Abstract
Background
The impact of loop electrosurgical excision procedure (LEEP) treatment for cervical precancerous lesions on subsequent acquisition of new human papillomavirus (HPV) infections is not well described.
Methods
Cumulative incidence rate ratios (IRR) for treated versus untreated women at 6- and 24-months of follow-up with 95% confidence intervals (95%CI) were calculated for infection by individual HPV genotypes, any HPV genotype, any carcinogenic HPV genotype, any non-carcinogenic HPV genotypes, and phylogenetic groups of HPV genotypes were compared between HPV-positive women who underwent colposcopy and were treated by LEEP (n = 195) and were untreated (n = 1,625) at entry into a two-year study.
Results
Treated women were 29% less likely than untreated women to acquire carcinogenic HPV genotypes at the 6-month follow-up (IRR = 0.71; 95%CI = 0.50–1.00) and 18% less likely at the 24-month follow-up (IRR = 0.82, 95%CI = 0.68–1.01). Treated women were, respectively, 56% and 40% less likely to acquire HPV genotypes of the α9 phylogenetic species (which includes HPV16) at 6-months (IRR = 0.44, 95%CI = 0.23–0.85) and 24-months (IRR = 0.60, 95%CI = 0.42–0.85).
Conclusion
LEEP may reduce the acquisition of certain carcinogenic HPV genotypes related to HPV16.
Keywords: human papillomavirus (HPV), loop electrosurgical excision procedure (LEEP), cervical intraepithelial neoplasia (CIN), cervical cancer, atypical squamous cell of undetermined significance (ASCUS), low-grade squamous intraepithelial lesion (LSIL)
INTRODUCTION
In conventional cervical cancer screening programs, women who screen positive are referred to colposcopic biopsy. Histologic diagnosis of cervical intraepithelial neoplasia grade 2 or 3 (CIN2/3) by colposcopically-directed biopsy triggers treatment, which in many countries is typically accomplished by loop electrosurgical excision procedure (LEEP). For safety, LEEP removes the epithelium and a small amount of underlying stroma from the entire cervical transformation zone, tissue that is uniquely susceptible to oncogenic transformation by HPV. Among women with more than one HPV-related cervical lesion, it is likely that LEEP removes both colposcopically apparent and unapparent lesions. Cervical infections by cancer-associated or carcinogenic human papillomavirus (HPV) genotypes cause virtually all cervical cancer (1;2) and the majority of CIN2/3 lesions (3); numerous studies have shown that successful treatment of CIN2/3 by LEEP is accompanied by the disappearance of the causal HPV genotype, and treatment failure is heralded by the detection of viral persistence of the causal HPV genotype during post-LEEP follow-up (4–9). On the basis of these data, follow-up testing for carcinogenic HPV is now accepted as an option for post-LEEP monitoring for recurrent disease (10;11).
However, few studies have examined the impact of LEEP on the acquisition of new HPV infections. It is unclear whether temporarily removing the tissue of susceptibility through excision, with subsequent epithelial healing, might also reduce acquisition of HPV. If so, LEEP could potentially decrease the risk of subsequent cervical cancer from HPV genotypes not present at the time of treatment of the first CIN2/3 lesion. To address this question of the effects of LEEP on the natural history of HPV, we conducted a retrospective analysis to compared rates of HPV acquisition between women treated by LEEP and a comparison group of HPV-positive, colposcopically-evaluated women who did not undergo LEEP during the enrollment period of the atypical squamous cells of undetermined significance (ASCUS) and low-grade squamous intraepithelial lesion (LSIL) triage study (ALTS) (12–16). We examined the impact of LEEP on the subsequent acquisition of new HPV infections, first for individual HPV genotypes and then for groups of HPV genotypes related by cancer risk or genetic similarities.
MATERIALS AND METHODS
Study Design and Population
ALTS (1997–2001) was a multi-site, randomized trial comparing three management strategies for women referred for ASCUS (n = 3,488) or LSIL (n = 1,572) conventional cytology (12–16). The National Cancer Institute and local institutional review boards approved the study and all participants provided written, informed consent.
At enrollment and follow-up visits, all women underwent a pelvic examination with collection of two cervical specimens; the first specimen in PreservCyt for ThinPrep cytology (Cytyc Corporation, Marlborough, MA; now Hologic) and the second in specimen transport medium (STM; Digene Corporation, Gaithersburg, MD; now Qiagen). Women in all 3 arms of the study were re-evaluated by cytology every 6 months for 2 years and sent to colposcopy if cytology was high-grade squamous intraepithelial lesion. An exit examination with colposcopy was scheduled for all women. We refer readers to other references for details on randomization, examination procedures, patient management, and laboratory and pathology methods (12).
HPV Genotyping
Testing for at least 27 HPV genotypes, and for most specimens, 38 HPV genotypes was performed using an L1-based PCR assay that employs a primer set designated PGMY09/11 on the STM specimen as previously described (17–19). For this analysis, HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68 (for which all specimens were assayed) were considered carcinogenic (20;21).
Pathology and Treatment
Clinical management was based primarily on the clinical center pathologists’ cytologic interpretations and histologic diagnoses as previously described (12–16). Referral smears, ThinPreps, and histology slides were also sent to the Pathology Quality Control Group (QC Pathology) based at the Johns Hopkins Hospital for review, including computer-assisted review, and secondary diagnoses as previously described (12–16). A histological diagnosis of cervical intraepithelial neoplasia grade 2 or more severe (≥CIN2) based on the clinical center pathology or ≥CIN3 based on the QC Pathology review triggered treatment by LEEP. Women with a persistent low-grade lesion at the time of the exit from the study were offered LEEP.
Statistical Methods
We restricted this analysis to women with test results only from the laboratory that tested for 38 HPV genotypes (approximately half of the enrollment specimens and all the follow-up specimens) because of analytical sensitivity differences between this laboratory and the laboratory that tested for 27 HPV genotypes on a subset of enrollment specimens (data not shown). Women who were tested for 38 HPV genotypes were more likely to referred into ALTS because of an ASCUS Pap smear (vs. LSIL) compared to women who were tested for 27 HPV genotypes (p < 0.001) but there was no significant differences in the lifetime number of sexual partners (0–3, 4–5, 6–9, and 10 or more partners) between the two groups to suggest that these women were more or less likely to acquire new HPV infections. We compared the acquisition of HPV genotypes in HPV-positive women following LEEP at baseline (n = 195) to the acquisition of HPV genotypes in HPV-positive women who underwent colposcopy but did not undergo LEEP (i.e., a diagnosis of <CIN2) at baseline (n = 1,625). This strategy allowed us to examine the impact of LEEP over the two-years of follow-up in ALTS. Women in either group who were treated by LEEP during follow-up were censored, and did not contribute observation time post-LEEP to either group.
Our analysis considered each woman’s possible acquisition of each of 38 HPV genotypes as units of analysis, under the assumption that the natural history of HPV genotypes is independent of whether other HPV genotypes are present (22;23). We excluded the observation for presence or absence during follow-up of genotypes that were positive at enrollment, because a subsequent positive could be considered a failure of LEEP, not a new acquisition of infection.
We also considered the acquisition of categories of HPV genotypes: all HPV genotypes, carcinogenic HPV genotypes, and non-carcinogenic HPV genotypes. In addition, we evaluated the acquisition of HPV genotypes according to phylogenetic species groups (24;25): α1/8/10 (HPV6, 11, 40, 42, 55), α3/4/15 (HPV57, 61, 62, 71, 72, 81, 83, 84, 89), α5 (HPV26, 51, 69, 82), α6 (HPV53, 56, 66), α7 (HPV18, 39, 45, 59, 68, 70), α9 (HPV16, 31, 33, 35, 52, 58, 67), and α11 (HPV64, 73). HPV40 (α8) and HPV54 (α13) were the only HPV genotypes in their species detected by the assay used and so were presented only as individual HPV genotypes.
The prevalence at baseline of individual HPV genotypes was assessed for each study group, with odds ratios calculated, and the Pearson χ2 test was used to test for difference in prevalence at baseline. Cumulative incidence rate ratios (IRR) for treated versus untreated women and 95 percent confidence intervals (95%CI) were calculated for individual HPV genotypes and categories of HPV genotypes as described above.
An obvious concern in this study design is that women treated by LEEP were more likely to have HPV genotypes at enrollment that are linked to risk of ≥CIN2. Because we were only studying subsequent new infections, the numbers of women who were eligible to acquire those high-risk infections during follow-up were reduced in the treated group. The IRR for each individual genotype were calculated as the ratios of new infections among those eligible, which adjusted for this issue. However, a weighted stratified analysis was needed for study of categories, which took into account the numbers of women eligible for new infections with each genotype in that category. The weights are shown in Table 1. The best example of the impact of this difference is for HPV16: 55.9% of women undergoing LEEP for ≥CIN2 versus 20.1% of untreated women were positive for HPV16, which means that only 44.1% of treated women versus 79.9% of untreated women could acquire HPV16 during follow-up if weights were not used in our analytic approach. Thus, HPV categories that include HPV16, i.e., all combined carcinogenic HPV and α9 phylogenetic species, would have been directly influenced by these differences in prevalence at baseline for HPV16.
Table 1.
The baseline prevalence of individual HPV genotypes and groups of HPV genotypes in women treated by loop electrosurgical excision procedure (LEEP) at enrollment and HPV-positive women who underwent colposcopy but did not undergo LEEP at enrollment. Weights were used to correct for differences between type within group. Pearson chi-square was used to test for statistical differences (<0.05) (highlighted in bold type) in the prevalence for individual HPV genotypes between treated and untreated women. OR, odds ratio for individual HPV genotypes in untreated versus treated women; n/a, not applicable
| Untreated (N = 1,625) | Treated (N = 195) | OR* | p | ||||
|---|---|---|---|---|---|---|---|
| Prevalence | Weight | Prevalence | Weight | ||||
| Individual HPV Genotypes | |||||||
| HPV6 | 4.6% | 1.05 | 3.1% | 1.03 | 1.5 | 0.3 | |
| HPV11 | 1.2% | 1.01 | 2.1% | 1.02 | 0.60 | 0.3 | |
| HPV16 | 20.1% | 1.25 | 55.9% | 2.27 | 0.20 | <0.001 | |
| HPV18 | 7.3% | 1.08 | 10.3% | 1.11 | 0.69 | 0.1 | |
| HPV26 | 0.9% | 1.01 | 0.0% | 1.00 | n/a | 0.2 | |
| HPV31 | 10.3% | 1.11 | 14.4% | 1.17 | 0.68 | 0.08 | |
| HPV33 | 4.9% | 1.05 | 7.7% | 1.08 | 0.62 | 0.1 | |
| HPV35 | 6.2% | 1.07 | 9.7% | 1.11 | 0.61 | 0.06 | |
| HPV39 | 9.4% | 1.10 | 9.7% | 1.11 | 0.96 | 0.9 | |
| HPV40 | 2.7% | 1.03 | 2.1% | 1.02 | 1.3 | 0.6 | |
| HPV42 | 6.9% | 1.07 | 3.1% | 1.03 | 2.3 | 0.04 | |
| HPV45 | 6.8% | 1.07 | 4.6% | 1.05 | 1.5 | 0.2 | |
| HPV51 | 11.0% | 1.12 | 13.9% | 1.16 | 0.77 | 0.2 | |
| HPV52 | 14.4% | 1.17 | 14.9% | 1.17 | 0.96 | 0.9 | |
| HPV53 | 9.9% | 1.11 | 10.3% | 1.11 | 0.96 | 0.9 | |
| HPV54 | 6.6% | 1.07 | 5.1% | 1.05 | 1.3 | 0.4 | |
| HPV55 | 3.5% | 1.04 | 4.6% | 1.05 | 0.75 | 0.4 | |
| HPV56 | 7.3% | 1.08 | 5.6% | 1.06 | 1.3 | 0.4 | |
| HPV57 | 0.1% | 1.00 | 0.0% | 1.00 | n/a | 0.6 | |
| HPV58 | 7.5% | 1.08 | 8.2% | 1.09 | 0.91 | 0.7 | |
| HPV59 | 8.5% | 1.09 | 6.7% | 1.07 | 1.3 | 0.4 | |
| HPV61 | 7.7% | 1.08 | 7.7% | 1.08 | 1.0 | 1 | |
| HPV62 | 9.3% | 1.10 | 5.6% | 1.06 | 1.7 | 0.09 | |
| HPV64 | 0.5% | 1.00 | 0.5% | 1.01 | 0.96 | 1 | |
| HPV66 | 7.1% | 1.08 | 6.2% | 1.07 | 1.2 | 0.6 | |
| HPV67 | 3.2% | 1.03 | 5.6% | 1.06 | 0.55 | 0.08 | |
| HPV68 | 4.3% | 1.05 | 4.6% | 1.05 | 0.93 | 0.8 | |
| HPV69 | 0.6% | 1.01 | 1.0% | 1.01 | 0.54 | 0.4 | |
| HPV70 | 5.6% | 1.06 | 6.2% | 1.07 | 0.90 | 0.8 | |
| HPV71 | 0.8% | 1.01 | 0.0% | 1.00 | n/a | 0.2 | |
| HPV72 | 1.7% | 1.02 | 2.1% | 1.02 | 0.81 | 0.7 | |
| HPV73 | 3.5% | 1.04 | 3.1% | 1.03 | 1.1 | 0.8 | |
| HPV81 | 3.7% | 1.04 | 2.6% | 1.03 | 1.5 | 0.4 | |
| HPV82 | 3.0% | 1.03 | 4.6% | 1.05 | 0.64 | 0.2 | |
| HPV82v | 0.7% | 1.01 | 0.0% | 1.00 | n/a | 0.2 | |
| HPV83 | 5.6% | 1.06 | 4.1% | 1.04 | 1.4 | 0.4 | |
| HPV84 | 4.6% | 1.05 | 3.6% | 1.04 | 1.3 | 0.5 | |
| HPV89 | 8.4% | 1.09 | 6.7% | 1.07 | 1.3 | 0.4 | |
| HPV Genotype Groups | |||||||
| α1/8/10 | 17.5% | n/a | 14.4% | n/a | 1.3 | 0.3 | |
| α3/4/15 | 34.8% | n/a | 26.7% | n/a | 1.5 | 0.02 | |
| α5 | 14.8% | n/a | 17.4% | n/a | 0.82 | 0.34 | |
| α6 | 21.8% | n/a | 20.5% | n/a | 1.1 | 0.7 | |
| α7 | 35.2% | n/a | 35.4% | n/a | 1.0 | 1.0 | |
| α9 | 53.7% | n/a | 87.2% | n/a | 0.17 | <0.001 | |
| α11 | 3.9% | n/a | 3.6% | n/a | 1.1 | 0.8 | |
| Carcinogenic HPV | 78.0% | n/a | 95.4% | n/a | 0.17 | <0.001 | |
| Non-Carcinogenic HPV | 60.8% | n/a | 52.8% | n/a | 1.4 | 0.03 | |
| Any HPV | 100% | n/a | 100% | n/a | n/a | n/a | |
Accordingly, strata were defined by a specific LEEP status and specific HPV genotype; the weight for a stratum was the ratio of the total number of women with the specific LEEP status of the stratum to the number of women contributing an observation to the stratum. For example, among the 1,625 untreated women, 326 of these women were positive for HPV16 at enrollment, so only the remaining 1,299 women contributed a record to the HPV16 stratum for untreated women, and the weight for this stratum was 1,625/1,299=1.25. Similarly, 109 of 195 treated women were positive for HPV16 at enrollment, so the observed number of infections in follow-up was multiplied by 195/(195−109) = 2.27.
SUDAAN software and subsequent calculations accounted for these weighting factors when estimating the IRR, defined as the ratio of the complement of the weighted Kaplan-Meier estimate (i.e., the cumulative incidence rate) from women treated by LEEP strata and the complement of the weighted Kaplan-Meier estimate from the women without LEEP. The variance for the log cumulative incidence ratio was obtained by applying the delta method (26), so for each group, the variance contribution was the ratio of the variance estimate calculated in SUDAAN, assuming stratified sampling with replacement, to the squared cumulative incidence estimate. This delta method result was used in the calculation of the asymmetric confidence interval for the cumulative incidence ratio.
To allow for correlation of the same woman acquiring multiple infections (which a priori we expected to be null), an analysis that considers each woman as a cluster was also conducted using SUDAAN software to calculate the weighted Kaplan-Meier estimates and the variance estimates. The variance for the log cumulative incidence ratio was calculated using the delta method, and the asymmetric confidence interval was calculated. A similar analysis was performed using SUDAAN and the proportional hazards model with cluster data. These alternative approaches yielded similar results to the approach that did not treat each woman as a cluster. Because they confirmed our prior assumption that the infections act independently, without auto-correlation within women, these estimates were not presented.
RESULTS
The prevalence at baseline of individual HPV genotypes detected for each study (HPV-positive) group are shown in Table 1. There were significant overall differences in the distribution of HPV genotypes (p < 0.001). As expected, HPV16 was detected significantly more frequently among treated versus untreated women (p < 0.001). Conversely, untreated women were more likely to have an HPV42 infection at enrollment compared to treated women (p = 0.04). Among women who underwent LEEP at baseline, HPV89 (cumulative incidence rate [CIR] = 13.45), HPV54 (CIR = 9.85), HPV62 (CIR = 9.69) HPV53 (CIR = 9.58), HPV59 (CIR = 9.40), and HPV84 (CIR = 9.08) were the most common HPV genotypes acquired over the 24-month follow-up. Among HPV-positive women who were not treated by LEEP at baseline, HPV62 (CIR = 10.95), HPV16 (CIR = 10.17), HPV52 (CIR = 9.90), HPV54 (CIR = 9.20), and HPV53 (CIR = 8.79) were the most common HPV genotypes acquired over the 24-month follow-up.
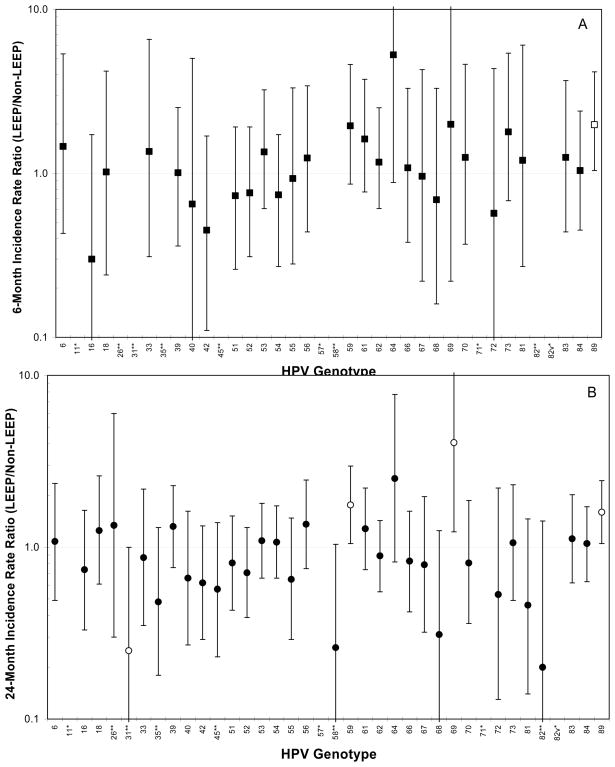
Figure 1 shows the cumulative IRR (treated versus untreated at baseline) for each individual HPV genotype at 6 months and 24 months of follow-up. Treated women were more likely than untreated women to acquire HPV89 at 6 months (IRR = 1.98, 95% = 1.04–3.78) and 24 months (IRR = 1.60, 95%CI = 1.05–2.44) of follow-up. Treated women were more likely than untreated women to acquire HPV59 (IRR = 1.76, 95%CI = 1.05–2.97) and HPV69 (IRR = 4.06, 95%CI = 1.23–13.40) at 24 months of follow-up. Treated women were marginally less likely than untreated women to acquire HPV31 (IRR = 0.25, 95%CI = 0.06–1.0) at 24 months of follow-up.
Figure 1.
The 6-month (A) (■) and 24-month (B) (●) cumulative incidence rate ratio (IRR) of individual HPV genotypes of women treated by loop electrosurgical excision procedure (LEEP) versus HPV-positive women who underwent colposcopy but did not undergo LEEP at enrollment. Open symbol indicates IRR was statistically different than 1.0. There was no 6-month acquisition of HPV11, 26, 31, 35, 45, 57, 58, 71, 82, and 82v and no 24-month acquisition of HPV11, 57, 71, and 82v among treated women. **No acquisition by the treated groups at 6-months, *No acquisition by the treated groups at 6-months and 24-months.
The prevalence at baseline of HPV genotypes grouped according to phylogenetic clade or cancer risk detected for each study HPV-positive group are shown in Table 1. HPV genotypes in the a9 phylogenetic species and those in the carcinogenic HPV risk group were much more common in LEEP patients than untreated patients (p < 0.001).
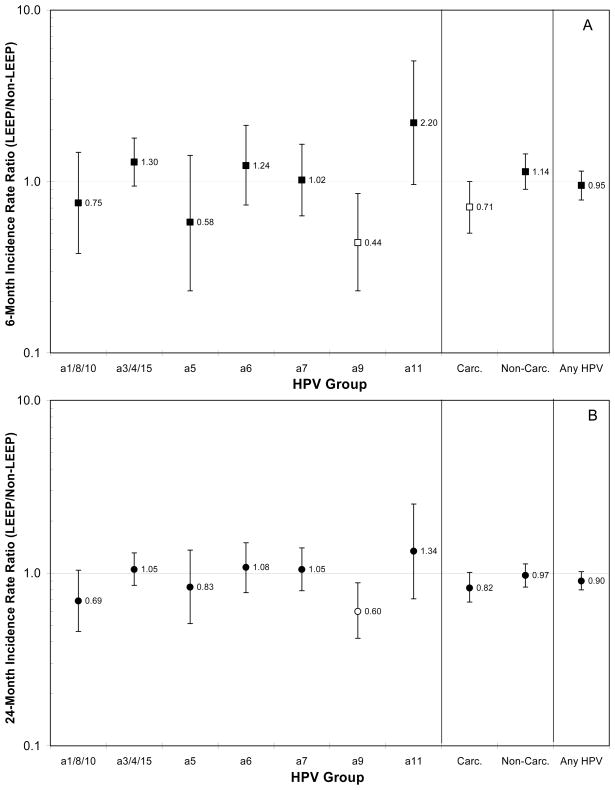
Figure 2 shows the cumulative IRR for HPV groups at 6 months and 24 months of follow-up. There was no difference between treated and untreated women for the 6-month cumulative incidence rate for the category of any HPV genotypes (IRR = 0.95, 95%CI = 0.78–1.15) or any non-carcinogenic HPV genotypes (IRR = 1.14, 95%CI = 0.90–1.45). Treated women were marginally less likely than untreated women to acquire any carcinogenic HPV genotypes (IRR = 0.71, 95%CI = 0.50–1.00) at 6 months of follow-up. There was no difference between treated and untreated women for the 24-month cumulative incidence rate for any HPV genotypes (IRR = 0.90, 95%CI = 0.80–1.02), any carcinogenic HPV genotypes (IRR = 0.82, 95%CI = 0.68–1.01), or any non-carcinogenic HPV genotypes (IRR = 0.97, 95%CI = 0.83–1.13).
Figure 2.
The 6-month (A) (■) and 24-month (B) (●) cumulative incidence rate ratio (IRR)of HPV groups (phylogenetic species, carcinogenic HPV vs. non-carcinogenic HPV, and any HPV) of women treated by loop electrosurgical excision procedure (LEEP) versus HPV-positive women who underwent colposcopy but did not undergo LEEP at enrollment. Open symbol indicates IRR was statistically different than 1.0.
Grouping HPV genotypes according to phylogenetic species and groups of species, HPV genotypes of the α9 species, which is composed predominately of carcinogenic HPV genotypes (with the exception of HPV67), was the only species that was significantly different in treated versus untreated women. Treated women were respectively 56%and 40% less likely than untreated women to acquire α9 HPV genotypes at 6 months of follow-up (IRR = 0.44, 95%CI = 0.23–0.85) and at 24 months of follow-up (IRR = 0.60, 95%CI = 0.42–0.85). Treated women were respectively 50%and 44% less likely than untreated women to acquire α9 HPV genotypes excluding HPV16 at 6 months of follow-up (IRR = 0.50, 95%CI = 0.26–0.97) and at 24 months of follow-up (IRR = 0.56, 95%CI = 0.38–0.81).
We did not observe meaningful differences in the IRR for α9 genotypes between the subgroups of women with different phylogenetic species groups of HPV genotypes prevalent at baseline (Table 2). The IRR for α9 genotypes for subgroups with different phylogenetic HPV genotypes at baseline ranged from 0.46 (for women with α1/α8/α10 genotypes at baseline) to 0.78 (for women with α3/α4/α15, α5, or α6 genotypes at baseline)
Table 2.
The 6-month and 24-month cumulative incidence rate (CIR) of α9 HPV genotypes for HPV-positive women treated by loop electrosurgical excision procedure (LEEP) versus HPV-positive women who underwent colposcopy but did not undergo LEEP at enrollment. The data were stratified by the HPV genotypes, categorized according to phylogenetic species group, that were prevalent at baseline. The incidence rate ratio (IRR) between the two groups of women is also presented. The α9 HPV genotypes include HPV16, 31, 33, 35, 52, 58, and 67.
| α9 Acquisition | |||||||
|---|---|---|---|---|---|---|---|
| Non-LEEP | LEEP | ||||||
| 6-month CIR | 24-month CIR | 6-month CIR | 24-month CIR | 6-month IRR | 24-month IRR | ||
| HPV Genotype at Baseline | |||||||
| α1/8/10 | 2.84 | 8.49 | 0.58 | 3.90 | 0.20 | 0.46 | |
| α3/4/15 | 1.68 | 6.17 | 0.64 | 4.83 | 0.38 | 0.78 | |
| α5 | 2.74 | 7.33 | 1.53 | 5.71 | 0.56 | 0.78 | |
| α6 | 2.35 | 6.86 | 0.59 | 5.35 | 0.25 | 0.78 | |
| α7 | 2.62 | 7.22 | 0.99 | 5.13 | 0.38 | 0.71 | |
| a9 | 2.23 | 6.36 | 1.13 | 3.97 | 0.51 | 0.62 | |
| α11 | 2.26 | 8.27 | 4.76 | 4.76 | 2.11 | 0.58 | |
There was no indication of difference in the self-reported number of new sexual partners during each follow-up visit (p = 0.2, p = 0.8, p = 0.2, and p = 0.3, Fisher’s exact, for 6-month, 12-month, 18-month, and 24-month follow-up, respectively) and in enrollment age between groups of women (p = 1.0, Kruskal-Wallis) (data not shown). Women who underwent LEEP at enrollment self-reported more person years of smoking (p = 0.006, Kruskal-Wallis) and marginally more person years of using oral contraceptives (p = 0.09, Kruskal-Wallis) than the untreated women, but there was no difference between groups in the self-reported use of condoms (p = 0.8, Kruskal-Wallis). Treated women had on average fewer visits (4.86 for treated vs. 4.96 for untreated, p = 0.001) but virtually all women had a 6 month visit (2 women in the LEEP group were censored before the 6-month follow-up visit). Most women in both groups were followed for 24 months: 93.3% for treated and 97.4% for untreated. Restricting our analysis to only women with 24 months of follow-up did not appreciably change our findings (data not shown).
DISCUSSION
We evaluated whether the removal of the cervical transformation zone by excision for CIN2/3 treatment affected subsequent acquisition of HPV infection. We found that excision had little impact on HPV acquisition in the subsequent 6-month and 24-month periods, except for the suggestion that carcinogenic HPV genotypes, particularly those of α9 phylogenetic species (which includes the most carcinogenic HPV genotype, HPV16), were slightly less likely to be acquired by treated women than by untreated women.
One important limitation of the study was less than complete comparability of the treated and untreated women. Our case and control group consisted of HPV-positive women who underwent colposcopy; the exposed had ≥CIN2 and were treated by LEEP, and the controls did not get treatment because either they had a biopsy diagnosis of <CIN2 or were not biopsied due to lack of worrisome lesions. We restricted our analysis to HPV-positive women in recognition that HPV-negative women at any point in time are likely to have different recent behavioral characteristics than HPV-positive ones, and different HPV exposure history, whether treated or not, and therefore have a different probability of acquiring new infections. Nonetheless, any differences between the two groups in behaviors related to acquiring infection could distort our finding.
Having prevalent HPV16 but no other α9 genotypes at baseline versus having noα9 genotype genotypes at baseline did not influence the cumulative incidence of non-HPV16 α9 genotypes (data not shown). This argues against the preponderance of HPV16 in the LEEP group causing significant biological immunity that might alter acquisition rates of genetically-related HPV genotypes. This is consistent with our previous report from ALTS that showed the natural history of an HPV infection tended to behave independently of whether or not there is co-infection with another HPV genotypes (22).
Nevertheless, we recognize that women who were diagnosed with CIN2/3 are inherently different from those who are not and may be more likely to engage in higher-risk behaviors, such as smoking (27–29), which are linked to having (30) and acquiring HPV (31). Moreover, we note that the prevalence at baseline of α9 genotypes was the most significant difference between study groups, which raises the possibility of a confounding effect due to these differences in theα9 prevalence at baseline leading to an apparent protective effect due to LEEP. The only truly comparable group to the treated group would be group of women with CIN2/3 who were not treated; such a comparison sub-group was not available in ALTS. As a sensitivity analysis, we repeated our analysis of α9 acquisition but restricted our control group to 197 HPV-positive women who were untreated at baseline but were subsequently diagnosed with CIN2/3 during follow-up. Many of these subsequent diagnoses undoubtedly represent missed prevalent disease because of the limitation in the sensitivity of colposcopy (32) and therefore more similar to the treated group in terms of risk behaviors for acquisition of HPV. The 6-month and 24-month IRRs for α9 HPV genotypes were 0.23 (95%CI = 0.12–0.47) and 0.32 (95%CI = 0.22–0.47), respectively, suggesting the observed reduction in α9 HPV genotype acquisition is not an artifact due to the selection of the control group.
However, we did not observe similar effects for the acquisition ofα11 HPV genotypes, the most closely related phylogenetic species to α9, nor for α7 HPV genotypes, the other major carcinogenic HPV genotype-populated phylogenetic species, raising some question about the veracity of this finding. It is difficult to formulate a biological rationale for why there would be an effect on one group (α9) but the others (α11 or α7) although there are clearly examples of phylogenetic species-specific differences in HPV natural history (25;33;34).
We can only speculate as to the biological causes if the phenomenon is real. One possibility is the LEEP, by damaging the cervical tissue, causes an acute inflammatory response that reduces the susceptibility to new HPV infections. Yet, there is no evidence to support such a mechanism or why it would be specific to HPV16-related genotypes.
We did not find significant differences in self-reported behaviors between the two groups that would explain our observations. Indeed, smoking, which has been linked to having HPV (30), was more common in the women who underwent baseline treatment than those who did not, which would tend to mute the possible protective effects of LEEP on HPV acquisition.
In conclusion, our study finds a reduction in acquisition of new carcinogenic HPV infections, particularly in α9, following LEEP. Our findings from this first study to address the impact of LEEP on genotype-specific HPV acquisition and raise the possibility that LEEP has secondary effect to treatment of precancer that reduce subsequent cancer risk. We cannot, however, draw a definitive conclusion about the protective effect of LEEP on HPV acquisition because of the limitations in the study design, and we urge caution against over-interpretation of this preliminary finding. The minor benefit, if true, must be weighed carefully against the costs of iatrogenic morbidity induced by treatment that potentially leads to adverse obstetric outcomes (35–37). Clearly, additional investigations of this question using other designs are needed.
Acknowledgments
ALTS was supported by the National Cancer Institute, National Institutes of Health Department of Health and Human Services contracts CN-55153, CN-55154, CN-55155, CN-55156, CN-55157, CN-55158, CN-55159 and CN-55105. Some of the equipment and supplies used in these studies were donated or provided at reduced cost by Digene Corporation, Gaithersburg, MD; Cytyc Corporation, Marlborough, MA; National Testing Laboratories, Fenton, MO; DenVu, Tucson, AZ; and TriPath Imaging, Inc., Burlington, NC, and Roche Molecular Systems Inc., Alameda, CA. We thank the ALTS Group Investigators for their help in planning and conducting the trial. The authors report no conflicts of interest. This research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute.
Reference List
- 1.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999 Sep;189(1):12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus genotypes associated with cervical cancer. N Engl J Med. 2003 Feb 6;348(6):518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 3.Schiffman MH, Bauer HM, Hoover RN, et al. Epidemiologic evidence showing that human papillomavirus infection causes most cervical intraepithelial neoplasia. J Natl Cancer Inst. 1993 Jun 16;85(12):958–64. doi: 10.1093/jnci/85.12.958. [DOI] [PubMed] [Google Scholar]
- 4.Kreimer AR, Guido RS, Solomon D, et al. Human papillomavirus testing following loop electrosurgical excision procedure identifies women at risk for posttreatment cervical intraepithelial neoplasia grade 2 or 3 disease. Cancer Epidemiol Biomarkers Prev. 2006 May;15(5):908–14. doi: 10.1158/1055-9965.EPI-05-0845. [DOI] [PubMed] [Google Scholar]
- 5.Arbyn M, Paraskevaidis E, Martin-Hirsch P, Prendiville W, Dillner J. Clinical utility of HPV-DNA detection: Triage of minor cervical lesions, follow-up of women treated for high-grade CIN: An update of pooled evidence. Gynecol Oncol. 2005 Dec;99(3 Suppl):S7–S11. doi: 10.1016/j.ygyno.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 6.Aerssens A, Claeys P, Beerens E, et al. Prediction of recurrent disease by cytology and HPV testing after treatment of cervical intraepithelial neoplasia. Cytopathology. 2008 May 28; doi: 10.1111/j.1365-2303.2008.00567.x. [DOI] [PubMed] [Google Scholar]
- 7.Prato B, Ghelardi A, Gadducci A, et al. Correlation of recurrence rates and times with posttreatment human papillomavirus status in patients treated with loop electrosurgical excision procedure conization for cervical squamous intraepithelial lesions. Int J Gynecol Cancer. 2008 Jan;18(1):90–4. doi: 10.1111/j.1525-1438.2007.00965.x. [DOI] [PubMed] [Google Scholar]
- 8.Bae JH, Kim CJ, Park TC, Namkoong SE, Park JS. Persistence of human papillomavirus as a predictor for treatment failure after loop electrosurgical excision procedure. Int J Gynecol Cancer. 2007 Apr 18; doi: 10.1111/j.1525-1438.2007.00945.x. [DOI] [PubMed] [Google Scholar]
- 9.Kreimer AR, Katki HA, Schiffman M, Wheeler CM, Castle PE. Viral determinants of human papillomavirus persistence following loop electrical excision procedure treatment for cervical intraepithelial neoplasia grade 2 or 3. Cancer Epidemiol Biomarkers Prev. 2007 Jan;16(1):11–6. doi: 10.1158/1055-9965.EPI-06-0710. [DOI] [PubMed] [Google Scholar]
- 10.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007 Oct;197(4):346–55. doi: 10.1016/j.ajog.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 11.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. Am J Obstet Gynecol. 2007 Oct;197(4):340–5. doi: 10.1016/j.ajog.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 12.Schiffman M, Adrianza ME. ASCUS-LSIL Triage Study. Design, methods and characteristics of trial participants. Acta Cytol. 2000 Sep;44(5):726–42. doi: 10.1159/000328554. [DOI] [PubMed] [Google Scholar]
- 13.Human papillomavirus testing for triage of women with cytologic evidence of low-grade squamous intraepithelial lesions: baseline data from a randomized trial. The Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS) Group. J Natl Cancer Inst. 2000 Mar 1;92(5):397–402. doi: 10.1093/jnci/92.5.397. [DOI] [PubMed] [Google Scholar]
- 14.ASCUS-LSIL Triage Study (ALTS) Group. A randomized trial on the management of low-grade squamous intraepithelial lesion cytology interpretations. Am J Obstet Gynecol. 2003 Jun;188(6):1393–400. doi: 10.1067/mob.2003.462. [DOI] [PubMed] [Google Scholar]
- 15.ASCUS-LSIL Triage Study (ALTS) Group. Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. Am J Obstet Gynecol. 2003 Jun;188(6):1383–92. doi: 10.1067/mob.2003.457. [DOI] [PubMed] [Google Scholar]
- 16.Solomon D, Schiffman M, Tarone R. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst. 2001 Feb 21;93(4):293–9. doi: 10.1093/jnci/93.4.293. [DOI] [PubMed] [Google Scholar]
- 17.Gravitt PE, Peyton CL, Alessi TQ, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000 Jan;38(1):357–61. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiffman M, Wheeler CM, Dasgupta A, Solomon D, Castle PE. A comparison of a prototype PCR assay and hybrid capture 2 for detection of carcinogenic human papillomavirus DNA in women with equivocal or mildly abnormal papanicolaou smears. Am J Clin Pathol. 2005 Nov;124(5):722–32. doi: 10.1309/E067-X0L1-U3CY-37NW. [DOI] [PubMed] [Google Scholar]
- 19.Peyton CL, Gravitt PE, Hunt WC, et al. Determinants of genital human papillomavirus detection in a US population. J Infect Dis. 2001 Jun 1;183(11):1554–64. doi: 10.1086/320696. [DOI] [PubMed] [Google Scholar]
- 20.Bosch FX, Manos MM, Munoz N, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995 Jun 7;87(11):796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 21.Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005 Apr;6(4):204. doi: 10.1016/s1470-2045(05)70086-3. [DOI] [PubMed] [Google Scholar]
- 22.Plummer M, Schiffman M, Castle PE, Maucort-Boulch D, Wheeler CM. A 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. J Infect Dis. 2007 Jun 1;195(11):1582–9. doi: 10.1086/516784. [DOI] [PubMed] [Google Scholar]
- 23.Liaw KL, Hildesheim A, Burk RD, et al. A prospective study of human papillomavirus (HPV) genotype 16 DNA detection by polymerase chain reaction and its association with acquisition and persistence of other HPV genotypes. J Infect Dis. 2001 Jan 1;183(1):8–15. doi: 10.1086/317638. [DOI] [PubMed] [Google Scholar]
- 24.de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur HH. Classification of papillomaviruses. Virology. 2004 Jun;20324(1):17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 25.Schiffman M, Herrero R, Desalle R, et al. The carcinogenicity of human papillomavirus genotypes reflects viral evolution. Virology. 2005 Jun;20337(1):76–84. doi: 10.1016/j.virol.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Lachin JM. Biostatistical Methods: The Assessment of Relative Risks. New York: John Wiley & Sons, Inc; 2000. [Google Scholar]
- 27.Plummer M, Herrero R, Franceschi S, et al. Smoking and cervical cancer: pooled analysis of the IARC multi-centric case--control study. Cancer Causes Control. 2003 Nov;14(9):805–14. doi: 10.1023/b:caco.0000003811.98261.3e. [DOI] [PubMed] [Google Scholar]
- 28.Castle PE, Wacholder S, Lorincz AT, et al. A prospective study of high-grade cervical neoplasia risk among human papillomavirus-infected women. J Natl Cancer Inst. 2002 Sep 18;94(18):1406–14. doi: 10.1093/jnci/94.18.1406. [DOI] [PubMed] [Google Scholar]
- 29.McIntyre-Seltman K, Castle PE, Guido R, Schiffman M, Wheeler CM. Smoking is a risk factor for cervical intraepithelial neoplasia grade 3 among oncogenic human papillomavirus DNA-positive women with equivocal or mildly abnormal cytology. Cancer Epidemiol Biomarkers Prev. 2005 May;14(5):1165–70. doi: 10.1158/1055-9965.EPI-04-0918. [DOI] [PubMed] [Google Scholar]
- 30.Vaccarella S, Herrero R, Snijders PJ, et al. Smoking and human papillomavirus infection: pooled analysis of the International Agency for Research on Cancer HPV Prevalence Surveys. Int J Epidemiol. 2008 Jun;37(3):536–46. doi: 10.1093/ije/dyn033. [DOI] [PubMed] [Google Scholar]
- 31.Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003 Feb 1;157(3):218–26. doi: 10.1093/aje/kwf180. [DOI] [PubMed] [Google Scholar]
- 32.Guido R, Schiffman M, Solomon D, Burke L. Postcolposcopy management strategies for women referred with low-grade squamous intraepithelial lesions or human papillomavirus DNA-positive atypical squamous cells of undetermined significance: a two-year prospective study. Am J Obstet Gynecol. 2003 Jun;188(6):1401–5. doi: 10.1067/mob.2003.456. [DOI] [PubMed] [Google Scholar]
- 33.Castle PE, Jeronimo J, Schiffman M, et al. Age-related changes of the cervix influence human papillomavirus genotype distribution. Cancer Res. 2006 Jan 15;66(2):1218–24. doi: 10.1158/0008-5472.CAN-05-3066. [DOI] [PubMed] [Google Scholar]
- 34.Smith JS, Lindsay L, Hoots B, et al. Human papillomavirus genotype distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007 Aug 1;121(3):621–32. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 35.Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet. 2006 Feb 11;367(9509):489–98. doi: 10.1016/S0140-6736(06)68181-6. [DOI] [PubMed] [Google Scholar]
- 36.Albrechtsen S, Rasmussen S, Thoresen S, Irgens LM, Iversen OE. Pregnancy outcome in women before and after cervical conisation: population based cohort study. BMJ. 2008 Sep 18;337:a1343. doi: 10.1136/bmj.a1343.:a1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arbyn M, Kyrgiou M, Simoens C, et al. Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: meta-analysis. BMJ. 2008 Sep 18;337:a1284. doi: 10.1136/bmj.a1284.:a1284. [DOI] [PMC free article] [PubMed] [Google Scholar]