Abstract
Iron-sulfur proteins play an essential role in many biologic processes. Hence, understanding their assembly is an important goal. In Escherichia coli, the protein IscA is a product of the isc (iron-sulfur cluster) operon and functions in the iron-sulfur cluster assembly pathway in this organism. IscA is conserved in evolution, but its function in mammalian cells is not known. Here, we provide evidence for a role for a human homologue of IscA, named IscA1, in iron-sulfur protein biogenesis. We observe that small interfering RNA knockdown of IscA1 in HeLa cells leads to decreased activity of two mitochondrial iron-sulfur enzymes, succinate dehydrogenase and mitochondrial aconitase, as well as a cytosolic iron-sulfur enzyme, cytosolic aconitase. IscA1 is observed both in cytosolic and mitochondrial fractions. We find that IscA1 interacts with IOP1 (iron-only hydrogenase-like protein 1)/NARFL (nuclear prelamin A recognition factor-like), a cytosolic protein that plays a role in the cytosolic iron-sulfur protein assembly pathway. We therefore propose that human IscA1 plays an important role in both mitochondrial and cytosolic iron-sulfur cluster biogenesis, and a notable component of the latter is the interaction between IscA1 and IOP1.
Introduction
Iron-sulfur proteins play essential roles in pathways that include the Krebs cycle, oxidative phosphorylation, gene regulation, and purine metabolism (1, 2). Their assembly presents particular challenges in that iron is readily oxidized and can generate free radicals. Hence, there are specific pathways that participate in the assembly of iron-sulfur clusters. In bacteria such as Escherichia coli, there is a pathway dedicated to the assembly of iron-sulfur clusters (3). The isc operon is the central genetic locus for this pathway, and it contains genes that encode for the proteins that include IscS, IscU, and IscA. The key elements of this pathway include a mechanism for obtaining sulfur through the enzymatic activity of a cysteine desulfurase, a mechanism for combining sulfur with iron on scaffold proteins, and a means for delivery of the resultant iron-sulfur clusters to target apoproteins. It appears that these key elements have been conserved through evolution. Thus, the E. coli cysteine desulfurase IscS has homologues in Saccharomyces cerevisiae and mammalian cells, indicative of a central conserved function. Moreover, homologues of IscU and IscA also exist in yeast and mammalian cells. The exact function of these is less certain. For example, IscU has been proposed to be a scaffold of iron-sulfur cluster assembly in E. coli, S. cerevisiae, and mammals (4–6). IscA has been proposed to serve as a scaffold for delivering iron-sulfur clusters to select target proteins in E. coli and S. cerevisiae or, alternatively, as an iron donor in E. coli (7–10); its role in mammalian cells is not known.
In eukaryotic cells, the situation is made more complex by the necessity of having to assemble both mitochondrial and cytosolic iron-sulfur clusters. In S. cerevisiae, there are proteins dedicated to the cytosolic iron-sulfur assembly (CIA)2 pathway, and these include Cfd, Nbp35, Nar1, and Cia1 (1). There is active investigation into this pathway in mammalian cells. Current evidence indicates an important role for human Nbp35 in cytosolic iron-sulfur protein maturation (11). We have recently provided evidence that the human homologue of Nar1, IOP1, plays a role in cytosolic iron-sulfur protein maturation (12). IOP1 is homologous to iron-only hydrogenases, enzymes found in anaerobic bacteria that generate hydrogen gas from reducing equivalents. IOP1 does not exhibit hydrogenase activity, but it does possess conserved cysteine residues that chelate iron-sulfur clusters in the bacterial iron-only hydrogenases, and indeed, yeast Nar1 is an iron-sulfur cluster protein (13). In this study, we continue to examine the pathway involved in cytosolic iron-sulfur protein assembly and now provide evidence for a role for a mammalian homologue of IscA. We propose that this protein, IscA1, participates in both cytosolic and mitochondrial iron-sulfur cluster biogenesis.
EXPERIMENTAL PROCEDURES
Yeast Two-hybrid Screen
The yeast two-hybrid screen (14) was performed with a Matchmaker GAL4 Two-hybrid System 3 kit (BD Biosciences). S. cerevisiae strain AH109 transformed with pGBKT7-IOP1 was mated with S. cerevisiae strain Y187 pretransformed with a human adult kidney Matchmaker Library (BD Biosciences), and positives were selected on −Ade/−His/−Leu/−Trp/+ 5 mm 3-aminotriazole media. A total of 1.1 × 107 clones were screened. In subsequent assays with selected bait and prey plasmids, AH109 transformed with select pGBKT7-IOP1-derived plasmids was transformed with select pGADT7-derived plasmids, grown on −Leu/−Trp media, and then examined for growth on −Ade/−His/−Leu/−Trp media.
Plasmids
pcDNA3-FLAG-IOP1 was constructed by subcloning the IOP1 coding sequence of pGEX-IOP1 (15) into pcDNA3-FLAG. pGBKT7-IOP1 was constructed by subcloning the IOP1 coding sequence of pcDNA3-FLAG-IOP1 into pGBKT7 (Clontech). pGBKT7-IOP1-(1–98) was constructed by digesting pGBKT7-IOP1 with XbaI and XhoI and blunting, followed by self-ligation. pGBKT7-IOP1-(121–476) was constructed by digesting pGBKT7-IOP1 with BamHI and XbaI and blunting, followed by self-ligation.
pACT2-IscA1-(48–129) was isolated from a yeast clone obtained from the two-hybrid screen. pcDNA3-HA-IscA1 was constructed by PCR amplifying the IscA1 coding sequence from MGC clone 4276 (ATCC) and subcloning it into pcDNA3-HA. pGAD-IscA1 was constructed by subcloning the IscA1 coding sequence of pcDNA3-HA-IscA1 into pGADT7 (Clontech). pGAD-IscA1-(48–129), pGAD-IscA1-(48–90), pGAD-IscA1-(48–64), and pGAD-IscA1-(64–90) were prepared by standard recombinant DNA techniques.
pEGFP-IscA1 was constructed by subcloning the IscA1 coding sequence of pcDNA3-HA-IscA1 into pEGFP-C2 (Clontech). pEGFP-IscA1-(48–129) and pEGFP-IscA1-(48–90) were prepared by transferring the appropriate IscA1 coding sequences of pGAD-IscA1-(48–129) and pGAD-IscA1-(48–90), respectively, into pEGFP-C2.
pcDNA5/FRT/TO-EGFP was constructed by subcloning the EGFP coding sequence of pEGFP-C2 into pcDNA5/FRT/TO. pcDNA5/FRT/TO-EGFP-IscA1 was constructed by subcloning the IscA1 coding sequence of pcDNA3-HA-IscA1 into pcDNA5/FRT/TO-EGFP such that IscA1 is fused in-frame to the C terminus of EGFP. pcDNA5/FRT/TO-EGFP- IscA1-(48–90) was constructed by transferring the appropriate IscA1 coding sequence of pGAD-IscA1-(48–90) into pcDNA5/FRT/TO-EGFP.
pcDNA3-HA-IscA1 C121S/C123S was prepared using a QuikChange mutagenesis kit. pGAD-IscA1 C121S/C123S and pEGFP-IscA1 C121S/C123S were constructed by subcloning the IscA1 coding sequence of pcDNA3-HA-IscA1 C121S/C123S into pGADT7 and pcDNA5/FRT/TO-EGFP, respectively. pGAD-IscA1 C57S and pEGFP-IscA1 C57S were prepared by overlapping PCR employing pcDNA3-HA-IscA1 as a template.
pcDNA5/FRT/TO-IscA1-(23–129), which lacks the mitochondrial targeting sequence and is resistant to the IscA1-A siRNA detailed below, was prepared by overlapping PCR. In this construct, an initiator ATG immediately precedes codon 23, and the sequence for codons 91–92 in this construct is CAATTG (where underlining indicates silent nucleotide changes).
pcDNA3-HA-Ciao1 was constructed by PCR amplifying the Ciao1 coding sequence from IMAGE clone 5219740 (ATCC) and subcloning it into pcDNA3-HA. pGADT7-Ciao1 was constructed by transferring the Ciao1 coding sequence into pGADT7. pcDNA3-HA-CFD was constructed by subcloning the CFD coding sequence of IMAGE clone 3633393 into pcDNA3-HA. pcDNA3-HA-NBP35 was constructed by subcloning the NBP35 coding sequence of IMAGE clone 6141112 into pcDNA3-HA.
pGEX-IscA1 and pMAL-IscA1 were constructed by subcloning the IscA1 coding sequence of pcDNA3-HA-IscA1 into pGEX-5X-1 and pMAL-5X-1, respectively. Authenticity of constructs was verified by sequencing.
siRNA
The sequences of oligonucleotides employed to prepare two independent siRNA duplexes targeting IscA1 were as follows (Dharmacon): IscA1-A, GAAAGCACAGCUAACACUUUU and AAGUGUUAGCUGUGCUUUCUU; IscA1-B, ACUUGUGGCUGUGGAGAAAUU and UUUCUCCACAGCCACAAGUUU. The siRNA duplex targeting IOP1 (IOP1-A) and control siRNA (Control-B) have been described (15).
Cell Culture, Transfection, and Extract Preparation
Flp-In T-REx HEK293 (Invitrogen), HeLa (ATCC), and COS-7 (ATCC) cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% (v/v) fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin. Stable transfectants of Flp-In T-REx HEK293 cells were generated by cotransfecting pcDNA5/FRT/TO or pcDNA5/FRT/TO-derived constructs with pOG44 using FuGENE followed by selection in 100 μg/ml hygromycin. Hygromycin-resistant clones were selected at 14 days.
Cytosolic and mitochondrial extracts were prepared by first pelleting and then resuspending cells in 100 mm Hepes, pH 7.4, containing 0.25 m sucrose, 0.01% digitonin, and mammalian cell extract protease inhibitor mixture (Sigma). After 10 min on ice, the suspension was centrifuged at 10,000 × g for 10 min at 4 °C. The supernatant was designated the cytosolic fraction. The cell pellets were further washed three times in 100 mm Hepes, pH 7.4, containing 0.25 m sucrose, with a 1-min centrifugation at 3,000 × g after each wash. The resulting pellet was then lysed in 100 mm Hepes, pH 7.4, containing 0.5% Triton X-100 and mammalian cell extract protease inhibitor mixture. After 10 min on ice, the lysate was centrifuged at 10,000 × g for 10 min at 4 °C. The supernatant from this centrifugation was designated the mitochondrial fraction.
Mitochondrial matrix, membrane, and intermembrane space fractions were prepared as described previously (16–19), with minor modifications. In brief, HeLa cells were washed twice in PBS and resuspended in 10 mm Tris/HCl, pH 7.4, 0.25 m sucrose, 0.1 mm EDTA, 1 mm phenylmethylsulfonyl fluoride (Buffer A) and homogenized with a Dounce homogenizer. The unbroken cells and nuclei were removed by three consecutive 5-min centrifugations at 700 × g at 4 °C. The remaining supernatant was centrifuged at 13,000 × g for 10 min at 4 °C, and the pellet served as the crude mitochondrial fraction.
To prepare the mitochondrial membrane fraction, the crude mitochondrial fraction was suspended in Buffer A supplemented with 0.1% Triton X-100 and briefly vortexed, and the solution was then centrifuged at 13,000 × g for 10 min at 4 °C to separate the soluble proteins of the matrix from the membrane-associated proteins.
To prepare the intermembrane space fraction, the crude mitochondrial fraction was suspended in Buffer A lacking sucrose to disrupt the mitochondrial outer membrane, and the solution was then centrifuged at 13,000 × g for 10 min at 4 °C to separate the supernatant (intermembrane space) and pellets. These pellets were further washed three times with the same buffer and treated with 0.4% Triton X-100 to disrupt the mitochondrial inner membrane. The solution was centrifuged at 13,000 × g for 10 min at 4 °C, and the supernatant was collected as the mitochondrial matrix.
Protein concentration measurements and siRNA transfections were performed as described (12). Lipofectamine 2000 (Invitrogen) was employed for siRNA and transient transfections. For siRNA transfections, cells were typically transfected every 72 h with 20 nm siRNA and harvested at 9 days post-transfection. Hydrogen peroxide was obtained from Sigma. The source of other chemicals has been described. Cell viability was assessed by trypan blue exclusion at day 9.
Antibodies, Coimmunoprecipitations, and Western Blotting
GST-IscA1 and MBP-IscA1 were purified by affinity chromatography on GSH-agarose or amylose agarose from E. coli transformed with pGEX-IscA1 or pMAL-IscA1, respectively. Polyclonal antibodies to GST-IscA1 were raised in rabbits and affinity-purified on MBP-IscA1 conjugated to agarose (Covance Research). Antibodies against green fluorescent protein (FL), hemagglutinin (F-7 and Y-11), HSP 60 (H-300), and presenilin-2 (H-76) were from Santa Cruz Biotechnology. Sources of all other antibodies have been described (12, 15).
COS-7 cells were cotransfected with pcDNA3-FLAG-IOP1 and pEGFP-derived plasmids encoding various fragments of IscA1 using FuGENE. Twenty four hours later, cells were lysed in Buffer B (100 mm Hepes, pH 7.4, 0.25 m sucrose, 0.1% Triton X-100) supplemented with mammalian protease inhibitor mixture (Sigma). The lysates were clarified by centrifugation at 14,000 × g for 10 min at 4 °C and then incubated with 10 μl of anti-FLAG (M2)-agarose for 1 h at 4 °C with rocking. The resins were washed three times with Buffer B and eluted with 2× SDS-PAGE loading buffer, and the eluates were subjected to SDS-PAGE. Western blotting was then performed using anti-green fluorescent protein antibodies as described (12).
HeLa cell cytosolic extracts were incubated with or without anti-IOP1 or anti-presenilin-2 antibodies for 1 h at 4 °C with rocking. The reactions were then incubated with 10 μl of protein G-agarose (Invitrogen) for an additional 1 h. The resins were washed three times with 100 mm Hepes, pH 7.4, 0.25 m sucrose and eluted with 2× SDS-PAGE loading buffer, and the eluates were subjected to SDS-PAGE. Western blotting was then performed using anti-IscA1 antibodies. Yeast extracts were prepared by vortexing yeast in the presence of glass beads (425–600 μm, Sigma) in 50 mm Tris, pH 8, 25 mm β-glycerol phosphate, 1 mm pyrophosphate, 1 mm EDTA, 0.1% SDS, 1 mm phenylmethylsulfonyl fluoride.
Enzyme Assays
Aconitase, citrate synthase, and lactate dehydrogenase activities were measured as described (12). Succinate dehydrogenase activity was measured as described (20) against 20 mm sodium succinate, 0.14 mm 2,6-dichloroindophenol, and 1 mm sodium cyanide in the dark. The decrease in absorbance at 600 nm was measured at 25 °C.
Real Time PCR
RNA isolation and real time PCR were performed as described (12, 15) using a SYBR Green Master mix (ABI). The following oligonucleotides were employed as primers: hIscA1 forward, 5′-TCAGCAGTAAACAAGATAAAACAACTTCT-3′, and hIscA1 reverse, 5′-GGTTCGGACACCAACTTTTACAC-3′. Dissociation curve analysis revealed a single peak. Relative quantification was performed employing the ΔΔCt method and β-actin as the endogenous control. Oligonucleotides for measuring β-actin using SYBR Green have been described (15).
Statistical Analysis
Student's t test and analysis of variance were used for statistical analysis. Significant differences were considered when p < 0.05.
RESULTS
To identify proteins that might interact with IOP1, we performed a yeast two-hybrid screen using IOP1 as a bait to screen a human adult kidney cDNA library. A clone that was isolated encoded for residues 48–129 of human IscA1 (Unigene Hs.449291) (21). IscA1 is a homologue of the E. coli protein IscA (3), and at the mRNA level it is expressed in multiple tissues, including heart, kidney, cerebellum, and liver (21). The bacterial protein IscA is a product of the isc operon, which is involved in the assembly of iron-sulfur clusters (3). The specific role of IscA in this pathway in E. coli has been a matter of substantial debate, and it has been proposed that IscA serves either as an iron donor or as a scaffold for iron-sulfur cluster assembly (9, 10). That being said, the importance of IscA in the bacterial iron-sulfur cluster assembly pathway and our previous work identifying a role for IOP1 in cytosolic iron-sulfur cluster assembly in mammalian cells lead us to investigate human IscA1 in more detail.
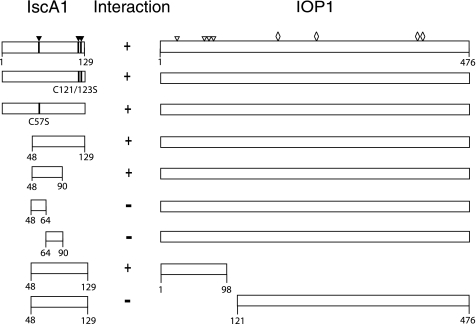
We performed mapping studies using the yeast two-hybrid system to characterize the interaction sites between IscA1 and IOP1 (Fig. 1). We first examined full-length IscA1 and found that it, like the IscA1 fragment originally identified in the two-hybrid screen (residues 48–129), interacts with IOP1. IscA1 contains three evolutionarily conserved cysteine residues, Cys-57, Cys-121, and Cys-123, that have been proposed to play roles in binding iron-sulfur clusters (3, 22). A C57S point substitution and a C121S/C123S double point substitution were both found to interact with IOP1 in the yeast two-hybrid screen, suggesting that this potential function in mammalian IscA1 is not essential for its interaction with IOP1, at least in this yeast two-hybrid assay. We found that IscA1-(48–90) also interacts with IOP1. Further subdivision of IscA1-(48–90) into IscA1-(48–64) and IscA1-(64–90) did not lead to any fragment that displayed interaction with IOP1. The negative results observed with these two IscA1 fragments are not due to an inability to obtain expression of the fragments, because both are detectable by Western blotting (supplemental Fig. 1).
FIGURE 1.
Yeast two-hybrid analysis of the IscA1-IOP1 interaction. Schematic representations of full-length IscA1 and IOP1, as well as fragments, are shown. Residue numbers are as indicated. Presence of an interaction is indicated by +, its absence by −. The three cysteine residues in IscA1 are indicated by closed triangles, and cysteine substitutions of IscA1 are as indicated. Open triangles indicate IOP1 cysteines predicted to chelate an iron-sulfur cluster in a ferredoxin-like domain at the N terminus of IOP1. Open diamonds indicate IOP1 cysteines that correspond to ligands of an H-cluster in iron-only hydrogenases.
IOP1 appears to contain two domains, a small N-terminal region with a potential ferredoxin-like domain and a larger C-terminal domain that is homologous to the domain that contains the catalytic H-cluster of bacterial iron-only hydrogenases (15). We divided IOP1 into two fragments containing these domains (amino acids 1–98 and 121–476, respectively), and we found that the smaller N-terminal domain interacts with IscA1-(48–129) in this yeast two-hybrid assay. We therefore conclude that the minimal interaction domain in IOP1 for IscA1 is IOP1 residues 1–98, whereas the minimal interaction domain in IscA1 for IOP1 is IscA1 residues 48–90.
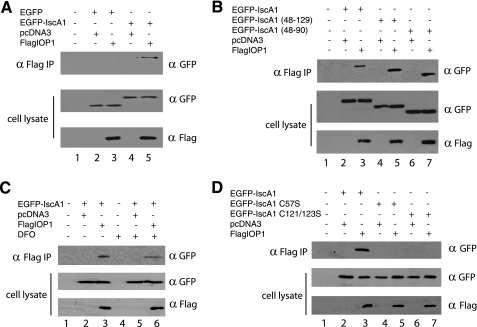
We next examined whether these proteins can interact in mammalian cells. We coexpressed FLAG-IOP1 with EGFP-IscA1 in COS cells, immunoprecipitated the former with anti-FLAG antibodies, and examined the immunoprecipitates by Western blotting. We find that IOP1 can coimmunoprecipitate with full-length IscA1 fused to EGFP (Fig. 2A, top panel, lane 5). Additional studies show that IOP1 can interact with either IscA1-(48–129) or IscA1-(48–90) fused to EGFP (Fig. 2B, top panel, lanes 5 and 7, respectively), consistent with the yeast two-hybrid studies.
FIGURE 2.
Coimmunoprecipitation analysis of IscA1-IOP1 interaction. COS cells were cotransfected with pcDNA3-FLAG-IOP1 and pEGFP-IscA1 and constructs encoding for either EGFP (A), EGFP fused to truncated versions of IscA1 (B), or EGFP fused to IscA1 with the indicated cysteine residue substitutions (D). The FLAG-IOP1 was immunoprecipitated with anti-FLAG antibodies, and the immunoprecipitates (IP) were examined for the absence or presence of IscA1 using anti-green fluorescent protein (GFP) antibodies. C, some cells were treated with 100 μm DFO for 16 h, as indicated.
To determine whether the interaction is iron-dependent, we next cotransfected COS cells with expression constructs for FLAG-IOP1 and EGFP-IscA1 and exposed some cells to the iron chelator desferrioximine (100 μm for 16 h). Immunoprecipitations and Western blotting were then performed as before. We find that the interaction between IOP1 and IscA1 is present, although importantly, it should also be noted that it is somewhat diminished (Fig. 2C, top panel, compare lanes 3 and 6). This led us to examine the interaction of the cysteine substitutions of IscA1 for their interaction with IOP1. We find that, in contrast to the results of the yeast two-hybrid studies, both the C57S and C121S/C123S substitutions both abolish the interaction with IOP1 (Fig. 2D, compare lanes 3, 5, and 7). Although this result might also seem at odds with the coimmunoprecipitation of EGFP-IscA1-(48–90) (which lacks Cys-121 and Cys-123) with FLAG-IOP1 (Fig. 2B), one possible explanation is that although the contact site in IscA1 for IOP1 might not directly involve these cysteine residues, the cysteines, and potentially any associated iron-sulfur clusters, could be essential for the correct conformation of the full-length protein, at least in mammalian cells. We performed additional experiments to determine whether FLAG-IOP1 might be able to also interact with the hemagglutinin-tagged versions of CFD, NBP35, and Ciao1, the human homologues of the yeast CIA proteins Cfd, Nbp35, and Cia1, respectively (1). These experiments failed to reveal interactions between IOP1 and these three proteins (supplemental Fig. 2). We also did not observe an interaction between IOP1 and Ciao1 using the yeast two-hybrid assay (supplemental Fig. 3).
We raised antibodies against full-length IscA1. These antibodies react appropriately with EGFP-IscA1 but not EGFP (Fig. 3A). When mitochondrial and cytosolic fractions are examined by Western blotting using these antibodies, we find that the majority of IscA1 is present in the mitochondria (Fig. 3B, top panel, lane 2), consistent with findings for the yeast homologues of IscA (7, 8, 22). We also fractionated mitochondria into a soluble matrix-containing fraction and a membrane fraction. We find that IscA1 cofractionates with the mitochondrial matrix marker HSP 60 (Fig. 3C, lanes 2 and 6) but not with cytochrome c, which is present in the mitochondrial membrane and intermembrane space (lanes 3 and 5, respectively). This supports a predominantly mitochondrial matrix localization for IscA1, as has been observed for S. cerevisiae Isa1 (7).
FIGURE 3.
Western blot analysis of IscA1. A, cellular extracts containing either EGFP or EGFP-IscA1 were analyzed by Western blotting using anti-green fluorescent protein (GFP) (left) or anti-IscA1 (right) antibodies. Positions of molecular mass markers are shown to the left. B, HeLa cell cytosolic (40 μg) or mitochondrial (2 μg) extracts were analyzed by Western blotting using anti-IscA1 antibodies. Extracts (10 μg) were also analyzed by Western blotting using anti-β-tubulin or anti-cytochrome c (Cyto C) antibodies. C, whole cell extracts, mitochondrial matrix, mitochondrial membrane, and intermembrane space (IMS) fractions were analyzed by Western blotting using anti-IscA1, anti-HSP 60, or anti-cytochrome c antibodies. D, top panel, HeLa cell cytosolic extracts were incubated with or without the indicated antibodies, which were then immunoprecipitated (IP) with protein G-agarose. The immunoprecipitates or the extract input (representing 4% of the total) were then examined by Western blotting using anti-IscA1 antibodies. Bottom panel, HeLa cell cytosolic extracts were incubated with anti-IscA1 antibodies, which were then immunoprecipitated with protein G-agarose. The immunoprecipitates or the extract input (representing 4% of the total) before or after immunoprecipitation were then examined by Western blotting using anti-IscA1 antibodies. E and F, HeLa cells were transfected with control (CON) or IscA1 siRNAs. E, total RNA was isolated from cells and analyzed by real time PCR for IscA1 mRNA (means ± S.D.), and normalized to that of β-actin. F, mitochondrial extracts (20 μg) were analyzed by Western blotting using antibodies against IscA1 or cytochrome c.
In these experiments, we also found evidence for lower but nonetheless detectable levels of IscA1 in the cytosolic fractions (Fig. 3B, top panel, lane 1). We prepared cytosolic fractions, immunoprecipitated endogenous IOP1, and then examined the immunoprecipitates by Western blotting using anti-IscA1 antibodies. As shown in Fig. 3D (top panel, lane 4), IscA1 is found to coimmunoprecipitate with IOP1. The specificity of this interaction is supported by controls in which the anti-IOP1 antibody or the cytosolic lysate is omitted (Fig. 3D, lanes 2 and 5, respectively) or a control in which an unrelated antibody was employed for the immunoprecipitation (lane 3). Additional studies show that immunodepletion of IOP1 from cytosolic extracts removes most of the IscA1 (Fig. 3D, lower panel), suggesting that most of the cytoplasmic IscA1 is associated with IOP1.
To assess a potential functional role of IscA1, we employed siRNA to knock down IscA1. Two independent siRNAs were examined, both of which were effective in reducing IscA1 mRNA levels (Fig. 3E). The first reduced IscA1 mRNA levels to 24.4 ± 13.8%, whereas the second reduced it to 32.6 ± 4.1%. Both of these siRNAs were also effective in reducing IscA1 protein levels (Fig. 3F, lanes 2 and 4), and it should be noted that this provides additional evidence for the specificity of this antibody. The first of these siRNAs (IscA1-A) was employed in the majority of the experiments to be described. We typically treated HeLa cells with consecutive siRNA doses every 72 h and harvested 9 days after the initial transfection.
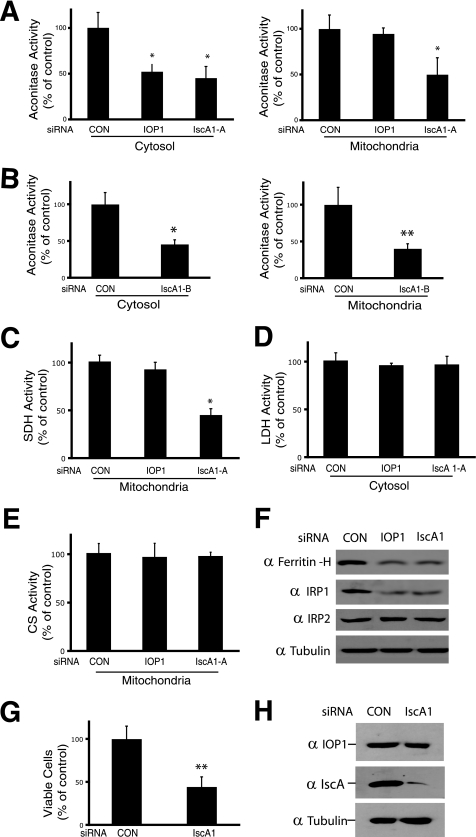
In initial experiments, we measured both cytosolic and mitochondrial aconitase activities. As shown in Fig. 4, A and B, respectively, we find that upon IscA1 knockdown, both cytosolic and mitochondrial aconitase activities were significantly decreased (to 45.3 ± 12.5 and 50 ± 18.3% of control levels, respectively, p < 0.01 in both cases). As a control, we also knocked down IOP1 and found that it decreased the activity of cytosolic aconitase (to 52.3 ± 7.7% of control) but not that of mitochondrial aconitase, consistent with our previous observations (12). We employed the second siRNA to IscA1 and found that it too diminishes the activity of both mitochondrial and cytosolic aconitase (Fig. 4B). We examined the activity of another iron-sulfur cluster mitochondrial enzyme, succinate dehydrogenase, and observed that IscA1 knockdown decreases the activity of this enzyme to 45.3% (p < 0.01) of control levels (Fig. 4C). IOP1 knockdown, in contrast, was without effect. To further examine the specificity of these effects, we monitored the activities of two enzymes that do not contain iron-sulfur clusters, lactate dehydrogenase (cytosolic) and citrate synthetase (mitochondrial). We found that IscA1 knockdown does not affect the activity of either (both p > 0.05 versus control siRNA treatment) (Fig. 4, D and E, respectively), providing evidence that IscA1 may play a specific role in the assembly of iron-sulfur cluster enzymes.
FIGURE 4.
Effects of IscA1 knockdown on cytosolic and mitochondrial aconitase. HeLa cells were transfected with the indicated siRNAs. A–E, cytosolic or mitochondrial extracts were assayed for aconitase, succinate dehydrogenase (SDH), lactate dehydrogenase (LDH), or citrate synthase (CS) activity as indicated. Shown are the means ± S.D., n = 3. *, p < 0.01; **, p < 0.05. F, total cellular extracts (20 μg) were analyzed by Western blotting using antibodies against ferritin-H, IRP1, IRP2, or β-tubulin. G, HeLa cells were transfected with the indicated siRNAs and counted by trypan blue exclusion after 9 days. Shown are the means ± S.D., n = 3. **, p < 0.05. H, total cellular extracts (20 μg) were analyzed by Western blotting using antibodies against IscA1, IOP1, or β-tubulin. CON, control.
IscA1 knockdown decreases the activity of both mitochondrial and cytosolic aconitase. The latter protein, upon loss of its iron-sulfur cluster, converts to IRP1 (iron regulatory protein 1), an RNA-binding protein that promotes adaptation to low iron levels (2). One such means is through its binding to the 5′-untranslated regions of the ferritin heavy and light chain mRNA, which in turn serves to inhibit protein translation and diminish the protein level of this intracellular iron storage protein. We examined ferritin heavy chain protein levels by Western blotting and found, indeed, that its levels decrease upon IscA1 knockdown to levels that are comparable with that induced by IOP1 knockdown (Fig. 4F, top panel). We also examined IRP1 levels and observed that its levels, as with IOP1 knockdown, also decrease upon IscA1 knockdown (Fig. 4F, 2nd panel from top). Decreased IRP1 levels are consistent with destabilization and enhanced degradation upon loss of its iron-sulfur cluster, as has been observed by us and other investigators (12, 23). IRP2 is 61% identical to IRP1 and can also bind IRE-containing mRNAs. It does not contain an iron-sulfur cluster nor does it display aconitase activity. The protein levels of IRP2, in contrast to that of IRP1, are not particularly changed by IscA1 knockdown (Fig. 4F, 3rd panel from top). In additional experiments, we were not able to observe an interaction between IRP1 and either IOP1 or IscA1 by coimmunoprecipitation (data not shown). We assessed cell viability and found that it was decreased upon IscA1 knockdown (Fig. 4G), similar to results obtained with IOP1 or Nfs1 knockdown (12, 24). We examined IOP1 protein levels and found that they did not change upon IscA1 knockdown (Fig. 4H).
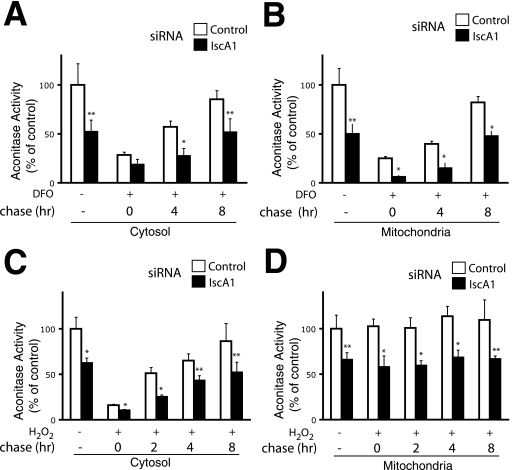
Iron-sulfur proteins are sensitive to a variety of agents, including iron-chelating agents and hydrogen peroxide (5, 25). We examined the effect of IscA1 on the recovery of mitochondrial and cytosolic aconitase activities in response to these stresses. In one set of experiments, we treated HeLa cells with DFO for 16 h, then refed the cells with media lacking DFO, and at various times monitored aconitase activity. As shown in Fig. 5, A and B, we find that DFO treatment markedly diminishes both cytosolic and mitochondrial aconitase activities. Removal of the DFO results in a time-dependent recovery of both activities, which reaches 85% of the untreated controls at 8 h in the case of cytosolic aconitase activity and 82% in the case of mitochondrial activity. As seen in the earlier experiments (Fig. 4), we find that IscA1 knockdown diminishes cytosolic and mitochondrial aconitase activity in the absence of DFO. Importantly, we find that upon DFO treatment and subsequent time points, both activities remained depressed upon IscA1 knockdown at almost all of the time points examined.
FIGURE 5.
Effects of IscA1 knockdown on recovery of aconitase activity from DFO and hydrogen peroxide treatments. HeLa cells were transfected with the indicated siRNAs. A and B, 8 days after the initial transfection, some cells were treated with 100 μm DFO for 16 h and then refed with media lacking DFO for the indicated times. C and D, 9 days after the initial transfection, some cells were treated with 200 μm H2O2 for 30 min and then refed with media lacking H2O2 for the indicated times. Cytosolic (A and C) or mitochondrial (B and D) extracts were assayed for aconitase activity. Shown are the means ± S.D., n = 3. *, p < 0.01; **, p < 0.05.
We next examined the effects of hydrogen peroxide, which damages the active site iron-sulfur cluster of aconitase (25). We exposed cells to 200 μm hydrogen peroxide for 30 min and then refed cells with media lacking hydrogen peroxide. As shown in Fig. 5C, we find that hydrogen peroxide markedly diminishes cytosolic aconitase activity, with its removal resulting in a time-dependent recovery of cytosolic aconitase activity. IscA1 knockdown reduces cytosolic aconitase activity in untreated cells and, significantly, also impairs recovery following hydrogen peroxide treatment. We assayed for mitochondrial aconitase but find that, in contrast to cytosolic aconitase (Fig. 5D), its activity is not appreciably diminished by hydrogen peroxide treatment; one possibility is that the concentration of hydrogen peroxide and/or time of exposure were not sufficiently long to allow entry of free radicals into the mitochondria in this particular series of experiments. As seen before (Fig. 4), we did observe that in the absence of hydrogen peroxide, IscA1 knockdown diminishes mitochondrial aconitase activity.
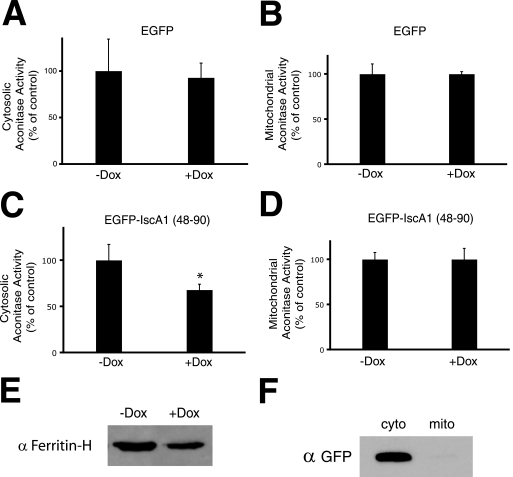
These results collectively suggest that IscA1 participates in the assembly of both mitochondrial and cytosolic aconitase activities. Furthermore, the interaction with IOP1 points to this interaction as potentially being important for this function in the CIA pathway. To further test this, we generated HEK293 cell lines stably transfected with a doxycycline-inducible transgene expressing either EGFP or EGFP-IscA1-(48–90). The latter interacts with IOP1 (Fig. 2B) and therefore might act as a dominant negative inhibitor. We induced expression of these proteins with doxycycline, and then we measured cytosolic aconitase activity. Under conditions in which EGFP overexpression has no effect on cytosolic aconitase activity (Fig. 6A), we find that overexpression of EGFP-IscA1-(48–90) results in decreased cytosolic aconitase activity (Fig. 6C) but not that of mitochondrial aconitase (Fig. 6D). We also observed decreased ferritin heavy chain protein levels, consistent with conversion of cytosolic aconitase to IRP1 activity (Fig. 6E). The cytosolic localization of this fusion protein (Fig. 6F), combined with its capacity to interact with IOP1 (Fig. 1 and Fig. 2B), suggests that it is indeed behaving as a dominant negative inhibitor of the cytosolic iron-sulfur cluster assembly pathway.
FIGURE 6.
Effects of overexpression of IscA1-(48–90) on cytosolic and mitochondrial aconitase activity. Flp-In TRex HEK293 cells stably transfected with pcDNA5/FRT/TO-EGFP (A and B) or pcDNA5/FRT/TO-EGFP-IscA1-(48–90) (C and D) were treated with or without 2 μg/ml of doxycycline (Dox) and then grown for an additional 72 h. Cytosolic (A and C) or mitochondrial (B and D) extracts were then assayed for aconitase activity. Shown are the means ± S.D., n = 3. *, p < 0.01. E, Western blot for ferritin-H of cytosolic extract (40 μg) of Flp-In TRex HEK293 cells stably transfected with pcDNA5/FRT/TO-EGFP and treated with or without doxycycline. F, cytosolic (cyto) (10 μg) or mitochondrial (mito) (10 μg) extracts of doxycycline-treated Flp-In TRex HEK293 cells stably transfected with pcDNA5/FRT/TO-EGFP were analyzed by Western blotting with antibodies against green fluorescent protein.
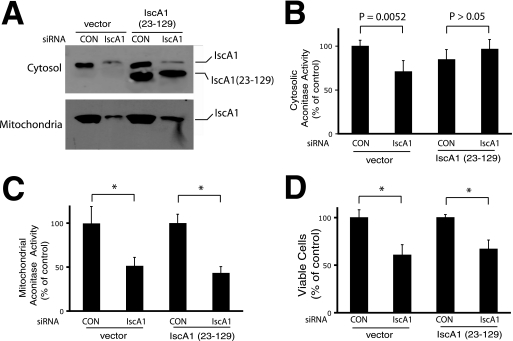
In an independent approach, we sought to determine whether a cytosol-localized version of IscA1 could rescue the defect in cytosolic iron-sulfur cluster assembly induced by IscA1 knockdown. We generated HEK293 cells stably transfected with a doxycycline-inducible transgene expressing IscA1R-(23–129) or with vector alone. IscA1R-(23–129) lacks a mitochondrial targeting sequence predicted to be present in IscA1 (21), and its coding sequence contains three nucleotide substitutions in the region targeted by the IscA1 siRNA. We induced cells with doxycycline and treated them with siRNA. We find that IscA1-(23–129) appears as a more rapidly migrating species as compared with the endogenous IscA1 (Fig. 7A, top panel, compare 1st and 3rd lanes) and that it is present in the cytosolic but not mitochondrial fractions (3rd lane, compare top and bottom panels). Importantly, it is resistant to IscA1 siRNA knockdown (Fig. 7A, top panel, compare 3rd and 4th lanes). Measurements of aconitase activity reveal that expression of this cytosolic localized IscA1-(23–129) can rescue IscA1 knockdown-induced loss of cytosolic aconitase activity (Fig. 7B, compare 1st two columns with last two columns) but not IscA1 knockdown-induced loss of mitochondrial aconitase activity (Fig. 7C). This cytosolic localized IscA1-(23–129) fails to rescue IscA1 knockdown-induced loss of cell viability (Fig. 7D), consistent with IscA1-dependent mitochondrial iron-sulfur cluster assembly being essential for viability. These results support a critical role for cytosolic IscA1 in cytosolic iron-sulfur cluster assembly.
FIGURE 7.
Effects of overexpression of siRNA-resistant IscA1-(23–129) on cytosolic and mitochondrial aconitase activity. Flp-In TRex HEK293 cells stably transfected with vector (pcDNA5/FRT/TO) or pcDNA5/FRT/TO-IscA1R-(23–129) were induced with doxycycline and treated with control (CON) or IscA1-A siRNA. Cells were harvested after 9 days. A, cytosolic (30 μg) and mitochondrial (10 μg) fractions were examined by Western blotting using anti-IscA1 antibodies. The positions of endogenous IscA1 and IscA1-(23–129) are as shown. B and C, cytosolic (B) or mitochondrial (C) extracts were assayed for aconitase activity. *, p < 0.01. Shown are the means ± S.D., n = 4. D, viability was assessed by counting cells using trypan blue exclusion. Shown are the means ± S.D., n = 3. *, p < 0.01.
DISCUSSION
The present results provide evidence that IscA1, the human homologue of the ancient protein IscA, plays a role in both mitochondrial and cytosolic iron-sulfur cluster assembly. In S. cerevisiae, the homologue of IscA1, Isa1, is a mitochondrial protein and has a mitochondrial targeting sequence (7, 22). Indeed, human IscA1 also is predicted to have a mitochondrial targeting sequence (21), and consistent with this, we find that most IscA1 is present in the mitochondrial fraction (Fig. 3B). This, in turn, raises the question of how this protein affects cytosolic iron-sulfur cluster assembly. One possibility is that IscA1 might participate directly in the synthesis of iron-sulfur clusters in proteins that themselves are essential for cytosolic iron-sulfur clusters. For example, the requirement of Isa1 for proper cytosolic iron-sulfur cluster assembly in yeast may be due to a role for Isa1 in the mitochondrial iron-sulfur cluster protein Yah1 (22). It is also possible that IscA1 might play a role in the assembly of iron-sulfur clusters in IOP1 and thereby indirectly affect cytosolic iron-sulfur cluster assembly; if this were the case, the binding of IscA1 to an N-terminal fragment of IOP1 (Fig. 1) raises the possibility that it may be involved in the assembly of the iron-sulfur cluster in the N-terminal ferredoxin-like domain of IOP1. Such a domain would be predicted to harbor a [2Fe-2S] cluster, distinct from the [4Fe-4S] cluster present in aconitase.
Yet another explanation, which is supported by the data presented here, is that IscA1 plays a role not only in the mitochondria but also directly in the cytosol through its interaction with IOP1. Indeed, this study provides the following evidence: (i) IscA1 is present in the cytosol; (ii) IscA1 can interact with IOP1, as assessed by coimmunoprecipitation of endogenous and overexpressed proteins as well as by yeast two-hybrid analysis; and (iii) an siRNA-resistant, cytosol-localized version of IscA1-(23–129) can rescue the defect in cytosolic aconitase activity induced by IscA1 knockdown. A possible scenario is that IscA1 might serve as a scaffold for delivery of iron-sulfur clusters to aconitases in both cytosolic and mitochondrial locales. The cysteines of IscA1 would presumably be important in this function, and although they are not essential per se for the IscA1-IOP1 interaction in the yeast two-hybrid assay, they do appear to be important in mammalian cells as assessed by the coimmunoprecipitation studies.
We have searched for but have yet to detect other interactions with IOP1 by coimmunoprecipitation. For example, Ciao1, the human homologue of Cia1, is a nuclear protein that plays an essential role in the yeast CIA pathway (26, 27), and it interacts with Nar1p in S. cerevisiae (27). However, we have yet to be able to detect an interaction between the mammalian homologues of these proteins, Ciao1 and IOP1, respectively, by coimmunoprecipitation or by yeast two-hybrid assay (supplemental Figs. 2 and 3, respectively). That being said, these as well as some of the other negative results mentioned previously should be interpreted with caution, because the proteins may be labile and the interactions weak. Additional studies will be needed in this regard.
It is notable that the cysteine substitutions of IscA1 interact with IOP1 in the yeast two-hybrid assay but not in the mammalian cell coimmunoprecipitation experiments. One possible explanation is different levels of stringency from these two fundamentally different assays. The yeast two-hybrid assay can allow weak interactions to be detectable (28), because it permits readout from a steady state and potentially transient level of interaction. The coimmunoprecipitation assay may have a higher level of stringency, because it requires stable association of proteins during the coimmunoprecipitation. Hence, it is possible that the cysteine mutations significantly decrease the affinity of IscA1 for IOP1 to a point where interaction is lost with the latter but not the former assay. It is also possible that the different fusion protein tags employed in the yeast two-hybrid (GAL4 DNA binding domain and GAL4 activation domain) and mammalian coimmunoprecipitation (FLAG and EGFP) assays may sterically affect the interaction between IscA1 and IOP1 in ways that may not be identical.
Two models for the assembly of cytosolic iron-sulfur proteins posit that there is a dedicated pathway utilizing cytosolic proteins or, alternatively, a pathway that parallels that which exists in the mitochondria but is present at lower protein levels (1, 2). The present data suggest features of both. IOP1 is a cytosolic protein that appears to be part of a dedicated cytosolic iron-sulfur cluster assembly pathway (12), yet it interacts with a protein, IscA1, that is predominantly mitochondrial and to a lesser extent cytosolic. In this regard, IscA1 has similarities to IscU, which is present in both the mitochondria and cytosol (5).
Acknowledgments
We thank Scott Sutherland for purifying the GST-IscA1 and MBP-IscA1 proteins and Adrian Flores for assistance in plasmid construction.
This work was supported by National Institutes of Health Grant R01-GM71459 (to F. S. L.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
- CIA
- cytosolic iron-sulfur assembly
- DFO
- desferrioximine
- EGFP
- enhanced green fluorescent protein
- siRNA
- small interfering RNA.
REFERENCES
- 1.Lill R., Mühlenhoff U. (2008) Annu. Rev. Biochem. 77, 669–700 [DOI] [PubMed] [Google Scholar]
- 2.Rouault T. A., Tong W. H. (2005) Nat. Rev. Mol. Cell Biol. 6, 345–351 [DOI] [PubMed] [Google Scholar]
- 3.Johnson D. C., Dean D. R., Smith A. D., Johnson M. K. (2005) Annu. Rev. Biochem. 74, 247–281 [DOI] [PubMed] [Google Scholar]
- 4.Gerber J., Neumann K., Prohl C., Mühlenhoff U., Lill R. (2004) Mol. Cell. Biol. 24, 4848–4857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tong W. H., Rouault T. A. (2006) Cell Metab. 3, 199–210 [DOI] [PubMed] [Google Scholar]
- 6.Agar J. N., Krebs C., Frazzon J., Huynh B. H., Dean D. R., Johnson M. K. (2000) Biochemistry 39, 7856–7862 [DOI] [PubMed] [Google Scholar]
- 7.Jensen L. T., Culotta V. C. (2000) Mol. Cell. Biol. 20, 3918–3927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pelzer W., Mühlenhoff U., Diekert K., Siegmund K., Kispal G., Lill R. (2000) FEBS Lett. 476, 134–139 [DOI] [PubMed] [Google Scholar]
- 9.Krebs C., Agar J. N., Smith A. D., Frazzon J., Dean D. R., Huynh B. H., Johnson M. K. (2001) Biochemistry 40, 14069–14080 [DOI] [PubMed] [Google Scholar]
- 10.Ding H., Clark R. J., Ding B. (2004) J. Biol. Chem. 279, 37499–37504 [DOI] [PubMed] [Google Scholar]
- 11.Stehling O., Netz D. J., Niggemeyer B., Rösser R., Eisenstein R. S., Puccio H., Pierik A. J., Lill R. (2008) Mol. Cell. Biol. 28, 5517–5528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song D., Lee F. S. (2008) J. Biol. Chem. 283, 9231–9238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balk J., Pierik A. J., Netz D. J., Mühlenhoff U., Lill R. (2004) EMBO J. 23, 2105–2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fields S., Song O. (1989) Nature 340, 245–246 [DOI] [PubMed] [Google Scholar]
- 15.Huang J., Song D., Flores A., Zhao Q., Mooney S. M., Shaw L. M., Lee F. S. (2007) Biochem. J. 401, 341–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daum G., Böhni P. C., Schatz G. (1982) J. Biol. Chem. 257, 13028–13033 [PubMed] [Google Scholar]
- 17.Hallberg E. M., Shu Y., Hallberg R. L. (1993) Mol. Cell. Biol. 13, 3050–3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang B. H., Plescia J., Dohi T., Rosa J., Doxsey S. J., Altieri D. C. (2007) Cell 131, 257–270 [DOI] [PubMed] [Google Scholar]
- 19.Minczuk M., Piwowarski J., Papworth M. A., Awiszus K., Schalinski S., Dziembowski A., Dmochowska A., Bartnik E., Tokatlidis K., Stepien P. P., Borowski P. (2002) Nucleic Acids Res. 30, 5074–5086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosen H., Rakita R. M., Waltersdorph A. M., Klebanoff S. J. (1987) J. Biol. Chem. 262, 15004–15010 [PubMed] [Google Scholar]
- 21.Cózar-Castellano I., del Valle Machargo M., Trujillo E., Arteaga M. F., González T., Martín-Vasallo P., Avila J. (2004) Biochim. Biophys. Acta 1700, 179–188 [DOI] [PubMed] [Google Scholar]
- 22.Kaut A., Lange H., Diekert K., Kispal G., Lill R. (2000) J. Biol. Chem. 275, 15955–15961 [DOI] [PubMed] [Google Scholar]
- 23.Fosset C., Chauveau M. J., Guillon B., Canal F., Drapier J. C., Bouton C. (2006) J. Biol. Chem. 281, 25398–25406 [DOI] [PubMed] [Google Scholar]
- 24.Biederbick A., Stehling O., Rösser R., Niggemeyer B., Nakai Y., Elsässer H. P., Lill R. (2006) Mol. Cell. Biol. 26, 5675–5687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brazzolotto X., Gaillard J., Pantopoulos K., Hentze M. W., Moulis J. M. (1999) J. Biol. Chem. 274, 21625–21630 [DOI] [PubMed] [Google Scholar]
- 26.Johnstone R. W., Wang J., Tommerup N., Vissing H., Roberts T., Shi Y. (1998) J. Biol. Chem. 273, 10880–10887 [DOI] [PubMed] [Google Scholar]
- 27.Balk J., Aguilar Netz D. J., Tepper K., Pierik A. J., Lill R. (2005) Mol. Cell. Biol. 25, 10833–10841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luban J., Goff S. P. (1995) Curr. Opin. Biotechnol. 6, 59–64 [DOI] [PubMed] [Google Scholar]