeLi (1.6 M in ether, 1.1 equiv), ether, −78 °C, 15 min; then tBuLi (1.7 M in pentane, 2.2 equiv), −78 °C, 15 min; then 15 (1.5 equiv), −78→ −40 °C, 40 min; d) IBX (3.0 equiv), DMSO, 23 °C, 1 h, 72 % over two steps; e) HF•py/THF (1:5), 23 °C, 12 h, 96 %; f) nBu3SnH (2.2 equiv), AcOH (2.2 equiv), Pd(PPh3)4 (0.05 equiv), benzene, 23 °C, 1 h, 90 %, 17:18 ca. 2:1 dr; g) MMPP (0.75 equiv), EtOH, 23 °C, 30 min; h) NaBH4 (1.0 equiv), MeOH/CH2Cl2 (1:1), −78 °C, 15 min, 73 % over two steps. MMPP=magnesium monoperoxophthalate.
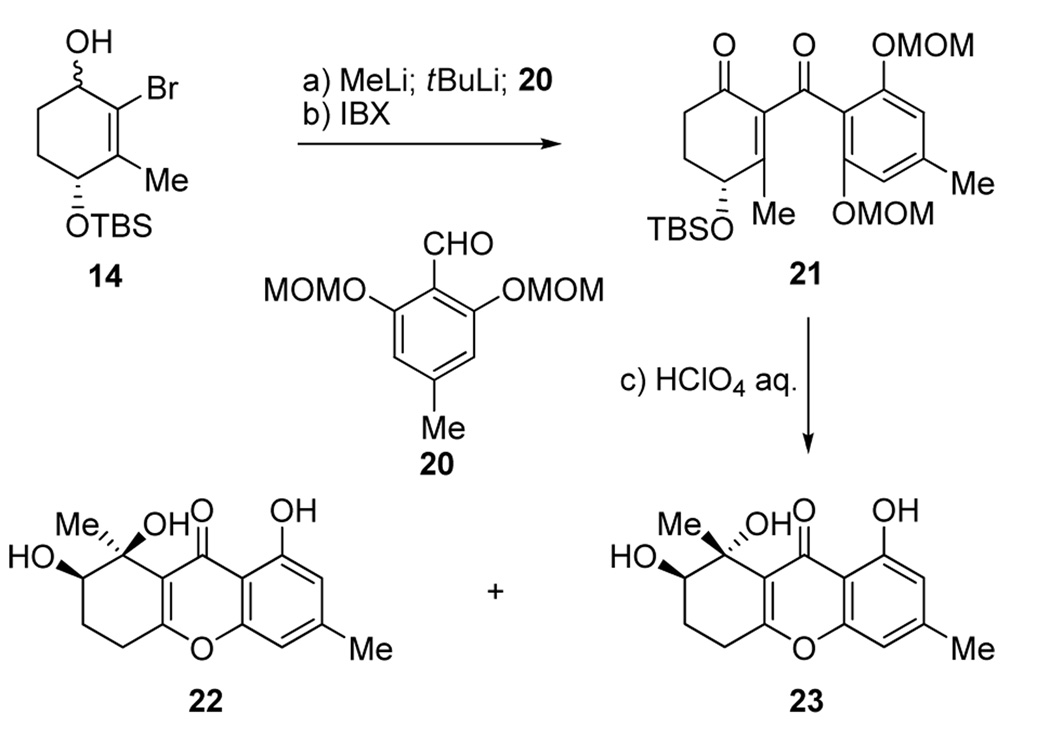
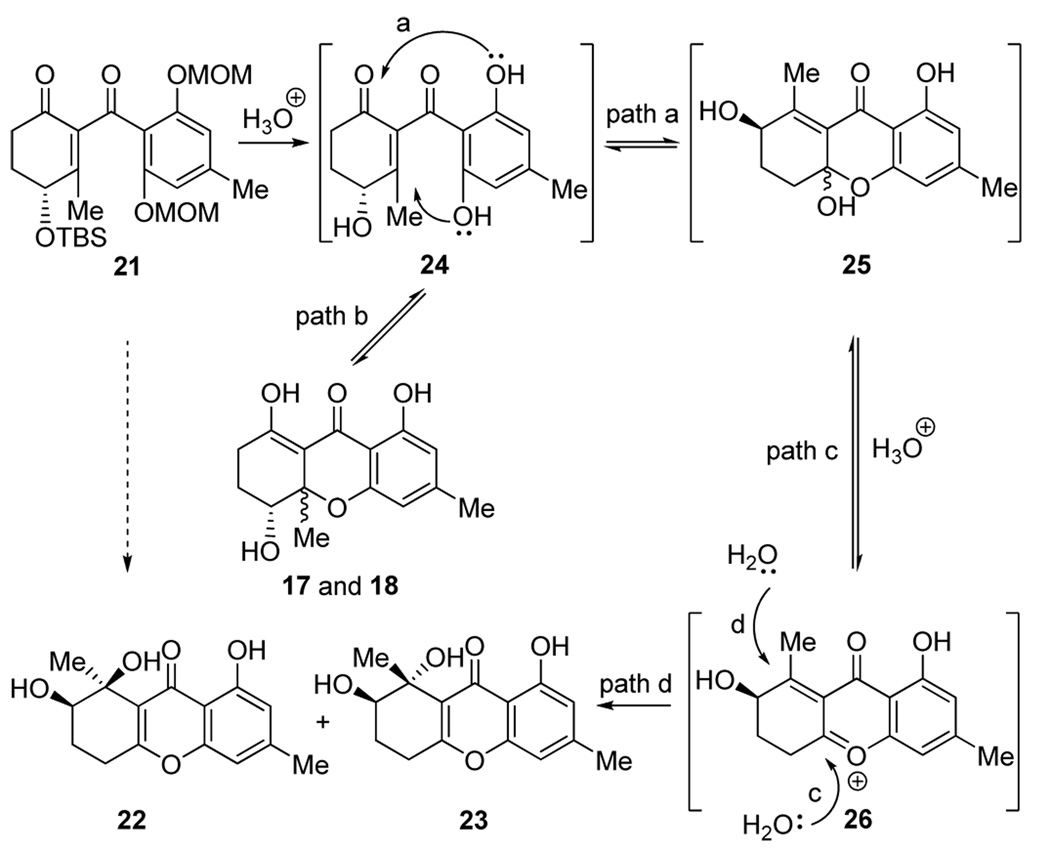
In what turned out to be a fortunate twist of fate, and aimin improving and streamlining our synthesis of diversonol (3), we decided to employ MOM protecting groups on the aromatic segment of the molecule (aldehyde 20, Scheme 3). We, therefore, proceeded to prepare, by the same route, intermediate 21 (Scheme 3), whose global deprotection under acidic conditions was expected to give compound 17 (Scheme 2) in one step, rather than two, and avoiding the organometallic reagent and catalyst employed in the first route. Upon exposure of 21 to aqueous HClO4, however, we did not observe any of the expected products (i.e. 17 and 18, Scheme 2), but instead obtained two new compounds (ca. 2:1 ratio) isomeric in nature to the previously obtained products (17 and 18) and to each other. Fortunately, one of these compounds (major, less polar, silica gel, hexanes: EtOAc 1:1) crystallized from hexanes: EtOAc (1:1) in beautiful colorless needles (m.p. 175–176 °C, EtOAc:hexanes 1:1). One of these crystals was subjected to X-ray crystallographic analysis (see ORTEP drawing, Figure 2),[8] which revealed its structure as 22, and hence, that of the other isomer as 23 (Scheme 3).
Scheme 3.
Construction of compounds 22 and 23. Reagents and conditions: a) MeLi (1.6 M in ether, 1.1 equiv), ether, −78 °C, 15 min; then tBuLi (1.7 M in pentane, 2.2 equiv), −78 °C, 15 min; then 20 (1.5 equiv), −78→ −40 °C, 40 min; b) IBX (3.0 equiv), DMSO, 22 °C, 1 h, 78 % over two steps; c) 1.0 M HClO4 aq. /THF (1:1), 40 °C, 2 h, 90 %, 22:23 ca. 2:1 dr.
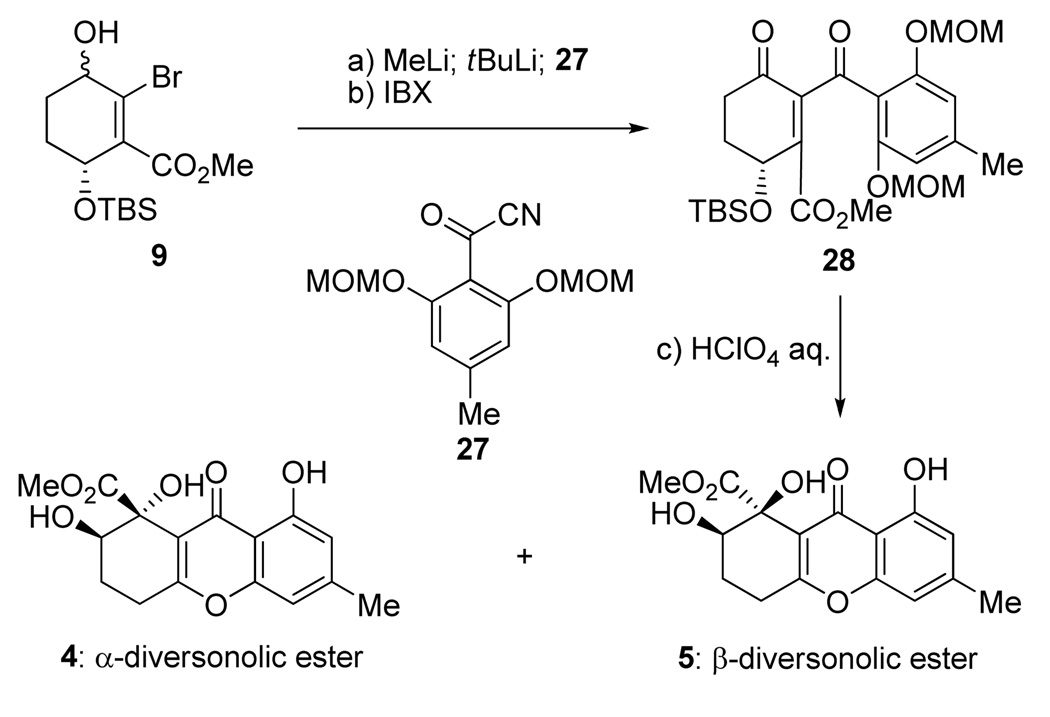
The proposed mechanism for the formation of compounds 22 and 23 from 21 through postulated intermediates 24–26 (Scheme 4) served as the basis for the total synthesis and structural revision of α- and β-diversonolic esters. Apparently, the initially formed structures undergo skeletal rearrangements to structures such as 22 and 23 under the acidic conditions employed for the deprotection. The similarity of the NMR data of 22 and 23 to those of the natural diversonolic esters led us to propose structures 4 and 5 as the revised structures of 1 and 2, respectively. Scheme 5 summarizes our syntheses of 4 and 5 starting with hydroxy bromo ester 9 and bis-MOM protected acyl cyanide 27 and proceeding through intermediate 28 (4:5 ca. 1:3 dr). The spectral properties of synthetic diversonolic esters 4 and 5 were consistent with their structures and matched those reported[1] for the naturally occurring substances. The structural connectivity of 4 and 5 was established by HMBC studies and their relative stereochemistry was assigned by nOe studies. The revised structures of α- and β-diversonolic esters (4 and 5) also explain the ambiguities in the spectral and chemical properties of these compounds described in the original isolation report.[1]
Scheme 4.
Postulated mechanism of the formation of 22 and 23.
Scheme 5.
Total synthesis of the revised structures of α- and β-diversonolic esters (4 and 5). Reagents and conditions: a) MeLi (1.6 M in ether, 1.1 equiv), ether, −78 °C, 15 min; then tBuLi (1.7 M in pentane, 2.2 equiv), −78 °C, 15 min; then 20 (1.5 equiv), −78→ −40 °C, 40 min; b) IBX (2.0 equiv), DMSO, 23°C, 1 h, 45 % over two steps; c) 1.0 M HClO4 aq./THF (1:1), 50 °C, 2 h, 80 %, 4:5 ca. 1:3 dr.
In a final twist, a naturally occurring compound named blennolide C, bearing the structure of 2, was reported[10] while this manuscript was in preparation. Although a natural product with the structure 1 has not yet been reported, it can be predicted to exist, especially as it is present as a monomeric unit in some dimeric natural products such as the rugulotrosins.[3]
Besides uncovering the true structures of α- and β-diversonolic esters and rendering them and diversonol as well as blennolide C readily available, the described chemistry opens an expedient entry into these and the more complex members of this class of natural products, including their enatiomerically pure forms and their analogs. Since both enantiomers of starting materials (6[11] and 13[12]) are known, the as yet undetermined absolute stereochemistry of all three natural products (3–5) should be easily discernable. Finally, biological investigations with the synthesized compounds may reveal interesting properties.
Supplementary Material
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
Footnotes
We thank Dr. R. K. Chadha, Dr. D. H. Huang, and Dr. G. Siuzdak for X-ray crystallographic, NMR spectroscopic, and mass spectrometric assistance, respectively. We also gratefully acknowledge Profs. Stefan Bräse and Robert J. Capon for providing the spectra of synthetic diversonol and natural rugulotrosins, respectively, and Drs. David J. Edmonds and Achim F. Lenzen for helpful discussions. This work was supported by the National Institute of Health, the Skaggs Institute for Chemical Biology, and a Bristol-Myers Squibb Graduate fellowship (to A. L.).
Contributor Information
Dr. K. C. Nicolaou, Department of Chemistry and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 USA), Fax: (+1) 858-784-2469, E-mail: kcn@scripps.edu
Ang Li, Department of Chemistry and Biochemistry, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093 (USA).
References
- 1.Holker JSE, O'Brien E, Simpson TJ. J. Chem. Soc Perkin Trans. 1. 1983:1365–1368. [Google Scholar]
- 2.Turner WB. J. Chem. Soc., Perkin Trans. 1. 1978:1621. [Google Scholar]
- 3.Stewart M, Capon RJ, White JM, Lacey E, Tennant S, Gill JH, Shaddock MP. J. Nat. Prod. 2004;67:728–730. doi: 10.1021/np034038b. [DOI] [PubMed] [Google Scholar]
- 4.Franck B, Gottschalk EM, Ohnsorge U, Baumann G. Angew. Chem. 1964;76:438–439. Angew. Chem. Int. Ed.1964, 3, 441–442. [Google Scholar]
- 5.Rezanka T, Sigler K. J. Nat. Prod. 2007;70:1487–1491. doi: 10.1021/np070079m. [DOI] [PubMed] [Google Scholar]
- 6.Nicolaou KC, Gray DLF, Montagnon T, Harrison ST. Angew. Chem. 2002;114:1038–1042. doi: 10.1002/1521-3773(20020315)41:6<996::aid-anie996>3.0.co;2-i. Angew. Chem. Int. Ed.2002, 41, 996–1000. [DOI] [PubMed] [Google Scholar]
- 7. Ducrot PH, Lallemand JY, Milat ML, Blen JP. Tetrahedron Lett. 1994;47:8797–8800. The spectra of 1 and 2 also showed great similarity to those of 18 and 17, respectively.
- 8.CDCC-689919, -689920, -689921, and -689922 contain the supplementary crystallographic data of compounds 2, 3, 18 and 22, respectively. These data can be obtained free of charge at www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2EZ, UK; fax: (+44) 1223-336-033; or deposit@ccdc.cam.ac.uk).
- 9.Nising CF, Ohnemüller UK, Bräse S. Angew. Chem. 2006;118:313–315. doi: 10.1002/anie.200502913. Angew. Chem. Int. Ed.2006, 45, 307–309. [DOI] [PubMed] [Google Scholar]
- 10. Zhang W, Krohn K, Ullah Z, Flörke U, Pescitelli G, Di Bari L, Antus S, Kurtán T, Rheinheimer J, Draeger S, Schulz B. Chem. Eur. J. 2008;14:4913–4923. doi: 10.1002/chem.200800035. These authors apparently recognized that the structure of the diversonoic ester needed to be revised. The spectroscopic data of synthesis 2 were identical to those reported for natural blennolide C.
- 11.a) Hua Z, Yu W, Su M, Jin Z. Org. Lett. 2005;7:1939–1942. doi: 10.1021/ol050339w. [DOI] [PubMed] [Google Scholar]; b) Audia JE, Boisvert L, Patten AD, Villalobos A, Danishefsky SJ. J.Org. Chem. 1989;54:3738–3740. [Google Scholar]
- 12.a) Nicolaou KC, Li H, Nold AL, Pappo D, Lenzen A. J. Am. Chem. Soc. 2007;129:10356–10357. doi: 10.1021/ja074297d. [DOI] [PubMed] [Google Scholar]; b) Xia J, Brown LE, Konopelski JP. J. Org. Chem. 2007;72:6885–6890. doi: 10.1021/jo071156l. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.