Abstract
Patients with schizophrenia (n = 41) and healthy comparison participants (n = 46) completed neuropsychological measures of intelligence, memory, and executive function. A subset of each group also completed magnetic resonance diffusion tensor imaging (DTI) studies (fractional anisotropy and cross-sectional area) of the uncinate fasciculus (UF) and cingulate bundle (CB). Patients with schizophrenia showed reduced levels of functioning across all neuropsychological measures. In addition, selective neuropsychological–DTI relationships emerged. Among patients but not controls, lower levels of declarative–episodic verbal memory correlated with reduced left UF, whereas executive function errors related to performance monitoring correlated with reduced left CB. The data suggested abnormal DTI patterns linking declarative–episodic verbal memory deficits to the left UF and executive function deficits to the left CB among patients with schizophrenia.
Schizophrenia invariably compromises various aspects of cognition most commonly measured and documented with standardized clinical neuropsychological tests (e.g., Bilder, Mukherjee, Reider, & Pandurangi, 1985; Goldberg et al., 1990). As with other dimensions of the disease, neuropsychological test patterns are often marked by considerable heterogeneity. Arguably most common, particularly for chronic but also for first-episode conditions (Bilder et al., 2000; Mohamed, Paulsen, O'Leary, Arndt, & Andreasen, 1999), is a test pattern marked by general diminution of scores across various broad domains of cognition (Blanchard & Neale, 1994; Braff et al., 1991). Most pronounced deficits are typically evident on measures of working memory, verbal memory, and learning and executive functions that serve to guide action and thought (Bilder et al., 2000; Saykin et al., 1991, 1994). These various patterns of neuropsychological decline appear to have a relatively stable course (Heaton et al., 2001) and are not attributable to medication (Blanchard & Neale, 1994) or changes in personality, motivation, or transient mental state. Rather, these neuropsychological deficits are thought to represent a central and enduring characteristic of the disease or what might be considered a signature disturbance in processing of information, elements of which might even predate illness onset (Cornblatt & Kelip, 1994; Cornblatt, Obuchowski, Schnur, & O'Brien, 1998; Erlenmeyer-Kimling et al., 2000; Hans et al., 1999).
Wernicke (1906) was the first to propose that schizophrenia might alter the connectivity of distributed brain networks that are diverse in function and work in concert to support various cognitive abilities and their constituent operations (see also McGuire & Frith, 1996). Two important anatomically and functionally connected networks, frontal–temporal and dorsolateral prefrontal–cingulate, have been implicated in the neuropsychological disturbance of schizophrenia (Bilder et al., 2000; Carter, MacDonald, Ross, & Stenger, 2001; Weinberger, Berman, & Illowsky, 1988; Weinberger, Berman, & Rec, 1986; Weinberger, Berman, Suddath, & Torrey, 1992; Woodruff et al., 1997). The frontal–temporal network includes the inferior frontal and anterior temporal areas reciprocally connected via the uncinate fasciculus (UF), considered the major fiber tract linking these two cortical regions (Ebeling & von Cramon, 1992; Highley et al., 2001; Petrides & Pandya, 1988; Ungerleider, Gaffan, & Pelak, 1989). An important function of this network is to bind stimuli into specific episodes that can be later retrieved and consciously recollected (Squire & Zola-Morgan, 1991). Another important network, the dorsolateral prefrontal–cingulate network, is reciprocally connected via the cingulate bundle (CB), particularly the anterior–agranular, motor-related portion of the cingulate gyrus, as well as the amygdala, nucleus accumbens, and medial dorsal thalamus (Goldman-Rakic, Selemon, & Schwartz, 1984; Pandya & Seltzer, 1982; Vogt, Rosene, & Pandya, 1979). An important property of this network is that it extracts information about task regularities and contingencies so that rules can be acquired to guide thought and action (Miller, 2000).
A new technological advancement in MRI, diffusion tensor imaging (DTI), provides a direct measure (beyond conventional MRI capacity) to assess the integrity of brain fiber tracts (Basser, Mattiello, & LeBihan, 1994), such as the UF and CB, that serve as structural connections for particular brain networks. DTI assesses white matter brain fiber tracts by examining the movement of water molecules as a function of the degree of density and coherence of local tissue components such as cell membranes, axons, and organelles (Basser et al., 1994; Basser & Pierpaoli, 1996; Papadakis et al., 1999). For example, the coherent orientation of axons constrains the movement of water molecules preferentially along the main pathways of fiber tracts. DTI uses a scalar measure, referred to as fractional anisotropy, to quantify the extent to which water diffuses in a discernible and coherent direction. Higher values reflect greater directionality and coherence of the fiber tract, whereas lower values reflect greater random movement of water and thus reduced directionality of the fiber tract. Fractional anisotropy values therefore provide an objective, anatomically derived measure of the structural and functional integrity of imaged white matter fiber tracts.
For patients with schizophrenia, DTI studies have demonstrated reduced fractional anisotropy of prefrontal and temporal white matter (Buschbaum et al., 1998), of whole white matter (Lim et al., 1999), and of the splenium of the corpus callosum (Foong et al., 2000). In addition, more recent studies have demonstrated reduced anatomical asymmetry of the left UF, along with bilaterally reduced fractional anisotropy and cross-sectional area of the CB, among patients in relation to controls (Kubicki et al., 2002, 2003). These and other related DTI abnormalities suggest that schizophrenia may be associated with abnormalities in the fiber tracts interconnecting anatomically and functionally distinct neural networks of higher order cognition (e.g., Hirsch, Rodriguez Moreno, & Kim, 2001; Mesulam, 1990). More specifically, the findings of Kubicki and colleagues (2002, 2003) provide anatomical evidence of disturbances in the frontal–temporal and dorsolateral prefrontal–cingulate networks, each an often-hypothesized anatomical locus of schizophrenic neuropsychological disturbance (e.g., Bilder et al., 2000; Carter et al., 2001; Weinberger et al., 1992).
The aims of the current study were twofold. First, we sought to examine and compare the performance of schizophrenic patients on neuropsychological measures thought to rely heavily on the integrity of either the UF or CB network. Second, we sought to examine, in a relatively small subset of individuals who had previously completed DTI studies (Kubicki et al., 2002, 2003), the relationship between neuropsychological performance on measures of memory and executive function and DTI measures (fractional anisotropy and cross-sectional area) of the UF and CB. The pivotal question investigated was whether previously demonstrated DTI abnormalities of the UF and CB in patients with schizophrenia are related to their performance on neuropsychological tests of functions that are dependent on the integrity of each of these fiber tracts.
We hypothesized that DTI abnormalities in the UF and CB reflect disturbances in two functionally and anatomically distinct neural networks linking the frontal–temporal and prefrontal–cingulate regions, respectively, and that these DTI abnormalities will correlate with different aspects of the neuropsychological performance of patients with schizophrenia. Specifically, we predicted that reduced scores on the Wechsler Memory Scale—Third Edition (WMS–III; Tulsky, Zhu, & Ledbetter, 1997; Wechsler, 1997), a neuropsychological measure of declarative–episodic memory involving encoding, retrieval, and recollection of new information, would correlate with UF but not CB abnormalities in patients with schizophrenia. We also predicted that reduced scores on the Wisconsin Card Sorting Test (WCST; Heaton, 1981), a neuropsychological measure of executive functioning, would correlate with CB but not UF abnormalities in patients with schizophrenia. These two neuropsychological measures, though likely to recruit a common pool of general working memory resources, are considered to index distinct sets of abilities, with the WMS–III probing the relationship between declarative memory and the UF, and the WCST probing the relationship between executive function and the CB.
Method
Participants
All of the participants were between the ages of 17 and 55 years, right-handed, native speakers of English, without histories of electroconvulsive therapy or neurological illness, and without alcohol or drug abuse in the past 5 years, as assessed by the Addiction Severity Index (McClellan et al., 1992). Diagnoses were ascertained with the Structured Clinical Interview for DSM–IV Axis I Disorders—Patient Edition (SCID–P; First, Spitzer, Gibbon, & Williams, 1997), along with chart review. All patients were part of an ongoing comprehensive, longitudinal study of schizophrenia, and all were receiving neuroleptic medication; the mean chlorpromazine equivalent daily dose was 613.70 mg (SD = 370.62). Mean duration of illness was 18.6 years (SD = 10.58).
Healthy comparison participants, recruited from newspaper advertisements, completed the Structured Clinical Interview for DSM–IV Axis I Disorders—Nonpatient Edition (SCID–NP; First et al., 1997) and were matched to patients on the basis of age, sex, handedness, and parents’ socioeconomic status. Mean age did not differ significantly, t(81) = 0.78, p = .439, between the patient group (45.5 years, SD = 8.43) and comparison group (43.9 years, SD = 8.55). After the study had been described to them, all of the participants provided written informed consent. In terms of the neuropsychological assessment, 66 healthy controls and 59 patients with schizophrenia completed the WCST, and 46 and 41 participants in each of these groups, respectively, completed the third edition of the Wechsler Adult Intelligence Scale (WAIS–III; Wechsler, 1997) and the WMS–III. Of this group, 14 healthy controls and 14 patients with schizophrenia also completed both DTI (Kubicki et al., 2002, 2003) and neuropsychological studies.
Procedure
Neuropsychological assessment
The neuropsychological assessment included the WMS–III and WCST along with the WAIS–III. We computed WAIS–III summary measures of intelligence (Full Scale, Verbal, and Performance IQs) and factor analytically derived index scores for Verbal Comprehension, Perceptual Organization, Working Memory, and Processing Speed. The WMS–III provided the following index scores: Auditory Immediate Memory, Visual Immediate Memory, Immediate Memory, Auditory Delayed Memory, Visual Delayed Memory, General–Delayed Memory, Delayed Auditory Recognition, and Working Memory.
Participants also completed a computerized version of the WCST, which measured the executive functions of planning, self-monitoring, and response regulation. A computer display contained four stimulus cards, below which test cards were presented one at a time. Participants sorted each test card by placing it, via mouse response, under one of the four stimulus cards. The to-be-sorted test cards differed along three dimensions: color (red, green, yellow, or blue), form (triangle, star, cross, or circle), and number, with each having from one to four triangles, stars, crosses, or circles. Test cards were to be sorted on the basis of one of these three dimensions. However, participants were not informed of the sorting principle; rather, they had to deduce it on the basis of the computer feedback of “right” or “wrong.” After participants had completed 10 consecutive correct trials, the sorting principle was then changed, unbeknown to the participants, who then had to learn by deducing the new sorting principle on the basis of only performance feedback. Three sorting principles (color, form, and number) were tested twice, forming a total of six categories that each required 10 consecutive responses for criterion. The WCST dependent measures were number of Categories Completed (0–6), number of Perseverative Errors, and number of Nonperseverative Errors.
Diffusion tensor imaging
As described elsewhere in detail (Kubicki et al., 2002), we applied line scan diffusion imaging to obtain fractional anisotropy maps allowing us to measure the integrity of fibers within the UF and CB. MRI scans used a quadrature head coil on a 1.5 Tesla GE Echospeed system (General Electric Medical Systems, Milwaukee, WI) that permits maximum gradient amplitudes of 40 mT/m. A set of three orthogonal T1-weighted images were used as localizers (sagittal, axial oblique aligned to the anterior commissure [AC-PC] line, and another sagittal oblique aligned to the interhemispheric fissure). In each session, six images were collected with high (1,000 s/mm2) diffusion weighting along six noncollinear directions. For low (5 s/mm2) diffusion weighting, two images were collected, providing an adequate sample in that diffusion-related changes were minimal. Scan parameters were as follows: 220- × 165-mm rectangular field of view, 128 × 128 scan matrix (256 × 256 image matrix), 4-mm slice thickness, 1-mm interslice distance, ±4-kHz receiver bandwidth, 64-ms echo time, 81-ms repetition time (effective repetition time: 2,592 ms), and 60 s per section scan time. The number of coronal slices acquired to cover the entire brain ranged from 31 to 35 depending on brain size. After reconstruction, the diffusion-weighted images were transferred to a SUN Microsystems workstation for calculation of eigenvalue, eigenvector, and fractional anisotropy maps of diffusion.
For the UF, we used one slice that cut the fiber tract in the densest portion. To take into account brain torque, we selected one slice separately for the left and right sides. For the CB, we selected eight consecutive slices, starting from the level of the genu of the corpus callosum, and then entered the mean values of the FA as well as the mean cross-sectional areas of the fiber over the measured distance into our statistical comparisons (see Figure 1). Figure 2 represents a coronal slice of the tensor map with an automatically created region of interest (ROI) for our analysis. The ROI was derived from automatic segmentation based on the fact that both UF and CB within the measured area are parallel to the AC-PC line and appear visible in the segmentation defined by the maximum diffusivity (lambda 1, the largest eigenvalue of the diffusion tensor; 1 × 10−3 mm2/s was used as the fixed threshold in all cases). In all cases, a point centered within each fiber tract (separately for left and right) was selected, and FA was calculated. In addition, the areas of these fibers, derived from maximum diffusivity segmentation, were calculated. We also calculated the degree of motion-related artifacts within the segmented ROI. This measure was defined as the number of lines missing in the raw line scan DTI data (six directed diffusion images), and its comparison between patients with schizophrenia and control participants revealed no statistically significant differences in number of lines missing.
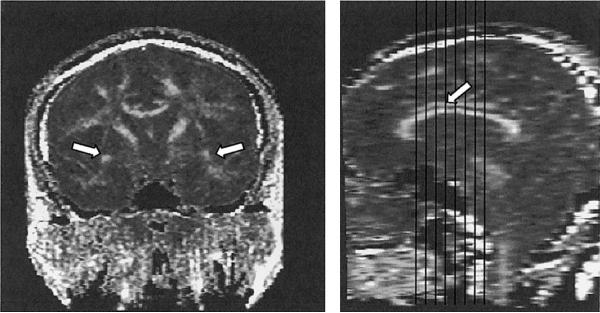
Figure 1.
Single coronal slice of the fractional anisotropy map on which the measurements for the uncinate fasciculus (white arrows) were performed (left) and demonstration of the extent of our cingulate bundle (structure above the corpus callosum marked with white arrow) measurements (right).
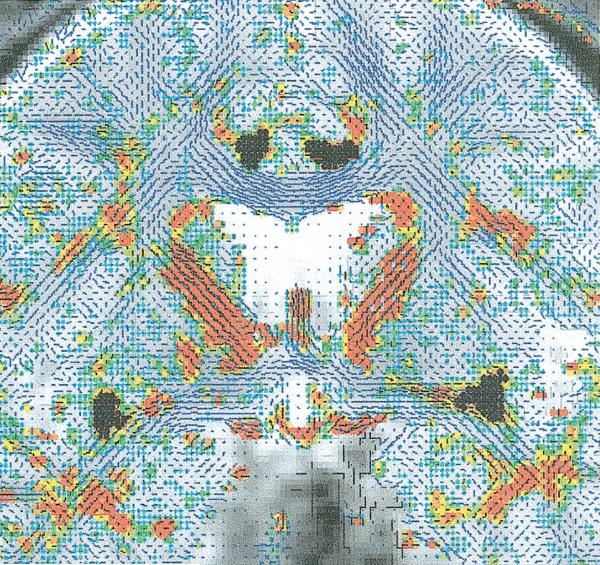
Figure 2.
Areas (highlighted in black) of the cingulate bundle (seen above the corpus callosum, higher here) and uncinate fasciculus (at the level of the anterior commissure, low here) derived from the automatic segmentation (as described in the Method section) used for the fractional anisotropy and cross-sectional area measurements.
Statistical Analyses
Multivariate analyses of covariance (MANCOVAs) with education as the covariate were used in group comparisons of the neuropsychological data. We computed neuropsychological and DTI correlations separately for each group using Pearson product–moment correlations, followed by partial correlations controlling for total brain size as measured by intracranial content. We then conducted parametric, hierarchical regression analyses to partition the total variance of the dependent variable, neuropsychological test score, among the independent variables, DTI measures of UF and CB.
To examine the unique contribution of UF and CB measures to neuropsychological test performance, we computed partial (rp) and semipartial (rsp) correlations in a series of hierarchical regression analyses, allowing us to evaluate significant univariate relationships by partitioning the total variance of the dependent variable (neuropsychological test score) among the independent variables (DTI measures of UF and CB). The squared partial correlation () represented the proportion of variance of a particular neuropsychological test score shared by a specific DTI-derived brain region (e.g., left UF) after the effects of the other DTI-derived brain regions (e.g., left CB) had been removed from both the neuropsychological and DTI measures (Cohen & Cohen, 1975). Calculation of this statistic allowed us to answer the question, “What proportion of the remaining neuropsychological variance (i.e., that which is not estimated by the other independent variables in the equation) is uniquely estimated by this DTI measure?”
In contrast, the square of the semipartial correlation () estimated the amount of neuropsychological variance uniquely shared with a particular DTI measure after the effects of all other DTI measures on that particular measure had been removed (Cohen & Cohen, 1975). It is labeled semipartial because the effects of the other independent variables have been removed from the independent variable but not from the dependent variable. In conjunction with the other linear regression statistics, partial and semipartial correlations provided a comprehensive picture of how DTI measures of the UF and CB relate to neuropsychological test scores when collinearity is controlled. In all regression analyses, the F-to-enter probability was .05, and the F-to-exclude probability was .10. Significance levels were two-tailed.
Results
Table 1 presents neuropsychological test summary scores for the control and patient groups for each of the broad cognitive domains sampled: intelligence, memory, working memory, and executive function. For each domain, MANCOVAs (covarying for education) revealed a significant overall multivariate effect for group. As can be seen in Table 1, patients with schizophrenia generally scored within the low-average range (80–89) on measures of intelligence and memory, and control participants scored, as expected, in the average range (90–109).
Table 1.
Neuropsychological Summary Scores for the Entire Sample and the DTI Subset of Patients With Schizophrenia and Control Participants
| Variable | Patients | Controls | DTI patients | DTI controls |
|---|---|---|---|---|
| Demographic information | ||||
| Age (years) | 40.73 ± 11.13 | 40.35 ± 10.01 | 40.73 ± 7.17 | 41.94 ± 6.58 |
| Education (years) | 12.48 ± 1.93 | 15.01 ± 2.19 | 12.00 ± 2.53 | 16.22 ± 2.71 |
| SES | 4.11 ± 1.54 | 2.27 ± 0.98 | 4.27 ± 0.70 | 2.17 ± 1.04 |
| Parents’ SES | 3.17 ± 1.15 | 2.61 ± 1.07 | 2.60 ± 1.18 | 2.39 ± 1.20 |
| WAIS–III IQ | ||||
| Full Scale | 83.12 ± 12.39 | 107.17 ± 13.44 | 82.85 ± 12.54 | 109.00 ± 10.30 |
| Verbal | 86.20 ± 12.73 | 109.28 ± 12.86 | 84.33 ± 14.65 | 109.47 ± 10.19 |
| Performance | 81.46 ± 10.47 | 103.33 ± 14.98 | 81.83 ± 10.32 | 107.80 ± 13.42 |
| WAIS–III index | ||||
| Verbal Comprehension | 88.41 ± 13.06 | 109.05 ± 11.64 | 87.62 ± 14.81 | 108.00 ± 9.78 |
| Perceptual Organization | 86.81 ± 13.98 | 106.79 ± 16.33 | 87.92 ± 13.74 | 110.53 ± 12.25 |
| Working Memory | 84.70 ± 13.24 | 108.57 ± 14.87 | 85.69 ± 14.66 | 107.60 ± 10.74 |
| Processing Speed | 78.84 ± 9.20 | 104.19 ± 15.28 | 77.92 ± 9.42 | 102.64 ± 13.96 |
| WMS–III memory quotient | ||||
| Immediate Memory | 79.86 ± 15.52 | 103.38 ± 16.38 | 73.09 ± 22.05 | 103.27 ± 18.53 |
| General Memory | 83.00 ± 15.10 | 103.79 ± 21.26 | 76.45 ± 20.63 | 104.13 ± 18.29 |
| WMS–III Index | ||||
| Auditory Immediate | 81.09 ± 20.42 | 105.86 ± 13.64 | 77.82 ± 27.44 | 107.80 ± 17.27 |
| Visual Immediate | 81.12 ± 18.50 | 99.83 ± 16.12 | 73.18 ± 21.93 | 97.53 ± 15.39 |
| Auditory Delayed | 84.53 ± 18.92 | 107.86 ± 11.13 | 80.82 ± 25.21 | 108.13 ± 12.41 |
| Visual Delayed | 82.74 ± 19.89 | 103.10 ± 15.97 | 76.18 ± 23.96 | 101.07 ± 19.54 |
| Working Memory | 88.03 ± 15.97 | 107.90 ± 16.33 | 87.40 ± 14.62 | 112.33 ± 15.02 |
| WCST | ||||
| Categories Completed | 3.20 ± 2.21 | 5.27 ± 1.45 | 4.21 ± 1.67 | 5.50 ± 0.76 |
| Perseverative Errors | 32.55 ± 22.59 | 13.12 ± 13.42 | 25.00 ± 17.03 | 10.93 ± 6.07 |
| Nonperseverative Errors | 19.27 ± 11.59 | 12.15 ± 9.76 | 16.50 ± 8.23 | 13.93 ± 10.86 |
Note. Values are means plus or minus standard deviations. DTI = diffusion tensor imaging; SES = socioeconomic status; WAIS–III = Wechsler Adult Intelligence Scale—Third Edition; WMS–III = Wechsler Memory Scale—Third Edition; WCST = Wisconsin Card Sorting Test.
On the WAIS–III Full Scale, Verbal, and Performance IQs, MANCOVAs revealed a highly significant effect for group, F(1, 79) = 24.51, p < .001, but no significant interaction involving scale type (Verbal or Performance IQ). The patient group scored lower on both Verbal IQ (M = 86.20, SD = 12.73) and Performance IQ (M = 81.46, SD = 10.47) summary measures than did the control group (M = 109.28, SD = 12.86, and M = 103.33, SD = 14.98, respectively). For the WAIS–III index scores, MANCOVAs revealed a significant effect for group, F(1, 76) = 27.46, p < .001, but did not reveal a significant Group × Index Score interaction.
A similar pattern of findings emerged on the WMS–III index measures of memory. MANCOVAs revealed a highly significant group effect for measures of Immediate Recall, F(1, 73) = 8.95, p < .01, and Delayed Recall, F(1, 73) = 12.50, p = .001, but there was no interaction involving either modality of presentation (auditory or visual) or time interval (immediate or delayed). The patient group scored lower on both Auditory Immediate Memory (M = 81.09, SD = 20.42) and Visual Immediate Memory (M = 81.12, SD = 18.50) than did the control group (M = 105.86, SD = 13.64, and M = 99.83, SD = 16.12, respectively). Likewise, for Delayed Recall, the patient group scored lower on both Auditory Delayed Memory (M = 84.53, SD = 18.92) and Visual Delayed Memory (M = 82.74, SD = 19.89) than did the control group (M = 107.86, SD = 11.13, and M = 103.10, SD = 15.97, respectively). Thus, on these summary measures of intelligence and memory, patients with schizophrenia showed clear evidence of reduced levels of performance, as reflected by the highly significant group effects observed even when controlling for group differences in education. However, they did not show evidence of a distinct profile on either the memory or intelligence summary measures.
On the WCST, a measure of executive function, a MANCOVA focusing on the dependent measures of Categories Completed, Perseverative Errors, and Nonperseverative Errors revealed a highly significant group effect, F(2, 124) = 7.75, p = .001. The patient group completed fewer categories and made more perseverative and nonperseverative errors than did the control group. In addition, the patient group showed evidence of a different pattern of WCST performance from that of the control group, as reflected by the highly significant Group × WCST Response Measure interaction, F(2, 124) = 8.40, p < .001. The nature of this interaction indicated that the patient group showed evidence of a disproportionate amount of perseverative errors. However, the groups also differed significantly in overall total responses (p < .001), with the patient group requiring more responses (M = 119.21, SD = 17.44) than the control group (M = 96.73, SD = 23.27). Because error rates could have been confounded by these group differences in total WCST responses (i.e., more responses and higher likelihood of errors), a second MANCOVA with total WCST responses as the covariate was performed. The MANCOVA results again demonstrated a significant group effect, F(2, 140) = 3.58, p < .05, and the interaction remained significant even when controlling for group differences in total WCST responses, F(2, 140) = 4.49, p < .05. These analyses indicated that reduced numbers of categories achieved might be attributed to perseverative errors in the patient group.
Table 1 also presents neuropsychological test scores for the subset of participants who completed DTI studies. As can be seen in Table 1, the subset of DTI patients with schizophrenia showed similarly reduced scores to those of the larger group for the WAIS–III summary measures of Full Scale IQ (M = 82.85, SD = 12.54), Verbal IQ (M = 84.33, SD = 14.65), and Performance IQ (M = 81.83, SD = 10.32), as well as for the WAIS–III index scores of Verbal Comprehension (M = 87.62, SD = 14.81), Perceptual Organization (M = 87.92, SD = 13.74), Working Memory (M = 85.69, SD = 14.66), and Processing Speed (M = 77.92, SD = 9.42). For the WMS–III summary measures, the DTI patients with schizophrenia also showed similarly reduced scores for Immediate (M = 73.09, SD = 22.05) and Delayed (M = 76.45, SD = 20.63) memory quotients as well as for Auditory Immediate Memory (M = 77.82, SD = 27.44), Visual Immediate Memory (M = 73.18, SD = 21.93), Auditory Delayed Memory (M = 80.82, SD = 25.21), Visual Delayed Memory (M = 76.18, SD = 23.96), and Working Memory (M = 87.40, SD = 14.62). Likewise, they exhibited means similar to those of the larger patient sample for WCST number of Categories Completed (M = 4.21, SD = 1.67), number of Perseverative Errors (M = 25.00, SD = 17.03), and number of Nonperseverative Errors (M = 16.50, SD = 8.23).
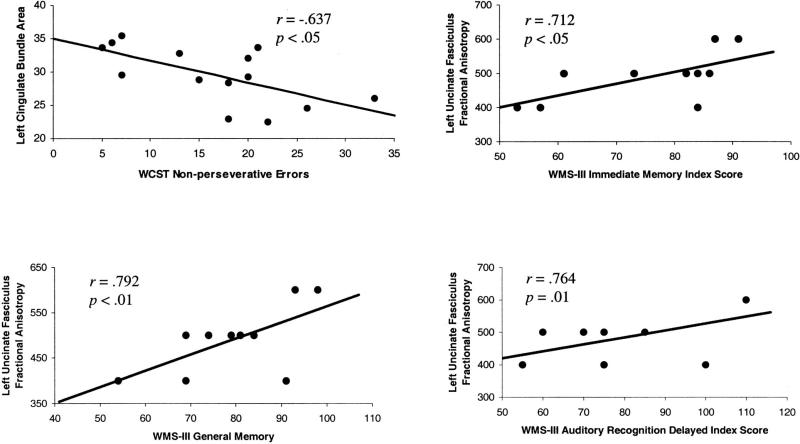
Table 2 presents Pearson product–moment correlations for DTI measures of UF and neuropsychological tests of learning and memory, as indexed by the WMS–III. Among patients with schizophrenia but not control participants, reduced left UF fractional anisotropy correlated significantly and positively with reduced scores on the following WMS–III indexes: Immediate Memory (r = .712, p = .021), Auditory Immediate Memory (r = .680, p = .030), General–Delayed Memory (r = .792, p = .006), and Delayed Auditory Recognition (r = .764, p = .01). For the WCST, reduced left CB area correlated significantly with increased number of Nonperseverative Errors (r =−.637, p = .014) among patients but not control participants. These correlations remained significant even when controlling for total brain size, as assessed by intracranial content. Thus, among the patient group, DTI-derived UF and CB measures correlated with performance on different neuropsychological measures. Scatter plots of these significant correlations are presented in Figure 3.
Table 2.
Pearson Correlations of WMS–III (n = 10) and WCST (n = 14) Scores for DTI-Derived Measures of the Uncinate Fasciculus and Cingulate Bundle Among Patients With Schizophrenia
| Uncinate fasciculus |
Cingulate bundle |
|||
|---|---|---|---|---|
| Variable | Left | Right | Left | Right |
| WMS–III memory quotient | ||||
| Immediate | .712* | .500 | .437 | .154 |
| General–Delayed | .792** | .483 | .353 | .208 |
| WMS–III index | ||||
| Auditory Immediate | .680* | .546 | .487 | .170 |
| Visual Immediate | .593 | .254 | .588 | .384 |
| Auditory Delayed | .772** | .496 | .558 | .290 |
| Visual Delayed | .610 | .464 | .519 | .431 |
| Auditory Delayed Recognition | .764* | .419 | .392 | .246 |
| Working Memory | .375 | .803** | .252 | –.577 |
| WCST | ||||
| Categories Completed | –.283 | –.053 | .383 | .153 |
| Perseverative Errors | .109 | –.056 | –.285 | .067 |
| Nonperseverative Errors | .262 | –.490 | –.637* | –.289 |
Note. WMS–III = Wechsler Memory Scale—Third Edition; WCST = Wisconsin Card Sorting Test.
p < .05.
p < .01.
Figure 3.
Scatter plots of Pearson product–moment correlations of Wechsler Memory Scale—Third Edition (WMS–III) and Wisconsin Card Sorting Test (WCST) scores and diffusion-tensor-imaging derived measures of the left uncinate fasciculus and the left cingulate bundle.
We next tested whether the significant correlations found only in the patient sample might reflect statistical evidence of a double dissociation between the left UF and declarative–episodic memory, on one hand, and the left CB and executive function, on the other hand. We entered both brain regions as predictors in a hierarchical regression analysis, first with WMS–III General Memory index and then with WCST Nonperseverative Errors as the dependent variable. For the WMS–III General Memory index, the left UF produced a significant R2 change of .652, F(2, 7) = 6.92, p = .022, in contrast to the nonsignificant R2 change of .014, F(1, 8) = 0.11, p = .747, accounted for by the left CB. Left UF and WMS–III General Memory index revealed a partial correlation value of .813 and a semipartial correlation value of .807, as compared with values of −.321 and −.196 for left CB and WMS–III General Memory index. These values indicated that the left UF uniquely accounted for 66% and 65% of the variance in WMS–III General Memory index scores. Further analyses demonstrated that only the left UF, β = .687, t(8) = 3.04, p = .019, contributed significantly to the WMS–III General Memory index.
By contrast, for WCST Nonperseverative Errors, the left CB produced a nearly significant R2 change of .370, F(2, 10) = 3.91, p = .056, in contrast to the nonsignificant R2 change of .069, F(1, 11) < 1, p > .35, accounted for by the left UF. Left CB and WCST Nonperseverative Errors revealed a partial correlation value of −.630 and a semipartial value of −.608, in comparison with values of .368 and .297 for left UF and WCST Nonperseverative Errors. These values indicated that the left CB uniquely accounted for 40% and 36% of the variance in WCST Nonperseverative Error scores. Likewise, the left CB, β = −.609, t(10) = −2.57, p = .028, but not the left UF, contributed significantly to WCST Nonperseverative Errors.
Finally, Pearson product–moment correlations revealed significant relationships between performance on other neuropsychological measures and UF fractional anisotropy values among the patient sample. Reduced right UF fractional anisotropy values correlated significantly with lower scores on the WMS–III Working Memory index (r = .803, p = .009) as well as on the WAIS–III measures of general intelligence (r = .640, p = .025), verbal intelligence (r = .638, p = .026), and verbal comprehension (r = .586, p = .045).
Discussion
Patients with schizophrenia scored significantly lower than control participants across neuropsychological summary measures of intelligence, declarative–episodic memory, working memory, and executive function. In addition, they showed a disproportionate number of perseverative errors relative to control participants on the WCST measure of executive function. By contrast, the current data suggest that memory performance was equally impaired for the patient sample across modality and time period, as well as for free recall and recognition.
Among the subset of participants who also completed DTI studies, a rather selective pattern of correlations emerged between particular neuropsychological measures and UF and CB values for patients only. That is, among patients but not control participants, left UF abnormalities correlated with deficits in declarative–episodic memory but not in executive functioning, whereas left CB abnormalities correlated with deficits in executive functioning but not in declarative–episodic memory. In addition, hierarchical regression analyses indicated a statistical dissociation between reduced DTI measures of the left UF and poorer declarative memory, on one hand, and reduced DTI measures of the left CB and poorer executive functions, on the other hand, in the patient sample only.
These results thus suggest a double dissociation between reduced DTI measures of the left UF and CB and deficits in declarative memory and executive function, respectively. Moreover, our hierarchical regression results arguably offer the strongest statistical evidence in support of a schizophrenic double dissociation between neuropsychological function and DTI measures of the left UF and left CB. However, only a small number of patients completed both DTI and neuropsychological measures; thus, these results, generated from hierarchical regression analyses, will need to be replicated in larger samples before any firm conclusions can be drawn about a double dissociation between function and DTI measures of left UF and CB integrity in schizophrenia. In addition, the significant correlations for the UF and neuropsychological tests were based on DTI fractional anisotropy values, whereas, for the CB, the DTI-derived area measure but not the fractional anisotropy measure correlated with WCST Nonperseverative Errors.
It is more likely that these pathways contribute to a diverse range of cognitive functioning. Among patients with schizophrenia, then, the effects of reduced DTI measures of the left UF and CB may extend beyond difficulties on tests of declarative–episodic memory and executive functioning. In fact, the current results indicate that, for the right UF, reduced fractional anisotropy correlated significantly with poorer scores on measures of working memory, general intelligence, verbal intelligence, and verbal comprehension in the patient sample. These correlations underscore the widely distributed nature of higher cognition in the brain and caution against drawing simple isomorphic relationships between function and anatomy.
How might these current DTI correlations relate to previous MRI and neuropsychological studies of schizophrenia? Previous MRI and neuropsychological studies have revealed correlations between reduced temporal lobe volume and deficits in declarative–episodic verbal memory, and between reduced prefrontal lobe volume and working memory deficits, within the same groups of patients with chronic schizophrenia (Hokama et al., 1995; Nestor et al., 1993; Nestor, Shenton, et al., 1998). The current DTI data represent measures of the integrity of anatomical structures and cannot be equated with the functional connectivity of the UF and CB. Nor can DTI distinguish among underlying mechanisms, such as axonal or myelination-related changes, that might account for reduced fractional anisotropy and reduced UF and CB volumes. However, our neuropsychological correlations demonstrate that declarative–episodic memory deficits in schizophrenia may be closely related to reduced structural integrity of the left UF, which links the inferior prefrontal cortex with anterior temporal regions to form a local network of a larger, wide-scale neural system of declarative–episodic memory (e.g., Kolb & Whishaw, 1995; Mesulam, 1990; Squire & Zola-Morgan, 1991). Likewise, disease-related prefrontal working memory disturbances may also contribute to deficits in executive function, as demonstrated by the strong correlation of WCST errors with reduced structural integrity of the left CB, an important pathway linking the dorsolateral prefrontal cortex to the cingulate gyrus as well as to the amygdala, medial dorsal thalamus, and nucleus accumbens (Goldman-Rakic et al., 1984; Pandya & Seltzer, 1982; Vogt et al., 1979).
Executive functioning encompasses two broad sets of mental processes: top-down control and performance monitoring. Imaging studies of healthy individuals have suggested that the executive functions of control of thought and action rely heavily on dorsolateral prefrontal circuits (MacDonald, Cohen, Stenger, & Carter, 2000; Smith & Jonides, 1999), whereas those of performance monitoring, especially in relation to sensitivity to errors, may be supported by the anterior cingulate cortex (Carter et al., 1998; Kiehl, Liddle, & Hopfinger, 2000). Event-related functional MRI studies involving patients with schizophrenia have demonstrated reduced error-related activity in the anterior cingulate and less performance adjustment after errors on a degraded continuous performance task (Carter et al., 2001).
In completing the WCST, used as a measure of executive function in the current study, the examinee must rely on ongoing performance feedback regarding errors to arrive at correct sorting rules. Learning these kinds of rules and contingencies may require a neural network to be dynamically set and flexibly tuned to select among existing codes and pathways and use “prediction error” signals to modify behavior in response to direct feedback (e.g., Dias, Robbins, & Roberts, 1996; Schultz, Dayan, & Montague, 1997). The CB may thus be centrally involved in the coordination of brain signals, including dopamine neurons responsive to errors across prefrontal and cingulate areas (Schultz & Dickinson, 2000). For patients with schizophrenia, their WCST difficulties may arise in part from the failure of the CB to orchestrate prefrontal and cingulate brain signals, particularly dopaminergic neurons coded for prediction errors, which are probably critical for rule acquisition and implementation.
In summary, patients with schizophrenia demonstrated a rather selective, nonoverlapping pattern of neuropsychological–DTI abnormalities, with declarative–episodic memory deficits correlating with reduced left UF fractional anisotropy and executive function deficits correlating with reduced left CB area. These abnormalities occurred within the context of an overall generalized diminution in cognition among patients, as demonstrated by the larger sample of participants who underwent neuropsychological assessments. Taken together, these data suggest that the often generalized schizophrenic neuropsychological impairment may also encompass underlying abnormalities in DTI measures of the integrity of discrete neural networks that each correspond to specific deficits in declarative–episodic memory and executive functioning. Thus, our findings may add to the development of a functional neuroanatomical basis for neuropsychological models of schizophrenia that point to a core disturbance in the synchronization and integration of local brain networks and wider scale neural systems (Andreasen et al., 1999; Nestor, Akdag, et al., 1998; Nestor et al., 2001).
Acknowledgments
This work was supported by a National Alliance for Research on Schizophrenia Young Investigator Award to Paul G. Nestor; a Kosciuszko Foundation Research Award to Marek Kubicki; National Institutes of Health Grants K02 MH 01110 and R01 MH 50747 to Martha E. Shenton and Grant R01 MH 40799 to Robert W. McCarley; Department of Veterans Affairs Merit Awards to Martha E. Shenton, Margaret Niznikiewicz, Paul G. Nestor, and Robert W. McCarley; and a National Center for Research Resources and Veterans Affairs Psychiatry/Neuroscience Research Fellowship Award to Melissa Frumin.
References
- Andreasen NC, Nopoulos P, O'Leary DS, Miller DD, Wassink T, Flaum M. Defining the phenotype of schizophrenia: Cognitive dysmetria and its neural mechanisms. Biological Psychiatry. 1999;46:908–920. doi: 10.1016/s0006-3223(99)00152-3. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophysiology Journal. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion tensor MRI. Journal of Magnetic Resonance. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Goldman RS, Robinson D, Reiter G, Bell L, Bates JA, et al. Neuropsychology of first-episode schizophrenia: Initial characterization and clinical correlates. American Journal of Psychiatry. 2000;157:549–559. doi: 10.1176/appi.ajp.157.4.549. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Mukherjee S, Reider RO, Pandurangi AK. Intellectual deficits in first-episode schizophrenia: Evidence for progressive deterioration. Schizophrenia Bulletin. 1985;18:437–448. doi: 10.1093/schbul/18.3.437. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Neale JM. The neuropsychological signature of schizophrenia: Generalized or differential deficit? American Journal of Psychiatry. 1994;151:40–48. doi: 10.1176/ajp.151.1.40. [DOI] [PubMed] [Google Scholar]
- Braff DL, Heaton R, Kuck J, Cullum M, Moranville J, Grant I, Zisool S. The generalized pattern of neuropsychological deficits in outpatients with chronic schizophrenia with heterogeneous Wisconsin Card Sorting Test results. Archives of General Psychiatry. 1991;48:891–898. doi: 10.1001/archpsyc.1991.01810340023003. [DOI] [PubMed] [Google Scholar]
- Buschbaum MS, Tang CY, Peled S, Gudbjartsson H, Lu D, Hazlett E, et al. MRI white matter diffusion anisotropy and PET metabolic rate in schizophrenia. NeuroReport. 1998;9:425–430. doi: 10.1097/00001756-199802160-00013. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick M, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998 May 1;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carter CS, MacDonald AW, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: An event related fMRI study. American Journal of Psychiatry. 2001;158:1423–1428. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P. Applied multiple regression/correlational analysis for the behavioral sciences. Erlbaum; Hillsdale, NJ: 1975. [Google Scholar]
- Cornblatt BA, Kelip JG. Impaired attention, genetics, and the pathophysiology of schizophrenia. Schizophrenia Bulletin. 1994;20:31–46. doi: 10.1093/schbul/20.1.31. [DOI] [PubMed] [Google Scholar]
- Cornblatt B, Obuchowski M, Schnur D, O'Brien JD. Hillside study of risk and early detection in schizophrenia. British Journal of Psychiatry. 1998;172(Suppl 33):26–32. [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: Effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behavioral Neuroscience. 1996;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Ebeling U, von Cramon DY. Topography of the uncinate fasicle and adjacent temporal fiber tracts. Acta Neurochirurgica (Wien) 1992;115:143–148. doi: 10.1007/BF01406373. [DOI] [PubMed] [Google Scholar]
- Erlenmeyer-Kimling L, Rock D, Simone RA, Janal M, Kestenbaum C, Cornblatt B, et al. Attention, memory, and motor skills as childhood predictors of schizophrenia-related psychoses: The New York High-Risk Project. American Journal of Psychiatry. 2000;157:1416–1422. doi: 10.1176/appi.ajp.157.9.1416. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM–IV Axis I Disorders. American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- Foong J, Maier M, Barker GJ, Brocklehurst S, Miller DH, Ron MA. In vivo investigation of white matter pathology in schizophrenia with magnetisation transfer imaging. Journal of Neurology, Neurosurgery, and Psychiatry. 2000;68:70–74. doi: 10.1136/jnnp.68.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Ragland D, Torrey EF, Gold JM, Bigelow LB, Weinberger DR. Neuropsychological assessment of monozygotic twins discordant for schizophrenia. Archives of General Psychiatry. 1990;4:1066–1072. doi: 10.1001/archpsyc.1990.01810230082013. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12:719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Hans S, Marcus J, Nuechterlein KH, Asarnow RF, Styr B, Auerbach JG. Neurobehavioral deficit at adolescence in children at risk for schizophrenia. Archives of General Psychiatry. 1999;56:741–748. doi: 10.1001/archpsyc.56.8.741. [DOI] [PubMed] [Google Scholar]
- Heaton RK. Wisconsin Card Sort manual. Psychological Assessment Resources; Odessa, FL: 1981. [Google Scholar]
- Heaton RK, Gladsjo JA, Palmer BW, Kuck J, Marcotte T, Jeste DV. Stability and course of neuropsychological deficits in schizophrenia. Archives of General Psychiatry. 2001;58:24–32. doi: 10.1001/archpsyc.58.1.24. [DOI] [PubMed] [Google Scholar]
- Highley JR, Walker MA, Esiri MM, McDonald B, Harrison PJ, Crow TJ. Schizophrenia and the frontal lobes: Post-mortem stereological study of tissue volume. British Journal of Psychiatry. 2001;178:337–343. doi: 10.1192/bjp.178.4.337. [DOI] [PubMed] [Google Scholar]
- Hirsch J, Rodriguez Moreno D, Kim KHS. Interconnected large-scale systems for three fundamental cognitive tasks revealed by functional MRI. Journal of Cognitive Neuroscience. 2001;13:389–405. doi: 10.1162/08989290151137421. [DOI] [PubMed] [Google Scholar]
- Hokama H, Shenton ME, Nestor PG, Kikinis R, Levitt JJ, Metcalf D, et al. Caudate, putamen, and globus pallidus volume in schizophrenia: A quantitative MRI study. Psychiatry Research: Neuroimaging. 1995;61:209–229. doi: 10.1016/0925-4927(95)02729-h. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: An event-related fMRI study. Psychophysiology. 2000;37:216–223. [PubMed] [Google Scholar]
- Kolb B, Whishaw IQ. Fundamentals of human neuropsychology. Freeman; New York: 1995. [Google Scholar]
- Kubicki M, Westin C, Frumin M, Nestor P, Salisbury D, Kikinis R, et al. Uncinate fasciculus findings in schizophrenia: A magnetic resonance diffusion tensor imaging study. American Journal of Psychiatry. 2002;159:813–820. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Nestor PG, Wible CG, Frumin M, Maier SE, et al. Cingulate fasciculus integrity disruption in schizophrenia: A magnetic resonance diffusion tensor imaging study. Biological Psychiatry. 2003;54:1171–1180. doi: 10.1016/s0006-3223(03)00419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KO, Hedehus M, Moseley M, de Crespigny A, Sullivan EV, Pfefferbaum A. Compromised white matter tract integrity in schizophrenia inferred from diffusion tensor imaging. Archives of General Psychiatry. 1999;56:367–374. doi: 10.1001/archpsyc.56.4.367. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000 June 9;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- McClellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Frith CD. Disordered functional connectivity in schizophrenia. Psychological Medicine. 1996;26:663–667. doi: 10.1017/s0033291700037673. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Annals of Neurology. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nature Reviews Neuroscience. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Mohamed S, Paulsen JS, O'Leary D, Arndt S, Andreasen N. Generalized cognitive deficits in schizophrenia. Archives of General Psychiatry. 1999;56:749–754. doi: 10.1001/archpsyc.56.8.749. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Akdag SJ, O'Donnell BF, Niznikiewicz M, Law S, Shenton ME, McCarley RW. Word recall in schizophrenia: A connectionist model. American Journal of Psychiatry. 1998;155:1685–1690. doi: 10.1176/ajp.155.12.1685. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Han SD, Niznikiewicz M, Salisbury D, Spencer K, Shenton ME, McCarley RW. Semantic disturbance in schizophrenia and its relationship to the cognitive neuroscience of attention. Biological Psychology. 2001;57:23–46. doi: 10.1016/s0301-0511(01)00088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor PG, Shenton ME, McCarley RW, Haimson J, Smith RS, O'Donnell B, et al. Neuropsychological correlates of MRI temporal lobe abnormalities in schizophrenia. American Journal of Psychiatry. 1993;150:1849–1855. doi: 10.1176/ajp.150.12.1849. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Shenton ME, Wible C, Hokama H, O'Donnell BF, Law S, McCarley RW. A neuropsychological analysis of schizophrenic thought disorder. Schizophrenia Research. 1998;29:217–225. doi: 10.1016/s0920-9964(97)00101-1. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Seltzer B. Intrinsic connections and architectonics of posterior parietal cortex in the rhesus monkey. Journal of Comparative Neurology. 1982;204:196–210. doi: 10.1002/cne.902040208. [DOI] [PubMed] [Google Scholar]
- Papadakis NG, Xing D, Houston GC, Smith JM, Smith MI, James MF, et al. A study of rotationally invariant and symmetric indices of diffusion anisotropy. Magnetic Resonance Imaging. 1999;17:881–892. doi: 10.1016/s0730-725x(99)00029-6. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Association fiber pathways to the frontal cortex from the superior temporal regions in the rhesus monkey. Journal of Comparative Neurology. 1988;273:52–66. doi: 10.1002/cne.902730106. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, et al. Neuropsychological function in schizophrenia: Selective impairment in memory and learning. Archives of General Psychiatry. 1991;48:618–624. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Archives of General Psychiatry. 1994;51:124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997 March 14;275:1593–1597. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annual Review of Neuroscience. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999 March 12;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991 September 20;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Tulsky D, Zhu J, Ledbetter MF. WAIS–III/WMS–III: Technical manual. Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Ungerleider LG, Gaffan D, Pelak VS. Projections from inferior temporal cortex to prefrontal cortex via the uncinate fasicle in rhesus monkeys. Experimental Brain Research. 1989;76:473–484. doi: 10.1007/BF00248903. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Rosene DL, Pandya DN. Thalamic and cortical afferents differentiate anterior from posterior cingulate cortex in the monkey. Science. 1979 April 13;204:205–207. doi: 10.1126/science.107587. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale—Third edition. Harcourt, Brace; San Antonio, TX: 1997. [Google Scholar]
- Weinberger DR, Berman KF, Illowsky BP. Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia: III. A new cohort and evidence for a monoaminergic mechanism. Archives of General Psychiatry. 1988;45:609–615. doi: 10.1001/archpsyc.1988.01800310013001. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Rec RF. Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia: I. Regional cerebral blood flow evidence. Archives of General Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Suddath R, Torrey EF. Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: A magnetic resonance imaging and regional cerebral blood flow of discordant monozygotic twins. American Journal of Psychiatry. 1992;149:890–897. doi: 10.1176/ajp.149.7.890. [DOI] [PubMed] [Google Scholar]
- Wernicke C. Grundrisse der psychiatrie [Foundations of psychiatry]. Theirne; Leipzig, Germany: 1906. [Google Scholar]
- Woodruff PW, Wright IC, Shuriquie N, Rushe T, Howard RJ, Graves M, Bullmore ET. Structural brain abnormalities in male schizophrenics reflect fronto-temporal dissociation. Psychological Medicine. 1997;27:1257–1266. doi: 10.1017/s0033291797005229. [DOI] [PubMed] [Google Scholar]