Abstract
We have previously reported that the epithelial cell-specific actin-binding protein villin directly associates with phosphatidylinositol 4,5-bisphosphate (PIP2) through three binding sites that overlap with actin-binding sites in villin. As a result, association of villin with PIP2 in hibits actin depolymerization and enhances actin cross-linking by villin. In this study, we demonstrate that these three PIP2-binding sites also bind the more hydrophilic phospholipid, lysophosphatidic acid (LPA) but with a higher affinity than PIP2 (dissociation constant (Kd) of 22 μm versus 39.5 μm for PIP2). More interestingly, unlike PIP2, the association of villin with LPA inhibits all actin regulatory functions of villin. In addition, unlike PIP2, LPA dramatically stimulates the tyrosine phosphorylation of villin by c-Src kinase. These studies suggest that in cells, selective interaction of villin with either PIP2 or LPA could have dramatically different outcomes on actin reorganization as well as phospholipid-regulated cell signaling. These studies provide a novel regulatory mechanism for phospholipid-induced changes in the microfilament structure and cell function and suggest that LPA could be an intracellular regulator of the actin cytoskeleton.
Introduction
Many actin-binding proteins are recruited to the plasma membrane to form lipid-protein interactions during cell signaling, membrane trafficking, and cell migration. Membrane recruitment of these microfilament proteins is mediated by membrane-targeting domains that recognize specific lipid molecules in the membranes (1). Phospholipids are implicated in the regulation of actin dynamics, cell growth, cell differentiation, cell survival, and cell motility (1). Phosphoinositides control the activity of various actin-associated proteins by promoting actin filament assembly and down-regulating actin disassembly at appropriate regions within the cell (1).
Villin is an epithelial cell-specific actin-binding protein that regulates actin dynamics, cell morphology, cell migration, and apoptosis, underscoring the significance of this protein to epithelial cell function (2). Villin belongs to a large family of actin-binding proteins that associate with phospholipids (3). Villin binds PIP22 with a Kd of 39.5 μm and a stoichiometry of 3, and we have previously identified three PIP2-binding domains in villin (3). Association of villin with PIP2 modifies its actin regulatory functions (3). The association of villin with PIP2 is also required for the catalytic activation of the other ligand of villin, phospholipase C-γ1 (4), which is required for the function of villin in cell migration (5, 6).
Lysophosphatidic acid (LPA) and its receptors are found in a wide variety of tissues and cell types, indicating their physiological significance to many biological functions (7). LPA is produced by platelets, fibroblasts, mesothelial cells, and adipocytes, and the physiological role of LPA in innate immunity, reproduction, vascular development, and nervous system functions is well recognized (7). However, aberrant LPA signaling is associated with malignant transformation (8). As a result, there is keen interest in identifying the regulatory elements that switch LPA from a physiological factor to a pathological factor.
Although most LPA research has focused on its extracellular action, there is considerable interest in the intracellular targets of LPA. Several intracellular targets of LPA have been identified, including the anti-inflammatory agent and nuclear receptor, peroxisome-proliferator-activated receptor γ (9); mechano-gated K+ channels (10); n-chimaerin (11); phosphatidylinositol 3-kinase (12); protein kinase C (13); and glycosylphosphatidylinositol-specific phospholipase D (14). Intracellular LPA also regulates the interaction of G-proteins with GTPase-activating proteins (15). More recent studies have identified an intranuclear role for LPA (16). These studies support the intracellular effect of LPA as a second messenger. In this report, we expand these findings by identifying another potential intracellular binding partner for LPA, namely villin. More interestingly, we report that both PIP2 and LPA compete for the same binding sites in villin but have opposite effects on actin reorganization. These findings suggest that phospholipids have the ability to function as potent modulators of actin reorganization and further that the substrate preference (PIP2 versus LPA) of the actin-binding protein can have very different outcomes in the cell. These studies provide a novel regulatory mechanism for determining not only the microfilament organization but also phospholipid signaling specificity regulated by actin and actin-binding proteins in vivo.
EXPERIMENTAL PROCEDURES
Recombinant Villin Protein and Peptides
Full-length recombinant human villin protein was generated as described previously (4). Human villin peptides encompassing the three PIP2-binding domains in villin have been described previously (3).
In Vitro Interaction of Villin with LPA
The binding of villin proteins and peptides with LPA was examined by measuring the quenching of the intrinsic tryptophan fluorescence of villin as described previously (3). Briefly, LPA at final concentrations between 0 and 1 mm was added to samples containing villin (0.5 mm), and fluorescence was recorded using a FluoroMax-3 spectrofluorometer. The excitation wavelength was 290 nm. The dissociation constant (Kd) was determined using the Microcal Origin software, and the stoichiometry of LPA binding to villin (p) and its association constant (Ka) were calculated using the equation of Stinson and Holbrook as described previously (3). The Hill coefficient (h) was calculated using the Hill equation as described previously (3).
Measurement of Actin-capping, -nucleating, -severing, and -bundling Activities of Villin
The actin-modifying activities of villin in the absence or presence of LPA were measured as described previously (3, 17, 18). Confocal measurement of F-actin bundling by villin was performed as described by Harris et al. (19). Briefly, 1 unit of Alexa Fluor 488-phalloidin in methanol was deposited on a 35-mm No. 0 glass-bottomed sterile plastic Petri dish (MatTek) and allowed to dry for 1 h. Prepolymerized actin in bundling buffer containing 2 mm EGTA was added either alone (control) or with villin (60 nm) or with villin (60 nm) and LPA (60 μm). Images were acquired with a ×40 objective on a confocal microscope (LSM 5 PASCAL, Carl Zeiss, Thornwood, NY).
Conformational Studies Using CD
CD measurements were made using full-length recombinant villin protein or PIP2-binding peptides of villin in the absence or presence of LPA (200 μm) as described previously (3).
In Vitro Phosphorylation of Recombinant Villin by c-Src Kinase
An in vitro kinase assay was performed using recombinant c-Src kinase as described previously (4). For densitometric analysis of Western blots, the value of highest band intensity was normalized as 1, and the normalized intensities of other bands were determined as described by Nam et al. (20).
RESULTS
Villin Binds LPA with a Higher Affinity than PIP2
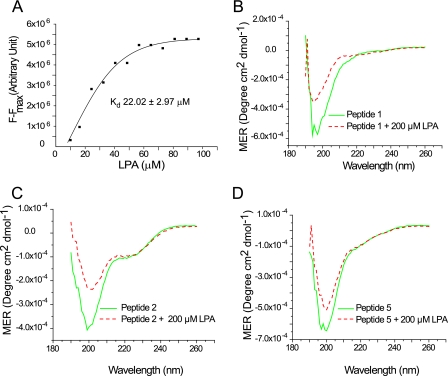
In this study, we demonstrate for the first time that villin directly associates with the more hydrophilic phospholipid, LPA. For kinetic analysis of villin and LPA binding, we monitored the quenching of the intrinsic tryptophan fluorescence of villin by LPA (supplemental Fig. 1A). Quenching of tryptophan fluorescence was plotted against the LPA concentration (in μm) and analyzed by the Microcal Origin software. Based on this analysis, we calculated a Kd value of 22.02 ± 2.97 μm, a stoichiometry of 1.72, a dissociation constant of 0.01 μm, and a Hill's coefficient of 1.08, suggesting no cooperativity between the two LPA molecules bound to villin (Fig. 1A). These data demonstrate that like PIP2, LPA interacts directly with villin but with a higher affinity than PIP2 (3), suggesting that in vivo, LPA could displace PIP2 bound to villin.
FIGURE 1.
Direct interaction of villin with LPA. A, kinetic analysis of villin fluorescence titration data. Binding curve plotting (F − Fmax) versus LPA was used to calculate the apparent dissociation constant (Kd) of villin and LPA as 22.02 ± 2.97 μm. This experiment is representative of three with similar results. Circular dichroism spectra of villin peptides, peptide 1 (B), peptide 2 (C), and peptide 5 (D) were recorded in the absence or presence of LPA (200 μm). This experiment is representative of three with similar results.
LPA Introduces Localized Changes in the Secondary Structure of Villin
We have previously demonstrated that the association of villin with PIP2 introduces significant localized changes in the secondary structure of the protein (3). To characterize LPA-induced changes in the secondary structure of villin, we elected to use three villin peptides encompassing the three PIP2-binding sites in villin (3). CD spectroscopy was performed in the “far-UV” spectral region (190–250 nm) in the absence or presence of LPA (200 μm). Like PIP2, LPA did not induce global changes in the conformation of full-length villin (supplemental Fig. 1B). Like PIP2, LPA induced significant changes in the secondary structure of the villin peptides (Fig. 1, B–D). Notably, these changes were qualitatively different. In contrast to more significant changes in the percentage of α-helix in the presence of PIP2, LPA induced more significant changes in the percentage of β-sheet with loss of α-helix structure (3). These data demonstrate that both LPA and PIP2 have the same binding sites in villin and further suggest localized changes in the secondary structure of villin when bound to LPA.
LPA Is a Potent Inhibitor of the Actin Regulatory Functions of Villin
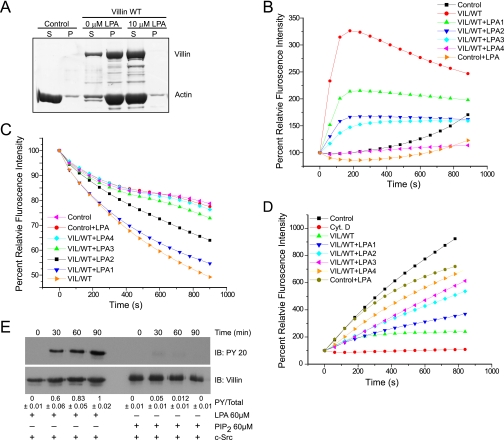
Peptide 5 overlaps with the F-actin-binding site in the villin headpiece; hence, we elected to determine the effect of LPA on the actin-bundling function of villin. Low speed centrifugation of F-actin filaments in the absence or presence of villin and in the absence or presence of LPA was used to measure actin bundling by villin. In control samples, in the absence of villin, all the F-actin appears in the supernatant fraction, demonstrating no cross-linking of actin filaments (Fig. 2A). In contrast, in the presence of full-length villin, the majority of the actin appears in the pellet fraction with the F-actin, consistent with the role of villin as an actin cross-linking protein. Low speed centrifugation of F-actin in the presence of villin and LPA (10 μm) resulted in the complete loss of F-actin bundles. To confirm these data, 3 μm F-actin was incubated without or with full-length villin in the absence or presence of LPA (10 μm) for 0–20 min, and F-actin was stained with Alexa Fluor 488-phalloidin. Images were acquired using a confocal microscope. In the absence of villin, most of the F-actin appears in the filamentous form, representing uncross-linked filaments. The addition of villin bundled F-actin filaments; further, the addition of 10 μm LPA prevented F-actin bundling by villin (supplemental Fig. 1C). Because the PIP2/LPA-binding sites in villin overlap with the actin-binding sites in villin, we speculate that LPA binding may completely displace the binding of F-actin to the villin headpiece, which could explain the loss of actin bundling by villin in the presence of LPA. We have previously reported that F-actin binding to the villin headpiece is required for actin cross-linking (17). Because LPA can associate with peptide 5 and inhibit villin-induced actin bundling, we suggest that PB5 represents one of the two LPA-binding sites in villin. Increasing concentrations of LPA (0–240 μm) also inhibited villin-induced actin nucleation (n = 3; p < 0.001; Fig. 2B). Similar to the effects of PIP2, LPA also inhibited the actin-depolymerizing activity of villin (n = 3; p < 0.01; Fig. 2C). Further, unlike PIP2, surprisingly, LPA also inhibited the actin-capping function of villin (n = 3; p < 0.01; Fig. 2D). Together these data demonstrate that LPA inhibits all the actin-modifying functions of villin. Thus, unlike PIP2, as a ligand of villin, LPA could function as a negative regulator of villin-mediated changes in actin dynamics within the cell.
FIGURE 2.
LPA inhibits the actin regulatory functions of villin. A, full-length villin (1.0 μm) was incubated with F-actin in the absence or presence of LPA (10 μm). The samples were centrifuged at 10,000 × g for 15 min, and actin distribution in the supernatant (S) and pellet (P) fractions was analyzed by 10% SDS-PAGE and GelCode Blue staining. Control refers to F-actin filaments in the absence of villin. This experiment is representative of four with similar results. B, LPA inhibits actin nucleation by villin. Pyrene-labeled G-actin (6 μm) was incubated with full-length villin protein (VIL/WT; 60 nm) in the absence or presence of different concentrations of LPA (LPA1, 60 μm; LPA2, 120 μm; LPA3, 200 μm; LPA4, 240 μm) in polymerization-inducing buffer. Fluorescence intensity was measured over time. Control and Control+LPA (240 μm) represent the polymerization of actin in the absence of villin. Values represent the mean of three independent experiments. C, LPA inhibits the actin-depolymerizing function of villin. The effect of LPA on actin depolymerization was recorded using recombinant full-length villin protein (VIL/WT) and different concentrations of LPA as described above. Values represent the mean of three independent experiments. D, LPA inhibited actin-capping activity of villin. To measure the actin-capping activity of villin in the absence or presence of LPA, 1.4 μm G-actin was nucleated by F-actin (290 nm) seeds in the absence (Control) or presence of VIL/WT. Different concentrations of LPA were used as described above. Values represent the mean of three independent experiments. Cyt D., cytochalasin D. E, recombinant villin was phosphorylated in vitro by c-Src in the absence or presence of LPA (60 μm) or PIP2 (60 μm) over a period of 90 min. This is a representative of three experiments with similar results. Densitometric analysis gave a phosphovillin:villin (PY/Total) ratio of 0.6 ± 0.06; 0.83 ± 0.05; and 1.0 ± 0.01, which corresponds to 60, 83, and 100% (n = 3; p < 0.01) increase in villin phosphorylation at 30, 60, and 90 min, respectively. IB, immunoblot. Tyrosine phosphorylation of villin was identified by Western analysis using a phosphotyrosine antibody (PY 20). Blots were stripped and reprobed for villin.
LPA Enhances the Tyrosine Phosphorylation of Villin
Tyrosine phosphorylation of actin-binding proteins of the villin family including gelsolin and adseverin is enhanced by PIP2 and LPA (21, 22). To characterize the effect of LPA and PIP2 on villin tyrosine phosphorylation, recombinant full-length villin was phosphorylated using recombinant c-Src kinase in the absence or presence of LPA or PIP2 (60 μm). As shown in Fig. 2E, c-Src kinase failed to phosphorylate recombinant villin irrespective of the presence or absence of PIP2, consistent with our previous observation (4). Interestingly, in the presence of LPA, there was a significant increase in the phosphorylation of villin protein. We interpret these data to suggest that the addition of LPA to villin changes the villin conformation favorably such that it can now be tyrosine-phosphorylated by c-Src kinase. It may be noted that in vitro phosphorylation of recombinant villin requires the presence of reducing agents (β-mercaptoethanol), suggesting that in vitro protein unfolding is required to make the phosphorylation sites accessible to c-Src (4). In the presence of β-mercaptoethanol, the addition of LPA significantly enhanced c-Src kinase-induced tyrosine phosphorylation of villin (supplemental Fig. 1D). We speculate that the secondary structure changes induced by these lipids could explain the changes in the tyrosine phosphorylation levels of villin by making the tyrosine residues either more accessible (as in the case of LPA) or inaccessible (as in the case of PIP2) for phosphorylation in vitro. These data suggest that based on the specific ligand, LPA versus PIP2, bound to villin, other functions of villin that require its tyrosine phosphorylation such as changes in cell morphology and cell migration could also be regulated in vivo (5, 23).
DISCUSSION
In this study, we characterize the significance of villin association with LPA, which can be extended to other actin-binding proteins that have been reported to directly bind LPA (21). Our data demonstrate that the PIP2-binding sites in villin overlap with the LPA-binding sites in villin, suggesting that in cells, these two phospholipids could compete for villin as a binding partner. Interestingly, a Kd value of 28.9 μm has been reported for binding of full-length fragminP with LPA, whereas the Kd values obtained for LPA binding to PIP2-binding peptides of gelsolin have provided a much lower Kd value of 920 nm (24). These previous findings validate our own observations that the PIP2-binding sites in the villin-gelsolin superfamily serve as binding sites for intracellular LPA. Further, these data suggest that one of the LPA-binding sites in villin may have a slightly higher affinity for LPA than noted for full-length villin. In thrombin-stimulated platelets, intracellular LPA concentrations between 47 and 77 μm have been reported (25). Further, a temporal correlation between actin polymerization and increased LPA concentration in thrombin-stimulated platelets has also been noted (25). These published studies suggest that intracellular LPA concentrations required to inhibit the actin-modifying activities of villin can be achieved in vivo.
Although both PIP2 binding and LPA binding to villin induce conformational changes in the villin protein, our studies suggest that these changes may be qualitatively different because LPA enhances tyrosine phosphorylation of villin, an effect that requires conformational changes in villin protein, whereas PIP2 has no effect (26). This is validated by our CD data (Fig. 1, B–D). Thus, the substrate preference (PIP2 versus LPA) of villin may also regulate phosphorylation-dependent functions of villin in vivo including changes in cell morphology and cell migration (2, 4). Our study then elucidates not only the functional similarities between LPA and PIP2 but also demonstrates the phospholipid-dependent biochemical differences that could have profoundly different effects in vivo on both actin dynamics as well as phospholipid signaling.
Another interesting observation made from these studies is that although full-length villin binds LPA with a stoichiometry of 2, in vitro data with villin peptides indicates that all three PIP2-binding peptides of villin could potentially bind LPA. From these data, we infer that (i) only two of these three sites may in fact be accessible for LPA binding in full-length villin protein; (ii) alternatively, two of these three sites may bind LPA with higher affinity; (iii) or that association of LPA with two of these three sites could induce a conformational change that either renders the third site inaccessible or lowers the affinity of this third site for LPA.
A substantial body of evidence demonstrates a clear involvement of PIP2 in actin cytoskeletal rearrangement (27, 28). Likewise, a role for LPA in cytoskeletal reorganization is well documented (29). We speculate that competition between PIP2 and LPA for villin binding could increase the levels of free PIP2 in the cell, which could result in cytoskeletal reorganization. Thus, in vivo villin could serve as a buffering agent for LPA. This may be physiologically relevant during inflammatory diseases or during epithelial cell injury when LPA synthesis within the cell may be elevated. It may be noted that mitochondrial LPA, nuclear LPA receptors, and targets of intracellular LPA, namely peroxisome-proliferator-activated receptor γ, are all highly expressed in the colon and play a key role in bacteria-induced inflammation (30, 31). By sequestering LPA, villin could contribute to the host defense and repair response. LPA regulates nuclear transcription factors that regulate metabolic functions and inflammation (9). Intracellular LPA also functions as a precursor for the production of other lipids (32) that regulate membrane fluidity (33). The selective interaction of intracellular LPA with villin could also modify these cellular functions.
Interaction of PIP2 or LPA with villin could not only regulate phospholipid signal transduction cascades but also define the intracellular compartment where these changes are induced. Interaction of villin with PIP2, for instance, could regulate cell surface actin reorganization, thus regulating cell motility, whereas interaction of villin with intracellular LPA could regulate nuclear function, thus regulating epithelial-to-mesenchymal transition, a function that has been attributed to villin (18).
LPA is synthesized in the endoplasmic reticulum or the mitochondria (34). There is no evidence to suggest that this intracellular LPA is involved in extracellular LPA signaling. Further, because charged phospholipids are also believed not to cross the plasma membrane, it is suggested that extracellular LPA is unlikely to find its way to the cytosol. Regulation of cell function by intracellular LPA has several advantages. Although LPA production in biological fluids appears to be unregulated because both the substrate and the enzyme pre-exist, the major advantage of intracellular LPA synthesis is that it is tightly regulated and could be generated as a result of cellular activation induced by various stimuli. Furthermore, it has been suggested that lipid phosphatases may prevent accumulation of intracellular LPA, providing another level of regulation for the cellular effects of intracellular LPA. This intracellular production of LPA may be particularly relevant to cellular pathology. The production of PA and conversion of PA to LPA may occur in cells following inflammatory disease or in carcinoma cells. Intense interest is aimed at ascertaining the regulatory elements that switch LPA from a physiological factor to a pathological factor. Changes in ligand levels (LPA) and changes in receptor levels (LPA2 and LPA3) have been reported. However, most studies have suggested that changes in either LPA levels or receptor levels are not sufficient to account for all the differential roles of LPA in tumor cells (35). It has been suggested that LPA must interact with other factors in cells to elicit its effect. In this study, we reveal that LPA can directly interact with villin, suggesting that it can modulate signaling pathways critical for the pathological role of LPA in tumorigenesis such as changes in actin dynamics, cell morphology, cell adhesion, cell migration, cell invasion, epithelial-mesenchymal transition, and apoptosis, all of which are regulated by villin (3). Identification of the biological activities of LPA in vivo has just begun, and undoubtedly, many of these issues will be resolved in the near future. In the meantime, we believe our study helps identify a potential intracellular target of LPA.
Acknowledgment
We thank Dr. Gabor Tigyi for sharing expertise on LPA with us and for valuable suggestions.
This work was supported, in whole or in part, by National Institutes of Health Grants DK-65006, DK-54755, and DK-81408 (to S. K.) through the NIDDK.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- PIP2
- phosphatidylinositol 4,5-bisphosphate
- LPA
- lysophosphatidic acid
- PA
- phosphatidic acid
- PPAR-γ
- peroxisome-proliferator-activated receptor γ
- WT
- wild type.
REFERENCES
- 1.Niggli V. (2006) in Aspects of the Cytoskeleton (Khurana S., ed) Vol. 37, Advances in Molecular and Cell Biology, pp. 222–244, Elsevier Science Publishing Co., Inc., New York [Google Scholar]
- 2.Khurana S., George S. P. (2008) FEBS Lett. 582, 2128–2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar N., Zhao P., Tomar A., Galea C. A., Khurana S. (2004) J. Biol. Chem. 279, 3096–3110 [DOI] [PubMed] [Google Scholar]
- 4.Panebra A., Ma S. X., Zhai L. W., Wang X. T., Rhee S. G., Khurana S. (2001) Am. J. Physiol. Cell Physiol. 281, C1046–1058 [DOI] [PubMed] [Google Scholar]
- 5.Tomar A., George S., Kansal P., Wang Y., Khurana S. (2006) J. Biol. Chem. 281, 31972–31986 [DOI] [PubMed] [Google Scholar]
- 6.Wang Y., Tomar A., George S. P., Khurana S. (2007) Am. J. Physiol. Cell Physiol. 292, C1775–1786 [DOI] [PubMed] [Google Scholar]
- 7.Rivera R., Chun J. (2008) Rev. Physiol. Biochem. Pharmacol. 160, 25–46 [DOI] [PubMed] [Google Scholar]
- 8.Murph M., Tanaka T., Liu S., Mills G. B. (2006) Clin. Cancer Res. 12, 6598–6602 [DOI] [PubMed] [Google Scholar]
- 9.McIntyre T. M., Pontsler A. V., Silva A. R., St. Hilaire A., Xu Y., Hinshaw J. C., Zimmerman G. A., Hama K., Aoki J., Arai H., Prestwich G. D. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 131–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chemin J., Patel A., Duprat F., Zanzouri M., Lazdunski M., Honoré E. (2005) J. Biol. Chem. 280, 4415–4421 [DOI] [PubMed] [Google Scholar]
- 11.Ahmed S., Lee J., Kozma R., Best A., Monfries C., Lim L. (1993) J. Biol. Chem. 268, 10709–10712 [PubMed] [Google Scholar]
- 12.Lauener R., Shen Y., Duronio V., Salari H. (1995) Biochem. Biophys. Res. Commun. 215, 8–14 [DOI] [PubMed] [Google Scholar]
- 13.Sando J. J., Chertihin O. I. (1996) Biochem. J. 317, 583–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Low M. G., Huang K. S. (1993) J. Biol. Chem. 268, 8480–8490 [PubMed] [Google Scholar]
- 15.Chettibi S., Lawrence A. J., Stevenson R. D., Young J. D. (1994) FEMS Immunol. Med. Microbiol. 8, 271–281 [DOI] [PubMed] [Google Scholar]
- 16.Waters C. M., Saatian B., Moughal N. A., Zhao Y., Tigyi G., Natarajan V., Pyne S., Pyne N. J. (2006) Biochem. J. 398, 55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.George S. P., Wang Y., Mathew S., Srinivasan K., Khurana S. (2007) J. Biol. Chem. 282, 26528–26541 [DOI] [PubMed] [Google Scholar]
- 18.Athman R., Louvard D., Robine S. (2003) Mol. Biol. Cell 14, 4641–4653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris E. S., Rouiller I., Hanein D., Higgs H. N. (2006) J. Biol. Chem. 281, 14383–14392 [DOI] [PubMed] [Google Scholar]
- 20.Nam J. O., Jeong H. W., Lee B. H., Park R. W., Kim I. S. (2005) Cancer Res. 65, 4153–4161 [DOI] [PubMed] [Google Scholar]
- 21.Meerschaert K., De Corte V., De Ville Y., Vandekerckhove J., Gettemans J. (1998) EMBO J. 17, 5923–5932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Corte V., Gettemans J., Vandekerckhove J. (1997) FEBS Lett. 401, 191–196 [DOI] [PubMed] [Google Scholar]
- 23.Tomar A., Wang Y., Kumar N., George S., Ceacareanu B., Hassid A., Chapman K. E., Aryal A. M., Waters C. M., Khurana S. (2004) Mol. Biol. Cell 15, 4807–4817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mintzer E., Sargsyan H., Bittman R. (2006) Biochim. Biophys. Acta 1758, 85–89 [DOI] [PubMed] [Google Scholar]
- 25.Watson S. P., McConnell R. T., Lapetina E. G. (1985) Biochem. J. 232, 61–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar N., Khurana S. (2004) J. Biol. Chem. 279, 24915–24918 [DOI] [PubMed] [Google Scholar]
- 27.Gilmore A. P., Burridge K. (1996) Nature 381, 531–535 [DOI] [PubMed] [Google Scholar]
- 28.Sakisaka T., Itoh T., Miura K., Takenawa T. (1997) Mol. Cell. Biol. 17, 3841–3849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kranenburg O., Poland M., van Horck F. P., Drechsel D., Hall A., Moolenaar W. H. (1999) Mol. Biol. Cell 10, 1851–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubuquoy L., Rousseaux C., Thuru X., Peyrin-Biroulet L., Romano O., Chavatte P., Chamaillard M., Desreumaux P. (2006) Gut 55, 1341–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalari S., Zhao Y., Spannhake E. W., Berdyshev E. V., Natarajan V. (2009) Am. J. Physiol. Lung Cell Mol. Physiol. 296, L328–L336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mills G. B., Moolenaar W. H. (2003) Nat. Rev. Cancer 3, 582–591 [DOI] [PubMed] [Google Scholar]
- 33.Kooijman E. E., Chupin V., Fuller N. L., Kozlov M. M., de Kruijff B., Burger K. N., Rand P. R. (2005) Biochemistry 44, 2097–2102 [DOI] [PubMed] [Google Scholar]
- 34.Aoki J. (2004) Semin Cell Dev. Biol. 15, 477–489 [DOI] [PubMed] [Google Scholar]
- 35.Sengupta S., Wang Z., Tipps R., Xu Y. (2004) Semin Cell Dev. Biol. 15, 503–512 [DOI] [PubMed] [Google Scholar]