Abstract
Current treatments for autoimmune disease are hampered by the non-specificity of immunomodulatory interventions, having to accept broad suppression of immunoresponsiveness with potential serious side effects, such as infection or malignancy. The development of antigen-specific approaches, downregulating pathogenic immune responses while maintaining protective immunity, would be a major step forward. One possible approach involves the targeting of physiologic regulatory mechanisms, such as inhibitory CD8 T cells that are now recognized to fine-tune many aspects of immune responses. CD8 T suppressor (Ts) cells may directly inhibit other T cells or condition antigen-presenting cells in such a way that immune amplification steps are dampened. The great promise of CD8 Ts cells lies in their potential to disrupt host-injurious immune responses in a very targeted fashion. For therapeutic purposes, such CD8 Ts cells could either be generated in vitro and transferred into the host or their numbers and activity could be modulated by treating the patient with established or novel immunomodulators. Emerging evidence supports the existence of several different subsets of CD8 Ts cells. While there is still considerable uncertainty about the precise molecular mechanisms through which CD8 Ts cells can reset immune responses to protect the host, their potential diagnostic and therapeutic use is intriguing and has focused renewed interest.
Suppressor/regulatory T cells from humble beginnings to star players of the immune team
Early evidence that the immune system not only causes but also prevents autoimmunity came from Nishizuka and colleagues in 1969[1]. These authors showed that neonatal thymectomy can lead to oophoritis in mice, suggesting that thymocyte depletion was involved in the evolution of organ-specific autoimmune disease. However, it was not until 1972, when Gershon et al published their landmark article about “ suppressor T cells ” paving the way for the concept that T cells are not only able to augment but also to suppress immune responses[2]. In these early years, the focus was mainly on CD8+ T cells. Six years later, Cantor and colleagues reported that CD8+ suppressor (Ts) cells might target class Ib Qa-1 surface protein[3]. In the eighties, interest in Ts cells dwindled and doubts were raised about whether T cells which such a functional profile truly existed. Technology was not advanced enough to isolate CD8+ Ts cells or to decipher their mechanisms of action. It was not until 1995 that Sakaguchi et al identified interleukin (IL)-2 receptor α-chain (CD25) molecules as a marker of natural suppressor CD4+ T cells. These cells are now commonly referred to as T regulatory or “Treg” cells. Sakaguchi and his colleagues established that CD4+CD25+ Treg were able to prevent autoimmunity in BALB/c mice[4]. This finding was the beginning of the renaissance of the suppressor cell and once again, research in this area flourished. Eventually, it became clear that Treg cells induced by specific antigen are capable of inhibiting immune reactions directed against other antigens in the same microenvironment. This observation widened the potential therapeutic applicability of Treg cells, broadening their potential use to clinical scenarios in which depression of immunity is desirable; particularly in autoimmunity and transplant tolerance induction. Conversely, a role for excessive Treg function in chronic persistent infection was suggested by data from Belkaid et al, demonstrating that natural CD4+CD25+ Treg cells accumulated at the sites of chronic Leishmania major infection, and favored the parasite’s persistence[5]. Today, Treg cells are recognized as an essential component of immune responses, in physiologic and pathogenic situations, giving rise to the concept that both insufficient and excessive function of Ts cells contributes to immunopathogenesis. This paradigm clearly leads us to explore the therapeutic potential of modulating Ts-cell function. Clinical settings requiring immunosuppression, such as the management of patients with autoimmune disease or with organ transplants, call for therapeutic measures amplifying Ts cells [6, 7]. Alternatively, in disease states such as malignancies, in which the immune system has failed to protect the host, it would be beneficial to downregulate Ts-cell functions. One of the most appealing aspects of utilizing inhibitory T cells as a target for immunotherapy derives from the possibility of generating antigen-specific Ts cells. That would allow for a much more targeted approach to inhibit unwanted immune responses while sparing all other immunoprotective functions.
Since Sakaguchi’s groundbreaking work, a growing portfolio of CD4+ Treg and CD8+ Ts cells has been described. After a decade of focusing on CD4 Tregs, inhibitory CD8+ T cell populations have recently gained attention. Today we know that there are many subsets of inhibitory CD8+ T cells, many of which may be of value as therapeutic targets in future immunotherapeutic approaches. Evidence has accumulated that specialized CD8+ T cells are key players in autoimmune disorders, transplantation, cancer, allergy, and infectious diseases. Notable examples are the observations of Karlsson et al showing the significance of CD8+CD25+Foxp3+ Ts cells in SIV (simian immunodeficiency virus) -infected cynomolgus macaques. As CD4+ Treg numbers decline, the frequencies of CD8+CD25+Foxp3+ cell numbers increase in the SIV-infected animals. Frequencies of inhibitory CD8+ T cells appeared to correlate with the high viral load, suggesting a role for CD8+ Ts cells in virus persistence, possibly by inhibiting antiviral immune reactions[8]. Another interesting role of CD8+ inhibitory cells seems to be in tolerizing the mother to the fetus. Shao et al have suggested the possibility that trophoblasts-activated CD8+ Ts cells might add to the regulation of the immune system during pregnancy[9]. Finally, CD8+ Ts cells are gaining a position in regulating the immune privilege that protects certain organs from immunologic attack. In support of this concept, Sugita et al have demonstrated that ocular pigment epithelial cells from the anterior segment of the eye can effectively induce CD8+CD25+Foxp3+ Ts cells[10].
In this review we will summarize the progress that has been made in defining and understanding the role of CD8+ Ts cells in autoimmune disease. Wherever possible we will emphasize human CD8+ Ts cells and define barriers for their clinical utilization. Commonalities in phenotypes and functions of inhibitory CD8+ T cells involved in preventing anti-tumor and anti-microbial responses while sustaining autoimmunity hold the promise of novel immunomodulatory therapies, applicable in several clinical arenas.
The growing universe of CD8+ Ts-cell subpopulations
Emerging evidence supports the concepts that multiple distinct CD8+ T-cell populations exist that are functionally characterized by downregulating immune responses. There is intense interest in understanding the molecular pathway through which inhibitory CD8+ T cells modulate other T-cell subsets, in particular CD4+ T cells, and how they can affect antigen-presenting cells (APC) to suppress instead of enhance immune recognition events. A frequently used approach in classifying CD8+Ts cells is to categorize them as natural versus adaptive/induced. Here, we have adopted this classification to provide an overview over the growing diversity of inhibitory CD8+ T cells. Natural CD8+ Ts cells originate from the thymus and are often considered to represent a separate T-cell lineage. Conversely, adaptive CD8+ Ts cells originate from the post-thymic T-cell pool and are induced through a variety of in vivo or in vitro stimuli. Adaptive CD8+ Ts cells can be further subclassified into those that have antigen specificity and those that display their immunoregulatory function in an antigen-non-specific fashion. Whereas antigen-specific CD8+ Ts cells, which represent the majority of adaptive CD8+ T cells, need stimulation through the T-cell receptor (TCR) to be induced, pathways bypassing the TCR are sufficient to induce nonantigen-specific CD8+ Ts cells (Table 1).
Table 1.
CD8 suppressor T cells
| Natural | |||
|---|---|---|---|
| Phenotype | Mechanism of Suppression | Occurrence | Reference |
| CD8+CD25+CTLA-4+GITR+Foxp3+ | contact dependent | in postnatal human thymus | [11] |
| CD8+CD122+ | IL-10 secretion | mouse | [12] |
| Adaptive/Induced: Antigen Specific | ||||
|---|---|---|---|---|
| Phenotype | Mechanism | Induction | Reference | |
| Contact- dependent | Other | |||
| CD8+CD25+Foxp3+CD28+GITR+CTLA-4+ | ● | stimulation of CD8+CD25-T cells with staphylococcal enterotoxin B and CD3/28 mAb | [16] | |
| CD8+CD25+Foxp3+ | ● | TGFβ1 and Anti-CD3/Anti-28 Ab | [17] | |
| CD8+CD28-Foxp3+CTLA-4+ | cell contact dependent upregulation of ILT3/4on APCs | stimulation with antigen pulsed APC | [30] | |
| CD8+CD103+ | ● | Anti-CD3/28 mAb (addition of Rapamycin enhanced cell number and suppressive ability) | [21,22] | |
| CD8+CD25+CTLA-4+Foxp3+ | ● | CCL-4 and TNF dependent | modified anti-CD3 mAb in vivo (DM I) and in vitro | [18,19,64] |
| CD8+CD25+LAG-3+Foxp3+CCL4+ | CCL4 secretion | BCG stimulation of PBMC | [20] | |
| CD8+TCRαβ+CD25+ | ● | autologous LPS-activated | [25] | |
| CTLA-4+Foxp3+ | DC | |||
| CD8+CD101+CD103+CD28− | ● | human intestinal epithelial cells | [23] | |
| CD8+CD11c+ | IDO-dependent suppression of CD4 effector cells | Anti-4-1 BB mAb | [47] | |
| CD8+IL-10+ | IL-10 secretion | CD40L-activated pDC | [26] | |
| CD8+CD25+Foxp3+ | TGFβ production | TGFβ secretion of ocular pigment epithelial cells | [10] | |
| CD8+CCR7+CD45RO+IL-10+ | IL-10 secretion | pDC from tumor ascites | [27] | |
| Adaptive/Induced: Non-Antigen Specific | |||
|---|---|---|---|
| Phenotype | Mechanism | Induction | Reference |
| CD8+CD28-Foxp3-CD56- | IL-10 secretion | incubation with IL-2 and IL-10 | [15] |
Naturally occurring CD8+ Ts cells
As reported by Cosmi et al, postnatal human thymus contains a defined population of suppressive CD8+ T cells. They express the phenotype CD8+CD25+CTLA-4+GTR+Foxp3+ and suppress in a contact-dependent manner via CTLA-4 and TGF-β1[11]. Whether such thymus-derived CD8+ Ts cells have a pathogenic role in autoimmunity is not known. They should have a broad repertoire of antigenic specificities, and it is difficult to envisage how a failure of natural CD8+ Ts cells could result in organ-specific autoimmune disease. Mouse natural CD8+ Ts cells have been phenotyped as CD8+CD122+ T cells and can be induced by coculture with pre-activated T cells. In general, they suppress via secreting the cytokine IL-10. They inhibit IFN-γ secretion of target cells in syngeneic mouse strains but not in allogeneic mouse strains, suggesting an MHC class I-dependent suppression[12].
Interfering with the thymic generation of natural Ts cells could have potential for broad-base immunomodulatory therapy, e.g. in hosts with autoimmunity. In terms of possible clinical application, this approach is certainly far away and requires much more knowledge about natural Ts cells, the rules of their induction, their half-lives, trafficking patterns, and mechanism of suppression.
Nonantigen-specific CD8+CD28− Ts cells
The current paradigm holds that adaptive/induced CD8+ inhibitory T cells can be divided into antigen-specific and nonantigen-specific suppressor cells. This distinction has important consequences for the therapeutic applicability of Ts-based cellular immunotherapy. Treating transplant recipients or patients with autoimmune disease with CD8+ Ts cells may entail generating a unique T cell for each patient, dictated by the antigen-specificity and MHC restriction. Vice versa, if CD8+ Ts cells can function in an antigen-nonspecific fashion then they could be useful in conditions involving a spectrum of antigens and could possibly be shared by different patients.
According to the work of Filaci et al, it is relatively easy to generate nonantigen-specific adaptive CD8+CD28-Foxp3-CD56- Ts cells. Culturing CD8+CD28- T cells in a cytokine cocktail containing IL-2, IL-10, and granulocyte macrophage-colony stimulating factor (GM-CSF) is sufficient to expand CD8+ Ts cells; antigen recognition is not needed. Induction of this cell, however, is monocyte dependent, possibly reflecting the requirement for GM-CSF. Such nonantigen-specific CD8+ Ts cells suppress APC-mediated antigen presentation, T-cell proliferation, and CTL-mediated cytotoxicity via IL-10 secretion, a cytokine that has been linked to anergy induction in activated T cells[13, 14] (Antigen nonspecific CD8 Ts cells are able to survive in culture for more than 1 month, broadening their potential therapeutic application. It has also been suggested that such CD8 Ts cells could possibly be extracted and cultured from patients during periods of disease remission and reinjected during disease exacerbation[13]. Recent findings from the same group suggest that during the induction phase, these cells downregulate the IL-7 receptor, distinguishing them from classical CD8+ memory T cells and eventually allowing separation of these two CD8+T-cell subsets. Another functional characteristic of such CD8+CD28-Foxp3-CD56- Ts cells important to consider for the development of immunomodulatory therapies is their resistance to corticosteroids[15].
Adaptive CD8+ Ts cells induced by stimulating through the T-cell receptor
In the growing universe of CD8+ Ts cells, adaptive CD8+ Ts cells are by far the dominant group (Fig. 1, Table 1). Most of the adaptive CD8+ Ts cells appear to suppress via cell contact-dependent mechanisms but for several of the described CD8+ Ts cells, the mechanisms through which they modulate the function of either APC or other T cells remain unresolved. A wide variety of protocols have been developed which permit induction of inhibitory CD8+ T cells.
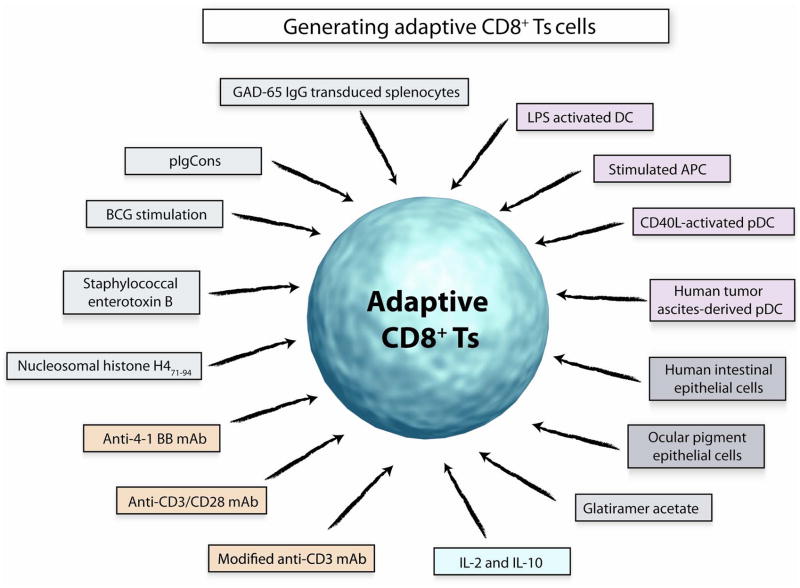
Fig 1. Generating adaptive CD8+ Ts cells.
Numerous modes of induction have been applied to generate CD8+ Ts cells in vitro. Coculturing with specialized APC or specially conditioned APC has been successfully applied. Investigators have used antigen-specific approaches to expand CD8+ Ts cells that have specific inhibitory function when re-exposed to such antigens. Antibody-mediated triggering of the TCR or targeting of regulatory receptors has been reported. Finally, cytokines, such as IL-2 and IL-10, can promote outgrowth of CD8 T cells that have suppressor functions. Whether all of these distinct CD8+ Ts cell subsets derive from a single or few precursors is unknown. Also, evidence suggests that their mechanisms of action through which they downregulate other T cells or APC are diverse.
CD8+CD25+Foxp3+CD28+GITR+CTLA-4+ cells can be induced by stimulation of CD8+CD25− T lymphocytes with staphylococcal enterotoxin B and CD3/28 mAb. Their mechanism of suppression seemed to be independent of CTLA-4 or Il-10 secretion[16]. Another population of CD8+CD25+Foxp3+ Ts cells has been described by Fan et al. Here, TGFβ1 and CD3/28 mAb were required to expand these cells; mechanisms of suppression involved cell-to-cell contact[17].
A third type of CD8+CD25+Foxp3+ was first observed in a clinical trial exploring the efficiency of low dose modified anti-CD3 mAb in the treatment of patients with type 1 diabetes mellitus. The authors succeeded in inducing a similar cell type in in vitro cultures, by culturing PBMC with the modified anti-CD3 mAb. There was a significant correlation between the in vivo and the in vitro studies concerning the inducibility of CD8+CD25+Foxp3+ cells[18, 19]. An alternative way to induce CD8+ Ts cells is to stimulate PBMC from primed donors with the mycobacterial vaccine Bacillus Calmette Guerin (BCG) yielding CD8+CD25+LAG-3+Foxp3+CCL4+ cells. The suppressive function of such CD8+ Ts cells was assigned to secretion of the chemokine CCL4 [20].
CD103 seems to be an important molecule in several of the CD8+ Ts cell subpopulations. Uss et al described that in vitro generated CD8+CD103+ T cells but not CD8+CD103− T cells possess suppressor activity, and are induced via anti-CD3/28 mAb. They strongly suppressed the proliferation of T cells in mixed lymphocyte cultures in a contact-dependent manner. Further, they were capable of producing IL-10[21]. This group then studied the influence of immunosuppressive drugs on these inhibitory CD8+ T cells. They showed in in vitro cocultures that rapamycin but not calcineurin, mycophenolate mofetil (MMF), or anti-CD25 mAb enhances the number of such cells and tends to increase their suppressive activity. Prednisolone appears to diminish frequencies of CD8+ CD103+ CD101+ cells[22]. Apparently, CD8+ Ts cells are amendable to pharmacologic therapy, and care has to be taken regarding how standard immunosuppressants affect their function.
CD8+CD103+CD101+ Ts cells have also been implicated in guarding maternal-fetal tolerance, a scenario with similarities to allograft transplantation. It has been proposed that CD8+CD103+ cells induced by human placental trophoblasts suppress B cell-dependent immune responses and their antibody production to protect the fetus[9]. A possible role for CD8+CD103+ Ts cells in regulating gastrointestinal immunity has been proposed by Allez et al. It was shown that intestinal epithelial cells (IEC) from healthy individuals can induce CD8+CD101+CD103+CD28− cells which have suppressor function and interact with IEC via gp180[23, 24]. Another appealing way to generate CD8+ Ts cells is by stimulating them with autologous LPS-activated dendritic cells. Ts cells induced under these conditions have been phenotyped as CD8+TCRαβ+CD25+CTLA-4+Foxp3+ and function by suppressing effector CD4+ T cells via cell-cell contact[25]. Allogeneic CD40L-activated pDC promote differentiation of naive CD8+ T cells into Ts cells. These CD8+ Ts cells inhibit the proliferation of naive allogeneic CD8+ T cells by IL-10 secretion[26]. pDC isolated from human ovarian carcinoma-associated ascites mediated the induction of CD8+CCR7+CD45RO+IL-10+ Ts cells which suppressed effector T-cell function by secreting IL-10[27].
An interesting mechanism of suppression was described by Chang et al. These authors showed that CD8+CD28− Ts cells induce negative regulatory receptors, rendering APC tolerogenic. Specifically, CD8+CD28− T cells were able to upregulate ILT3 and ILT4 on monocytes and dendritic cells and downregulate costimulatory molecules CD80 and CD86, profoundly modulating antigen-presenting function. Such CD8+CD28− Ts cells emerged in cultures driven with either allogeneic, xenogeneic, or antigen-pulsed APC, opening the possibility that they could be generated in a number of different scenarios[28] [29, 30]. In a recent publication these authors have described that inhibitory ILT3high expression on APC not only functions to render APC tolerogenic, but is also involved in the differentiation of antigen-specific CD8+ Ts cells. Speculations are made if ILT3 might be responsible for differentiating CD8+ Ts cells from CTL. In support of this notion, an ILT3-Fc protein fusion construct showed strong immunosuppressive ability in in vitro and in vivo experiments. ILT3 was proven to prevent tumor transplant rejection in humanized SCID mice (mice injected with human PBMC), even encouraging tumor growth by inhibiting the CD4+ T-cell immune responses. Similar observations were made by transplanting human pancreatic islet cells in humanized NOD/SCID mice in which diabetes was induced by administering streptozotocin. ILT3-Fc-treated mice did not reject the islet cells, in contrast to control mice which rejected the transplant. Furthermore, ILT3-Fc treatment resulted in marked expansion of CD8+CD28− Ts cells [31, 32]. The enormous clinical potential of this novel immunomodulatory intervention needs to be emphasized. Because ILT3-Fc acts selectively on activated and not on resting T cells, this agent could potentially introduce selectivity and mediate antigen-specific immunosuppression.
In different studies, another class of CD8+ Ts cells MHC class I molecule has been implicated in suppressing responsiveness of CD4+ T cells. The important molecules in this case are the Qa-1 molecule in mice, and its analog, the HLA-E molecule in humans. MHC class I restricted CD8+ Ts cells are able to recognize these molecules on the surface of CD4+ T cells. This TCR Qa-1 molecule interaction results in the cytolysis of the CD4+ T cell [33–35].
In essence, a multiplicity of culture conditions, and possibly also in vivo interventions, may be able to induce CD8+ Ts cell populations (Fig. 1). The potential to generate such cells in vitro could possess great therapeutic relevance as it would allow developing novel immunomodulatory therapies based on transferring functionally selected T cells into patients. The major restrictions are to be able to grow a sufficient number of cells and the barrier of having to adapt the cell-based therapy to the individual host. Infusion of allogeneic cells would create new and unacceptable problems. The fact that CD8+ Ts cells can be expanded in vitro through relatively uncomplicated means is encouraging. Selectivity for an antigen would allow extremely targeted intervention in a host, leaving all other T-cell responses intact while paralyzing those against a single antigen. In many autoimmune diseases the specific antigens are not yet known, creating further hurdles.
Clinical significance of CD8+ Ts cells and their role in autoimmunity
CD8+ Ts cells have now an established place in the immune system’s complex networks that ultimately determine the balance between host protection and the avoidance of self attack. Several features make them attractive not only for the conceptual understanding of immunity but for its therapeutic modulation; including the ability to expand their numbers, to store and transfer them, to make them travel to relevant sides of immunity and inflammation, and their longevity. However, despite being long-lived cells, T cells do, of course, have a limited lifespan which needs to be considered in cell-based therapies. For reasons insufficiently understood, CD8+ T cells age much more quickly than their CD4+ counterparts[36]. How this applies to CD8+ Ts cells is unknown, but this characteristic does affect the potential availability of such cells in patients during the 6th-8th decades of life. It is also unknown whether CD8+ Ts cells can impose permanent suppression of immunity, or only achieve transient downregulation; a feature relevant when treating patients with organ transplants or autoimmune disease. Current therapies for autoimmune disorders are limited by the unwanted side effect of broad immunosuppression and by the inability to reset the immune system in such a way that autoreactivity can be controlled long-term. In the management of autoimmune syndromes, the application of CD8+ Ts cells is particularly attractive as it could overcome many of the shortfalls of current immunosuppressive treatments. This concept is further strengthened by accumulating evidence that CD8+ Ts cells have beneficial effects in autoimmune disease and that patients with ongoing autoimmunity lack sufficient numbers of effective CD8+ Ts cells.
Rheumatoid arthritis
Rheumatoid arthritis (RA) is a systemic inflammatory disease, which primarily manifests with chronic destructive inflammation in the synovial lining of joints but can also target other organ systems. Recent evidence suggests that RA shortens the life expectancy of patients by accelerating the process of atherosclerosis, now also understood as an immuno-inflammatory condition[37, 38]. The current disease model proposes that arthritogenic antigens elicit a chronic immune response with participation of the innate and adaptive arms of the immune system. Consequences of chronic antigen stimulation include the proliferation of the synovium in an uncontrolled manner and the accumulation of ectopic germinal centers containing B and T lymphocytes [39, 40], as well as formation of new blood vessels and the secretion of proinflammatory cytokines in the tissue environment of the inflamed synovium [41].
Data have accumulated that chronic antigenic stimulation may not be the only disease mechanism of RA[42]. Rather, premature aging of the immune system with the accumulation of aged and proinflammatory effector T cells has also been implicated [43]. Functional abnormalities in hematopoietic stem cells from RA patients have given rise to the concept that immune regeneration processes are impaired[44]. Overall, these considerations are critically important as they would influence how cell-based therapy in RA should be targeted. Memory T cells in the joint may only be one relevant target.
Glucocorticoids first introduced in the late 1940’s have revolutionized the therapy of RA. Their effectiveness in RA may actually relate to the diversity of inhibitory effects they have in the innate and adaptive immune system. Further progress was made with the introduction of the disease-modifying agents, and more recently with the arrival of biologics. Monoclonal antibodies (mAb) or engineered soluble receptor molecules are now used to interfere with T-cell function, B-cell function and, mostly, with cytokine production in RA. While the armamentarium of therapeutics in RA has rapidly grown, we are still unable to cure the disease, and patients have to stay on immunosuppressants for years. Therefore, the future goal is to find immunotherapy that can achieve safe and permanent downregulation of disease-relevant immune responses. Tapping into the physiologic power of CD8+ Ts cells might be the next step in modulating the immune system in RA.
Data have been presented that CD8+CD28−CD56+ T cells have suppressive function in RA. These Ts cells tolerize APC, prevent the activation of naive CD4+ T cells, and inhibit CD4+ T-cell response in vivo. To test their immunoregulatory potential, CD8+CD28−CD56+ Ts cells were transferred into NOD-SCID chimeras engrafted with human synovial tissue. Transferred CD8+ Ts cells effectively suppressed the inflammatory activity in the synovial lesions with a concomitant decrease in cytokine production [45]. In a separate study, rheumatoid synovitis in NOD-SCID chimeras could be successfully treated by infusing tissue-derived autologous CD8+ T cells. Production of IL-1β, IFN-γ, TNF-α, and all other cytokines implicated in sustaining rheumatoid inflammation in the joint were markedly diminished after the transfer of IL-16+ CD8 T cells. Treatment with IL-16 mimicked the anti-inflammatory actions of the CD8+ Ts cells, and anti-IL-16 abrogated the activities of the inhibitory CD8+[46]. These studies have emphasized the potential of utilizing CD8+ T cells to downregulate the inflammatory reactions underlying rheumatoid synovitis. Further studies need to address whether CD8+ Ts cells have long-term effects, whether they have selective anti-inflammatory actions, and whether they could be expanded in vivo through targeted immunomodulation (Fig. 2).
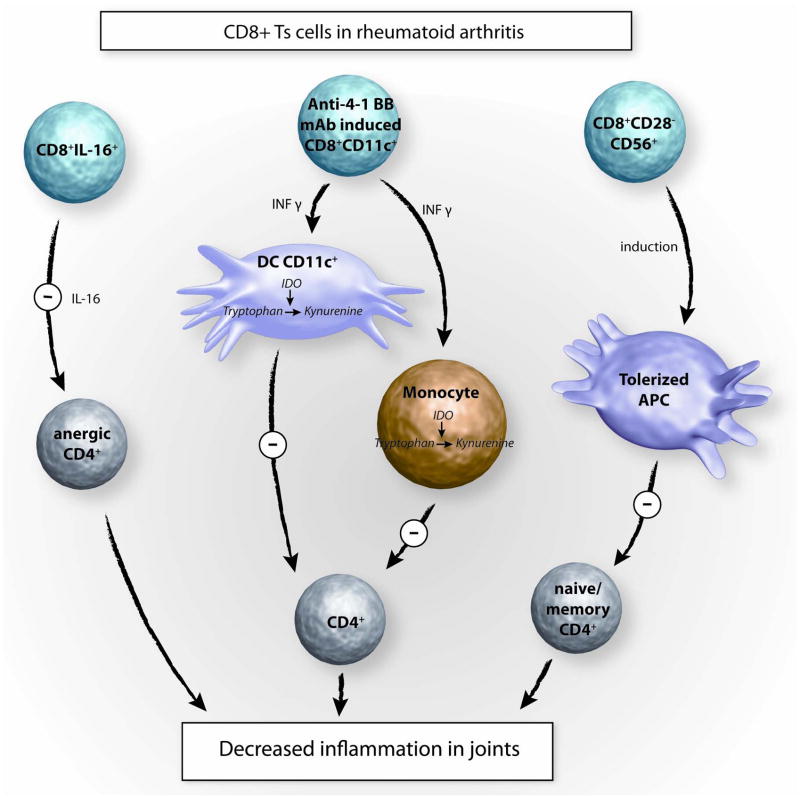
Fig 2. CD8+Ts cells in rheumatoid arthritis.
Three distinct immunomodulatory interventions have been described that can suppress the joint inflammation associated with RA. Adoptively transferred IL-16-secreting CD8+ Ts cells are believed to cause anergy in inflammatory CD4+ effector T cells. In an alternative approach, CD8+CD28−CD56+ Ts cells were transferred into NOD-SCID chimera mice engrafted with human synovial tissue. This cell-based therapy was highly effective in inhibiting inflammatory pathways mediated by APC and effector T cells. In a murine model system of chronic joint inflammation, CD8+CD11c+ Ts cells were induced by treatment with anti-4-1 BB mAb. Mechanisms of immunosuppression are believed to involve the induction of IDO (=indoleamine 2,3-dioxygenase ) in dendritic cells (DC) and monocytes through the secretion of IFN- γ. IDO-producing monocytes and DC can suppress effector CD4+ T cells in murine collagen type II-induced arthritis.
In mice, this strategy has been successfully applied by Seo at al who have generated CD8+ Ts cells by stimulation with anti- 4-1 BB mAb. Anti-4-1 BB mAb induced the clonal expansion of CD8+CD11c+ T cells which themselves secreted IFN-γ, which in turn induced IDO expression in DC and macrophages. DC and macrophages conditioned by the CD8+ Ts cells suppressed CD4+ T-cell responses in collagen type II-induced arthritis[47]. So far, the potential therapeutic benefit of anti-4-1BB has not been tested in humans (Fig. 2).
Systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is a life-threatening multisystem autoimmune disorder which preferentially affects females, often during the early decades of life. Patients can present with polyarthritis, Raynaud’s phenomenon, renal disease, hematological manifestations, and skin inflammation. Pulmonary, cardiac, gastrointestinal, and neurological involvement may occur and cause life-threatening complications. A typical immune defect in SLE patients is the production of autoantibodies, many of which target intracellular and nuclear antigens. Immunocomplex formation and deposition are considered critical steps in the pathogenesis. Steroids are used routinely in the management of SLE, mostly complemented with other broad immunosuppressants. The prominent role of autoantibodies has focused interest on B-cell dysfunction, but it would be equally important to reset T cells, which promote autoimmunity. The chronic nature of this disease requires safer and more efficient immunomodulatory interventions than currently are available.
Evidence for impaired Ts-cell function in SLE has come from the work of Filaci et al. CD8+ Ts cells were generated form SLE patients during exacerbation, during remission, and from healthy controls by culturing blood mononuclear cells with IL-2 and GM-CSF. While the numbers of outgrowing CD8+ T cells seemed to be rather similar between active and non-active patients and controls, functional capacities to suppress were distinct. Suppressive activity was tested by coculturing CD8+ T cells with autologous PBMC activated by anti-CD3 antibodies. CD8+ Ts cells collected from patients during exacerbation were not able to suppress, a function which was well maintained by patients in remission[48]. The finding that CD8+ Ts cells from healthy controls and SLE patients in remission were similarly able to suppress T-cell function suggests that CD8+ Ts cell defects in SLE may only be transient and directly related to disease activity.
Evidence has accumulated that novel immunomodulatory interventions may actually successfully treat SLE by targeting CD8+ Ts cells, at least in murine models. Based on binding characteristics of murine anti-DNA antibodies, Singh, Hahn, and colleagues have created a synthetic peptide, called pConsensus (pCons), which they have employed to tolerize lupus-prone (NZB × NZW) F1 female (BWF1) mice[49, 50]. Tolerized mice expressed an increased number of CD8+CD28− Ts cells which suppressed anti-DNA Ab production and could counteract the immune attack causing glomerulonephritis. In mechanistic studies, such CD8+CD28− Ts cells exhibited their anti-inflammatory activity through TGF-β-dependent inhibition of CD4+ T-cell and B-cell immune responses[51, 52]. Together with this group, Ferrera et al transfected somatic B cells with a plasmid encoding human IgG1 and pCons Ig (=pIgCons). Such B cells were then infused into mice and promoted the expansion of CD8+CD28− Ts cells which were able to decrease proteinuria in hypergammaglobulinemic mice, as well as suppress CD4+ T-cell proliferation via secretion of TGF-β [53] (Fig. 3).
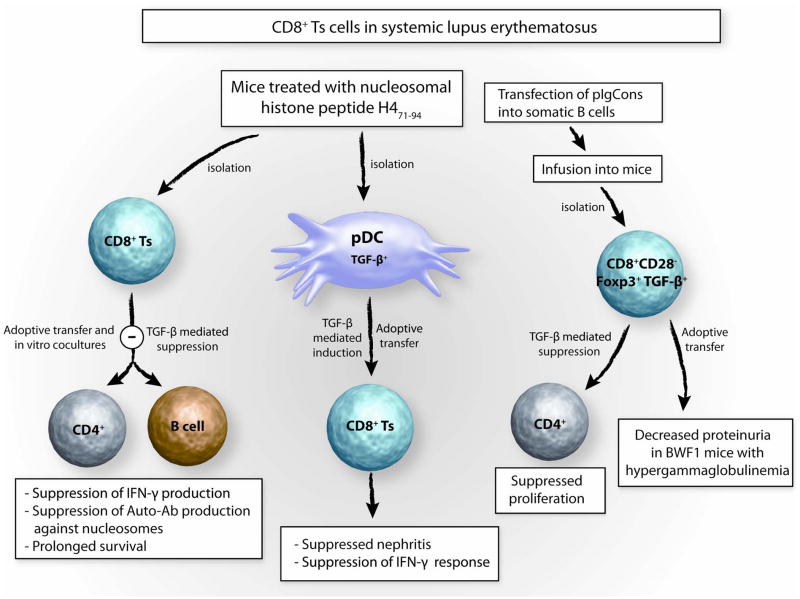
Fig 3. CD8+Ts cells in systemic lupus erythematosus.
The potential therapeutic applicability of anti-inflammatory CD8 Ts in SLE has been explored in detail in murine models of SLE. Injection of nucleosomal histone peptide H4 71-94 into lupus- prone mice induces CD8+ suppressor T cells which suppress autoantibody and IFN- γ production in cocultures with T and B cells of uninjected lupus prone mice. TGF-β has been implicated in the suppressive action. Further, s.c. injection of nucleosomal histone peptide H4 71-94 into lupus prone mice tolerizes DC, which release TGF-β and induce CD8+ suppressor T cells inhibiting nephritis. Treating mice with pIgCons (= plasmid encoding the Ig Consensus peptide) induces CD8+CD28−Foxp3+ suppressor T cells which suppress CD4+ T-cell proliferation and upon adoptive transfer into hypergammaglobulinemic mice reduce progression of renal disease.
In a study by Kang et al, inhibitory CD8+ T cells were generated upon stimulation with a naturally occurring nucleosomal histone peptide, H4 71-94. These CD8+ Ts cells were isolated to test them functionally in adoptive transfer experiments as well as explore their mechanisms of action in in vitro experiments. A single adoptive transfer was sufficient to suppress antibody production and nephritis for up to 2 months. When cocultured with lupus CD4+ T cells and B cells, CD8+ Ts cells efficiently downregulated antibody production and reduced IFN-γ production. These suppressive effects were mediated through TGF-β [54]. In a follow-up study, Kang et al isolated plasmacytoid dendritic cells (pDC) from mice treated with the nucleosomal histone peptide H471-94. These pDC produced high amounts of TGF-β and upon adoptive transfer induced CD8+ Ts cells. Remarkably, pDC conditioned by the immunization with the nucleosomal histone peptide enhanced the suppressive ability of inhibitory CD8+ T cells [55]. These data have opened the possibility that every stage of the SLE disease process, ranging from the prenephretic phase to advanced disease, is amendable to the action of CD8+ Ts cells (Fig. 3).
Utilizing synthetic peptides or histone peptides lends elegance to these novel approaches. Theoretically, with a unique design synthetic peptides could be adapted to the need of individual SLE patients. Potentially, the peptide-mediated induction of CD8+ Ts cells could provide a relatively safe means of downregulating defective immune responses while maintaining protective immune responses in SLE.
Multiple sclerosis
Multiple sclerosis (MS) is an immune-mediated demyelinating disease of the central nervous system (CNS) which causes neurological disability in young adults. The therapeutic armamentarium includes steroids, cyclosporine, methotrexate, azathioprine, interferon, and intravenous immune globulin (IVIG). Currently, no curative interventions are available and often patients progress despite all means of nonspecific immunosuppression. Like other autoimmune diseases, MS is a chronic disease with an almost unpredictable course. The strict target tropism for the CNS has supported the concept that CNS-specific antigens are the instigators, with the breakdown in immune tolerance possibly elicited by other immune responses, such as anti-pathogen responses.
Glatiramer acetate (GA) is a polymer of four amino acids found in myelin basic protein. Introduction of GA into MS therapy was guided by the idea that tolerance to myelin basic protein could be reestablished. GA has been shown to reduce the number and severity of disease exacerbations in MS patients [56]. Experimental evidence suggests that CD8+ Ts cells may be critical in controlling tissue-destructive immunity in MS. Specifically; data have accumulated associating the therapeutic benefits of glatiramer acetate with the induction of CD8+ Ts cells. In MS patients on GA therapy, Tennakoon et al observed higher frequencies of inhibitory CD8+ T cells with a higher ability to suppress CD4+ T cells. Data support the notion that such CD8+ Ts cells can directly kill CD4+ T cells in a HLA-E-dependent manner. It has been proposed that GA-induced CD8+ Ts cells recognize GA on the surface of target cells where it is bound by MHC class I molecules[ 57]. This is the first example of utilizing a specific antigenic moiety to induce CD8+ Ts cells which upon re-exposure eliminate antigen-presenting target cells (Fig. 4).
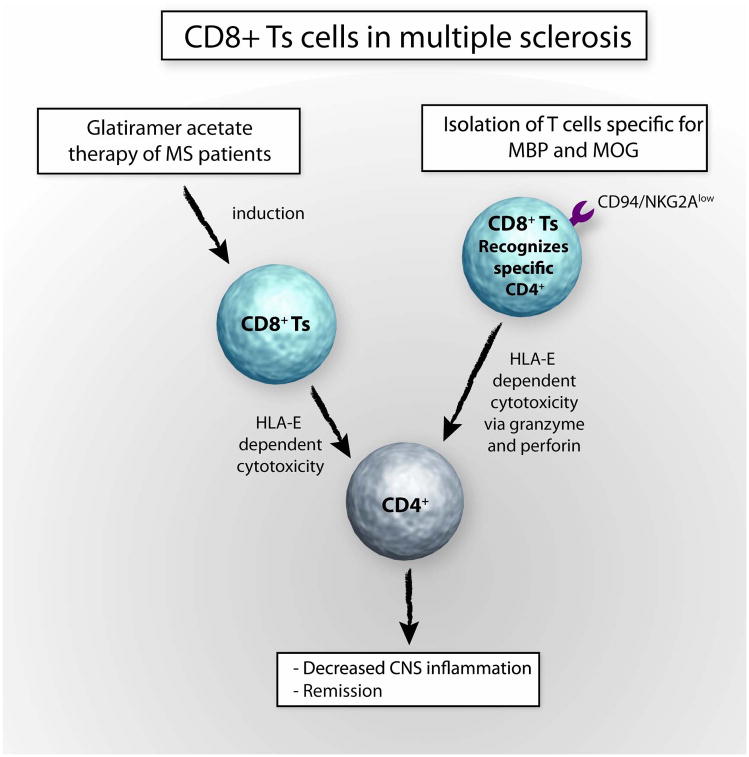
Fig 4. CD8+Ts cells in multiple sclerosis.
The principle of applying immunomodulatory therapy to promote CD8+ Ts cell function in vivo has been successfully utilized in patients with MS. Glatiramer acetate (GA) therapy induces CD8+ Ts cells which have been found to directly kill CD4+ T cells. The mechanism of suppression is contact dependent and HLA class I-restricted. Additional evidence for the potential therapeutic use of CD8+ Ts cells in MS has derived from studies exploring CD8+ Ts cells recognizing myelin basic protein (MBP) and myelin oligodendrocyte glycoprotein (MOG). Such CD8 Ts cells appear to be able to kill CD4+ T cells via granzyme and perforin in a HLA-E-dependent manner. In patients with flaring MS, such CD8+ Ts cells seem to be CD94/NKG2Ahigh, which decreases their cytotoxic ability but blocking such inhibitory receptors with anti-CD94 and anti-NKG2A mAb may restore their cytolytic function.
Additional support for a disease-relevant regulatory role of CD8+ Ts cells in MS comes from the work of Correale et al. These authors compared the rates of CD8+ inhibitory T cells in blood and CSF in patients during a MS flare and remission. Numbers of CD8+ Ts cells which recognized and killed activated myelin-reactive CD4+ T cells were decreased in patients with flare ups. Expression of CD94/NKG2A on CD8+ Ts cells resulted in decreased killing rates, emphasizing that the receptor profile on CD8+ Ts cells may closely control their suppressive actions. Cytotoxicity could be regained by addition of anti-CD94 and anti-NKG2A mAb. The authors concluded that surface molecules on CD8+ Ts cells have a direct impact on the functionality and need to be taken into account when assessing CD8 Ts cells in vivo and in vitro[58]. Targeting such regulatory receptors on CD8+ T cells by in vivo therapy may emerge as a potential new way of reactivating disease-inhibitory CD8+T cells (Fig. 4).
Inflammatory bowel disease (IBD)
Ulcerative colitis and Crohn’s disease represent the two major autoimmune inflammatory syndromes injuring the gastrointestinal tract. While distinct disorders with unique pathogenic defects, they share some epidemiologic features. They also have in common that they occur at the interface between the powerful gastrointestinal immune system and the enormous flora of gut-residing bacteria. IBD patients frequently have autoimmune manifestations in other organ systems, providing fertile ground to explore immunomodulatory therapies that can alter the immune system overall.
In a murine model for IBD, naturally occurring CD8+CD28− Ts lymphocytes can prevent experimentally induced gut inflammation. In this model, IBD was induced by injecting CD4+CD45RBhigh cells into RAG-2-deficient mice, and this process was amendable to suppression through CD8+CD28− T cells. In vitro studies revealed that CD8+CD28− T cells from wild-type mice were able to efficiently reduce IFN-γ production of CD4+ T cells in an allogeneic mixed lymphocyte culture. Secretion of IL-10 was identified as the underlying suppressive mechanism. Other mechanisms including TGF-β secretion may also contribute to the prevention of colitis[59].
In an alternate model of spontaneous ileitis in TNFΔ ARE mice, Ho et al could successfully ameliorate disease with CD8+CD103high T cells. These CD8+ Ts cells diminished proliferation of CD4+ T cells through a TGF-β-dependent mechanism. Noticeably, CD8+CD103 highCD44low T cells express L-selectin, enabling them to access secondary lymphoid tissues. It has therefore been proposed that they may exhibit their disease-suppressive action within lymph nodes modulating T-cell priming rather than acting within the inflammatory intestinal sites [60].
Intestinal epithelial cells may have a role in directly regulating the number and the activation status of CD8+ Ts cells. Brimnes et al have described a CD8+ Ts cell, which can be triggered by intestinal epithelial cells. An insufficiency in this downregulatory role may be relevant in IBD. A direct comparison of lamina propria CD8+ T cells from patients with IBD and healthy controls demonstrated effective suppression of IgG production by the control lamina propria CD8+ T cells but a lack of suppression by the IBD lamina propria cells [61].
Diabetes mellitus type I
Organ-specific autoimmunity leading to the destruction of insulin producing β-cells in the pancreatic islets of Langerhans causes type I diabetes mellitus (DM). The current disease model proposes that a breakdown in self tolerance results in the production of β-cell-damaging antibodies and that tissue injury is amplified by cellular mechanisms involving a multitude of effector cells. Important autoantigens are glutamic acid decarboxylase (GAD) and insulin andinsulinoma-associated protein 2 (IA-2). With a checkpoint position in maintaining self tolerance, T cells and their dysregulation play a central role in the immunopathogenesis of type I DM. As the loss of pancreatic islet cells is currently considered to be irreversible, immunodulatory therapy would have to be introduced very early in the disease process, essentially during the preclinical phase. This imposes a major emphasis on being able to identify individuals at risk to begin immunosuppression prior to destruction of insulin-producing cells. In that sense, type I DM distinguishes itself from many other autoimmune syndromes, e.g. SLE in which cell-injurious immune reactivations are drawn out over long periods. Untreated type I DM is fatal but insulin therapy has made this a chronic disease. Insulin-deficient individuals, despite major advances in insulin supplementation therapy, are still prone to develop serious side effects from insufficient glucose control. Micro and macroangiopathic complications are the most significant in reducing life quality and life span of type I DM patients. Because most patients lose their insulin-producing cells during their childhood, immunosuppression in this autoimmune disease is particularly challenging. Amplifying the number and activity of physiologic immunoregulatory cells, such as CD8+ Ts cells emerges as an appealing goal.
Production of autoantibodies potentially damaging to insulin-producing β-cells is not exclusively found in diabetic individuals but can occur in normal donors. James et al have proposed that CD8+CD45RA+CD27− Ts cells can regulate CD4+ T-cell response to the autoantigen GAD65 in healthy individuals. This control seems to fail in type I DM patients. In vitro GAD65-specific CD4+ T cells from healthy individuals can be strongly inhibited by autologous CD8+ T cells. Cell contact-dependent mechanisms have been implicated and although IL-10 secretion seems to play an important role in the suppression, it does not seem to be secreted by CD8+ Ts cells. Rather, it has been proposed that IL-10 is secreted by the APC as a result of a conditioning process [62] (Fig. 5).
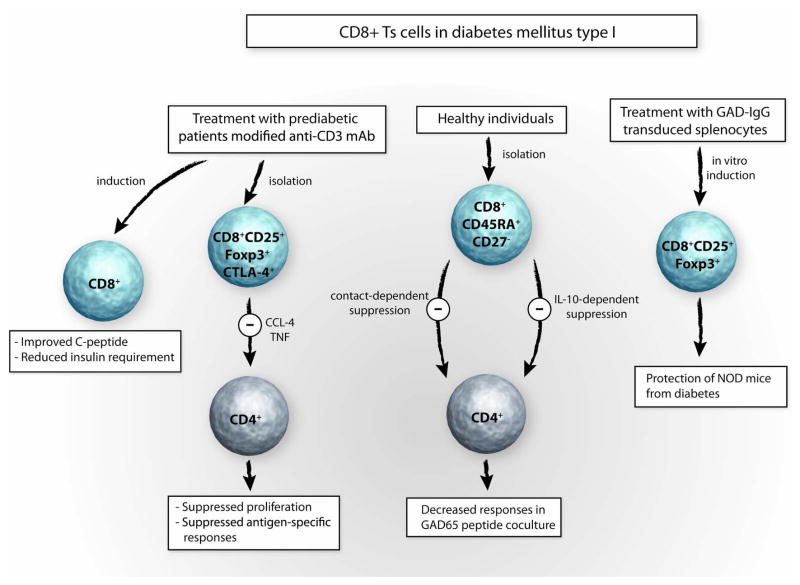
Fig 5. CD8+Ts cells in diabetes mellitus type I.
Utilizing endogenous pathways of immunosuppression has particular appeal for patients with diabetes mellitus type 1 as they are often in their childhood and prone to suffer long-term side effects from nonspecific immunosuppressive therapy. Treating patients with recent onset of diabetes type I with a single course of modified anti-CD3 mAb induces CD8+ T cells in vivo. This experimental therapy was associated with improvement of C-peptide levels and reduction of insulin requirements. In in vitro studies CD8+CD25+Foxp3+ T suppressor cells isolated from patients treated with modified anti-CD3 mAb suppress CD4+ T-cell proliferation in a CCL-4- and TNF-dependent manner. Also, CD8+CD45RA+CD27− Ts cells isolated form healthy individuals can inhibit the function of CD4+ T cells, which respond to the GAD65 peptide. In a model of murine diabetes, CD8+CD25+Foxp3+ Ts cells induced by vaccinating with GAD-IgG transduced splenocytes protect NOD mice from diabetes.
In a groundbreaking study, Herold and colleagues have explored therapeutic effects of treating patients with new onset insulin-dependent diabetes mellitus with a one course administration of humanized modified anti-CD3 mAb called teplizumab (hOKT3γ1(Ala-Ala))[18]. The rationale of this study was to reset the patient’s immune system in such a way that immune-mediated B-cell destruction could be stopped or even reverted. Original work in this area had focused on utilizing antibodies to the T-cell marker CD3 to disrupt T-cell dependent immune responses and open a window of opportunities to eliminate memory T-cell responses to autoantigens. Utilization of murine OKT3/anti-CD3 mAb to prevent transplant rejection was associated with too many side effects [63, 64]This prompted the development of humanized and modified anti-CD3 mAb which could modulate T-cell function in the absence of causing a cytokine storm or massively depleting T cells[65, 66]. After reassuring studies in which the administration showed positive results in NOD mouse models, Herold et al initiated a study in patients with new onset type I diabetes in which hOKT3γ1(Ala-Ala) was given over a course of 12 or 14 days. To monitor the insulin reserve of enrolled patients, they were regularly tested for C-peptide responses. The study demonstrated that the C-peptide and therefore insulin secretion improved for almost 2 years after a single course of treatment[18]. This beneficial outcome was also reflected in the lower HbA1c levels of the treated group. In mechanistic studies Bisikirska et al could correlate the in vivo effects with an increase in the numbers of CD8+ CD25+Foxp3+CTLA-4+ Ts cells circulating in the blood. All patients treated with hOKT3γ1 (Ala-Ala) demonstrated an increase in CD8+CD25+ CTLA-4+Foxp3+ Ts cells. Such CD8 Ts cells were able to inhibit the stimulation of CD4+ T cells in an in vitro coculture system[19]. Again, cell-cell contact seemed to be necessary for the CD8+ Ts cells to deploy their inhibitory functions. In a recent publication, these authors suggest that the inhibitory effects of the CD8+ Ts cells generated by hOKT3γ1 (Ala-Ala) could be partially eliminated by anti-CCL-4 and anti-TNF, suggesting the suppressor mechanism to be CCL-4 and TNF dependent[67] (Fig. 5). Encouraging results were also reported from experiments in diabetes-prone mice. In vitro retroviral transduction of splenocytes from nonobese diabetic (NOD) donor mice with a GAD65-IgG fusion construct resulted in marked induction of CD8+CD25+Foxp3+ Ts cells, which were shown to prevent diabetes and to suppress in a contact-dependent manner. In subsequent experiments, CD8−CD25−GAD-IgG transduced splenocytes were injected into recipient female NOD mice where they could not prevent hyperglycemia, compared to control mice which were injected with total GAD-IgG transduced splenocytes. These experiments were interpreted as showing that depletion of CD8+ or CD8+CD25+ Ts cells from the transferred population significantly increased the incidence of diabetes. Injection of non-depleted, total GAD-Ig transduced splenocytes into NOD mice reduced the frequency of insulinitis significantly, compared to the depleted CD8−CD25−GAD-IgG-treated group. This data support the notion that CD8 Ts cells have protective functions in vivo [68] (Fig. 5).
Conclusion/Expert Opinion
The concept that T cells are capable of downregulating immunity is now firmly established. Both within the CD4 and the CD8 T-cell subsets, specialized populations apply sophisticated mechanisms to inhibit early and late steps of immune responses. The current review has summarized the increasing information on CD8 Ts cells and their potential applications in managing autoimmune diseases. Some general principles emerge from the available data:
- CD8 Ts cells are a heterogeneous group of subsets. Whether all these subsets derive from a common precursor or whether different types of CD8 T cells can differentiate into suppresser cells is not known.
- Natural CD8 Ts cells originating in the thymus seem to exist in mice and humans.
- In vitro CD8 T cells with suppressive function can be induced by antigen-specific and nonspecific means. Antigen nonspecific CD8 Ts cells could have broad therapeutic application, potentially useful in many clinical settings.
- While present in many CD8 Ts cell populations, expression of the transcription factor Foxp3 appears not to be a condition sine qua non for inhibitory CD8 T cells.
- Like in CD4 T regulatory cells, CD8 Ts cells employ their suppressive function by releasing cytokines or by directly altering the functional status of T cells or APC. Secreted products and cell surface receptors facilitating suppression are only partially understood.
- Accumulating evidence suggests that patients with autoimmune disease have lower numbers of or less functional CD8 Ts cells. Examples include patients with IBD, SLE, and MS.
- The potential to generate antigen-specific inhibitory T cells opens the door for novel therapeutic applications that could avoid the shortfalls of current immunosuppressive therapies.
- Barriers for the use of antigen-specific CD8 Ts cells include the lack of knowledge about disease-inducing antigens and the need to generate a specific CD8 Ts cells for each patient.
- Immunomodulatory therapies already in use may work by inducing CD8 Ts cells. Notable examples are GA therapy in MS and the immunosuppressive benefits associated with modified anti-CD3 antibodies in patients with type Idiabetes.
- Spontaneous and therapy-induced frequencies of CD8 Ts cells may be useful in monitoring disease activity and responses to immunomodulation.
- Immediate steps to take in translating the knowledge about CD8 Ts cells into clinical medicine include optimization of isolating precursor cells from patients and generating large numbers; exploring their potential use in localized inflammatory lesions and injecting CD8 locally; and testing the effects of established and novel immunomodulatory drugs on the frequencies and functions of CD8 Ts cells.
Acknowledgments
This work was funded in part by grants from the National Institutes of Health (RO1 AR42527, RO1 AR41974, R01 AI44142, R01 AI57266, RO1 EY11916, R01 AG15043, and HL058000) and a grant from the Vasculitis Foundation. The authors thank Tamela Yeargin for editing the manuscript and Ajaya K. Vonumu for making the figures.
References
- 1.Nishizuka Y, Sakakura T. Thymus and reproduction: sex-linked dysgenesia of the gonad after neonatal thymectomy in mice. Science. 1969 Nov 7;166(906):753–5. doi: 10.1126/science.166.3906.753. [DOI] [PubMed] [Google Scholar]
- 2.Gershon RK, Cohen P, Hencin R, Liebhaber SA. Suppressor T cells. J Immunol. 1972 Mar;108(3):586–90. [PubMed] [Google Scholar]
- 3.Cantor H, Hugenberger J, McVay-Boudreau L, Eardley DD, Kemp J, Shen FW, et al. Immunoregulatory circuits among T-cell sets. Identification of a subpopulation of T-helper cells that induces feedback inhibition. J Exp Med. 1978 Oct 1;148(4):871–7. doi: 10.1084/jem.148.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995 Aug 1;155(3):1151–64. [PubMed] [Google Scholar]
- 5.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002 Dec 5;420(6915):502–7. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 6••.Kapp JA, Bucy RP. CD8+ suppressor T cells resurrected. Hum Immunol. 2008 Nov;69(11):715–20. doi: 10.1016/j.humimm.2008.07.018. Excellent review on CD8+ Ts cells, their potential mechanism of suppression, and their role in transplant rejection. [DOI] [PubMed] [Google Scholar]
- 7.Kapp JA, Honjo K, Kapp LM, Xu X, Cozier A, Bucy RP. TCR transgenic CD8+ T cells activated in the presence of TGFbeta express FoxP3 and mediate linked suppression of primary immune responses and cardiac allograft rejection. Int Immunol. 2006 Nov;18(11):1549–62. doi: 10.1093/intimm/dxl088. [DOI] [PubMed] [Google Scholar]
- 8.Karlsson I, Malleret B, Brochard P, Delache B, Calvo J, Le Grand R, et al. FoxP3+ CD25+ CD8+ T-cell induction during primary simian immunodeficiency virus infection in cynomolgus macaques correlates with low CD4+ T-cell activation and high viral load. J Virol. 2007 Dec;81(24):13444–55. doi: 10.1128/JVI.01466-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shao L, Jacobs AR, Johnson VV, Mayer L. Activation of CD8+ regulatory T cells by human placental trophoblasts. J Immunol. 2005 Jun 15;174(12):7539–47. doi: 10.4049/jimmunol.174.12.7539. [DOI] [PubMed] [Google Scholar]
- 10.Sugita S, Futagami Y, Horie S, Mochizuki M. Transforming growth factor beta-producing Foxp3(+)CD8(+)CD25(+) T cells induced by iris pigment epithelial cells display regulatory phenotype and acquire regulatory functions. Exp Eye Res. 2007 Nov;85(5):626–36. doi: 10.1016/j.exer.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Cosmi L, Liotta F, Lazzeri E, Francalanci M, Angeli R, Mazzinghi B, et al. Human CD8+CD25+ thymocytes share phenotypic and functional features with CD4+CD25+ regulatory thymocytes. Blood. 2003 Dec 1;102(12):4107–14. doi: 10.1182/blood-2003-04-1320. [DOI] [PubMed] [Google Scholar]
- 12.Rifa’i M, Shi Z, Zhang SY, Lee YH, Shiku H, Isobe K, et al. CD8+CD122+ regulatory T cells recognize activated T cells via conventional MHC class I-alphabetaTCR interaction and become IL-10-producing active regulatory cells. Int Immunol. 2008 Jul;20(7):937–47. doi: 10.1093/intimm/dxn052. [DOI] [PubMed] [Google Scholar]
- 13.Filaci G, Fravega M, Negrini S, Procopio F, Fenoglio D, Rizzi M, et al. Nonantigen specific CD8+ T suppressor lymphocytes originate from CD8+CD28- T cells and inhibit both T-cell proliferation and CTL function. Hum Immunol. 2004 Feb;65(2):142–56. doi: 10.1016/j.humimm.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Groux H, Bigler M, de Vries JE, Roncarolo MG. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J Exp Med. 1996 Jul 1;184(1):19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fenoglio D, Ferrera F, Fravega M, Balestra P, Battaglia F, Proietti M, et al. Advancements on phenotypic and functional characterization of non-antigen-specific CD8+CD28- regulatory T cells. Hum Immunol. 2008 Nov;69(11):745–50. doi: 10.1016/j.humimm.2008.08.282. [DOI] [PubMed] [Google Scholar]
- 16.Mahic M, Henjum K, Yaqub S, Bjornbeth BA, Torgersen KM, Tasken K, et al. Generation of highly suppressive adaptive CD8(+)CD25(+)FOXP3(+) regulatory T cells by continuous antigen stimulation. Eur J Immunol. 2008 Mar;38(3):640–6. doi: 10.1002/eji.200737529. [DOI] [PubMed] [Google Scholar]
- 17.Fan TM, Kranz DM, Flavell RA, Roy EJ. Costimulatory strength influences the differential effects of transforming growth factor beta1 for the generation of CD8+ regulatory T cells. Mol Immunol. 2008 May;45(10):2937–50. doi: 10.1016/j.molimm.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 18.Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, et al. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005 Jun;54(6):1763–9. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19••.Bisikirska B, Colgan J, Luban J, Bluestone JA, Herold KC. TCR stimulation with modified anti-CD3 mAb expands CD8+ T cell population and induces CD8+CD25+ Tregs. J Clin Invest. 2005 Oct;115(10):2904–13. doi: 10.1172/JCI23961. Provides evidence that the mechanism-of-action of modified anti-CD3 mAb involves the induction of CD8+ Ts cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joosten SA, van Meijgaarden KE, Savage ND, de Boer T, Triebel F, van der Wal A, et al. Identification of a human CD8+ regulatory T cell subset that mediates suppression through the chemokine CC chemokine ligand 4. Proc Natl Acad Sci U S A. 2007 May 8;104(19):8029–34. doi: 10.1073/pnas.0702257104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uss E, Rowshani AT, Hooibrink B, Lardy NM, van Lier RA, ten Berge IJ. CD103 is a marker for alloantigen-induced regulatory CD8+ T cells. J Immunol. 2006 Sep 1;177(5):2775–83. doi: 10.4049/jimmunol.177.5.2775. [DOI] [PubMed] [Google Scholar]
- 22••.Uss E, Yong SL, Hooibrink B, van Lier RA, ten Berge IJ. Rapamycin enhances the number of alloantigen-induced human CD103+CD8+ regulatory T cells in vitro. Transplantation. 2007 Apr 27;83(8):1098–106. doi: 10.1097/01.tp.0000259555.29762.f0. This study exemplifies that some of our current immunosuppressive medications might function by targeting CD8+ Ts cells. [DOI] [PubMed] [Google Scholar]
- 23.Allez M, Brimnes J, Dotan I, Mayer L. Expansion of CD8+ T cells with regulatory function after interaction with intestinal epithelial cells. Gastroenterology. 2002 Nov;123(5):1516–26. doi: 10.1053/gast.2002.36588. [DOI] [PubMed] [Google Scholar]
- 24.Yio XY, Mayer L. Characterization of a 180-kDa intestinal epithelial cell membrane glycoprotein, gp180. A candidate molecule mediating t cell-epithelial cell interactions. J Biol Chem. 1997 May 9;272(19):12786–92. doi: 10.1074/jbc.272.19.12786. [DOI] [PubMed] [Google Scholar]
- 25.Jarvis LB, Matyszak MK, Duggleby RC, Goodall JC, Hall FC, Gaston JS. Autoreactive human peripheral blood CD8+ T cells with a regulatory phenotype and function. Eur J Immunol. 2005 Oct;35(10):2896–908. doi: 10.1002/eji.200526162. [DOI] [PubMed] [Google Scholar]
- 26.Gilliet M, Liu YJ. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J Exp Med. 2002 Mar 18;195(6):695–704. doi: 10.1084/jem.20011603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei S, Kryczek I, Zou L, Daniel B, Cheng P, Mottram P, et al. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res. 2005 Jun 15;65(12):5020–6. doi: 10.1158/0008-5472.CAN-04-4043. [DOI] [PubMed] [Google Scholar]
- 28.Ciubotariu R, Colovai AI, Pennesi G, Liu Z, Smith D, Berlocco P, et al. Specific suppression of human CD4+ Th cell responses to pig MHC antigens by CD8+CD28- regulatory T cells. J Immunol. 1998 Nov 15;161(10):5193–202. [PubMed] [Google Scholar]
- 29.Li J, Liu Z, Jiang S, Cortesini R, Lederman S, Suciu-Foca N. T suppressor lymphocytes inhibit NF-kappa B-mediated transcription of CD86 gene in APC. J Immunol. 1999 Dec 15;163(12):6386–92. [PubMed] [Google Scholar]
- 30.Chang CC, Ciubotariu R, Manavalan JS, Yuan J, Colovai AI, Piazza F, et al. Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol. 2002 Mar;3(3):237–43. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 31.Vlad G, Cortesini R, Suciu-Foca N. CD8+ T suppressor cells and the ILT3 master switch. Hum Immunol. 2008 Nov;69(11):681–6. doi: 10.1016/j.humimm.2008.08.286. [DOI] [PubMed] [Google Scholar]
- 32••.Vlad G, D’Agati VD, Zhang QY, Liu Z, Ho EK, Mohanakumar T, et al. Immunoglobulin-like transcript 3-Fc suppresses T-cell responses to allogeneic human islet transplants in hu-NOD/SCID mice. Diabetes. 2008 Jul;57(7):1878–86. doi: 10.2337/db08-0054. Provides a novel approach towards the treatment of diabetes type I building on the powerful immunomodulatory role of ILT3 in transplantation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chess L, Jiang H. Resurrecting CD8+ suppressor T cells. Nat Immunol. 2004 May;5(5):469–71. doi: 10.1038/ni0504-469. [DOI] [PubMed] [Google Scholar]
- 34.Lu L, Werneck MB, Cantor H. The immunoregulatory effects of Qa-1. Immunol Rev. 2006 Aug;212:51–9. doi: 10.1111/j.0105-2896.2006.00418.x. [DOI] [PubMed] [Google Scholar]
- 35.Sarantopoulos S, Lu L, Cantor H. Qa-1 restriction of CD8+ suppressor T cells. J Clin Invest. 2004 Nov;114(9):1218–21. doi: 10.1172/JCI23152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Czesnikiewicz-Guzik M, Lee WW, Cui D, Hiruma Y, Lamar DL, Yang ZZ, et al. T cell subset-specific susceptibility to aging. Clin Immunol. 2008 Apr;127(1):107–18. doi: 10.1016/j.clim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung CP, Avalos I, Raggi P, Stein CM. Atherosclerosis and inflammation: insights from rheumatoid arthritis. Clin Rheumatol. 2007 Aug;26(8):1228–33. doi: 10.1007/s10067-007-0548-7. [DOI] [PubMed] [Google Scholar]
- 38.Kaplan MJ. Cardiovascular disease in rheumatoid arthritis. Curr Opin Rheumatol. 2006 May;18(3):289–97. doi: 10.1097/01.bor.0000218951.65601.bf. [DOI] [PubMed] [Google Scholar]
- 39.Weyand CM. Immunopathologic aspects of rheumatoid arthritis: who is the conductor and who plays the immunologic instrument? J Rheumatol Suppl. 2007 Jul;79:9–14. [PubMed] [Google Scholar]
- 40.Weyand CM, Goronzy JJ. Ectopic germinal center formation in rheumatoid synovitis. Ann N Y Acad Sci. 2003 Apr;987:140–9. doi: 10.1111/j.1749-6632.2003.tb06042.x. [DOI] [PubMed] [Google Scholar]
- 41.Knedla A, Neumann E, Muller-Ladner U. Developments in the synovial biology field 2006. Arthritis Res Ther. 2007;9(2):209. doi: 10.1186/ar2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goronzy JJ, Weyand CM. Rheumatoid arthritis. Immunol Rev. 2005 Apr;204:55–73. doi: 10.1111/j.0105-2896.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 43.Weyand CM, Goronzy JJ. T-cell-targeted therapies in rheumatoid arthritis. Nat Clin Pract Rheumatol. 2006 Apr;2(4):201–10. doi: 10.1038/ncprheum0142. [DOI] [PubMed] [Google Scholar]
- 44.Colmegna I, Diaz-Borjon A, Fujii H, Schaefer L, Goronzy JJ, Weyand CM. Defective proliferative capacity and accelerated telomeric loss of hematopoietic progenitor cells in rheumatoid arthritis. Arthritis Rheum. 2008 Apr;58(4):990–1000. doi: 10.1002/art.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davila E, Kang YM, Park YW, Sawai H, He X, Pryshchep S, et al. Cell-based immunotherapy with suppressor CD8+ T cells in rheumatoid arthritis. J Immunol. 2005 Jun 1;174(11):7292–301. doi: 10.4049/jimmunol.174.11.7292. [DOI] [PubMed] [Google Scholar]
- 46.Klimiuk PA, Goronzy JJ, Weyand CM. IL-16 as an anti-inflammatory cytokine in rheumatoid synovitis. J Immunol. 1999 Apr 1;162(7):4293–9. [PubMed] [Google Scholar]
- 47.Seo SK, Choi JH, Kim YH, Kang WJ, Park HY, Suh JH, et al. 4-1BB-mediated immunotherapy of rheumatoid arthritis. Nat Med. 2004 Oct;10(10):1088–94. doi: 10.1038/nm1107. [DOI] [PubMed] [Google Scholar]
- 48.Filaci G, Bacilieri S, Fravega M, Monetti M, Contini P, Ghio M, et al. Impairment of CD8+ T suppressor cell function in patients with active systemic lupus erythematosus. J Immunol. 2001 May 15;166(10):6452–7. doi: 10.4049/jimmunol.166.10.6452. [DOI] [PubMed] [Google Scholar]
- 49.Singh RR, Ebling FM, Sercarz EE, Hahn BH. Immune tolerance to autoantibody-derived peptides delays development of autoimmunity in murine lupus. J Clin Invest. 1995 Dec;96(6):2990–6. doi: 10.1172/JCI118371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hahn BH, Singh RR, Wong WK, Tsao BP, Bulpitt K, Ebling FM. Treatment with a consensus peptide based on amino acid sequences in autoantibodies prevents T cell activation by autoantigens and delays disease onset in murine lupus. Arthritis Rheum. 2001 Feb;44(2):432–41. doi: 10.1002/1529-0131(200102)44:2<432::AID-ANR62>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 51.Hahn BH, Singh RP, La Cava A, Ebling FM. Tolerogenic treatment of lupus mice with consensus peptide induces Foxp3-expressing, apoptosis-resistant, TGFbeta-secreting CD8+ T cell suppressors. J Immunol. 2005 Dec 1;175(11):7728–37. doi: 10.4049/jimmunol.175.11.7728. [DOI] [PubMed] [Google Scholar]
- 52•.Singh RP, La Cava A, Wong M, Ebling F, Hahn BH. CD8+ T cell-mediated suppression of autoimmunity in a murine lupus model of peptide-induced immune tolerance depends on Foxp3 expression. J Immunol. 2007 Jun 15;178(12):7649–57. doi: 10.4049/jimmunol.178.12.7649. This work builds on a series of appealing studies establishing the potential clinical use of peptide-induced immunotolerance as a novel way of treating SLE. The paper provides further insight for the important contribution of pConsensus-induced CD8+ Ts cells and the role of Foxp3 expression. [DOI] [PubMed] [Google Scholar]
- 53.Ferrera F, Hahn BH, Rizzi M, Anderson M, Fitzgerald J, Millo E, et al. Protection against renal disease in (NZB × NZW)F(1) lupus-prone mice after somatic B cell gene vaccination with anti-DNA immunoglobulin consensus peptide. Arthritis Rheum. 2007 Jun;56(6):1945–53. doi: 10.1002/art.22700. [DOI] [PubMed] [Google Scholar]
- 54.Kang HK, Michaels MA, Berner BR, Datta SK. Very low-dose tolerance with nucleosomal peptides controls lupus and induces potent regulatory T cell subsets. J Immunol. 2005 Mar 15;174(6):3247–55. doi: 10.4049/jimmunol.174.6.3247. [DOI] [PubMed] [Google Scholar]
- 55.Kang HK, Liu M, Datta SK. Low-dose peptide tolerance therapy of lupus generates plasmacytoid dendritic cells that cause expansion of autoantigen-specific regulatory T cells and contraction of inflammatory Th17 cells. J Immunol. 2007 Jun 15;178(12):7849–58. doi: 10.4049/jimmunol.178.12.7849. [DOI] [PubMed] [Google Scholar]
- 56.Weber MS, Hohlfeld R, Zamvil SS. Mechanism of action of glatiramer acetate in treatment of multiple sclerosis. Neurotherapeutics. 2007 Oct;4(4):647–53. doi: 10.1016/j.nurt.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tennakoon DK, Mehta RS, Ortega SB, Bhoj V, Racke MK, Karandikar NJ. Therapeutic induction of regulatory, cytotoxic CD8+ T cells in multiple sclerosis. J Immunol. 2006 Jun 1;176(11):7119–29. doi: 10.4049/jimmunol.176.11.7119. [DOI] [PubMed] [Google Scholar]
- 58.Correale J, Villa A. Isolation and characterization of CD8+ regulatory T cells in multiple sclerosis. J Neuroimmunol. 2008 Mar;195(1–2):121–34. doi: 10.1016/j.jneuroim.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 59.Menager-Marcq I, Pomie C, Romagnoli P, van Meerwijk JP. CD8+CD28-regulatory T lymphocytes prevent experimental inflammatory bowel disease in mice. Gastroenterology. 2006 Dec;131(6):1775–85. doi: 10.1053/j.gastro.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ho J, Kurtz CC, Naganuma M, Ernst PB, Cominelli F, Rivera-Nieves J. A CD8+/CD103high T cell subset regulates TNF-mediated chronic murine ileitis. J Immunol. 2008 Feb 15;180(4):2573–80. doi: 10.4049/jimmunol.180.4.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brimnes J, Allez M, Dotan I, Shao L, Nakazawa A, Mayer L. Defects in CD8+ regulatory T cells in the lamina propria of patients with inflammatory bowel disease. J Immunol. 2005 May 1;174(9):5814–22. doi: 10.4049/jimmunol.174.9.5814. [DOI] [PubMed] [Google Scholar]
- 62•.James EA, Kwok WW. CD8+ suppressor-mediated regulation of human CD4+ T cell responses to glutamic acid decarboxylase 65. Eur J Immunol. 2007 Jan;37(1):78–86. doi: 10.1002/eji.200636383. Provides a new concept towards understanding how CD8+ Ts cells might be able to protect healthy individuals from type I diabetes. [DOI] [PubMed] [Google Scholar]
- 63.Chatenoud L. OKT3-induced cytokine-release syndrome: prevention effect of anti-tumor necrosis factor monoclonal antibody. Transplant Proc. 1993 Apr;25(2 Suppl 1):47–51. [PubMed] [Google Scholar]
- 64.Chatenoud L. Humoral immune response against OKT3. Transplant Proc. 1993 Apr;25(2 Suppl 1):68–73. [PubMed] [Google Scholar]
- 65.Alegre ML, Peterson LJ, Xu D, Sattar HA, Jeyarajah DR, Kowalkowski K, et al. A non-activating “humanized” anti-CD3 monoclonal antibody retains immunosuppressive properties in vivo. Transplantation. 1994 Jun 15;57(11):1537–43. [PubMed] [Google Scholar]
- 66.Xu D, Alegre ML, Varga SS, Rothermel AL, Collins AM, Pulito VL, et al. In vitro characterization of five humanized OKT3 effector function variant antibodies. Cell Immunol. 2000 Feb 25;200(1):16–26. doi: 10.1006/cimm.2000.1617. [DOI] [PubMed] [Google Scholar]
- 67.Ablamunits V, Bisikirska BC, Herold KC. Human regulatory CD8 T cells. Ann N Y Acad Sci. 2008 Dec;1150:234–8. doi: 10.1196/annals.1447.000. [DOI] [PubMed] [Google Scholar]
- 68.Wang R, Han G, Song L, Wang J, Chen G, Xu R, et al. CD8+ regulatory T cells are responsible for GAD-IgG gene-transferred tolerance induction in NOD mice. Immunology. 2009 Jan;126(1):123–31. doi: 10.1111/j.1365-2567.2008.02884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]