Abstract
Loss of imprinting (LOI) is the reactivation of the silenced allele of an imprinted gene, leading to perturbation of monoallelic expression. We tested the hypothesis that LOI of PLAGL1, a representative maternally imprinted gene, occurs through an all-or-none process leading to a mixture of fully imprinted and nonimprinted cells. Herein using a quantitative RT-PCR-based experimental approach, we measured LOI at the single cell level in human trophoblasts and demonstrated a broad distribution of LOI among cells exhibiting LOI, with the mean centered at ∼100% LOI. There was a significant (P < 0.01) increase in expression after 2 days of 5-aza-2′-deoxycytidine (AZA) treatment and a significant (P < 0.01) increase in LOI after both 1 and 2 days of AZA treatment, while the distribution remained broad and centered at ∼100% LOI. We propose a transcriptional pulsing model to show that the broadness of the distribution reflects the stochastic nature of expression between the two alleles in each cell. The mean of the distribution of LOI in the cells is consistent with our hypothesis that LOI occurs by an all-or-none process. All-or-none LOI could lead to a second distinct cell population that may have a selective advantage, leading to variation of LOI in normal tissues, such as the placenta, or in neoplastic cells.
INTRODUCTION
Genomic imprinting is the silencing of one parental allele in the zygotes of gametes leading to monoallelic expression of the gene in the offspring (1). Several epigenetic processes such as DNA methylation and histone modification regulate this sex-dependent pattern of gene expression (1). Most of the imprinted genes in mammals control tissue growth (2). The most predominant hypothesis to explain such conservation is the ‘parental conflict hypothesis’ (3). This hypothesis proposes that the purpose of the imprinting is to assure appropriate allocation of limited maternal resources to each conceptus. Perturbations of genomic imprinting, i.e. loss of imprinting (LOI), have been implicated in multiple human diseases, including reproductive abnormalities and cancer (4–7). In previous work, we have demonstrated variation of LOI for many paternally or maternally expressed genes among human placentas (8). In this study, we examined the mechanism of LOI by measuring cell-to-cell variation in imprinting status.
PLAGL1 encodes a zinc finger protein that is thought to function as a transcription factor, inducing apoptosis and cell cycle arrest at G1 phase (9). PLAGL1 is a paternally expressed (maternally imprinted) gene that belongs to an imprinting cluster located on chromosome 6q24 (10). It is polymorphically imprinted in different tissues; monoallelic expression has been shown in various human tissues (placenta, muscle, lung), while it is biallelically expressed in peripheral blood leukocytes (11,12). Dysregulation of PLAGL1 has been observed in ovarian and breast cancer cells, while paternal uniparental disomy of 6q24 has been implicated in transient neonatal diabetes mellitus (13–15). We selected PLAGL1 as our reference gene to study the mechanism of LOI, because PLAGL1 was among the most highly expressed imprinted genes that we had assayed in our previous work and our cell line was heterozygous for the readout polymorphism, a prerequisite for the LOI measurement.
PLAGL1 has two promoters, but only one is active in human placentas (11). The inactive promoter is neither imprinted nor methylated. The active promoter is silenced from the maternal allele by differential methylation in primary human cells at all or the majority of 51 CpG sites compared with lack of methylation at all or the majority of the sites in the paternal allele (16). The same type of pattern is seen in cell lines, but with more variation in methylation between individual subclones (14). We chose a readout polymorphism (rs9373409) in the 5′-UTR which is represented in all splice variants (16) and has a minor allele frequency >22% in all populations.
Stochasticity in transcription has been observed for many genes in both prokaryotic and eukaryotic cells (17–19). In previous work, we have shown that stochastic transcription of biallelically expressed genes in human cells can lead to cell-to-cell variation in mRNA copy number by as much as 1000-fold (20), and to imbalanced transcription between two alleles within single cell (21). Gene expression noise has a significant effect on many biological processes, contributing to phenotypic variability of genetically identical organisms and determining cellular fate following viral infection (22–26). To be noted, the measurements of LOI in PLAGL1 at the single cell level take place in the context of significant transcriptional noise.
Herein, we test the hypothesis that LOI is an all-or-none phenomenon at the single cell level, wherein partial LOI in tissue would reflect the fraction of cells with complete LOI. We quantify expression of the paternal and maternal alleles in single cells from a human placental trophoblast cell line heterozygous for a readout polymorphism in PLAGL1 mRNA (8). The PLAGL1 gene is known to be regulated by DNA methylation and histone modification (14,27). By treating the cell line with 5-aza-2′-deoxycytidine (AZA) or Trichostatin A (TSA), we were able to examine the mechanism of LOI at the single cell level under different perturbations.
MATERIALS AND METHODS
Cell culture
Primary cytotrophoblasts were extracted from human term placentas as described earlier (28). Primary cytotrophoblasts and the placental trophoblast cell line HTR-8/SV neo (HTR8) (29) were cultured at 37°C in DMEM and RPMI 1640 media (Gibco), respectively, supplemented with 10% fetal bovine serum (Hyclone), 100 U/ml penicillin and 100 µg/ml streptomycin (Invitrogen).
Cell treatment
HTR8 cells were treated separately with 0.5 µM AZA or 20 nM TSA. Fresh cell media and antibiotics were added every 24 h and treated cells were collected after 24 and 48 h.
Cell synchronization
HTR8 cells were synchronized at the G1/S border using double treatment of thymidine as described earlier (30). Briefly, 2 mM of thymidine was added in growing cells for 12 h to accumulate the majority of the cells at G1/S. Thymidine was replaced by fresh media for 12 h and was added back to the media for 12 h. Finally, the thymidine was removed and cells were collected every 2 h for up to 8 h. Cell synchronization was validated by FACS using a LSRII system (BD Bioscience) to separate treated cells stained by Vybrant®DyeCycle™ violet (Invitrogen) following standard protocols.
Nucleic acid extraction and cDNA synthesis
Nucleic acids extraction, DNase treatment and conversion of total RNA to single-stranded cDNA were performed as described earlier (8).
Quantitative PCR of total RNAs
cDNAs were amplified with gene-specific primers. The primers are listed in Supplementary Table S1. The expression levels of PLAGL1, ZNF331 and ACTB genes were determined by quantitative PCR (qPCR) using the LightCycler480™ (Roche). All qPCR assays were carried out in triplicate in a reaction containing: buffer (50 mM Tris–OH + HCl, pH 7.5; 50 mM KOAc; 2% glycerol, 0.1 mg/ml BSA); 4 mM Mg(OAc)2; 0.2 mM each dNTPs (dUTP replacing dTTP); 0.2 μM primers; 0.25× SYBR Green (Invitrogen); 5 U/µl AmpliTaq Gold (Applied Biosystems); 20 ng single-stranded cDNA template; final volume 20 µl. Cycling conditions for all genes were: 95.0°C for 10 min, followed by 50 cycles of 95.0°C for 30 s, 65.0°C for 30 s and 72.0°C for 30 s. The crossing point, Cp, was automatically calculated from the amplification curve without human intervention.
Single cell sorting
HTR8 cells and primary cytotrophoblasts were sorted directly into 384-well PCR plates as described earlier (21). Briefly, single cells were sorted into 384-well PCR plates (Roche) using the MoFlo high-speed cell sorter. Each well contained 5 µl cell lysis buffer [4 mM magnesium acetate (Sigma), 0.05% NP40 (Sigma), 0.8 U/µl Protector RNAse Inhibitor (Roche Applied Sciences)]. After sorting, the plates were immediately placed on dry ice and stored at −70°C for future use.
LOI assay and measurements on total RNA and single cells
LOI is a measurement of expression of the silenced allele, which may be calculated as:
where the |ΔCp| refers to the absolute difference between the allele-specific Cp values on cDNA level corrected for the specificity of the allele-specific PCR (8).
The conditions for the measurement of the LOI levels in total RNA level have been described elsewhere (8). The sequences of the primers used for the amplification of the area bracketing the readout polymorphism and the quantitative allele-specific PCR (qASPCR) are listed in Supplementary Table S1. The SNP reference numbers for PLAGL1 and ZNF331 are rs9373409 and rs8109631, respectively.
To measure the levels of LOI at the single cell level, a two-step hemi-nested RT-PCR protocol was carried out. For the first RT-PCR step, an aliquot of 5 µl of 2xAccuRT PCR reaction mix [2xAccuRT buffer, 4 mM magnesium acetate, 0.2 µM primer sets, control oligonucleotides (2000 copies/well for PLAGL1 or 200 copies/well for ZNF331; the sequences of the control oligonucleotides are given in Supplementary Table S1), 0.2 mM each dNTP (dUTP) and 0.375 U/µl AccuRT with aptamer (an aptamer-based hot-start, magnesium-activated thermostable DNA polymerase kindly provided by Dr Tom Myers of Roche Molecular Systems)] was added into each well of a cell-sorted PCR plate. The cycling conditions were: 65.0°C for 30 min (reverse transcription), followed by 15 cycles of 95.0°C for 15 s and 60.0°C for 50 s. For the second PCR step, 2 µl of the PCR products from the first PCR were mixed with 8 µl of PCR reaction mix [2 ×Lightcycler Probe Master Mix, 0.2 µM final concentrations of allele-specific primer sets and 0.1 µM final concentration of Roche LNA probe]. All the reactions were run in duplicate. The sequences of the primers used for the qASPCR are listed in Supplementary Table S1. We used the Roche LNA probes #74 and #50 for PLAGL1 and ZNF331 genes, respectively. The cycling conditions were: 95°C for 10 min, followed by 50 cycles of 95°C for 10 s, 65°C for 20 s and 72°C for 10 s.
Accuracy of the LOI measurements
The error of our LOI measurements was determined by performing the qASPCR assay using serial dilutions of a genomic DNA mixture from two homozygous individuals for the PLAGL1 SNP (rs9373409, A/G). The DNA mix contained G:A copies in a 9:1 ratio (10% LOI). Genomic DNA was diluted from 6000 copies to 6 copies per reaction. All the reactions were repeated six times.
Statistical analysis
The statistical analyses were performed using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA). The Fisher exact test was used to determine the significance of the percentage of AZA-treated cells exhibiting LOI compared with the untreated control. The chi-square test was utilized to compare the LOI percentage and the expression of PLAGL1 in RNA from treated versus untreated cells. The mean and variance of LOI for cells exhibiting LOI was obtained by bootstrapping using R version 2.90. The simulation of the distributions of LOI in the all-or-none LOI and gradient LOI models was computed in R version 2.90. The Kolmogorov–Smirnov test for comparison of the experimental and simulated distributions was also performed in R version 2.90.
Model
The model proposes that each allele of a gene at a specific time point can be either in an active state, during which transcription is very efficient, or an inactive state, in which transcription is hindered. The synthesized mRNA is proposed to follow an exponential decay. The amount of total mRNA from each allele (denoted as A1 or A2) at a certain time ti is given by
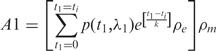 |
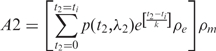 |
where p(t, λ) is a hypergeometric random function determining the transcription state at time t : 1 = active or 0 = inactive with the pulsing frequency λ; ρe is a Gaussian random variable from 0% to 100% for the transcription efficiency of each pulse; k is the parameter to control the mRNA exponential decay rate; ρm is a Gaussian random variable to estimate the measurement error. We set ΔCp = 0.5 as the SD of ρm.
The simplest simulation assumed that every pulse gave 100% transcription efficiency so that ρe = 1. For the all-or-none LOI model, 10% of the cells were set to follow transcriptional pulsing on both alleles with pulsing frequency λ1 = λ2 = 0.2 and 90% of the cells to exhibit pulsing only for the major allele with pulsing frequency λ1 = 0.2. For the partial LOI model, we set the minor allele pulsing frequency to be λ2 = 0.02, 10-fold less than the pulsing frequency of the major allele. Using the equations above, we carried out simulations of 200 pulsing steps in 2000 cells.
Furthermore, additional simulations were carried out with consideration of transcription efficiency ρe as a Gaussian random variable at the single cell level (Supplementary Figure S1A) or with consideration of only the cells in the top 25% of total mRNA copies (Supplementary Figure S1B). Both simulations showed similar distributions to that seen for the simple model.
RESULTS
We tested the hypothesis that LOI was an all-or-none phenomenon at the single cell level using the maternally imprinted gene PLAGL1. Figure 1 illustrates the experimental design for studying the effect of treatment of single HTR8 trophoblasts with AZA. Because of cell-to-cell variability in gene expression, PLAGL1 expression could only be measured in a subset of the cells (center panels). LOI in the PLAGL1 gene in the expressing cells was measured by examining allele-specific expression in the presence and absence of AZA (right panels).
Figure 1.
Illustration of heterogeneity in the expression and LOI of the PLAGL1 gene. Individual trophoblasts (shown as ovals), either nontreated (upper panel) or treated with AZA (lower panel) were tested for the expression level of the PLAGL1 gene. We detected PLAGL1 expression only in a subset of the cells (labeled with Y). The cells expressing PLAGL1 showed different LOI levels [shown as a color gradient from dark red (0% LOI) to light yellow (100% LOI), with a numerical bias toward LOI].
Genomic imprinting is regulated primarily by DNA methylation and histone modification. We treated the trophoblasts either with AZA, a DNMT1 inhibitor or TSA, an HDAC inhibitor, and looked at the impact of these drugs on the PLAGL1 expression and LOI profile on total RNA. Table 1 shows the relative expression levels of PLAGL1 and the percent LOI together with confidence limits for the allele-specific PCR triplicate measurements. There was a significant (P < 0.01) increase in expression after 2 days of AZA treatment and a significant (P < 0.01) increase in LOI after both 1 and 2 days of AZA treatment. TSA treatment resulted in no significant changes in expression or LOI.
Table 1.
LOI% and expression of PLAGL1 on the total RNA level
| LOI% | Relative expressiona (× 1000) | |
|---|---|---|
| (low95%, up95%) | (low95%, up95%) | |
| Without treatment | ||
| Primary cytotrophoblasts/AG | 3.4 (2.8, 4.1) | |
| HTR8/AG | 2.3 (0.4, 4.2) | 2.1 (1.6, 2.7) |
| AZA treatment of HTR8/AG | ||
| 1 day | 8.4 (5.4, 12.9)** | 3.4 (2.8, 4.2)* |
| 2 days | 12.7 (9.8, 16.4)** | 6.0 (4.9, 7.3)** |
| TSA treatment of HTR8/AG | ||
| 1 day | 2.5 (2.0, 3.0) | 3.8 (2.4, 6.0) |
| 2 days | 4.4 (3.9, 4.9)* | 3.2 (2.5, 4.0) |
aNormalized against ACTB.
Treated versus untreated (chi-square): **P < 0.01; *P < 0.1.
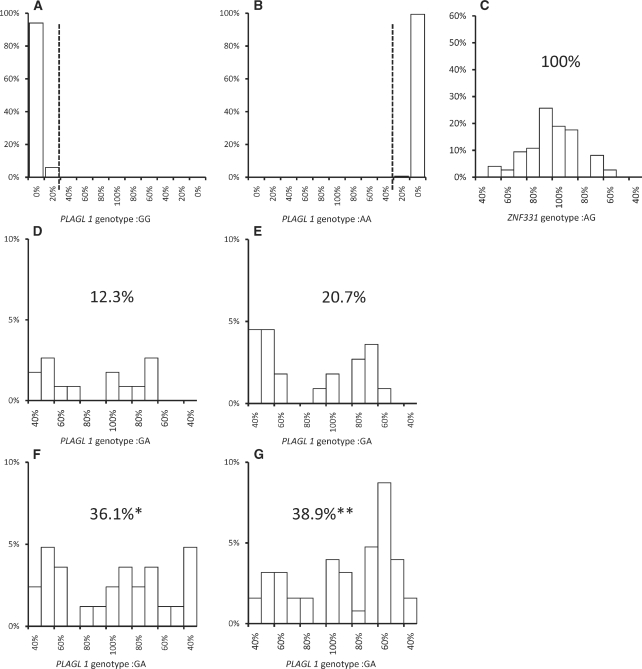
Single cell measurements are presented in Figure 2. Figure 2A and B present measurement controls for primary cytotrophoblasts from individuals homozygous for the two alleles of the PLAGL1 readout polymorphism. Because the LOI measurement method cannot detect LOI in readout polymorphism homozygotes, measured LOI must reflect allele-specific PCR measurement error. All their calculated LOI values were between 0% and 35%. To exclude all contributions from monoallelic expressing cells, we present the distribution of heterozygous cells exhibiting LOI within the range of 35–100%. The means and the variances for the distributions were computed by a bootstrapping method. We found that the mean PLAGL1 LOI measurements of the AZA treated cells at 0, 1 and 2 days were 87%, 97.2% and 92.3%, respectively, while the SDs were 7.4%, 7.3% and 5.8%, respectively. To explore possible bias in the 35% cutoff, we repeated the same analyses using cutoffs of 10 and 20% (Supplementary Table S2). For all the AZA-treated samples, the mean LOI with each cutoff was centered at ∼100% with SDs of 5–9%.
Figure 2.
Single cell LOI distributions of PLAGL1 and ZNF331 in human trophoblasts. Histograms show the percentage of cells exhibiting a given LOI. In order to present data with positive and negative values of ΔCp based on a single allele and limit LOI to 0–100%, data with negative ΔCp were calculated using the alternative allele. (A–B) LOI for primary PLAGL1 homozygous trophoblast controls. (C) LOI for ZNF331 heterozygotes (scale limited to 35–100% LOI). (D–G) LOI of PLAGL1 heterozygotes with significant LOI (35–100%). (D) Primary cytotrophoblasts; (E) Untreated HTR8 cells. (F) HTR8 cells treated 1 day with AZA. (G) HTR8 cells treated 2 days with AZA. The Fisher’s exact test was used to determine significance of the percentage of cells exhibiting LOI compared with the untreated control. (*P < 0.05; **P < 0.01).
Figure 2C depicts the analysis of LOI for ZNF331, which is not imprinted in HTR8 cells (31), and whose expression was between 2- and 4-fold greater than that of PLAGL1. The mean LOI and standard deviation of the mean for the nonimprinted gene ZNF331 were 98.6% and 2.2%, respectively. The distributions of LOI measured for both genes in cells within the selected range were centered at ∼100% LOI.
The PCR reaction for PLAGL1 was reproducibly able to detect six copies of duplex DNA template (Supplementary Table S3). When examining PLAGL1 at the single cell level, mRNA expression could only be detected in 40% of the cells. To test whether expression of PLAGL1 was dependent on the cell cycle phase, we compared the PLAGL1 expression levels between cells with no synchronization and synchronized to G1/S phase. The synchronization was confirmed by FACS analysis. We found that there was no significant difference at the expression levels at any time points (P = 0.51, 0.41, 0.77, 0.54 for 2, 4, 6 and 8 h, respectively) after synchronization. Thus, the results in Figure 2D–G were limited to cells expressing mRNA above the limit of detection. Figure 2D depicts a LOI histogram for primary cytotrophoblasts. Although the distribution of cells exhibiting LOI was wider than the distribution seen in Figure 2C, the results still suggested a distribution centered at ∼100% LOI.
Similar to the primary cytotrophoblasts, untreated HTR8 cells showed a similar wide distribution of LOI (Figure 2E). To follow up on the LOI results seen in Table 1, HTR8 cells were treated with AZA for 1 or 2 days. The percentage of cells exhibiting LOI increased significantly (P < 0.05 and P < 0.01 at 1 and 2 days, respectively), while the distribution remained wide and centered at ∼100% LOI (Figure 2F and G). This distribution is consistent with our hypothesis that LOI may occur by an all-or-none process.
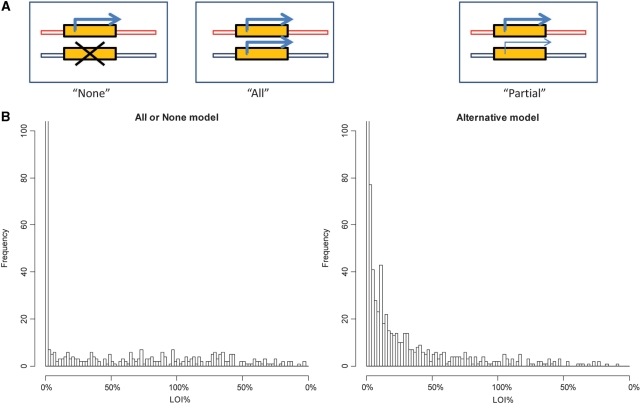
We examined two possible models for the interpretation of the single cell data. The first is the all-or-none LOI model during which cells either are fully imprinted or have completely lost their imprinting; the second is the partial LOI model where the silenced allele exhibits incomplete activation (Figure 3A). In order to distinguish between the models, we developed a mathematical model based on transcriptional pulsing from the two alleles, which simulated the variations of the mRNA synthesis at the single cell level (19). Simulations for both models (Figure 3B) used the equations described in Materials and Methods section. The shapes of the computed distributions were independent of pulse size, threshold for detection or PCR error (Supplementary Figure S1). The distribution of LOI observed in our experiments (Figure 2D–G) fit the all-or-none LOI model (left side of Figure 2B). The Kolmogorov–Smirnov test showed a statistically significant difference between experiment and simulation based on the alternative model (P < 0.05 for each of 100 simulations), but no significant difference based on the all-or-none model (P > 0.05 for each of 100 simulations).
Figure 3.
Models for the distributions of LOI in single cells. Simulations of single cell LOI distributions in 2000 cells were presented as histograms of cell counts with different percentage of LOI. The format for data presentation is the same as in Figure 2. (A) In the all-or-none model, we defined two distinct populations of cells: fully imprinted or having completely lost imprinting in the minor allele. In the partial LOI model, the transcription of the minor allele is less efficient than the major allele in each cell. (B) Simulated LOI distributions in single cells for each model (left side: all-or-none model; right side: partial LOI model).
DISCUSSION
We observed a low but significant level of LOI in both primary cytotrophoblasts and the cell line HTR8 (Table 1). In order to examine the mechanism of LOI, we tested the effects of two drugs that have been shown to affect epigenetic silencing. TSA affects histone acetylation and was previously shown to increase PLAGL1 in cancer cell lines (14). Our results indicated only a small effect on expression, suggesting that regulation of PLAGL1 by histone acetylation is less important in placental trophoblasts. In contrast, treatment with the methylation inhibitor AZA substantially increased both expression and LOI.
If LOI were a function of the degree of methylation, this LOI could reflect heterogeneity in methylation among individual cells leading to cells with different degrees of LOI. We hypothesized, however, that LOI was an all-or-none phenomenon, with LOI reflecting only the fraction of cells expressing both alleles. Testing of this hypothesis requires a functional assay of single cell LOI based on transcriptional profiling.
We examined the effect of AZA treatment on expression and LOI at the single cell level. PLAGL1 was expressed at low levels (∼0.2% of ACTB expression levels, see Table 1), with expression unaffected by synchronization of the cells. Expression increased with AZA treatment. Our single cell measurements showed highly heterogeneous LOI distributions in both human primary cytotrophoblasts and HTR8 cells. The AZA treatment increased the number of cells exhibiting high LOI, while the heterogeneity among single cells remained the same. The median LOI remained close to 100%, consistent with our hypothesis that LOI was an all-or-none phenomenon. It should be noted that a process with many steps (e.g. loss of methylation at individual sites) would be consistent with all-or-none behavior if there is a one rate-determining step that governs the switch from imprinted to nonimprinted expression.
We examined the possibility that the PCR reaction contributed significantly to the wide distribution in LOI seen at the single cell level. However, the rise in the variance with serial dilution of template could be accounted for by the expected variability in pipetting small numbers of molecules. Thus, we proposed that the large variation in single cell LOI measurements reflected the stochastic nature in expression between the two alleles and among the single cells. ZNF331 (Figure 2C), which is expressed at a 2- to 4-fold higher level in total RNA than PLAGL1, was detectable in all the cells yet showed significant cell-to-cell LOI variation. The fact that PLAGL1 mRNA levels in 60% of the cells were below the detection limit suggested an even greater cell-to-cell variation in expression, possibly due to transcriptional pulsing (32). Herein, we proposed a transcription pulsing model to show that transcriptional pulsing could also contribute to chromosome to chromosome variation in expression which would be reflected in a wide distribution of LOI among cells that are expressing both alleles. Stochastic expression by transcriptional pulsing will not affect the observed mean LOI at 100%, which is the important parameter for supporting the all-or-none hypothesis for LOI for PLAGL1 in trophoblasts. All-or-none LOI leads to a second distinct cell population which could have a selective advantage, leading to widespread LOI in normal tissues, such as the placenta (8) or in neoplastic cells (11).
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
National Institutes of Health (NO1 AI50028 and P01 ES09584); U.S. Environmental Protection Administration (R827039). Funding for open access charge: National Institutes of Health.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr Tom Myers from Roche Molecular Systems (Alameda, CA, USA) for generously providing our lab with AccuRT DNA polymerase plus aptamer. We thank the Mount Sinai flow cytometry shared resource facility for single cell sorting.
REFERENCES
- 1.Tilghman SM. The sins of the fathers and mothers: genomic imprinting in mammalian development. Cell. 1999;96:185–193. doi: 10.1016/s0092-8674(00)80559-0. [DOI] [PubMed] [Google Scholar]
- 2.Reik W, Davies K, Dean W, Kelsey G, Constancia M. Imprinted genes and the coordination of fetal and postnatal growth in mammals. Novartis Found Symp. 2001;237:19–31. doi: 10.1002/0470846666.ch3. discussion 31–42. [DOI] [PubMed] [Google Scholar]
- 3.Badcock C, Crespi B. Imbalanced genomic imprinting in brain development: an evolutionary basis for the aetiology of autism. J. Evol. Biol. 2006;19:1007–1032. doi: 10.1111/j.1420-9101.2006.01091.x. [DOI] [PubMed] [Google Scholar]
- 4.Byrne J, Cama A, Reilly M, Vigliarolo M, Levato L, Boni L, Lavia N, Andreussi L. Multigeneration maternal transmission in Italian families with neural tube defects. Am. J. Med. Genet. 1996;66:303–310. doi: 10.1002/(SICI)1096-8628(19961218)66:3<303::AID-AJMG13>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 5.Jelinic P, Shaw P. Loss of imprinting and cancer. J. Pathol. 2007;211:261–268. doi: 10.1002/path.2116. [DOI] [PubMed] [Google Scholar]
- 6.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat. Rev. Genet. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMinn J, Wei M, Schupf N, Cusmai J, Johnson EB, Smith AC, Weksberg R, Thaker HM, Tycko B. Unbalanced placental expression of imprinted genes in human intrauterine growth restriction. Placenta. 2006;27:540–549. doi: 10.1016/j.placenta.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Lambertini L, Diplas AI, Lee MJ, Sperling R, Chen J, Wetmur J. A sensitive functional assay reveals frequent loss of genomic imprinting in human placenta. Epigenetics. 2008;3:261–269. doi: 10.4161/epi.3.5.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilanges B, Varrault A, Mazumdar A, Pantaloni C, Hoffmann A, Bockaert J, Spengler D, Journot L. Alternative splicing of the imprinted candidate tumor suppressor gene ZAC regulates its antiproliferative and DNA binding activities. Oncogene. 2001;20:1246–1253. doi: 10.1038/sj.onc.1204237. [DOI] [PubMed] [Google Scholar]
- 10.Bliek J, Verde G, Callaway J, Maas SM, De Crescenzo A, Sparago A, Cerrato F, Russo S, Ferraiuolo S, Rinaldi MM, et al. Hypomethylation at multiple maternally methylated imprinted regions including PLAGL1 and GNAS loci in Beckwith-Wiedemann syndrome. Eur. J. Hum. Genet. 2008;17:611–619. doi: 10.1038/ejhg.2008.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamiya M, Judson H, Okazaki Y, Kusakabe M, Muramatsu M, Takada S, Takagi N, Arima T, Wake N, Kamimura K, et al. The cell cycle control gene ZAC/PLAGL1 is imprinted–a strong candidate gene for transient neonatal diabetes. Hum. Mol. Genet. 2000;9:453–460. doi: 10.1093/hmg/9.3.453. [DOI] [PubMed] [Google Scholar]
- 12.Mackay DJ, Coupe AM, Shield JP, Storr JN, Temple IK, Robinson DO. Relaxation of imprinted expression of ZAC and HYMAI in a patient with transient neonatal diabetes mellitus. Hum. Genet. 2002;110:139–144. doi: 10.1007/s00439-001-0671-5. [DOI] [PubMed] [Google Scholar]
- 13.Temple IK, Shield JP. Transient neonatal diabetes, a disorder of imprinting. J. Med. Genet. 2002;39:872–875. doi: 10.1136/jmg.39.12.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdollahi A, Pisarcik D, Roberts D, Weinstein J, Cairns P, Hamilton TC. LOT1 (PLAGL1/ZAC1), the candidate tumor suppressor gene at chromosome 6q24-25, is epigenetically regulated in cancer. J. Biol. Chem. 2003;278:6041–6049. doi: 10.1074/jbc.M210361200. [DOI] [PubMed] [Google Scholar]
- 15.Bilanges B, Varrault A, Basyuk E, Rodriguez C, Mazumdar A, Pantaloni C, Bockaert J, Theillet C, Spengler D, Journot L. Loss of expression of the candidate tumor suppressor gene ZAC in breast cancer cell lines and primary tumors. Oncogene. 1999;18:3979–3988. doi: 10.1038/sj.onc.1202933. [DOI] [PubMed] [Google Scholar]
- 16.Valleley EM, Cordery SF, Bonthron DT. Tissue-specific imprinting of the ZAC/PLAGL1 tumour suppressor gene results from variable utilization of monoallelic and biallelic promoters. Hum. Mol. Genet. 2007;16:972–981. doi: 10.1093/hmg/ddm041. [DOI] [PubMed] [Google Scholar]
- 17.Blake WJ, KAErn M, Cantor CR, Collins JJ. Noise in eukaryotic gene expression. Nature. 2003;422:633–637. doi: 10.1038/nature01546. [DOI] [PubMed] [Google Scholar]
- 18.Elowitz MB, Levine AJ, Siggia ED, Swain PS. Stochastic gene expression in a single cell. Science. 2002;297:1183–1186. doi: 10.1126/science.1070919. [DOI] [PubMed] [Google Scholar]
- 19.Raj A, Peskin CS, Tranchina D, Vargas DY, Tyagi S. Stochastic mRNA synthesis in mammalian cells. PLoS Biol. 2006;4:e309. doi: 10.1371/journal.pbio.0040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu J, Biswas SI, Sealfon SC, Wetmur JG, Jayaprakash C, Hayot F. Power-laws in interferon-β mRNA distribution in virus infected dendritic cells. Biophys. J. 2009 doi: 10.1016/j.bpj.2009.05.067. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu J, Sealfon SC, Hayot F, Jayaprakash C, Kumar M, Pendleton AC, Ganee A, Fernandez-Sesma A, Moran TM, Wetmur JG. Chromosome-specific and noisy IFNB1 transcription in individual virus-infected human primary dendritic cells. Nucleic Acids Res. 2007;35:5232–5241. doi: 10.1093/nar/gkm557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blake WJ, Balazsi G, Kohanski MA, Isaacs FJ, Murphy KF, Kuang Y, Cantor CR, Walt DR, Collins JJ. Phenotypic consequences of promoter-mediated transcriptional noise. Mol. Cell. 2006;24:853–865. doi: 10.1016/j.molcel.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Pascal V, Stulberg MJ, Anderson SK. Regulation of class I major histocompatibility complex receptor expression in natural killer cells: one promoter is not enough! Immunol. Rev. 2006;214:9–21. doi: 10.1111/j.1600-065X.2006.00452.x. [DOI] [PubMed] [Google Scholar]
- 24.Rosenfeld N, Young JW, Alon U, Swain PS, Elowitz MB. Gene regulation at the single-cell level. Science. 2005;307:1962–1965. doi: 10.1126/science.1106914. [DOI] [PubMed] [Google Scholar]
- 25.Volfson D, Marciniak J, Blake WJ, Ostroff N, Tsimring LS, Hasty J. Origins of extrinsic variability in eukaryotic gene expression. Nature. 2006;439:861–864. doi: 10.1038/nature04281. [DOI] [PubMed] [Google Scholar]
- 26.Weinberger LS, Burnett JC, Toettcher JE, Arkin AP, Schaffer DV. Stochastic gene expression in a lentiviral positive-feedback loop: HIV-1 Tat fluctuations drive phenotypic diversity. Cell. 2005;122:169–182. doi: 10.1016/j.cell.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Varrault A, Bilanges B, Mackay DJ, Basyuk E, Ahr B, Fernandez C, Robinson DO, Bockaert J, Journot L. Characterization of the methylation-sensitive promoter of the imprinted ZAC gene supports its role in transient neonatal diabetes mellitus. J. Biol. Chem. 2001;276:18653–18656. doi: 10.1074/jbc.C100095200. [DOI] [PubMed] [Google Scholar]
- 28.Ma Y, Kadner SS, Guller S. Differential effects of lipopolysaccharide and thrombin on interleukin-8 expression in syncytiotrophoblasts and endothelial cells: implications for fetal survival. Ann. NY Acad. Sci. 2004;1034:236–244. doi: 10.1196/annals.1335.025. [DOI] [PubMed] [Google Scholar]
- 29.Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp. Cell Res. 1993;206:204–211. doi: 10.1006/excr.1993.1139. [DOI] [PubMed] [Google Scholar]
- 30.Galgano PJ, Schildkraut CL. G1/S phase synchronization using double thymidine synchronization. CSH Protoc. 2006;2006 doi: 10.1101/pdb.prot4487. doi:10.1101/Pdb.prot4487. [DOI] [PubMed] [Google Scholar]
- 31.Diplas AI, Lambertini L, Lee MJ, Sperling R, Lee YL, Wetmur J, Chen J. Differential expression of imprinted genes in normal and IUGR human placentas. Epigenetics. 2009;4:235–240. doi: 10.4161/epi.9019. [DOI] [PubMed] [Google Scholar]
- 32.Chubb JR, Trcek T, Shenoy SM, Singer RH. Transcriptional pulsing of a developmental gene. Curr. Biol. 2006;16:1018–1025. doi: 10.1016/j.cub.2006.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.