Abstract
The oncogene v-myb of avian myeloblastosis virus (AMV) encodes a transcription factor (v-Myb) that transforms myelomonocytic cells by deregulating the expression of specific target genes. v-myb has acquired its oncogenic potential by truncation as well as by a number of point mutations of its cellular progenitor c-myb. As a result of these changes, the target gene spectrum v-Myb differs from that of c-Myb. We recently showed that the chicken mim-1 gene, a c-Myb target gene that is not activated by v-Myb harbors a powerful cell type-specific and Myb-inducible enhancer in addition to a Myb-responsive promoter. We now show that Myb-dependent activation of the mim-1 gene is accompanied by extensive remodeling of the nucleosomal architecture at the enhancer. We found that the mim-1 enhancer region also harbors a promoter whose activity is stimulated by Myb and which directs the transcription of an apparently non-coding RNA. Furthermore, our data show that the oncogenic mutations of AMV have disrupted the ability of v-Myb to induce remodeling of chromatin structure at the mim-1 enhancer. Together, our results demonstrate for the first time directly that Myb induces alterations of the nucleosomal organization at a relevant target site and provide new insight into the functional consequences of the oncogenic amino acid substitutions.
Introduction
Myb proteins constitute a family of transcription factors playing key roles during proliferation and differentiation of various cell types (1, 2). v-Myb was originally identified as the protein encoded by the oncogene v-myb of avian myeloblastosis virus (AMV)3 (3). v-myb is a truncated and mutated derivative of chicken c-myb, which is highly expressed in immature hematopoietic cells and is down-regulated during terminal differentiation. A large body of work has demonstrated that c-myb plays a crucial role in the development of the hematopoietic system; in addition, deregulation of c-myb has been implicated in the development of colon and breast cancer as well as leukemia (4).
v-Myb and c-Myb are DNA-binding proteins that recognize a specific sequence motif (5) and activate promoters containing this motif (6–9). A variety of approaches has been used to identify Myb target genes, such as differential screening of cells transformed by a temperature-sensitive mutant of v-Myb (7), differential display of RNA from cells expressing estrogen-inducible or dominant-interfering versions of v-Myb or c-Myb (10–12) or microarray-based assays of cells overexpressing different Myb family members (13, 14). Although a growing number of Myb target genes are now known, how they contribute to the biological effects of Myb is not fully understood.
Myb sequences have been transduced into two retroviruses, AMV and E26. The oncogenic potential of v-Myb is mainly caused by N- and C-terminal truncations that have occurred during retroviral transduction. In addition, the v-Myb protein of AMV harbors several amino acid substitutions that were acquired during the passage of the virus and strongly enhance its oncogenic potential (15). These “oncogenic” substitutions have raised considerable interest because they exert drastic effects on the activity of v-Myb and the spectrum of its target genes (14, 16). Some of the oncogenic substitutions are located in the DNA binding domain and affect the interaction of v-Myb with DNA (17, 18) and other proteins (19–21). Several amino acid substitutions, which are located around the transactivation domain of v-Myb, have also been implicated in defining the spectrum of v-Myb target genes (16); however, how they exert their effects is unknown.
The chicken mim-1 gene is one of the most thoroughly studied Myb target genes. Within the hematopoietic system, mim-1 is only transcribed in myelomonocytic cells and reaches extremely high expression levels (7). Because of its lineage-restricted expression and the strong activation by Myb, the mim-1 gene is an interesting model to study how Myb activates its target genes. Previous work has shown that the mim-1 promoter harbors Myb and C/EBP binding sites and that Myb synergizes with C/EBP family members to activate mim-1 expression (22, 23). Additional insight into mim-1 gene activation was provided by the identification of a Myb-responsive enhancer located 2-kb upstream of the mim-1 promoter (24). We have recently shown that the transcription factor C/EBPβ induces opening of the compact chromatin structure at the enhancer in a step preceding the transcription of the gene (25). These observations suggested that the mim-1 enhancer plays an important role during the activation of the gene and have made mim-1 an interesting model to study the stepwise activation of a gene by Myb.
Here, we show that Myb induces extensive remodeling of the nucleosomal architecture at the enhancer. Surprisingly, we found that the mim-1 enhancer region also harbors a Myb-inducible promoter which drives transcription of an apparently non-coding RNA. Furthermore, we show that the oncogenic mutations of AMV have disrupted the ability of v-Myb to remodel the enhancer chromatin. Our results demonstrate for the first time that Myb affects the nucleosomal organization at a relevant target site and provide new insight into the impact of the oncogenic amino acid substitutions.
EXPERIMENTAL PROCEDURES
Cells
HD11 cells are MC29-transformed chicken macrophages and were grown in basal Iscoves' medium supplemented with 8% fetal calf serum and 2% chicken serum. HD11-E cells express MybREV in a doxycyclin-inducible manner and have been described (18). HD11-A, HD11-E/A, and HD11-EP cells, expressing doxycyclin-inducible v-MybAMV, v-MybE/A, and v-MybEP, were generated as described by Ivanova et al. (18). Northern and Western blotting was performed as described (22). ChEST254k22 RNA was detected by RT-PCR with primers: 5′-AGATGAGTCATGCTGCATGC-3′ and 5′-CTTCCCCTGTGACAGGAT-3′. Total RNA was isolated and transcribed into cDNA using commercial kits.
In Vivo Footprinting and Chromatin Immunoprecipitation
In vivo dimethylsulfate footprinting and chromatin immunoprecipitation were done as described (25).
Reporter Genes, Expression Vectors, and Transfections
Luciferase reporter genes containing the mim-1 promoter (p-240-luc) and the mim-1 enhancer (pGL3-tk81-mimwt) have been described (7, 24). tk-mim(181–394)-luc and tk-mim(307–612)-luc contain only the indicated part of the enhancer (numbers refer to Ref. 24). In plasmid tk-mim-Ets, an Ets site was changed from CAGGAATC to CAGGTTTC. In plasmids tk-mim-450 and tk-mim-500 C/EBP sites were changed from TGTTGCCCAATG to TGTTGCCCTTTG and from TATTGCCCAACA to TACAGCCCATCA. Promoterless mim-1 enhancer/luciferase constructs were generated by cloning the full-length enhancer (nucleotides 1–795, Ref. 24) in both orientations into the XhoI site of pGL3-basic (Promega). pCMVβ was from Clontech. Expression vectors pCDAMVv-myb, pCDE26v-myb, and pCDE/Av-myb have been described (18, 24) and encode v-MybAMV, v-MybREV, and v-MybE/A, respectively. Expression vectors for v-MybEEA, v-MybEAE, v-MybEpA, and v-MybEP were generated by using specific restriction sites to exchange parts of the coding sequences between v-MybREV and v-MybAMV. DNA transfections, preparation of cell extracts, and reporter assays were performed as described (22).
Micrococcal Nuclease Digestion
Approximately 108 cells were formaldehyde-fixed for 15 min at room temperature with growth medium containing 1% formaldehyde and quenched with 125 mm glycine for 5 min. Further steps were done on ice. After washing with phosphate-buffered saline, the cells were suspended in 30 ml of nuclei preparation buffer (300 mm sucrose; 10 mm Tris-HCl, pH 7.5, 15 mm NaCl, 60 mm KCl, 0.1 mm EGTA, 0.15 mm spermidine, 0.5 mm spermine, 0.1% Nonidet P-40, 0.5 mm phenylmethylsulfonyl fluoride (PMSF) and incubated for 3 min. Nuclei were pelleted at 600 × g for 10 min and suspended at a concentration of 107 nuclei per 500 μl in nuclei preparation buffer lacking Nonidet P-40 and PMSF. MNase digestion was carried out in 500 μl by adding increasing concentrations of MNase and 10 μl of 100 mm CaCl2 and incubating for 15 min at 37 °C. The reaction was stopped by adding 500 μl of lysis buffer (50 mm Tris-HCl, pH 8.0, 20 mm EDTA, 1% SDS, 500 μg/ml proteinase K) and kept at 65 °C overnight before the phenol-chloroform DNA isolation. As control, purified genomic DNA was treated for 15 min at 37 °C with increasing concentrations of MNase in 150 μl of 10 mm Tris-HCl, pH 7.5 with addition of 20 μl of 10 mm CaCl2. The reaction was stopped by adding 15 μl of 50 mm EDTA, and the DNA was precipitated with ethanol. 35 μg of MNase-treated genomic DNA were digested with the desired restriction enzyme and analyzed by Southern blotting.
RESULTS
Amino Acid Substitutions of v-Myb Decrease Its Activity at the mim-1 Enhancer but Not at the mim-1 Promoter
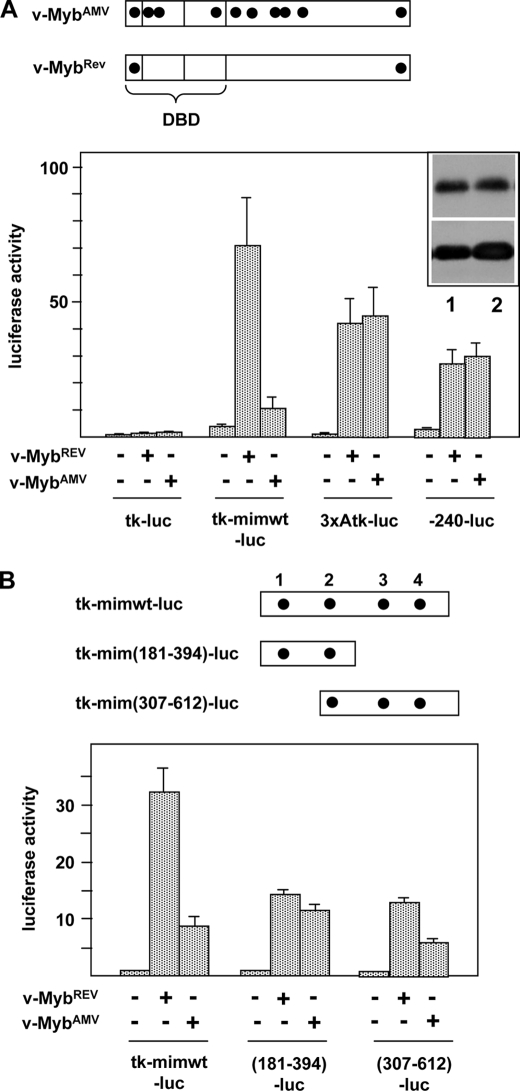
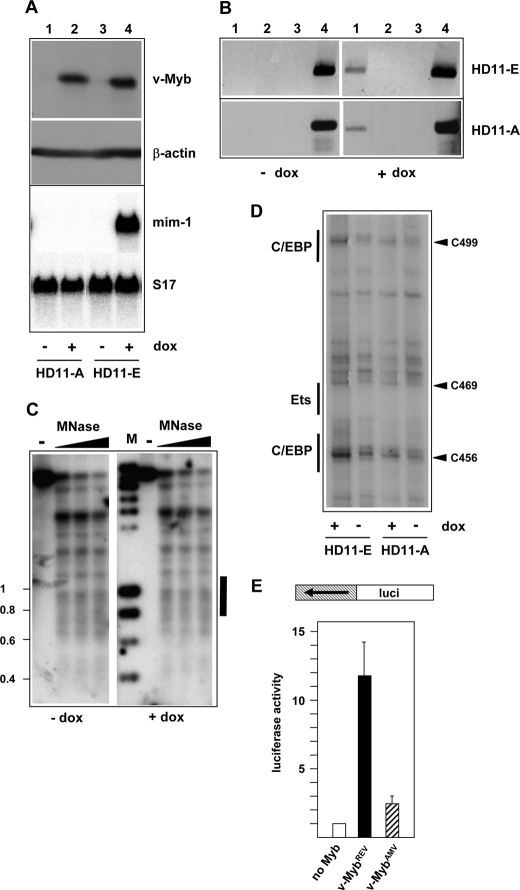
During the characterization of the mim-1 enhancer, we noted that a v-Myb protein in which most of the AMV-specific amino acid substitutions had been reverted (v-MybREV) stimulated the enhancer activity to much higher levels than v-MybAMV. By contrast, reporter genes containing the mim-1 promoter or the thymidine kinase promoter fused to several Myb binding sites were activated by both forms of v-Myb equally well (Fig. 1A). The observation that v-MybAMV was unable to fully stimulate the mim-1 enhancer was interesting and led us to explore the role of Myb at the enhancer in more detail.
FIGURE 1.
Activity of v-MybAMV and v-MybREV at the mim-1 enhancer. A, top, schematic illustration of the structure of v-MybAMV and v-MybREV. Black dots mark point mutations relative to c-Myb. DBD, DNA binding domain. Bottom, HD11 cells were transfected with the indicated luciferase reporter genes and expression vectors for v-MybAMV, v-MybREV, or empty expression vector. 24 h after transfection, the cells were analyzed for luciferase and β-galactosidase activity. The columns show the average luciferase activity normalized against the activity of the cotransfected β-galactosidase plasmid. Thin lines show S.D. The upper part of the inset at the top right corner shows a Western blot of v-MybREV (lane 1) and v-MybAMV (lane 2) of the transfected cells and β-actin as loading control. B, top, schematic illustration of truncated enhancer constructs. Numbers refer to Chayka et al. (24). Myb binding sites are marked by black dots. Bottom, luciferase reporter genes containing the full-length or truncated mim-1 enhancer upstream of the tk promoter were transfected and analyzed as in A.
The mim-1 enhancer harbors 4 Myb binding sites distributed over its entire length. Mutation of one of these sites (referred to as MBS3) located in the 3′-part of the enhancer strongly affects the Myb responsiveness of the enhancer (24). To investigate whether the differential stimulation by v-MybREV and v-MybAMV was mediated by a subregion of the enhancer, we used reporter genes containing only the 5′- or the 3′-part of the enhancer and investigated their activation by both versions of Myb. Interestingly, as shown in Fig. 1B, the increased responsiveness to v-MybREV was mainly due to sequences in the 3′-part of the enhancer: The 3′-part of the enhancer was activated more strongly by v-MybREV than by v-MybAMV, whereas the 5′-part was activated similarly by both Myb versions.
v-MybREV Induces Chromatin Remodeling of the mim-1 Enhancer
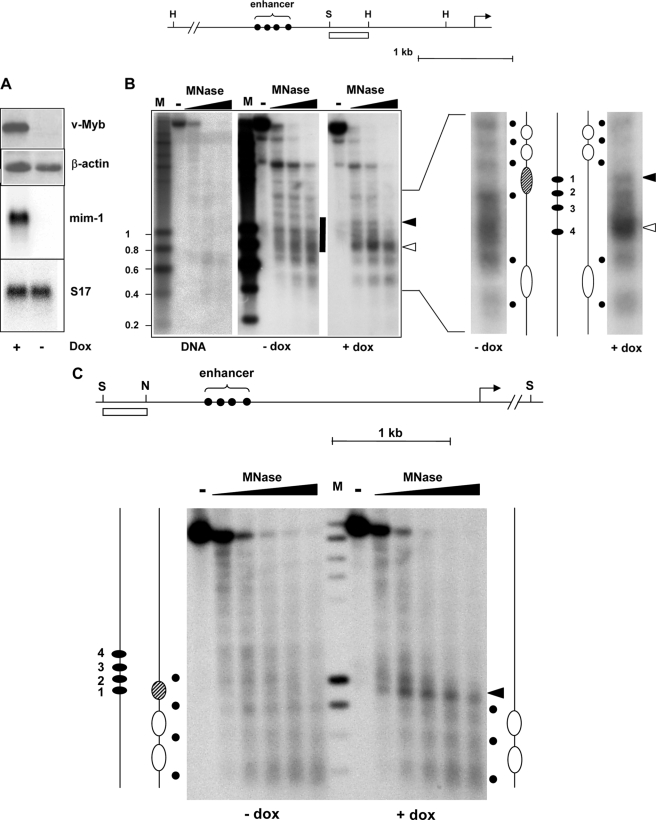
To explore the influence of Myb on the chromatin at the enhancer we used HD11-E cells that express v-MybREV and mim-1 in a doxycyclin-inducible manner (Fig. 2A). We performed MNase digestions of nuclei from HD11-E cells before and after treatment with doxycyclin. MNase induces double-strand breaks in DNA preferentially in the linker regions between nucleosomes and can therefore be used to explore the nucleosomal organization of a particular DNA region. Fig. 2B shows that in the absence of v-MybREV, MNase digestion of HD11-E chromatin resulted in a series of bands with an approximate spacing of 200 basepairs in the region upstream of the mim-1 enhancer, suggesting the presence of positioned nucleosomes in this region. In the enhancer region, there was a broad distribution of MNase cuts with no distinct bands, suggesting a more irregular spacing of nucleosomes. After induction of v-MybREV the MNase digestion pattern was altered and revealed pronounced differences (see black and white arrowheads) in the enhancer region. A direct comparison of the MNase pattern of HD11-E cells grown with or without doxycyclin is shown in the right part of Fig. 2B. A significant increase of the MNase sensitivity (marked by a white arrowhead) co-localized with the 3′-part of the enhancer. There was also a significant change in the 5′-part of the enhancer; in particular an additional MNase sensitive site appeared (see black arrowhead), suggesting that a nucleosome was either disrupted or altered such that the DNA became accessible to MNase. The alteration in the digestion pattern in this region was confirmed by using a hybridization probe derived from upstream of the enhancer (Fig. 2C). In this case, a prominent MNase cut appeared at the position of the disrupted/structurally altered nucleosome after induction of v-MybREV. These results demonstrated for the first time a Myb-dependent remodeling of the nucleosomal architecture at a physiological target region.
FIGURE 2.
Myb affects the nucleosomal organization at the mim-1 enhancer. A, top, Western blots of HD11-E cells grown with or without doxycyclin. Blots were stained with antibodies against v-Myb and β-actin. Bottom, Northern blots of the same cells hybridized with probes specific for mim-1 or ribosomal protein S17 mRNAs. B, strategy used for mapping micrococcal nuclease sensitive sites at the mim-1 enhancer is illustrated at the top. The arrow marks the transcriptional start site. Myb binding sites in the enhancer region are marked by black dots. Relevant restriction sites: H, HindIII; S, SacI. The white bar marks the region that was used as hybridization probe. Nuclei from HD11-E cells grown in the absence or presence of doxycyclin or isolated genomic DNA were treated without (−lanes) or with increasing concentrations of micrococcal nuclease. DNA was digested with HindIII and analyzed by Southern blotting using the probe shown at the top. M, size markers. The enhancer region is marked by a bar. The black and white arrowheads mark changes in the digestion pattern induced by v-Myb expression. The right part of the figure shows two lanes of the digestion pattern at higher magnification to permit their direct comparison. The deduced positions of nucleosomes and linkers are indicated by large ovals and black dots. The hatched nucleosome appears to be disrupted in the presence of v-Myb. The positions of the Myb binding sites (1–4) are marked by black ovals. C, top part illustrates strategy to map micrococcal nuclease sensitive sites using a hybridization probe derived from upstream of the enhancer. The transcriptional start site of mim-1 is marked by an arrow. Myb binding sites in the enhancer region are marked by black dots. Relevant restriction sites: S, SpeI; N, NdeI. The white bar marks the region that was used as hybridization probe. Nuclei from HD11-E cells grown in the absence or presence of doxycyclin were treated without or with increasing concentrations of micrococcal nuclease, as indicated. DNA isolated from the nuclei was digested with SpeI and analyzed by Southern blotting using the probe shown at the top. The positions of nucleosomes and Myb sites are marked as in B. The nucleosome that appears to be disrupted by v-Myb is marked by hatching. The black arrowhead points to the same position of increased MNase sensitivity that is also marked by a black arrowhead in B.
In Vivo DMS Footprinting Reveals Increased Occupancy of Several Transcription Factor Binding Sites at the mim-1 Enhancer
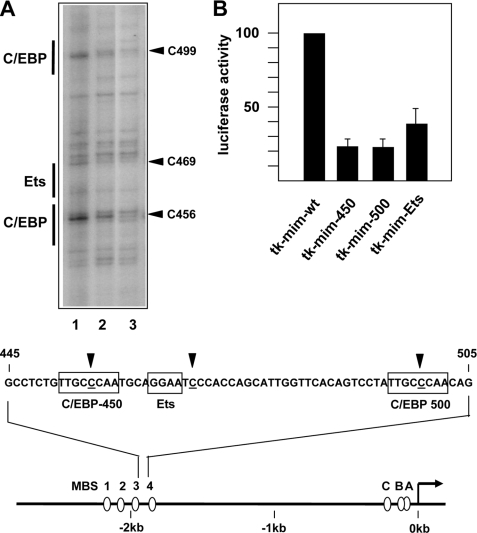
To investigate whether the Myb-induced nucleosomal remodeling of the enhancer caused increased binding of other transcription factors we performed in vivo DMS footprinting experiments. In these experiments, total cells or isolated DNA are treated with DMS. Protein-DNA interactions may either increase or decrease the local accessibility of specific bases to DMS and can therefore be detected by comparing the sensitivity of specific guanine bases toward modification by DMS. To visualize DMS-modified bases a cleavage reaction is performed, which introduces a strand break at the modified base. Lanes 1 and 2 of Fig. 3A illustrate the major differences in DMS sensitivity between HD11-E cells grown in the presence or absence of doxycyclin. There were clear differences of the DMS sensitivity at positions 456, 469, and 499. Positions 456 and 499 are located within potential C/EBP binding sites (C/EBP consensus sequence: TT/GNNNNAA) whereas position 469 is located immediately next to a potential Ets binding site (Ets consensus sequence: GGA(A/T)). These data showed that the occupancy at two C/EBP sites and an Ets site in the 3′-part of the enhancer was increased in the presence of v-MybREV. Note that these sites are all located in the part of the enhancer, which showed the most pronounced increase in MNase sensitivity. Outside of the enhancer region shown in Fig. 3A, we did not detect consistent alterations of the DMS sensitivity induced by Myb. We mutated the C/EBP and Ets binding sites to assess their functionality. Mutation in all cases significantly diminished the activity of the enhancer (Fig. 3B), indicating that these binding sites are functional.
FIGURE 3.
In vivo footprinting of the mim-1 enhancer. A, DMS footprinting studies of HD11-E cells grown in the presence (lane 1) or absence (lane 2) of doxycyclin. Lane 3 shows DMS-treated isolated genomic DNA as control. The footprinting gel encompasses the part of the mim-1 enhancer between Myb binding sites 3 and 4, which shows the most pronounced increase in MNase sensitivity. The nucleotide sequence of this region is shown at the bottom. The numbering refers to Chayka et al. (25). Nucleotides showing altered DMS sensitivity in the presence or absence of doxycyclin are highlighted by arrows, and C/EBP and Ets binding sites are marked. The bottom part of the figure shows schematically the mim-1 promoter and enhancer region and the positions of Myb binding sites A-C (at the promoter) and 1–4 (at the enhancer). B, equal amounts of reporter genes containing the tk promoter and the wild-type mim-1 enhancer (tk-mimwt) or derivatives of this plasmid carrying a mutation of Ets or C/EBP sites were transfected into HD11 cells. The cells were co-transfected with the β-galactosidase plasmid pCMVβ to control the transfection efficiency. Cells were harvested 24 h after transfection and analyzed for luciferase and β-galactosidase activities. The columns show the average luciferase activity normalized with respect to the activity of the co-transfected β-galactosidase plasmid. Thin lines show S.D. The activity of the reporter gene containing the wild-type enhancer was set to 100%.
The mim-1 Enhancer Functions as a Dual Enhancer/Promoter Element
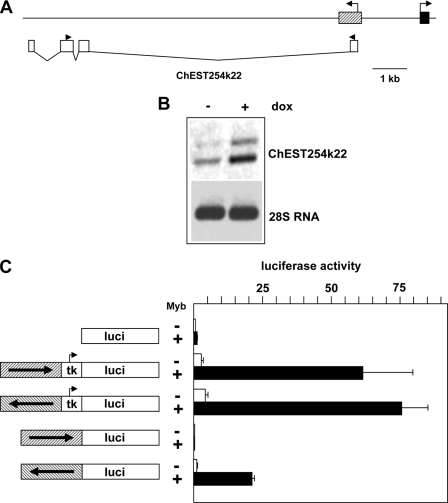
During the characterization of the mim-1 enhancer, we noted a chicken EST sequence (ChEST254k22, GenBankTM entry: BU240796.1) that overlaps with the enhancer. ChEST254k22 is derived from a spliced RNA whose transcription is initiated within the mim-1 enhancer region located 2-kb upstream of the mim-1 promoter and proceeds away from the body of the mim-1 gene into the intergenic region, as shown schematically in Fig. 4A. ChEST254k22 RNA appears to be a non-coding RNA. The sequence predicts several short open reading frames without homology to known proteins. Although the longest open reading could encode a polypeptide of ∼70 amino acids, its 5′-end conforms only poorly to the consensus sequence for translational start codons (26). Furthermore, the RNA would be expected to be degraded by nonsense-mediated decay, because there are two introns 3′ to the stop codon of the longest open reading frame (27). Based on RNA secondary structure predictions, ChEST254k22 RNA also appears not to be a microRNA precursor.
FIGURE 4.
The mim-1 enhancer region harbors a Myb-inducible promoter. A, schematic illustration of the mim-1 upstream region. The first exon of the mim-1 gene and the mim-1 enhancer are shown as black and hatched boxes, respectively. The white boxes shown below indicate the positions of the exons of ChEST254k22 RNA. The black arrowheads above the white boxes mark the positions of the primers used for RT-PCR. B, RNA isolated from HD11-E cells grown for 12 h in the absence or presence of doxycyclin was reverse transcribed and subjected to a PCR reaction using ChEST254k22 specific primers (top panel). The bottom panel shows PCR reactions of the reverse-transcribed samples using primers specific for 28 S ribosomal RNA. C, luciferase reporter genes shown schematically at the left were cotransfected with or without expression vector for v-MybREV into HD11 cells. The cells were additionally co-transfected with the β-galactosidase plasmid pCMVβ to control the transfection efficiency. Cells were harvested after 24 h and analyzed for luciferase and β-galactosidase activities. The columns show the average luciferase activity normalized with respect to the activity of the co-transfected β-galactosidase plasmid. Thin lines show S.D.
We failed to detect ChEST254k22 RNA by Northern blotting, suggesting that it is transcribed at low levels or has a short half-life; however, we detected the RNA by RT-PCR, as illustrated in Fig. 4B. PCR amplification resulted in two DNA fragments differing by ∼150 bp. Sequencing showed that they were both derived from ChEST254k22. The large fragment was derived from a partially spliced RNA, because it contained the small intron between the second and third exons. Interestingly, the expression of both RNA species was stimulated by Myb, suggesting that the mim-1 enhancer region also harbors a Myb-inducible promoter. To address this possibility, we analyzed the effect of v-MybREV on luciferase reporter genes containing the mim-1 enhancer but no additional basal promoter. As shown in Fig. 4C, the enhancer region lacked promoter activity when its orientation resembled the situation in the mim-1 gene (i.e. the 3′-end of the enhancer facing toward the luciferase-coding region). By contrast, in the opposite orientation the enhancer had significant promoter activity; moreover, this activity was Myb-inducible.
The finding that the mim-1 enhancer region is a dual promoter/enhancer element was reminiscent of the situation at the chicken lysozyme gene where an enhancer was shown to act as a LPS-inducible promoter for an RNA transcribed away from the body of the lysozyme gene (28). It was demonstrated that transcription through the enhancer was required for the subsequent LPS-induced remodeling of the enhancer chromatin. Because transcription of ChEST254k22 RNA is initiated on the 3′-side of the enhancer and proceeds through the enhancer we were interested to know whether Myb-induced remodeling of the nucleosomal architecture at the mim-1 enhancer was transcription dependent. To address this question, we used a clone of the HD11 cell-line (referred to as 10.4) constitutively expressing a v-MybREV/estrogen receptor fusion protein (10). The fusion protein is inactive in the absence of estrogen but is quickly activated upon addition of estrogen, leading to the transcription of several Myb-inducible genes (29–31). We confirmed that estrogen treatment of 10.4 cells induced the same changes in the MNase digestion pattern that were observed after doxycyclin treatment of HD11-E cells (Fig. 5A). Fig. 5B, shows that Myb-induced remodeling at the mim-1 enhancer chromatin could already be detected 2 h after addition of estrogen. In particular, the strong increase of the MNase sensitivity of the 3′-part of the enhancer (marked by an arrowhead) was clearly detectable after 2 h. By contrast, mim-1 mRNA was not detectable after 2 h of estrogen treatment but was strongly expressed after 12 h (Fig. 5C). This indicated that the Myb-induced remodeling of the enhancer chromatin preceded the activation of the gene.
FIGURE 5.
v-Myb-induced chromatin remodeling at the mim-1 enhancer is transcription dependent. A and B, micrococcal nuclease digestion of nuclei isolated from 10.4 cells grown for 12 or 2 h without or with estradiol or in the presence of DRB, as indicated below the panels. The experiments were carried out as described in the legend to Fig. 2B. The black and white arrowheads mark changes in the MNase digestion pattern induced by v-Myb. C, Northern blot analysis of mim-1 expression in 10.4 cells grown in the absence of estradiol or treated with 2 μm estradiol for 2 or 12 h.
Because the v-MybREV/estrogen receptor fusion protein already exists in 10.4 cells, its activation by estrogen does not require ongoing transcription; this permitted us to ask whether remodeling of the mim-1 enhancer chromatin by Myb also occurred in the absence of transcription. To address this issue we treated 10.4 cells for 2 h with estrogen in the presence of the transcriptional elongation inhibitor 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole (DRB). It has previously been shown that short term DRB treatment has no influence on RNA polymerase association and transcription factor binding (32). DRB treatment has also been used to demonstrate that remodeling of the enhancer/promoter element of the lysozyme gene is transcription dependent (28). The result of the MNase digestion experiment (Fig. 5B, third panel) clearly showed that DRB treatment suppressed the chromatin remodeling at the enhancer, indicating that remodeling of the enhancer by Myb is transcription dependent.
v-MybAMV Is Unable to Remodel the Nucleosomal Architecture of the mim-1 Enhancer
Because v-MybAMV activated the enhancer less efficiently than v-MybREV, we wished to know whether or not v-MybAMV was able to remodel the nucleosomal structure of the enhancer in similar manner as v-MybREV. We, therefore, used a cell line, designated as HD11-A, which expresses v-MybAMV upon induction with doxycyclin. Fig. 6A shows a side-by-side comparison of HD11-E and HD11-A cells to demonstrate that they express similar amounts of both versions of v-Myb. As expected, the mim-1 gene was not activated in HD11-A cells after treatment with doxycyclin, in contrast to HD11-E cells in which the gene was highly expressed (Fig. 6A). To exclude that v-MybAMV failed to activate the mim-1 gene because v-MybAMV was not recruited to the mim-1 enhancer, we performed a chromatin immunoprecipitation experiment. This experiment showed that there was no significant difference between the recruitment of v-MybREV and v-MybAMV to the enhancer (Fig. 6B). We then performed a MNase digestion experiment using nuclei isolated from HD11-A cells before and after doxycyclin treatment (Fig. 6C). In contrast to v-MybREV-expressing cells there were no changes in the digestion pattern upon induction with doxycyclin. This indicated that v-MybAMV, unlike v-MybREV, was not able to remodel the nucleosomal structure of the mim-1 enhancer. We also performed in vivo DMS footprinting experiments using HD11-A cells to see whether or not v-MybAMV was able to induce similar changes in binding site occupancy as v-MybREV. Fig. 6D shows that the DMS sensitivity at positions 456, 469 and 499 was increased much less by v-MybAMV than by v-MybREV. Finally, we assessed the ability of v-MybAMV to increase the promoter activity of the mim-1 enhancer region and found that v-MybAMV stimulated the promoter activity of the enhancer only weakly (Fig. 6E). This supports the notion that transcription initiated at the dual enhancer/promoter element is involved in the remodeling of the enhancer chromatin. ChEST254k22 RNA was also not induced by v-MybAMV in HD11-A cells (data not shown).
FIGURE 6.
Oncogenic amino acid substitutions abolish the ability of v-Myb to induce chromatin remodeling at the mim-1 enhancer. A, top panels show Western blots of HD11-A and HD11-E cells stained with antibodies against v-Myb and β-actin. The cells were grown in the presence or absence of doxycyclin. The bottom panel show a Northern blot of the same cells hybridized with probes specific for mim-1 and ribosomal protein S17 mRNAs. B, chromatin immunoprecipitation of HD11-E and HD11-A cells grown in the presence or absence of doxycyclin. Immunoprecipitation was carried out using Myb-specific antiserum (lane 1), non-immune serum (lane 2), or no antiserum (lane 3). Lane 4 show input controls. PCR reactions were carried out with primers specific for the mim-1 enhancer. C, micrococcal nuclease digestion of nuclei isolated from HD11-A cells grown in the absence or presence of doxycyclin. The experiment was carried out as described in the legend to Fig. 2B. The black bar on the right marks the position of the mim-1 enhancer. D, DMS footprinting analysis of HD11-E and HD11-A cells. The same region of the mim-1 enhancer was analyzed as in Fig. 3. E, promoter activity of the mim-1 enhancer region in the absence of Myb (white bar) or after co-transfection of expression vector for v-MybREV (black bar) or v-MybAMV (hatched bar). The reporter construct used is shown schematically at the top, and the experiment was carried out as described in Fig. 4C.
Amino Acid Substitutions in the Central Region of v-Myb Contribute to the Differential Activity of v-MybAMV and v-MybREV
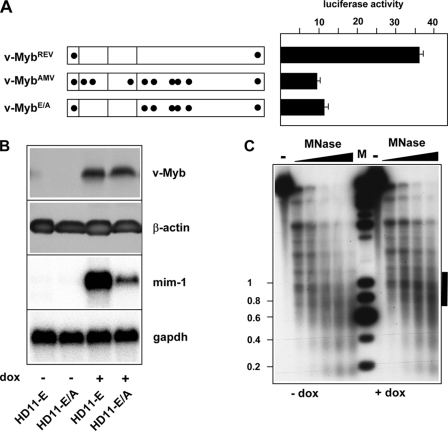
Next, we were interested to know which of the amino acid substitutions were responsible for the lack of remodeling activity of v-MybAMV. Previously, it was shown that reversion of the amino acid substitutions in the DNA binding domain of v-MybAMV was sufficient to enable the protein to activate the mim-1 gene (16). We therefore expected that a v-Myb construct in which these substitutions had been reverted (referred to as v-MybE/A) would stimulate the mim-1 enhancer as efficiently as v-MybREV. However, as shown in Fig. 7A, this was not the case. This suggested that the differential activities of v-MybAMV and v-MybREV were not only due to the differences in their DNA binding domains, but that the amino acid substitutions in the central region of v-Myb played a significant role. Consistent with the result of the reporter gene assay shown in Fig. 7A, we found that the level of mim-1 mRNA in HD11 cells expressing a doxycyclin-inducible version of v-MybE/A was very low (Fig. 7B).
FIGURE 7.
Amino acid substitutions in the C-terminal part of v-MybAMV contribute to the inability of v-MybAMV to induce nucleosomal remodeling at the mim-1 enhancer. A, v-Myb constructs are shown schematically on the left. Amino acid substitutions (relative to c-Myb) are marked by black dots. HD11 cells were transfected with expression vectors encoding the different v-Myb proteins, the luciferase reporter gene containing the wild-type mim-1 enhancer fused to the tk promoter and the β-galactosidase plasmid pCMVβ. Cells were harvested 24 h after transfection and analyzed for luciferase and β-galactosidase activity. The columns show the average luciferase activity normalized with respect to the activity of the co-transfected β-galactosidase plasmid. Thin lines show S.D. B, expression of v-Myb and mim-1 mRNA in HD11-E and HD11-E/A cells. The top two panels show Western blots stained with antibodies against v-Myb or β-actin. The bottom panels show a Northern blot hybridized sequentially with probes specific for mim-1 or gapdh mRNAs. C, micrococcal nuclease digestion of nuclei isolated from HD11-E/A cells grown in the absence or presence of doxycyclin. The experiment was carried out as described in the legend to Fig. 2A. The black bar on the right marks the position of the mim-1 enhancer.
v-MybE/A was almost unable to remodel the nucleosomal organization at the mim-1 enhancer, as there were only slight changes in the MNase digestion pattern shown in Fig. 7C. This suggested that the amino acid substitutions located near the central part of v-MybAMV played a major role for the inability of the protein to remodel the nucleosomal architecture at the mim-1 enhancer.
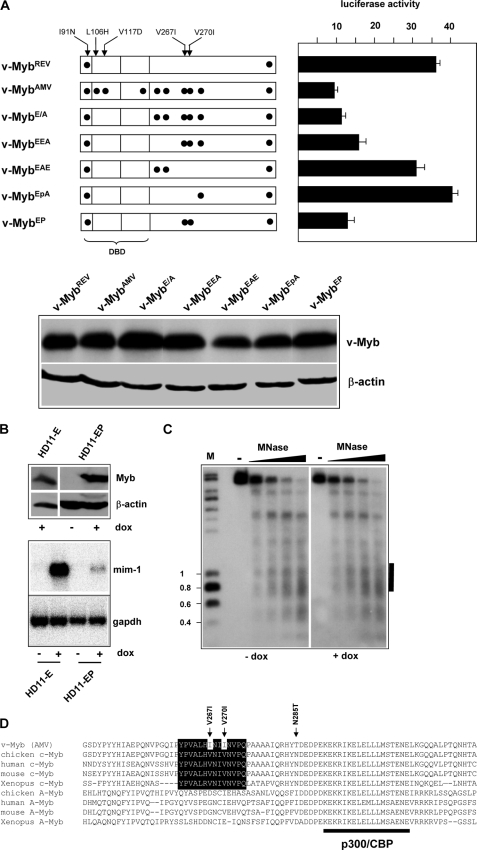
We were therefore interested to study the role of the amino acid substitutions in the central part of v-Myb in more detail. As illustrated in Fig. 8A, we generated various constructs carrying only subsets of these substitutions and initially analyzed their ability to stimulate mim-1 enhancer activity in reporter gene experiments. The most pronounced effect was observed in case of the V267I and V270I double mutant (referred to as v-MybEP), which reduced the activity of the protein at the enhancer to virtually the same level as v-MybAMV. To see if the V267I and V270I double mutant protein had also lost the ability to remodel the nucleosomal architecture of the enhancer we generated HD11 cells stably expressing a doxycyclin-inducible version of v-MybEP. Fig. 8B shows that v-MybEP was expressed in these cells at similar levels as v-MybREV in the presence of doxycyclin but that it only extremely weakly activated the endogenous mim-1 gene. This confirmed that the change of valines 267 and 270 to isoleucines had a major impact on the activity of the protein. Fig. 8C illustrates the result of a MNase digestion experiment of HD11-EP cells. It is evident that v-MybEP had also lost the ability to remodel the chromatin at the mim-1 enhancer. We concluded that both valine to isoleucine substitutions were sufficient to disrupt the ability of v-MybREV to remodel the nucleosomal structure at the enhancer.
FIGURE 8.
Amino acid substitutions V267I and V270I abolish the ability of Myb to remodel the nucleosomal architecture of the mim-1 enhancer. A, v-Myb constructs are shown schematically on the left. Amino acid substitutions (relative to c-Myb) are marked by black dots. HD11 cells were transfected with expression vectors encoding the different v-Myb proteins, the luciferase reporter gene containing the wild-type mim-1 enhancer fused to the tk promoter and the β-galactosidase plasmid pCMVβ. Cells were harvested 24 h after transfection and analyzed for luciferase and β-galactosidase activity. Columns show the average luciferase activity normalized with respect to the activity of the co-transfected β-galactosidase plasmid. Thin lines show S.D. The bottom shows a Western blot of HD11 cells transfected with different v-Myb constructs to demonstrate comparable expression of the different proteins. B, expression of v-Myb and mim-1 mRNA in HD11-E and HD11-EP cells. The top two panels show Western blots stained with antibodies against v-Myb or β-actin. The bottom panels show a Northern blot hybridized sequentially with probes specific for mim-1 or gapdh mRNAs. C, micrococcal nuclease digestion of nuclei isolated from HD11-EP cells grown in the absence or presence of doxycyclin. The experiment was carried out as described in the legend to Fig. 2B. The black bar on the right marks the position of the mim-1 enhancer. D, amino acid sequence comparison of the region around the p300/CBP binding site of different c-Myb and A-Myb proteins. v-Myb specific amino acid substitutions are marked, and the conserved patch around the V267I and V270I substitutions is highlighted.
It was surprising that two conservative amino acid substitutions had such a pronounced effect whereas other less conservative substitutions had virtually no effects. Fig. 8D compares the amino acid sequences around the transactivation domains of different Myb family members. Residues 290–308 have been identified as the p300/CBP interaction surface of c-Myb (33, 34). It is therefore not surprising that this region is also well conserved in A-Myb, which also interacts with p300 (35). The amino acids around valine 267 and 270 appear to form a conserved patch, which is distinct from the p300/CBP binding region as it is not conserved in A-Myb. We observed that the valine to isoleucine substitution at positions 267 and 270 do not affect the ability of the protein to bind to p300 in vitro or to cooperate with p300 in vivo (data not shown), further supporting the notion that the conserved patch around both valines is not part of the p300/CBP binding site. It is therefore likely that this region forms the binding site for another, as yet unknown interaction partner whose activity is crucial for the remodeling of the mim-1 enhancer.
DISCUSSION
c-Myb plays a key role as a transcription factor regulating the proliferation and differentiation of the immature cells of most hematopoietic lineages. A substantial number of c-Myb-regulated genes have been identified to date; however, the mechanisms by which Myb controls their expression have been explored in detail only in a few cases. Because its discovery as the first direct Myb target gene (7) the chicken mim-1 gene has been an interesting model to address how Myb activates gene expression in a lineage-specific manner. Furthermore, the amino acid substitutions that have made v-myb a very powerful oncogene have disrupted the ability of v-Myb to activate the mim-1 gene. The mim-1 gene is therefore also an attractive model to explore the functional consequences of the oncogenic amino acid substitutions of v-Myb.
We have addressed the role of Myb at a cell type-specific mim-1 enhancer which was recently identified in our laboratory (24). Our results confirm that the mim-1 enhancer is a bona fide target of Myb. Together with previous work, which has shown that Myb directly acts on the mim-1 promoter (7, 22, 36), this demonstrates that the activation of the mim-1 gene by Myb is orchestrated by two distinct cis-acting elements. This, in turn, might explain the extremely strong stimulatory effect of Myb on mim-1 expression. Our work also provides novel insight into the events that lead to the activation of the mim-1 enhancer. We discovered that Myb induces extensive chromatin remodeling of the mim-1 enhancer region. This is the first direct evidence that Myb induces alterations of the nucleosomal organization at a physiological target region. The Myb-induced remodeling of the enhancer takes place before transcription of the mim-1 gene is detectable. Furthermore, we have demonstrated that Myb-induced nucleosomal remodeling is accompanied by increased occupancy at functionally relevant C/EBP and Ets binding sites. We have recently shown that C/EBPβ induces the initial opening of the chromatin at the mim-1 enhancer in a step that occurs prior to the actual transcription of the gene and is independent of Myb (25). Taken together with the work reported here this suggests a stepwise mechanism for activation of the enhancer in which C/EBPβ is responsible for the initial opening of the chromatin structure and Myb induces subsequent remodeling events which lead to additional binding of transcription factors and, finally, to the transcription of the mim-1 gene.
A second interesting aspect of our work is the finding that the Myb-induced nucleosomal remodeling at the mim-1 enhancer is transcription-dependent. We have found that the enhancer region also harbors a promoter whose activity is stimulated by Myb and which appears to direct transcription of a spliced but presumably non-coding RNA through the enhancer into the intergenic region upstream of the mim-1 gene. Together, these observations raise the interesting possibility that transcription through the enhancer region facilitates chromatin remodeling by Myb. This is consistent with the observation that v-MybAMV, which only weakly stimulates the promoter activity of the enhancer region, also does not induce chromatin remodeling. The presence of a dual enhancer/promoter element in the mim-1 upstream region is reminiscent of the situation at the −1.9-kb enhancer of the chicken lysozyme gene: Lefevre et al. (28) showed that a LPS-inducible promoter resides in the enhancer region, which initiates the transcription of a non-coding RNA. Transcription through the −1.9 kb lysozyme enhancer was shown to trigger displacement of CTCF from a binding site and facilitate subsequent chromatin remodeling of the enhancer. Our studies, together with those of Lefevre et al. (28), identify a mechanism of transcription-dependent alteration of the chromatin architecture as an important step in the activation of an enhancer. It is thought that the majority of the transcriptional input of higher eukaryotes does not encode for proteins (37). The ability of intergenic transcription to alter the regulatory properties of cis-acting elements, as shown here for the mim-1 enhancer, might therefore provide a general mechanism of gene regulation. We do not know whether the spliced RNA derived from the mim-1 upstream region itself has a function. Long (i.e. longer than 200 nucleotides) non-coding RNAs have been implicated in different processes, including epigenetic regulation or the modulation of protein function (37). Whether the spliced RNA transcribed from the mim-1 upstream region plays such a role must be addressed by future work.
Finally, our work also provides new insight into the consequences of the amino acid substitutions of the v-Myb protein of AMV. These substitutions are thought to have arisen during the repeated passage of the virus in leukemic chickens, which selected for its increased oncogenicity (15). Our work shows that v-MybAMV, as a result of these substitutions, is unable to induce nucleosomal remodeling of the mim-1 enhancer. The amino acid substitutions have also abolished the ability to stimulate transcription from the dual enhancer/promoter element, consistent with the notion that transcription initiated in the enhancer region facilitates Myb-induced chromatin remodeling of the enhancer. Mo et al. (21) found that v-MybAMV, unlike c-Myb, was unable to bind to the N-terminal histone H3 tail and that reversion of the amino acid substitutions in the DNA binding domain was sufficient to restore histone H3 binding activity. By contrast, we showed (see Fig. 7) that reversion of these amino acid substitutions was not sufficient to allow remodeling of the nucleosomal structure at the mim-1 enhancer, indicating that the lack of histone H3 binding activity is not the main reason for the inability of v-MybAMV to induce nucleosomal remodeling. Our data show that the replacement of valine 267 and 270 by isoleucine has dramatic effects on the activity of the protein as they completely abolish the ability to induce nucleosomal remodeling at the mim-1 enhancer. This is particularly striking as both amino acid substitutions are conservative; valine and isoleucine have hydrophobic side chains that differ only by one methyl group. By comparing the amino acid sequences of different Myb family members we have found that these substitutions are located in a conserved patch close to, but distinct from the binding site for p300/CBP. Unfortunately, no information is available on the structure of Myb in this region. If the amino acids in this conserved patch are exposed on the surface of Myb, they might form a hydrophobic interaction site for another protein whose activity is crucial for nucleosomal remodeling. However, it is also possible that these residues are buried inside of the protein and that the additional methyl groups induce structural alterations in another part of v-Myb. It will be interesting to address these possibilities in future work.
The mim-1 gene is thought to be part of a differentiation program induced by c-Myb during granulocytic differentiation. By suffering the point mutations v-Myb was apparently selected for its failure to activate this differentiation program, thereby raising its oncogenic potential. This is consistent with the idea that v-Myb regulates only a subset of genes regulated by c-Myb (14). Microarray analyses of human cells transfected with versions of Myb carrying subsets of the oncogenic amino acid replacements have revealed a group of c-Myb-regulated genes whose activation was dependent on the mutant status of the transactivation region similar to the mim-1 gene (14). Possibly, these genes represent a class of c-Myb target genes whose activation requires major changes in the nucleosomal architecture, as exemplified by the mim-1 enhancer.
In summary, our work shows for the first time that Myb induces alterations of the nucleosomal architecture at a physiological target region and implicates a conserved patch of amino acid sequences located close to but distinct from the p300/CBP binding region of Myb in nucleosomal remodeling. In addition, our results provide novel insight into the stepwise activation of the mim-1 enhancer by Myb and demonstrate for the first time that Myb is involved in non-coding RNA transcription at a dual enhancer/promoter element.
Acknowledgments
We thank B. Berkenfeld and J. Hülskötter for technical assistance and S. Melnik and C. Bonifer for advice on in vivo footprinting.
This work was supported in part by the Deutsche Forschungsgemeinschaft and by fellowships from the Graduate School of Chemistry (GSC-MS) (to O. C. and A. P.).
- AMV
- avian myeloblastosis virus
- MNase
- micrococcal nuclease
- LPS
- lipopolysaccharide
- DRB
- 5,6-dichloro-1- β-d-ribofuranosylbenzimidazole.
REFERENCES
- 1.Lipsick J. S., Wang D. M. (1999) Oncogene 18, 3047–3055 [DOI] [PubMed] [Google Scholar]
- 2.Oh I. H., Reddy E. P. (1999) Oncogene 18, 3017–3033 [DOI] [PubMed] [Google Scholar]
- 3.Klempnauer K. H., Ramsay G., Bishop J. M., Moscovici M. G., Moscovici C., McGrath J. P., Levinson A. D. (1983) Cell 33, 345–355 [DOI] [PubMed] [Google Scholar]
- 4.Ramsay R. G., Gonda T. J. (2008) Nat. Rev. Cancer 8, 523–534 [DOI] [PubMed] [Google Scholar]
- 5.Biedenkapp H., Borgmeyer U., Sippel A. E., Klempnauer K. H. (1988) Nature 335, 835–837 [DOI] [PubMed] [Google Scholar]
- 6.Klempnauer K. H., Arnold H., Biedenkapp H. (1989) Genes Dev. 3, 1582–1589 [DOI] [PubMed] [Google Scholar]
- 7.Ness S. A., Marknell A., Graf T. (1989) Cell 59, 1115–1125 [DOI] [PubMed] [Google Scholar]
- 8.Weston K., Bishop J. M. (1989) Cell 58, 85–93 [DOI] [PubMed] [Google Scholar]
- 9.Ibanez C. E., Lipsick J. S. (1990) Mol. Cell. Biol. 10, 2285–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burk O., Klempnauer K. H. (1991) EMBO J. 10, 3713–3719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang G., White J. R., Argent-Katwala M. J., Allinson C. G., Weston K. (2005) Oncogene 24, 1375–1384 [DOI] [PubMed] [Google Scholar]
- 12.Chen J., Kremer C. S., Bender T. P. (2006) Oncogene 25, 2758–2772 [DOI] [PubMed] [Google Scholar]
- 13.Rushton J. J., Davis L. M., Lei W., Mo X., Leutz A., Ness S. A. (2003) Oncogene 22, 308–313 [DOI] [PubMed] [Google Scholar]
- 14.Liu F., Lei W., O'Rourke J. P., Ness S. A. (2006) Oncogene 25, 795–805 [DOI] [PubMed] [Google Scholar]
- 15.Dini P. W., Eltman J. T., Lipsick J. S. (1995) J. Virol. 69, 2515–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Introna M., Golay J., Frampton J., Nakano T., Ness S. A., Graf T. (1990) Cell 63, 1289–1297 [DOI] [PubMed] [Google Scholar]
- 17.Brendeford E. M., Myrset A. H., Hegvold A. B., Lundin M., Gabrielsen O. S. (1997) J. Biol. Chem. 272, 4436–4443 [DOI] [PubMed] [Google Scholar]
- 18.Ivanova O., Brass D., Klempnauer K. H. (2007) Nucleic Acids Res. 35, 7237–7247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leverson J. D., Ness S. A. (1998) Mol. Cell 1, 203–211 [DOI] [PubMed] [Google Scholar]
- 20.Tahirov T. H., Sato K., Ichikawa-Iwata E., Sasaki M., Inoue-Bungo T., Shiina M., Kimura K., Takata S., Fujikawa A., Morii H., Kumasaka T., Yamamoto M., Ishii S., Ogata K. (2002) Cell 108, 57–70 [DOI] [PubMed] [Google Scholar]
- 21.Mo X., Kowenz-Leutz E., Laumonnier Y., Xu H., Leutz A. (2005) Genes Dev. 19, 2447–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burk O., Mink S., Ringwald M., Klempnauer K. H. (1993) EMBO J. 12, 2027–2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ness S. A., Kowenz-Leutz E., Casini T., Graf T., Leutz A. (1993) Genes Dev. 7, 749–759 [DOI] [PubMed] [Google Scholar]
- 24.Chayka O., Kintscher J., Braas D., Klempnauer K. H. (2005) Mol. Cell. Biol. 25, 499–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plachetka A., Chayka O., Wilczek C., Melnik S., Bonifer C., Klempnauer K. H. (2008) Mol. Cell. Biol. 28, 2102–2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozak M. (1986) Cell 44, 283–292 [DOI] [PubMed] [Google Scholar]
- 27.Chang Y. F., Imam J. S., Wilkinson M. F. (2007) Annu. Rev. Biochem. 76, 51–74 [DOI] [PubMed] [Google Scholar]
- 28.Lefevre P., Witham J., Lacroix C. E., Cockerill P. N., Bonifer C. (2008) Mol. Cell 32, 129–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burk O., Worpenberg S., Haenig B., Klempnauer K. H. (1997) EMBO J. 16, 1371–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Worpenberg S., Burk O., Klempnauer K. H. (1997) Oncogene 15, 213–221 [DOI] [PubMed] [Google Scholar]
- 31.Schlichter U., Burk O., Worpenberg S., Klempnauer K. H. (2001) Oncogene 20, 231–239 [DOI] [PubMed] [Google Scholar]
- 32.Mitchell J. A., Fraser P. (2008) Genes Dev. 22, 20–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zor T., De Guzman R. N., Dyson H. J., Wright P. E. (2004) J. Mol. Biol. 337, 521–534 [DOI] [PubMed] [Google Scholar]
- 34.De Guzman R. N., Goto N. K., Dyson H. J., Wright P. E. (2006) J. Mol. Biol. 355, 1005–1013 [DOI] [PubMed] [Google Scholar]
- 35.Facchinetti V., Loffarelli L., Schreek S., Oelgeschläger M., Lüscher B., Introna M., Golay J. (1997) Biochem. J. 324, 729–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mink S., Kerber U., Klempnauer K. H. (1996) Mol. Cell. Biol. 16, 1316–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattick J. S. (2005) Science 309, 1527–1528 [DOI] [PubMed] [Google Scholar]