Abstract
In cystic fibrosis (CF), dysfunction of the cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channel disrupts epithelial ion transport and perturbs the regulation of intracellular pH (pHi). CFTR modulates pHi through its role as an ion channel and by regulating transport proteins. However, it is unknown how CFTR senses pHi. Here, we investigate the direct effects of pHi on recombinant CFTR using excised membrane patches. By altering channel gating, acidic pHi increased the open probability (Po) of wild-type CFTR, whereas alkaline pHi decreased Po and inhibited Cl− flow through the channel. Acidic pHi potentiated the MgATP dependence of wild-type CFTR by increasing MgATP affinity and enhancing channel activity, whereas alkaline pHi inhibited the MgATP dependence of wild-type CFTR by decreasing channel activity. Because these data suggest that pHi modulates the interaction of MgATP with the nucleotide-binding domains (NBDs) of CFTR, we examined the pHi dependence of site-directed mutations in the two ATP-binding sites of CFTR that are located at the NBD1:NBD2 dimer interface (site 1: K464A-, D572N-, and G1349D-CFTR; site 2: G551D-, K1250M-, and D1370N-CFTR). Site 2 mutants, but not site 1 mutants, perturbed both potentiation by acidic pHi and inhibition by alkaline pHi, suggesting that site 2 is a critical determinant of the pHi sensitivity of CFTR. The effects of pHi also suggest that site 2 might employ substrate-assisted catalysis to ensure that ATP hydrolysis follows NBD dimerization. We conclude that the CFTR Cl− channel senses directly pHi. The direct regulation of CFTR by pHi has important implications for the regulation of epithelial ion transport.
Introduction
The ATP-binding cassette (ABC)3 transporter cystic fibrosis transmembrane conductance regulator (CFTR) (1) is a multifunctional protein best known as a regulated Cl− channel (2). CFTR is assembled from five domains: two membrane-spanning domains (MSDs) that form an anion-selective pore, two nucleotide-binding domains (NBDs) that bind and hydrolyze ATP to control channel gating, and a unique regulatory domain (RD), whose phosphorylation by PKA is critical for CFTR activation (2, 3). CFTR is principally expressed in the apical membrane of epithelia throughout the body where it plays a fundamental role in fluid and electrolyte movements (4). Malfunction of CFTR causes the common genetic disease cystic fibrosis (CF) (4).
Previous studies demonstrate that the regulation of intracellular pH (pHi) is defective in CF epithelial cells (e.g. Ref. 5). They also reveal that expression of recombinant CFTR in heterologous cells modulates pHi (e.g. Ref. 6). Analysis of the literature suggests that CFTR modulates pHi in three main ways. First, CFTR itself directly transports HCO3− ions with a modest permeability (PHCO3−/PCl− ∼0.26 (7)). Second, CFTR regulates the Na+/H+ exchanger isoform 3 (NHE3), which contributes to Na+-dependent HCO3− reabsorption in pancreatic duct epithelia. CFTR stabilizes NHE3 expression at the cell surface and inhibits NHE3 activity by a cAMP-dependent mechanism when pancreatic HCO3− secretion is stimulated (8). Of note, the regulation of NHE3 by CFTR involves the association of CFTR and NHE3 with the scaffolding protein EBP50 to form a macromolecular complex (8). Third, CFTR regulates the Cl−/HCO3− (anion) exchanger (AE), which plays a central role in pancreatic HCO3− secretion. CFTR regulation of AE requires the cell surface expression and cAMP-dependent phosphorylation of CFTR, but not its transport of anions (6). Interestingly, Ko et al. (9) demonstrated that CFTR and members of the SLC26 family of AEs coordinate their activities through the interaction of the, phosphorylated RD of CFTR with the STAS (sulfate transporter and antisigma-factor antagonist) domain of SLC26 transporters. Thus, CFTR modulates pHi through its roles as an ion channel and regulator of transport proteins.
A key unresolved question is how CFTR senses changes in pHi. As described above, CFTR might detect pHi changes indirectly through its interactions with NHE3 and SLC26 transporters. Consistent with this idea, Reddy et al. (10) demonstrated that pHi modulates indirectly the CFTR Cl− conductance of sweat duct epithelia by altering the enzymatic activities of the protein kinases and phosphatases that control the phosphorylation status of CFTR. However, the ATPase activity of some ABC transporters (e.g. the transporter associated with antigen processing (TAP)) is pHi-dependent (11), while the ATPase activity of an NBD1-RD-CFTR fusion protein is optimal at neutral pH (12). These data raise the possibility that pHi might regulate directly CFTR activity. To test this hypothesis, we used excised inside-out membrane patches from cells expressing recombinant wild-type human CFTR. When we observed that pHi has multiple effects on the single-channel activity of CFTR, we used CFTR constructs containing site-directed mutations to explore the molecular mechanisms of CFTR regulation by pHi.
EXPERIMENTAL PROCEDURES
Cells and CFTR Expression
In this study we used mammalian cells heterologously expressing CFTR constructs. Where available, we used cells stably expressing CFTR constructs. These included (i) mouse mammary epithelial cells (C127 cells) expressing wild-type human CFTR, the CFTR variant ΔR-S660A (13) or the CF mutant G1349D (14), (ii) Fischer rat thyroid epithelial cells expressing the CF mutant G551D (15), and (iii) NIH-3T3 cells expressing the CFTR construct K1250M (16). To study the CFTR variants K464A, D572N, and D1370N, we employed the vaccinia virus/bacteriophage T7 hybrid expression system to transiently express CFTR variants in HeLa cells as described previously (17, 18). As a control, we verified that the single-channel behavior of wild-type human CFTR in different mammalian cells was equivalent (for further information, see supplemental “Results” and Figs. 1 and 2).
HeLa cells were purchased from the American Type Culture Collection (Manassas, VA). C127 cells expressing wild-type CFTR were a generous gift of Dr. C. R. O'Riordan (Genzyme, Framingham, MA), whereas Fischer rat thyroid cells were a generous gift of Drs. L. J. V. Galietta and O. Zegarra-Moran (Istituto Giannina Gaslini, Genova, Italy). Other cells and the vaccinia virus expression plasmid pTM1-CFTR4-expressing CFTR mutants were generous gifts of Professor M. J. Welsh (University of Iowa, Iowa City, IA). Cells were cultured and used as described (14–16, 19).
Electrophysiology
CFTR Cl− channels were recorded in excised inside-out membrane patches using an Axopatch 200A patch-clamp amplifier and pCLAMP software (both from MDS Analytical Technologies, Union City, CA) as described (20).
The pipette (extracellular) solution contained 140 mm N-methyl-d-glucamine, 140 mm aspartic acid, 5 mm CaCl2, 2 mm MgSO4, and 10 mm TES, pH 7.3, with Tris ([Cl−], 10 mm). The control bath (intracellular) solution contained 140 mm N-methyl-d-glucamine, 3 mm MgCl2, 1 mm CsEGTA, 5 mm Tris, and 5 mm Bis-Tris, pH 7.3, with HCl ([Cl−], 147 mm; free [Ca2+], <10−8 m) and was maintained at 37 °C; voltage was −50 mV. To ensure that the Cl− concentration of different intracellular solutions was identical, all solutions were first titrated to pH 7.3 with HCl before we added either H2SO4 to titrate pH to acidic values or Tris/NaOH to titrate to alkaline values. By using Bis-Tris (pKa 6.5) and Tris (Trizma® base; pKa 8.1) instead of TES (pKa 7.5) as the biological buffer, we buffered intracellular solutions over a wide range of pHi values (pHi 5.8–8.8).
After excision of inside-out membrane patches, we added PKA (75 nm) and ATP (1 mm) to the intracellular solution within 5 min of patch excision to activate CFTR. Once CFTR Cl− channels were fully activated, we varied the pHi value and/or [ATP] of the intracellular solution depending on the experimental protocol. Because MgATP controls CFTR channel gating (e.g. Refs. 21 and 22), we maintained the [MgATP] constant in different pHi solutions by adding different amounts of Na2ATP (for further information, see supplemental “Experimental Procedures” and Table 1). To minimize the rundown of CFTR Cl− channels in excised membrane patches, we added PKA to all intracellular solutions. However, for studies of some CFTR constructs and wild-type CFTR in the absence of Mg2+ ions, it was also necessary to increase the [ATP] to 1 or 3 mm, respectively, to sustain channel activity.
To investigate how pHi modulates CFTR Cl− currents, we used membrane patches containing large numbers of active channels. For all other studies, we used membrane patches containing ≤5 active channels. We determined the number of channels in a membrane patch from the maximum number of simultaneous channel openings observed during the course of an experiment. To minimize errors when counting the number of active channels, we employed the strategies described by Cai et al. (14). For further information, see supplemental “Experimental Procedures.” In all experiments, intracellular solutions titrated to different pHi values were compared with the average of pre- and postintervention control periods titrated to pHi 7.3, which contained the same concentrations of MgATP (or Mg2+-free ATP) and PKA.
To investigate the effects of voltage on the CFTR Cl− channel at different pHi, we bathed membrane patches in symmetrical Cl−-rich solutions (extracellular pH (pHe) 7.3; pHi 7.3, 8.3, 8.8, or 6.3). From a holding voltage of −50 mV, we stepped voltage from −120 to +80 mV in 20-mV increments of 30 s. At each test voltage, we measured the single-channel current amplitude (i) and open probability (Po) of CFTR (see below). We calculated the OH− ion concentration ([OH−]) of different intracellular solutions directly from pHi values (pHi 7.3, [OH−] = 0.2 μm; pHi 8.3, [OH−] = 2 μm; pHi 8.8, [OH−] = 6.3 μm).
CFTR Cl− currents were initially recorded on digital audiotape using a digital tape recorder (Biologic Scientific Instruments, model DTR-1204; Intracel Ltd., Royston, UK) at a bandwidth of 10 kHz. On playback, records were filtered with an eight-pole Bessel filter (model 902LPF2; Frequency Devices, Inc., Ottawa, IL) at a corner frequency of 500 Hz and acquired using a Digidata 1200 interface (MDS Analytical Technologies) and pCLAMP at sampling rates of 2.5 kHz (macroscopic currents) or 5 kHz (single-channel currents). For the purpose of illustration, current records were filtered at 500 Hz and digitized at 1 kHz.
To analyze the effects of pHi on CFTR Cl− currents, we determined the average CFTR Cl− current (ICFTR) at a specific pHi by averaging all the data points collected at that pHi and subtracting basal currents recorded in the absence of ATP and PKA. To plot the relationship between pHi or [Hi+], and CFTR Cl− current, current values at different pHi or [Hi+] were expressed as a percentage of the control CFTR Cl− current recorded at pHi 7.3. To measure i, Gaussian distributions were fit to current amplitude histograms.
For NPo, Po, and burst analyses, lists of open and closed times were created using a half-amplitude crossing criterion for event detection as described under supplemental “Experimental Procedures.” Burst analysis was performed as described by Carson et al. (16) using membrane patches that contained a single active channel and a tc (the time that separates interburst closures from intraburst closures) determined from analyses of closed time histograms (supplemental Table 2). The mean interburst interval (IBI or TIBI) was calculated using Equation 1,
where Tb = (mean burst duration) × (open probability within a burst). Mean burst duration (MBD or TMBD) and open probability within a burst (Po(burst)) were determined directly from experimental data using pCLAMP software. Po was calculated from open and closed times as described (23). However, for G551D and G1349D, because the number of active channels in a membrane patch was unknown, we measured NPo instead of Po.
To calculated the apparent voltage-dependent dissociation constant (Kd) for OH− ion inhibition of CFTR and estimate the location of the binding site for OH− ions within the CFTR pore, we employed the methods described by Sheppard and Robinson (20). For further information, see supplemental “Experimental Procedures.”
Reagents
With the exception of PKA purified from bovine heart (Promega, Southampton, UK), chemicals were purchased from Sigma-Aldrich (Gillingham, UK). Stock solutions of ATP were prepared immediately before each experiment. When studying CFTR channel gating at ATP = 10 mm, we added MgSO4 (7 mm) to the intracellular solution. For nominally Mg2+-free intracellular solutions, we omitted MgCl2 (3 mm) from the intracellular solution, titrated to pH 7.3 with HCl, and added EDTA (1 mm) to buffer free [Mg2+] to <6 nm (22) before adjusting pHi to 6.3 with H2SO4 and to pH 7.3 and 8.3 with Tris.
Statistics
Results are expressed as means ± S.E. of n observations. To compare sets of data, we used Student's t test. Differences were considered statistically significant when p < 0.05.
RESULTS
Multiple Effects of pHi on CFTR Activity
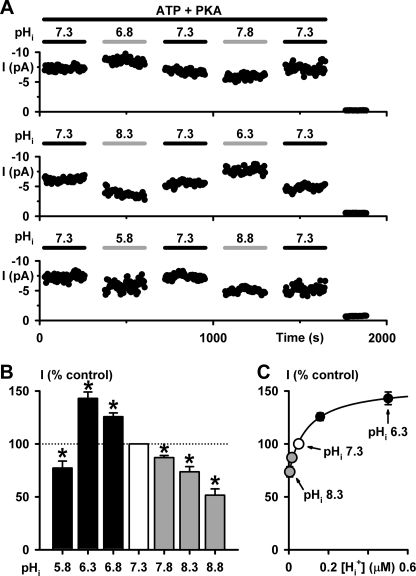
To investigate whether pHi regulates directly the CFTR Cl− channel, we studied CFTR Cl− currents in membrane patches excised from C127 cells expressing wild-type human CFTR. After CFTR activation by PKA-dependent phosphorylation at pHi 7.3, we varied pHi over the range pHi 5.8 to 8.8 and recorded CFTR Cl− currents while keeping the MgATP and PKA concentrations constant (for further information, see supplemental “Experimental Procedures” and Table 1. Fig. 1A demonstrates that changing pHi altered the magnitude of CFTR Cl− current in a readily reversible manner. At acidic pHi, CFTR Cl− currents were enhanced robustly at pHi 6.8 and 6.3, but decreased at pHi 5.8 (Fig. 1B). By contrast, at alkaline pHi, CFTR Cl− currents decreased steadily (Fig. 1B). To examine the pHi sensitivity of CFTR, we explored the relationship between CFTR Cl− current and the proton concentration ([H+]) of pHi solutions. Over the pHi range pHi 6.3–8.3, the data were best fit by a hyperbolic function with a half-maximal stimulatory concentration (Ks) at pHi 7.06 (Fig. 1C). We interpret these data to suggest that CFTR activity is regulated by pHi and that this regulation involves the protonation of CFTR.
FIGURE 1.
pHi modulates CFTR Cl− current. A, time courses of wild-type CFTR Cl− current in excised inside-out membrane patches. In this and subsequent figures, unless otherwise indicated, ATP (0.3 mm) and PKA (75 nm) were continuously present in intracellular solutions, which were adjusted to the indicated pHi; voltage was −50 mV, and there was a large Cl− concentration gradient across the membrane ([Cl−]int, 147 mm; [Cl−]ext, 10 mm). For the purpose of illustration, time courses have been inverted, and upward deflections represent inward currents. B, effects of pHi on CFTR Cl− currents. Data are means ± S.E. (n = 6) expressed as a percentage of the control current at pHi 7.3. The asterisks indicate values that are significantly different from the control (p < 0.05). C, relationship between intracellular proton concentration ([Hi+]) and CFTR activity. Data from B are plotted versus [Hi+]. The continuous line is the fit of a hyperbolic function to the mean data (y = y0 + ax/(b + x), where y0 = 70.9, a = 84.7, and b = 8.8 × 10−8; r = 0.99). Error bars are smaller than symbol size except where shown.
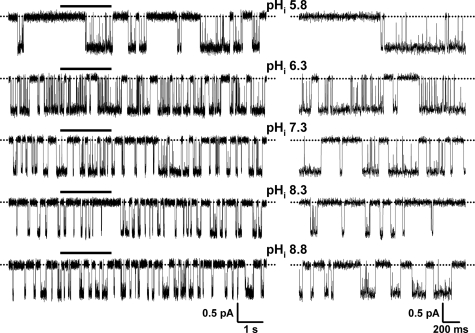
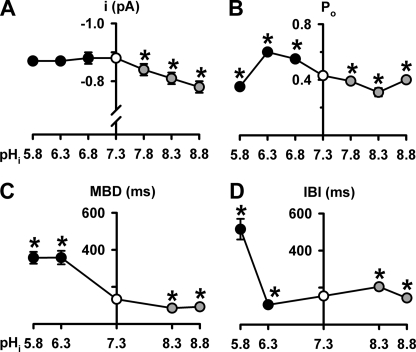
In principle, pHi might alter CFTR Cl− current in one or more of three ways: (i) by regulating the number of active channels (N), (ii) by modulating current flow through open channels, and (iii) by controlling channel gating and, hence, Po. To understand how pHi regulates CFTR, we investigated the effects of pHi on the single-channel activity of CFTR. Fig. 2 shows representative recordings of a single wild-type CFTR Cl− channel at different pHi. Visual inspection of these records suggests that varying pHi had striking effects on CFTR channel gating, little effect on single-channel current amplitude (i), and none on N. However, when we measured i, we found that i was unaltered at acidic pHi but diminished progressively at alkaline pHi (Fig. 3A), suggesting that OH− ions inhibit the CFTR Cl− channel.
FIGURE 2.
The single channel activity of wild-type CFTR at different pHi. Representative recordings show the activity of a single wild-type CFTR Cl− channel at different pHi. Dotted lines indicate where the channel is closed, and downward deflections correspond to channel openings. The 2-s portions indicated by bars are shown on an expanded time scale to the right of each 10-s recording.
FIGURE 3.
pHi alters the single-channel activity of CFTR. A–D, single channel current amplitude (i), open probability (Po), mean burst duration (MBD), and interburst interval (IBI) of wild-type CFTR at different pHi values. Data are means ± S.E. (n = 4–14). The asterisks indicate values that are significantly different from the control value (p < 0.05). Note the break in the ordinate in A.
The pattern of gating of wild-type CFTR at pHi 7.3 is characterized by bursts of channel openings interrupted by brief flickery closures and separated by longer closures between bursts (e.g. Ref. 24 and Fig. 2, third trace from the top). To quantify the pHi dependence of channel gating, we measured Po and performed an analysis of bursts. Over the pHi range pHi 6.3–8.3, Po decreased steadily as pHi increased (Fig. 3B). However, between pHi 6.3 and 5.8, Po fell sharply, whereas between pHi 8.3 and 8.8, it rose, returning to a value similar to that of pHi 7.8 (Fig. 3B). At acidic pHi, MBD increased dramatically, whereas at alkaline pHi, MBD decreased (Fig. 3C). By contrast, Fig. 3D demonstrates that the relationship between pHi and IBI is complex. At acidic pHi, IBI decreased at pHi 6.3, but increased markedly at pHi 5.8, whereas at alkaline pHi, IBI increased at pHi 8.3, but was unchanged at pHi 8.8 (Fig. 3D). Thus, pHi has intricate effects on CFTR channel gating. However, the effects of pHi on the single-channel behavior of CFTR account for the pHi dependence of CFTR Cl− currents (supplemental Table 3). Based on the control experiments described under supplemental “Results”, we suggest that pHi regulates directly the CFTR Cl− channel.
OH− Ions Occlude the CFTR Pore
Fig. 3A demonstrates that the i of CFTR diminishes progressively with alkalinization of the intracellular solution, suggesting that OH− ions might inhibit the CFTR Cl− channel by acting as an open-channel blocker (25). To test this hypothesis, we investigated the effects of membrane voltage on the i of wild-type CFTR at alkaline pHi. We reasoned that negative voltages would drive OH− ions from the intracellular solution into the CFTR Cl− channel where they might bind, occlude the channel pore, and block Cl− permeation. Conversely, positive voltages would drive Cl− ions into the CFTR Cl− channel from the extracellular solution, flushing blocking OH− ions from the channel pore and relieving inhibition.
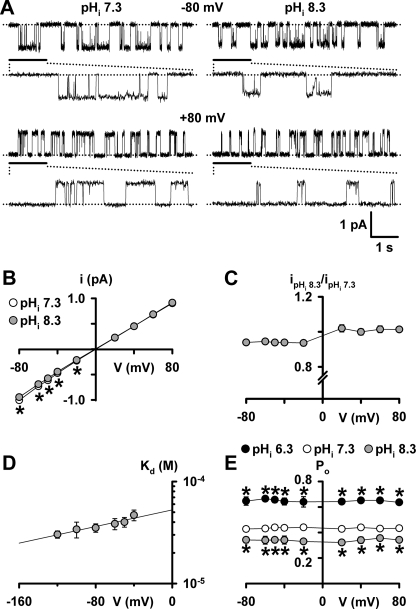
Fig. 4A shows representative recordings of a single CFTR Cl− channel at −80 and +80 mV, Fig. 4B shows the single-channel current-voltage (I-V) relationship of wild-type CFTR at pHi 7.3 and 8.3, and Fig. 4C shows the voltage dependence of the fraction of i inhibited at pHi 8.3. Fig. 4, A–C, demonstrates that at negative voltages there was a small, but significant, decrease in the i of wild-type CFTR at pHi 8.3 compared with that at pHi 7.3. By contrast, at positive voltages the inhibition of i was completely relieved (Fig. 4, A–C) (similar results were observed at pHi 8.8 over the voltage range −80 to +80 mV (data not shown)). Block of i at negative voltages, but relief at positive voltages suggests that OH− ions occlude the intracellular vestibule of the CFTR pore.
FIGURE 4.
Voltage-dependent inhibition of current flow through the CFTR pore by OH− ions. A, representative recordings show the effects of alkalinizing the intracellular solution to pHi 8.3 on the activity of a single wild-type CFTR Cl− channel at −80 mV (top) and +80 mV (bottom). The recording conditions were identical to those used in Figs. 1 and 2 with the exception that the membrane patch was bathed in symmetrical 147 mm Cl− solutions. Dotted lines indicate where the channel is closed, and downward deflections at −80 mV and upward deflections at +80 mV correspond to channel openings. The 1-s portions indicated by bars are shown on an expanded time scale below each 5-s recording. B, single-channel current-voltage (I-V) relationships of wild-type CFTR at pHi 7.3 (white circles) and pHi 8.3 (gray circles) using the conditions described in A. C, voltage dependence of the fraction of i inhibited at pHi 8.3. D, relationship between the voltage-dependent dissociation constant (Kd) and voltage at alkaline pHi (pHi 8.3 and pHi 8.8). E, voltage dependence of Po at pHi 6.3, pHi 7.3, and pHi 8.3. Data are means ± S.E. (B, C, and E, n = 6; D, n = 3–9, except −120 mV, where n = 2). In B and D, the continuous lines are the fits of first-order regression functions to the data. In B and E, the asterisks indicate values that are significantly different from the control (p < 0.05). Error bars are smaller than symbol size except where shown.
To determine the potency with which OH− ions block the CFTR pore, we calculated the apparent voltage-dependent dissociation constant (Kd) for OH− ion inhibition of wild-type CFTR. Fig. 4D demonstrates that apparent Kd(V) values for CFTR inhibition by OH− ions are weakly voltage-dependent. The data also indicate that the potency of CFTR inhibition by OH− ions (apparent Kd (0 mV) = 54 ± 6 μm; n = 9) approaches that of the best-studied CFTR blocker, the sulfonylurea glibenclamide (Kd (0 mV) = 37 μm) (20).
To estimate the location of the binding site for OH− ions within the CFTR pore, we calculated the electrical distance across the membrane sensed by OH− ions using the Woodhull equation (26), as described under supplemental “Experimental Procedures.” Using the data in Fig. 4D and assuming a single binding site for OH− ions, z′ = 0.23 ± 0.03 (n = 9), measured from the inside of the membrane over the voltage range −120 to −40 mV. These data suggest that the penetration of OH− ions into the transmembrane electric field from the intracellular side is 23%, similar to that of SCN− ions (20%) (27). Thus, at alkaline pHi, OH− ions cause open-channel block of the CFTR Cl− channel by occupying a superficial site within the intracellular vestibule of the CFTR pore.
In contrast to the effects of pHi on i (Figs. 3A and 4, B and C), the pHi dependence of Po was voltage-independent (Fig. 4E). At all voltages tested, the Po of wild-type CFTR was diminished at pHi 8.3, but augmented at pHi 6.3. The data also suggest that inhibition of CFTR Cl− current at alkaline pHi results predominantly from a diminution of Po rather than a decrease in i (supplemental Table 3).
pHi Modulates the Function of the NBDs and RD
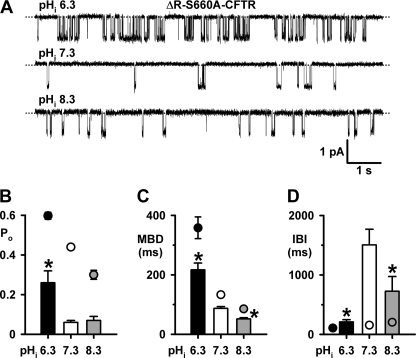
CFTR channel gating is controlled by the NBDs and RD (2, 3). Therefore, we reasoned that pHi might modulate CFTR activity by altering the function of these domains. To test this hypothesis, we adopted two strategies; first, we employed the CFTR construct ΔR-S660A that deletes a large part of the RD and is not regulated by PKA-dependent phosphorylation (13) to explore separately how pHi influences the function of the RD and NBDs. Second, we investigated the effects of pHi on the ATP dependence of CFTR channel gating. For these experiments, we studied CFTR potentiation at pHi 6.3 and CFTR inhibition at pHi 8.3.
Fig. 5A shows representative recordings of a single ΔR-S660A-CFTR Cl− channel at different pHi, whereas Fig. 5, B–D quantifies the effects of pHi on channel gating. Consistent with previous studies (13), at pHi 7.3 the Po of ΔR-S660A-CFTR was attenuated markedly compared with that of wild-type CFTR because its MBD was reduced 0.4-fold, while its IBI was prolonged 9-fold (Fig. 5, B–D). Acidifying to pHi 6.3 potentiated the Po of wild-type CFTR 0.4-fold, but that of ΔR-S660A-CFTR 3.3-fold (Fig. 5B). Fig. 5, C and D, demonstrates that the marked potentiation of ΔR-S660A-CFTR Po at pHi 6.3 was primarily caused by a 0.9-fold decrease in IBI, but it was also enhanced by a 1.5-fold increase of MBD. Alkalinizing to pHi 8.3 decreased the Po of wild-type CFTR 0.3-fold, but was without effect on the Po of ΔR-S660A-CFTR (Fig. 5B). An explanation for this striking difference between wild-type and ΔR-S660A-CFTR is shown in Fig. 5D. Between pHi 7.3 and 8.3, the IBI of wild-type CFTR increased 0.3-fold, whereas that of ΔR-S660A-CFTR decreased 0.5-fold (Fig. 5D). Fig. 5D also reveals that the relationship between IBI and pHi is linear for wild-type CFTR, but bell-shaped for ΔR-S660A-CFTR over the pHi range 6.3–8.3. We interpret the failure of pHi 8.3 to attenuate ΔR-S660A-CFTR channel gating to suggest that alkaline pHi might inhibit CFTR channel gating, at least in part, by modulating RD function. However, the robust potentiation of ΔR-S660A-CFTR channel gating at pHi 6.3 argues that acidic pHi likely enhances CFTR channel gating by acting on sites distinct from the RD.
FIGURE 5.
Acidic pHi potentiates the activity of ΔR-S660A-CFTR. A, representative recordings show the effects of pHi on the activity of a single ΔR-S660A-CFTR Cl− channel. Dotted lines indicate where the channel is closed, and downward deflections correspond to channel openings. B, C, and D, effects of pHi on the Po, MBD, and IBI of ΔR-S660A (columns) and wild-type CFTR (circles). Data are means ± S.E. (B, n = 6 for all data; C and D, ΔR-S660A-CFTR, n = 3; wild-type-CFTR, n ≥ 6); asterisks indicate ΔR-S660A-CFTR values that are significantly different from the pHi 7.3 control (p < 0.05).
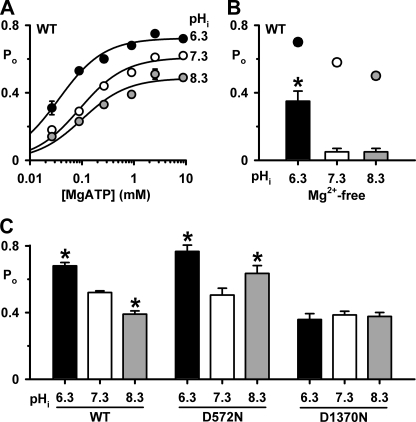
To investigate whether pHi influences NBD function, we studied the ATP dependence of CFTR channel gating. Fig. 6A shows the relationship between [MgATP] and Po for wild-type CFTR at pHi 6.3, 7.3, and 8.3. At each pHi tested, as the [MgATP] increased, Po values enlarged. However, altering pHi influenced both the MgATP sensitivity and channel activity of wild-type CFTR (Fig. 6A). These differences are best illustrated by considering values of KD (the [MgATP] required for half-maximal Po, which describes the apparent affinity of CFTR for MgATP) and Po max (the maximum Po) determined from Michaelis-Menten fits to the mean data (pHi 6.3: KD = 36 ± 5 μm, Po max = 0.72 ± 0.01, r2 = 0.99; pHi 7.3: KD = 90 ± 14 μm, Po max = 0.61 ± 0.02, r2 = 0.99; pHi 8.3: KD = 100 ± 24 μm, Po max = 0.49 ± 0.02, r2 = 0.97; Fig. 6A). Two conclusions are apparent from these data. First, acidifying pHi enhances markedly the apparent MgATP affinity of CFTR, whereas alkalinizing pHi has little effect. Second, acidifying pHi augments channel activity, whereas alkalinizing pHi inhibits CFTR.
FIGURE 6.
pHi modulates the MgATP dependence of CFTR channel gating. A, relationship between [MgATP] and Po of wild-type CFTR at the indicated pHi values. The continuous lines are Michaelis-Menten fits to mean data. B and C, effects of pHi on the Po of wild-type (WT), D572N-, and D1370N-CFTR in the presence of ATP (3 mm in B or 1 mm in C). In B, wild-type CFTR data were acquired in the presence (circles) and absence (columns) of Mg2+ (3 mm), whereas in C, wild-type, D572N-, and D1370N-CFTR data were acquired in the continuous presence of Mg2+ (3 mm). All data are means ± S.E. (n = 5–7); asterisks indicate values that are significantly different from the control value (p < 0.05).
The Role of Mg2+ Ions in Determining the pHi Sensitivity of the NBDs
Mg2+ ions are a pre-requisite for the hydrolysis of ATP that drives CFTR channel closure (21, 22). To learn whether pHi modulates CFTR channel gating by affecting the interaction of Mg2+ ions with the NBDs, we examined the single-channel activity of CFTR in the absence of Mg2+ ions at different pHi (Figs. 6B and supplemental Fig. 3A). At pHi 7.3 removal of Mg2+ ions attenuated markedly the Po of CFTR (Fig. 6B). Nevertheless, acidification to pHi 6.3 enhanced strongly Po, whereas alkalinization to pHi 8.3 was without effect (Fig. 6B). These data suggest that the presence of Mg2+ ions is critical for the inhibition of CFTR channel gating at alkaline pHi. By contrast, the potentiation of CFTR channel gating at pHi 6.3 is both dependent on, and independent of, Mg2+ ions.
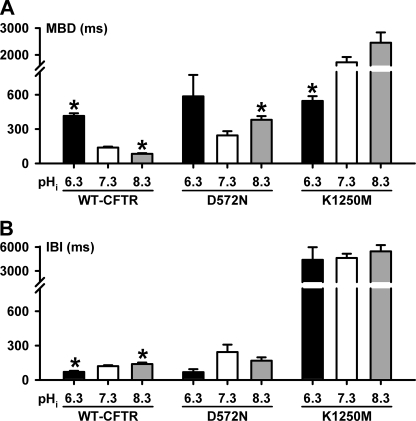
To understand better the role of Mg2+ ions in determining the pHi sensitivity of the NBDs, we studied Asp-572 and Asp-1370, the Walker B aspartates that coordinate Mg2+ ions in ATP binding sites 1 and 2 of the ATP-driven NBD dimerization model of CFTR channel gating (3, 28). Previous studies have demonstrated that the mutations D572N- and D1370N-CFTR abolish Mg2+ binding to the NBDs (21, 22). Figs. 6C and 8 and supplemental Fig. 3, B and C, demonstrate that the gating behavior of D572N- and D1370N-CFTR Cl− channels at different pHi diverges from that of wild-type CFTR in several important respects. First, at pHi 7.3, the Po of D572N-CFTR was the same as wild-type CFTR, whereas that of D1370N-CFTR was reduced (Fig. 6C). Second, at pHi 6.3, the Po of D572N-CFTR was potentiated markedly because IBI was decreased 0.7-fold and MBD was increased 1.4-fold (Figs. 6C and 8 and supplemental Fig. 3B). By contrast, for D1370N-CFTR at pHi 6.3, gating behavior and, hence, Po were unchanged (Fig. 6C and supplemental Fig. 3C). Third, in striking contrast to wild-type CFTR, at pHi 8.3 D572N-CFTR channel gating was enhanced because MBD was increased 0.6-fold and IBI decreased 0.3-fold, whereas that of D1370N-CFTR was unaltered (Figs. 2 and 8 and supplemental Fig. 3, B and C). As a result, at pHi 8.3, the Po of wild-type CFTR decreased, that of D1370N-CFTR was unchanged, whereas that of D572N-CFTR increased (Fig. 6C).
FIGURE 8.
Burst analysis of D572N- and K1250M-CFTR. A and B, MBD and IBI of D572N- and K1250M-CFTR at different pHi values; wild-type CFTR data are shown for comparison. Data are means ± S.E. (D572N- and K1250M-CFTR, n = 3; wild-type-CFTR, n ≥ 6). The asterisks indicate values that are significantly different from the pHi 7.3 control (p < 0.05). Note the breaks in the ordinates.
Several conclusions can be drawn from the data in Fig. 6C. First, the potentiation of D572N-CFTR channel activity at pHi 8.3 suggests that the binding of Mg2+ ions at site 1 is essential for the inhibition of CFTR channel gating at alkaline pHi. Second the lack of effect of pHi on D1370N argues that the pHi sensitivity of CFTR channel gating is dependent on Mg2+ binding to site 2. This suggests that pHi might exert its effects on CFTR channel gating by altering the ATPase activity of site 2.
ATP Binding Site 2 Is a Crucial Determinant of the pHi Sensitivity of CFTR Channel Gating
Structural (29–32) and functional (16, 21) studies suggest that the phosphate groups of ATP are key determinants of ATP binding to sites 1 and 2. The Walker A lysine residues Lys-464 (site 1) and Lys-1250 (site 2), which bind the γ-phosphate of ATP, are highly conserved and critical for ATP binding and hydrolysis (16, 21, 29). Moreover, the LSGGQ motifs contain the highly conserved glycine residues Gly-551 (site 2) and Gly-1349 (site 1) that clamp the phosphate tail of ATP during ATP-driven NBD dimerization (29, 30, 32). Of note, the CF mutations G551D and G1349D perturb severely CFTR channel gating (14, 33). Using CFTR constructs bearing site-directed mutations, we examined the roles of the residues Lys-464, Lys-1250, Gly-551, and Gly-1349 in determining the pHi sensitivity of CFTR channel gating.
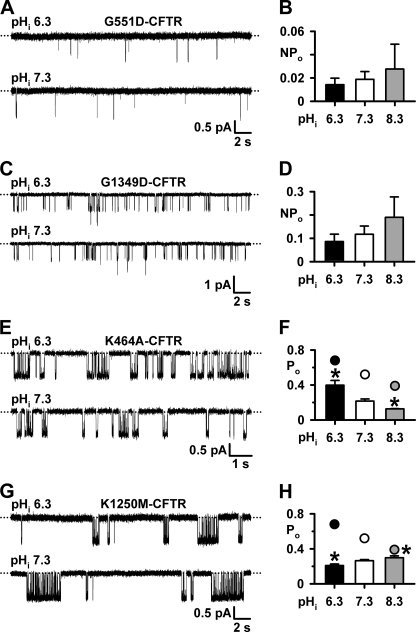
Consistent with previous studies (14, 33), G1349D-CFTR and especially G551D-CFTR attenuated strongly CFTR channel gating at pHi 7.3, with brief, poorly resolved channel openings separated by very long-lasting closures (Fig. 7, A and C). Fig. 7, A–D, demonstrates that the gating behavior and, hence, NPo of G551D- and G1349D-CFTR were unaffected by pHi. Because tight dimerization of the NBDs is a prerequisite for channel opening (28) and because G551D and G1349D perturb severely channel gating (14, 33), we speculate that the effects of pHi on CFTR channel gating are dependent on the formation of an NBD1:NBD2 dimer.
FIGURE 7.
Role of ATP phosphate binding in CFTR regulation by pHi. A, C, E, and G, representative recordings show the effects of pHi on the activity of G551D-, G1349D-, K464A-, and K1250M-CFTR Cl− channels in the presence of ATP (1 mm). Dotted lines indicate where channels are closed, and downward deflections correspond to channel openings. B, D, F, and H, effects of pHi on the NPo of G551D- and G1349D-CFTR and Po of K464A- and K1250M-CFTR. Data are means ± S.E. (n = 6, except G551D- and G1349D-CFTR at pHi = 8.3, where n = 3). The asterisks indicate values that are significantly different from the control value (p < 0.05). In F and H, wild-type CFTR data (circles) are shown for comparison.
Fig. 7, E and G, show the hallmark effects of mutating the Walker A lysines in NBD1 and NBD2 on CFTR channel gating (16, 21). At pHi 7.3, bursts of K464A-CFTR channel openings were separated by prolonged channel closures, whereas dramatically prolonged bursts of K1250M-CFTR channel openings were separated by very long-lived channel closures (Figs. 7, E and G, and 8). Although Po values of K464A-CFTR were less than those of wild-type CFTR at all pHi values tested, the effects of acidic and alkaline pHi on K464A- and wild-type CFTR were similar (Fig. 7, E and F). This suggests that K464A-CFTR does not change the pHi sensitivity of CFTR. By contrast, the pHi sensitivity of K1250M-CFTR differed strikingly from that of wild-type CFTR. At pHi 6.3, the Po of K1250M-CFTR was reduced because MBD was decreased 0.7-fold, whereas IBI was unchanged (Figs. 7, G and H, and 8). At pHi 8.3, the Po of K1250M-CFTR was increased, albeit slightly, as a result of a small increase in MBD and no change in IBI (Figs. 7H and 8). Fig. 7H also reveals that the Po of K1250M-CFTR was similar to that of wild-type CFTR at pHi 8.3, but greatly diminished at pHi 7.3 and 6.3. Thus, the pHi sensitivity of K1250M-CFTR is the converse of that of wild-type CFTR.
Taken together, our data argue that ATP binding site 2 is a crucial determinant of the pHi sensitivity of the CFTR Cl− channel. They also suggest that MgATP binding at site 1 might be important for mediating the inhibitory effects of alkaline pHi and cooperating with site 2 to drive channel gating.
DISCUSSION
In this study we investigated whether pHi regulates directly CFTR activity and the responsible mechanisms. Our data demonstrate that acidic pHi stimulates CFTR Cl− currents by potentiating channel gating, whereas alkaline pHi attenuates CFTR Cl− currents predominantly by inhibiting channel gating, but also by obstructing Cl− flow through the CFTR pore. Using CFTR constructs, we demonstrate that ATP-binding site 2 is a primary determinant of the pHi sensitivity of CFTR. Our data suggest that the mechanism by which pHi regulates CFTR is distinct from other ion channels. Instead of protons modifying channel gating by interacting with residues within (e.g. ClC-0 (34)) or close to the intracellular vestibule (e.g. Kir1.1 (35)) of the channel pore, in CFTR protons modulate channel gating by altering the function of ATP-binding site 2 in the NBDs.
Molecular Mechanisms of CFTR Regulation by pHi
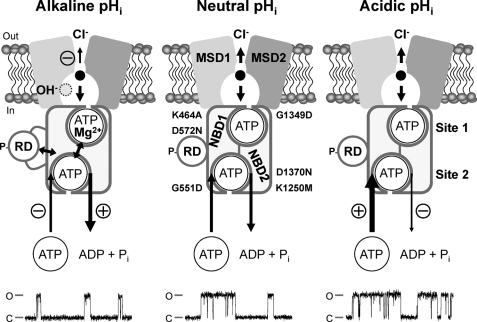
Our data demonstrate that pHi has multiple effects on the CFTR Cl− channel. They also reveal that pHi modulates CFTR function by altering the activity of each of the domains from which CFTR is assembled. In Fig. 9 we summarize the molecular mechanisms by which pHi regulates current flow through the CFTR pore and the control of channel gating by the NBDs and RD.
FIGURE 9.
Multiple mechanisms of CFTR regulation by pHi. The simplified models show an open CFTR Cl− channel with a phosphorylated RD and NBDs assembled to form a head-to-tail dimer with ATP molecules bound at ATP binding sites 1 and 2. Each ATP binding site is formed by the Walker A and B motifs of one NBD and the LSGGQ motif of the other NBD. The locations of the site-directed mutations examined in this study are shown. The models show the direct effects of H+ and OH− ions on the function of the individual domains of CFTR at alkaline, neutral, and acidic pHi. Single-channel recordings are shown beneath each model. For the purpose of illustration, the recordings have been inverted, and upward deflections correspond to channel openings. MSD, membrane-spanning domain; P, phosphorylation of the RD; Pi, inorganic phosphate. In and Out denote the intra- and extracellular sides of the membrane, respectively, whereas C and O indicate the closed and open-channel states, respectively. The long arrows represent the regulation of CFTR channel gating by ATP-driven NBD dimerization; arrow thickness and symbols indicate reaction speed (thick (+), fast (potentiation); thin (−), slow (inhibition)). Double-headed arrows denote interactions between the NBDs and RD and cross-talk between ATP-binding sites 1 and 2. Short arrows within the CFTR pore denote Cl− ion flow; arrow thickness and symbols indicate ease of flow (thick, unobstructed; thin (−), obstructed). See “Discussion” and Refs. 2, 3, and 25 for further information.
OH− ions inhibit CFTR function in several ways. First, OH− ions are open-channel blockers of the CFTR pore that disrupt Cl− flow by interacting with a superficial site within the intracellular vestibule of the CFTR pore. Second, studies of the CFTR construct ΔR-S660A suggest that OH− ions might inhibit CFTR channel gating, at least in part, by modulating RD function. Third, OH− ions inhibit CFTR function by regulating the control of channel gating by ATP-driven NBD dimerization (3, 21). The decrease in channel activity at alkaline pHi argues that OH− ions might destabilize ATP binding at sites 1 and 2, promoting dissociation of the NBD dimer and channel closure. However, our data also suggest that the binding of Mg2+ ions at site 1 is essential for CFTR inhibition by OH− ions.
In contrast to the effects of OH− ions, H+ ions mediate their effects on CFTR predominantly by modulating the control of channel gating at the NBDs. Our studies argue that ATP-binding site 2 is a crucial determinant of the pHi sensitivity of CFTR. The data suggest that H+ ions enhance the affinity with which MgATP binds at site 2 and stabilize ATP binding at this site with the result that the frequency of channel openings is increased and their duration prolonged. Thus, pHi regulates directly CFTR function. Importantly, this regulation occurs within the physiological pHi range (36), consistent with previous work demonstrating a significant role for CFTR in the regulation of pHi (e.g. Ref. 5, 6, 37, and 38).
Open-channel Block of the CFTR Pore by OH− Ions
At negative voltages OH− ions caused a small reduction of the i of CFTR. Given the low concentration of OH− ions in our test solutions (see “Experimental Procedures”), the potency of OH− ion inhibition of CFTR is surprisingly strong, exceeding that of many open-channel blockers of CFTR (e.g. diphenylamine-2-carboxylate and flufenamic acid (39)). Interestingly, the location of the OH− ion-binding site within the transmembrane electric field (23%) is similar to that of SCN− ions (20%) (27). These data argue that OH− and SCN− ions interact with a superficial site(s) within the intracellular vestibule of the CFTR pore (Fig. 9). The shallow penetrance of OH− and SCN− ions into the CFTR pore contrasts with that of large organic anions (e.g. glibenclamide (20)), which penetrate deep into the intracellular vestibule to interact with a site located halfway across the electric field of the membrane from the inside. Site-directed mutagenesis of Lys-95, located toward the extracellular end of the first transmembrane segment, reveals that the positive charge at Lys-95 is essential for channel block by glibenclamide and four other open-channel blockers of CFTR with unrelated chemical structures (40). However, studies of the kinetics of channel block (41–43) demonstrate that the interaction of open-channel blockers with CFTR is complex and likely to involve multiple binding sites within the intracellular vestibule of the CFTR pore. One of these binding sites might overlap with that of OH− ions.
Intricate Effects of pHi on CFTR Channel Gating
Our data reveal that pHi has complex effects on CFTR channel gating. Over the pHi range 6.3 to 8.3, the Po of wild-type CFTR decreases steadily because of reciprocal changes in IBI and MBD. This suggests that between pHi 6.3 and 8.3, the effects of pHi on MBD and IBI are coordinated. By contrast, our data suggest that in strongly acidic and alkaline intracellular solutions, MBD values are stable, whereas those of IBI appear unstable.
At pHi 5.8 the IBI of wild-type CFTR was prolonged strikingly, indicating that channel opening is very difficult at strongly acidic pHi. At pHi 5.8, MgATP2− is partially protonated at the γ-phosphate to become MgHATP− (44). This suggests that protonated ATP at pHi 5.8 might interfere with the formation of H-bonds between CFTR and the γ-phosphate of ATP, attenuating ATP binding and impeding channel opening. However, we speculate that protonation of phosphate groups in other parts of the CFTR gating pathway or on the RD at strongly acidic pHi might also contribute to this result.
Interestingly, at pHi 8.8 the IBI of wild-type CFTR was attenuated with the result that the Po of wild-type CFTR at pHi 8.8 enlarged to become equivalent to that at pHi 7.8. Because negative surface charge increases with alkalinization of the intracellular solution, the diminution of IBI at pHi 8.8 might represent a change in RD conformation or the interaction of the RD with other domains of CFTR (Fig. 9). For example, manipulation of the surface charge on the RD might mimic the effects of RD phosphorylation and disrupt interactions between the RD and NBDs leading to channel activation (13, 45, 46). Alternatively, increased negative surface charge at alkaline pHi might alter the balance between stimulatory and inhibitory RD phosphorylation sites to favor channel activation (47).
In contrast to the effects of strong alkaline pHi on wild-type CFTR, our studies of the ΔR-S660A-CFTR Cl− channel at pHi 8.3 argue that alkaline pHi inhibits CFTR channel gating, at least in part, by modulating RD function. The reason why ΔR-S660A-CFTR is not inhibited at pHi 8.3 is that its prolonged IBI is attenuated, not extended, at this pHi value. Further studies are required to understand the underlying mechanisms. However, it is interesting to note that for both ΔR-S660A-CFTR and the ATP-binding site 1 mutant D572N-CFTR, the relationship between pHi and IBI is bell-shaped, not linear, between pHi 6.3 and 8.3 (Figs. 5D and 8B). We interpret these data to suggest that the pHi dependence of IBI reflects complex interactions between the RD and NBDs.
Use of the ATP-driven NBD Dimerization Model of CFTR Channel Gating to Explain the Effects of pHi
Like other ABC transporters, the two NBDs of CFTR likely form a head-to-tail dimer that sandwiches ATP molecules within two binding sites (termed site 1 and site 2) located at the NBD1:NBD2 interface (3, 21). In CFTR, site 1 has no ATPase activity, but site 2 cyclically hydrolyzes ATP to drive channel gating (3, 21). In this asymmetric gating model, ATP-induced dimerization at site 1 likely occurs before that at site 2, which in turn precedes CFTR opening (48).
Structural studies of ABC transporters (29–32) demonstrate that within the ATP-binding sites the cis NBD anchors the phosphate tail of an ATP molecule by a network of hydrogen bonds (H-bonds) and hydrogen-mediated salt bridges from the Walker A motif. By contrast, in the trans NBD, only H-bonds from the LSGGQ motif clamp the γ-phosphate of ATP. These data suggest that perturbation of the LSGGQ motif might have a greater impact on NBD dimerization than disruption of the Walker A motif. Accordingly, the Walker A lysine mutant K1250A-CFTR is an ATP-dependent channel with moderate Po (16), whereas the LSGGQ motif mutant G551D-CFTR is an ATP-independent channel with extremely low Po (14, 33). Thus, the loss of pHi sensitivity by G551D- and G1349D-CFTR suggests that correct alignment of the NBD1:NBD2 dimer is required for the potentiation of CFTR channel gating by acidic pHi and inhibition by alkaline pHi.
Based on several lines of evidence, we suggest that ATP-binding site 2 is a crucial determinant of the pHi sensitivity of CFTR. First, acidifying pHi enhances markedly the apparent MgATP affinity of CFTR, whereas alkalinizing pHi has little effect. Vergani et al. (49) argue that Kd values obtained from the [MgATP] dependence of the opening rate of CFTR are a reasonable estimate of the Kd value for ATP binding to site 2. Moreover, using a simple kinetic model of CFTR channel gating, Winter et al. (24) demonstrated that the opening rate of CFTR, not its closing rate, is ATP-dependent. These considerations suggest that the pHi sensitivity of the apparent MgATP affinity of CFTR likely reflect the effects of H+ ions on the interaction of MgATP with site 2.
Second, our studies of CFTR channel gating in the absence of Mg2+ ions demonstrate that Mg2+ ions are critical for channel inhibition at alkaline pHi and modulate channel potentiation at acidic pHi. Because MgATP binding at both ATP binding sites is a prerequisite for channel opening (50), these data suggest that pHi has effects on both ATP binding sites. However, Mg2+ ions are also a prerequisite for the hydrolysis of ATP that closes the channel (50). This argues that the potentiation of channel gating at pHi 6.3 is evidence for H+ ions mediating their effects, at least in part, via site 2.
Third, H+ ions potentiate the gating behavior of CFTR constructs bearing site-directed mutations in ATP-binding site 1 (K464A- and D572N-CFTR). By contrast, H+ ions are either without effect (D1370N-CFTR) or inhibit (K1250M-CFTR) the gating behavior of site-directed mutations in ATP-binding site 2. As discussed above, the failure of H+ ions to potentiate G551D- (site 2) and G1349D-CFTR (site 1) is likely a consequence of the profound disruption of NBD dimerization and, hence, channel gating by these constructs. Taken together, the simplest interpretation of our data is that ATP-binding site 2 is a crucial determinant of the pHi sensitivity of the CFTR Cl− channel (Fig. 9). This suggests that H-bond formation at site 2 enhances channel activity by accelerating NBD dimerization and stabilizing the ATP bound conformation of the NBD1:NBD2 dimer. Consistent with this idea, acidic pH enhanced greatly the formation of an engineered TAP1 homodimer (11).
Mechanism of ATP Hydrolysis at Site 2
A common feature of the ATP binding sites of the ABC transporters CFTR and TAP is their asymmetric ATPase activity (11, 29). In an engineered human TAP1 homodimer, the ATP-binding site with ATPase activity contains a catalytic dyad composed of a histidine residue in the H-loop and a glutamic acid residue distal to the Walker B motif (11, 31). However, instead of hydrolyzing ATP by general base catalysis, this dyad employs substrate-assisted catalysis (SAC) for this reaction (11, 31). In SAC, the interaction of the substrate, ATP, with catalytic water via its γ-phosphate is stabilized by an H-bond between the dyad histidine and the γ-phosphate of ATP (31). A key advantage of SAC over general base catalysis is that it prevents ATP hydrolysis occurring before NBD dimerization (31). In this way so-called “futile ATP hydrolysis” by ABC transporters is avoided.
It is intriguing to consider whether CFTR hydrolyzes ATP at site 2 by SAC. In support of this idea, site 2 but not site 1 contains a catalytic dyad like that found in human TAP1 (11, 29). Although the duration of bursts of channel openings are not determined solely by the ATPase activity of the NBDs (51), the effects of pHi on the MBD of CFTR (Fig. 3C) suggest that alkaline pHi might enhance ATP hydrolysis at site 2, whereas acidic pHi has the converse effect. This raises the interesting possibility that the pHi sensitivity of the ATPase activity at site 2 of CFTR might be similar to the ATP-binding site of an engineered TAP1 homodimer that uses SAC to hydrolyze ATP (11). Finally, in SAC, OH− ion attack of the γ-phosphate is the rate-limiting step for ATP hydrolysis (11, 31). Taken together, the data suggest that SAC might provide an efficient way to couple NBD dimerization and ATP hydrolysis in the CFTR Cl− channel. Future studies should explore this possibility.
The pHi Sensitivity of CFTR Reveals Cross-talk between ATP Binding Sites 1 and 2
An interesting aspect of our data is the pHi sensitivity of K1250M-CFTR, which is the reverse of that of wild-type CFTR. This observation suggests that there might be hidden minor pHi effects, masked by the major effects of pHi on wild-type CFTR. If K1250M-CFTR severely disrupts the function of site 2 (16), these hidden minor pHi effects might originate from site 1. Our data also reveal that D572N-CFTR has a very unusual response to pHi: exaggerated potentiation of channel gating at acidic pHi and potentiation, not inhibition, at alkaline pHi. In ABC transporters, the Walker B motif is located adjacent to the highly conserved D-loop motif (30, 31). Structural studies of ABC transporters (30, 31) suggest that in CFTR the D-loop of NBD1 (site 1) might (i) interact with the Walker A motif of NBD2 (site 2) and (ii) sense the functional state of site 1 via the H-loop motif of NBD1. In support of this idea, the effects of acidic and alkaline pHi on D572N-CFTR are reminiscent of the enhanced activity of P574H-CFTR, a CF mutant affecting a residue in the Walker B-D-loop region of NBD1 (52). Thus, the D-loop might mediate cross-talk between the ATP binding sites of CFTR and influence the pHi sensitivity of CFTR.
Identification of pHi-sensitive Amino Acids in CFTR
The changes in CFTR channel gating at pHi 6.3 and 8.3 suggest that histidine (pKa ∼ 6.1) and cysteine (pKa ∼ 8.3) might serve as pHi-titratable amino acid residues. Examination of the NBD1:NBD2 dimer identifies a number of histidine and cysteine residues located close to the ATP binding sites. Site 1 contains two histidine residues in its LSGGQ motif (His-1348 and His-1350) and a cysteine residue Cys-491 (1, 29). Because Cys-491 is located spatially close to the Walker B aspartate, Asp-572, of site 1 (29), it might modulate the role of Asp-572 in CFTR channel gating by sensing alkaline pHi. Interestingly, the H-loop of site 2 contains histidine (His-1402) and cysteine (Cys-1400) residues that are lacking in the H-loop of site 1 (29). This raises the interesting possibility that Cys-1400 and His-1402 might act as a pHi sensor for site 2. However, the potential roles of other histidine and cysteine residues in the control of CFTR channel gating should not be overlooked. For example, the sulfhydryl-modifying reagent N-ethylmaleimide rapidly and irreversibly potentiates CFTR channel gating by covalently modifying Cys-832 in the RD (53). Moreover, analyzes of the pHi dependence of other ion channels (e.g. Ref. 35) suggest that other amino acid residues might act as pHi sensors for CFTR channel gating.
Physiological Significance
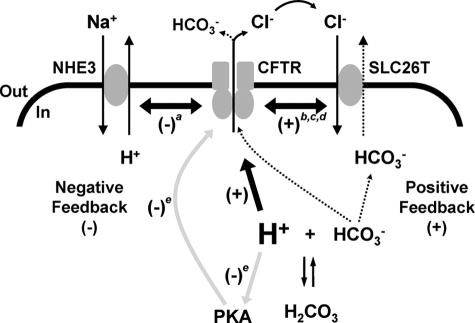
Reddy et al. (10) demonstrated that pHi regulates indirectly the CFTR Cl− conductance of sweat duct epithelia by altering the activity of protein kinases and phosphatases that control RD phosphorylation. By contrast, our data reveal that pHi regulates directly CFTR channel gating. Taken together, these data suggest that CFTR activity is regulated by pHi via two different control mechanisms (Fig. 10). First, at the cell membrane localized pHi fluctuations are detected directly by CFTR, leading to prompt changes in its behavior, coordinated with alterations in the activity of NHE3 and SLC26 transporters. Second, when there are global changes in pHi, the effects of pHi on the enzymes controlling the phosphorylation status of CFTR regulate CFTR indirectly.
FIGURE 10.
Coordination of the activities of CFTR, NHE3, and SLC26 transporters during acid-base regulation in epithelia. The simplified models show ion transport by NHE3, CFTR, and SLC26 transporters in the apical membrane of an epithelial cell. Dotted arrows indicate the pathways for HCO3− secretion from the epithelial cell. The thick black arrow denotes the direct regulation of CFTR by protons observed in this study, whereas the thick double-headed black arrows represent regulatory interactions between CFTR and either NHE3 or SLC26 transporters (see Refs. 6, 8, 9, and 38). The gray arrows indicate the indirect regulation of CFTR by protons through their control of the enzymatic activity of PKA. SLC26T, SLC26 transporter; labels a, b, c, d, and e indicate data from Ahn et al. (8), Lee et al. (6), Ko et al. (38), (9), and Reddy et al. (10); (+), stimulation; (−), inhibition. In and Out denote the intra- and extracellular sides of the membrane, respectively. See “Discussion” for further information.
Fig. 10 also suggests how the direct and indirect mechanisms of CFTR regulation by pHi might be integrated in the control of HCO3− secretion by epithelial cells. HCO3− secretion is driven by the coordinated activity of CFTR and electrogenic SLC26 transporters at the apical membrane (9). Cl− that exits the cell through CFTR is harnessed by SLC26 transporters to drive HCO3− secretion. This efflux of HCO3− acidifies pHi locally in the vicinity of the cell membrane (7), enhancing CFTR channel gating and, hence, Cl− secretion (Fig. 10). Because CFTR activity is reciprocally coupled to that of SLC26 transporters (9), our data suggest that the two transporters form a positive feedback loop to promote HCO3− secretion (Fig. 10). To break this positive feedback loop, a further CFTR control mechanism is required. This is provided by pHi regulating indirectly RD phosphorylation by controlling enzymatic activity through a negative feedback loop (Fig. 10).
The two-loop model of CFTR regulation by pHi (Fig. 10) provides a mechanism to control the magnitude and duration of epithelial HCO3− secretion. This model might also be exploited to understand better how CF causes aberrant HCO3− secretion (37, 38), abnormal pHi regulation (5, 6), and defects in acidic pHi-induced apoptosis (54) and bacterial killing (55). Thus, by investigating the effects of pHi on CFTR, we understand better its mechanism of action, role in fluid and electrolyte transport, and malfunction in CF.
Acknowledgments
We thank L. J. V. Galietta, C. R. O'Riordan, and M. J. Welsh for generous gifts of reagents and L. Schmitt and our departmental colleagues for valuable discussions.
This work was supported by the Cystic Fibrosis Trust.
This article was selected as a Paper of the Week.

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Experimental Procedures,” “Results,” Equations 2 and 3, references, Tables 1–5, and Figs. 1–4.
- ABC
- ATP-binding cassette
- AE
- Cl−/HCO3− (anion) exchanger
- Bis-Tris
- 2-[Bis(2-hydroxyethyl)imino]-2-(hydroxymethyl)-1,3-propanediol
- CF
- cystic fibrosis
- CFTR
- cystic fibrosis transmembrane conductance regulator
- i
- single-channel current amplitude
- IBI
- interburst interval
- MBD
- mean burst duration
- N
- number of active channels
- NBD
- nucleotide-binding domain
- NHE3
- Na+/H+ exchanger isoform 3
- PKA
- protein kinase A
- Po
- open probability
- RD
- regulatory domain
- SAC
- substrate-assisted catalysis
- TAP
- transporter associated with antigen processing
- TES
- N-tris[hydroxymethyl]methyl-2-aminoethanesulfonic acid
- Tris
- tris(hydroxymethyl)aminomethane.
REFERENCES
- 1.Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L., Drumm M. L., Iannuzzi M. C., Collins F. S., Tsui L. C. (1989) Science 245, 1066–1073 [DOI] [PubMed] [Google Scholar]
- 2.Sheppard D. N., Welsh M. J. (1999) Physiol. Rev. 79, S23–S45 [DOI] [PubMed] [Google Scholar]
- 3.Gadsby D. C., Vergani P., Csanády L. (2006) Nature 440, 477–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welsh M. J., Ramsey B. W., Accurso F., Cutting G. R. (2001) in The Metabolic and Molecular Basis of Inherited Disease (Scriver C. R., Beaudet A. L., Sly W. S., Valle D. eds) pp. 5121–5188, McGraw-Hill Inc., New York [Google Scholar]
- 5.Elgavish A. (1991) Biochem. Biophys. Res. Commun. 180, 342–348 [DOI] [PubMed] [Google Scholar]
- 6.Lee M. G., Wigley W. C., Zeng W., Noel L. E., Marino C. R., Thomas P. J., Muallem S. (1999) J. Biol. Chem. 274, 3414–3421 [DOI] [PubMed] [Google Scholar]
- 7.Poulsen J. H., Fischer H., Illek B., Machen T. E. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 5340–5344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahn W., Kim K. H., Lee J. A., Kim J. Y., Choi J. Y., Moe O. W., Milgram S. L., Muallem S., Lee M. G. (2001) J. Biol. Chem. 276, 17236–17243 [DOI] [PubMed] [Google Scholar]
- 9.Ko S. B., Zeng W., Dorwart M. R., Luo X., Kim K. H., Millen L., Goto H., Naruse S., Soyombo A., Thomas P. J., Muallem S. (2004) Nat. Cell Biol. 6, 343–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy M. M., Kopito R. R., Quinton P. M. (1998) Am. J. Physiol. 275, C1040–C1047 [DOI] [PubMed] [Google Scholar]
- 11.Ernst R., Koch J., Horn C., Tampé R., Schmitt L. (2006) J. Biol. Chem. 281, 27471–27480 [DOI] [PubMed] [Google Scholar]
- 12.Howell L. D., Borchardt R., Cohn J. A. (2000) Biochem. Biophys. Res. Commun. 271, 518–525 [DOI] [PubMed] [Google Scholar]
- 13.Rich D. P., Berger H. A., Cheng S. H., Travis S. M., Saxena M., Smith A. E., Welsh M. J. (1993) J. Biol. Chem. 268, 20259–20267 [PubMed] [Google Scholar]
- 14.Cai Z., Taddei A., Sheppard D. N. (2006) J. Biol. Chem. 281, 1970–1977 [DOI] [PubMed] [Google Scholar]
- 15.Zegarra-Moran O., Romio L., Folli C., Caci E., Becq F., Vierfond J. M., Mettey Y., Cabrini G., Fanen P., Galietta L. J. (2002) Br. J. Pharmacol. 137, 504–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carson M. R., Travis S. M., Welsh M. J. (1995) J. Biol. Chem. 270, 1711–1717 [DOI] [PubMed] [Google Scholar]
- 17.Elroy-Stein O., Fuerst T. R., Moss B. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 6126–6130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rich D. P., Anderson M. P., Gregory R. J., Cheng S. H., Paul S., Jefferson D. M., McCann J. D., Klinger K. W., Smith A. E., Welsh M. J. (1990) Nature 347, 358–363 [DOI] [PubMed] [Google Scholar]
- 19.Anderson M. P., Rich D. P., Gregory R. J., Smith A. E., Welsh M. J. (1991) Science 251, 679–682 [DOI] [PubMed] [Google Scholar]
- 20.Sheppard D. N., Robinson K. A. (1997) J. Physiol. 503, 333–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vergani P., Nairn A. C., Gadsby D. C. (2003) J. Gen. Physiol. 121, 17–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikuma M., Welsh M. J. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 8675–8680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lansdell K. A., Cai Z., Kidd J. F., Sheppard D. N. (2000) J. Physiol. 524, 317–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winter M. C., Sheppard D. N., Carson M. R., Welsh M. J. (1994) Biophys. J. 66, 1398–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H., Sheppard D. N. (2009) BioDrugs 23, 203–216 [DOI] [PubMed] [Google Scholar]
- 26.Woodhull A. M. (1973) J. Gen. Physiol. 61, 687–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tabcharani J. A., Rommens J. M., Hou Y. X., Chang X. B., Tsui L. C., Riordan J. R., Hanrahan J. W. (1993) Nature 366, 79–82 [DOI] [PubMed] [Google Scholar]
- 28.Vergani P., Lockless S. W., Nairn A. C., Gadsby D. C. (2005) Nature 433, 876–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis H. A., Buchanan S. G., Burley S. K., Conners K., Dickey M., Dorwart M., Fowler R., Gao X., Guggino W. B., Hendrickson W. A., Hunt J. F., Kearins M. C., Lorimer D., Maloney P. C., Post K. W., Rajashankar K. R., Rutter M. E., Sauder J. M., Shriver S., Thibodeau P. H., Thomas P. J., Zhang M., Zhao X., Emtage S. (2004) EMBO J. 23, 282–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith P. C., Karpowich N., Millen L., Moody J. E., Rosen J., Thomas P. J., Hunt J. F. (2002) Mol. Cell 10, 139–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanekop N., Zaitseva J., Jenewein S., Holland I. B., Schmitt L. (2006) FEBS Lett. 580, 1036–1041 [DOI] [PubMed] [Google Scholar]
- 32.Hopfner K. P., Karcher A., Shin D. S., Craig L., Arthur L. M., Carney J. P., Tainer J. A. (2000) Cell 101, 789–800 [DOI] [PubMed] [Google Scholar]
- 33.Bompadre S. G., Sohma Y., Li M., Hwang T. C. (2007) J. Gen. Physiol. 129, 285–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zifarelli G., Murgia A. R., Soliani P., Pusch M. (2008) J. Gen. Physiol. 132, 185–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schulte U., Hahn H., Konrad M., Jeck N., Derst C., Wild K., Weidemann S., Ruppersberg J. P., Fakler B., Ludwig J. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 15298–15303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roos A., Boron W. F. (1981) Physiol. Rev. 61, 296–434 [DOI] [PubMed] [Google Scholar]
- 37.Choi J. Y., Muallem D., Kiselyov K., Lee M. G., Thomas P. J., Muallem S. (2001) Nature 410, 94–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ko S. B., Shcheynikov N., Choi J. Y., Luo X., Ishibashi K., Thomas P. J., Kim J. Y., Kim K. H., Lee M. G., Naruse S., Muallem S. (2002) EMBO J. 21, 5662–5672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCarty N. A., McDonough S., Cohen B. N., Riordan J. R., Davidson N., Lester H. A. (1993) J. Gen. Physiol. 102, 1–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Linsdell P. (2005) J. Biol. Chem. 280, 8945–8950 [DOI] [PubMed] [Google Scholar]
- 41.Cai Z., Lansdell K. A., Sheppard D. N. (1999) Br. J. Pharmacol. 128, 108–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou Z., Hu S., Hwang T. C. (2002) J. Gen. Physiol. 120, 647–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Z. R., Zeltwanger S., McCarty N. A. (2004) J. Membr. Biol. 199, 15–28 [DOI] [PubMed] [Google Scholar]
- 44.Sigel H., Griesser R. (2005) Chem. Soc. Rev. 34, 875–900 [DOI] [PubMed] [Google Scholar]
- 45.Ostedgaard L. S., Baldursson O., Vermeer D. W., Welsh M. J., Robertson A. D. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 5657–5662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker J. M., Hudson R. P., Kanelis V., Choy W. Y., Thibodeau P. H., Thomas P. J., Forman-Kay J. D. (2007) Nat. Struct. Mol. Biol. 14, 738–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilkinson D. J., Strong T. V., Mansoura M. K., Wood D. L., Smith S. S., Collins F. S., Dawson D. C. (1997) Am. J. Physiol. 273, L127–L133 [DOI] [PubMed] [Google Scholar]
- 48.Scott-Ward T. S., Cai Z., Dawson E. S., Doherty A., Da Paula A. C., Davidson H., Porteous D. J., Wainwright B. J., Amaral M. D., Sheppard D. N., Boyd A. C. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 16365–16370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vergani P., Basso C., Mense M., Nairn A. C., Gadsby D. C. (2005) Biochem. Soc. Trans. 33, 1003–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dousmanis A. G., Nairn A. C., Gadsby D. C. (2002) J. Gen. Physiol. 119, 545–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramjeesingh M., Li C., Garami E., Huan L. J., Galley K., Wang Y., Bear C. E. (1999) Biochemistry. 38, 1463–1468 [DOI] [PubMed] [Google Scholar]
- 52.Sheppard D. N., Ostedgaard L. S., Winter M. C., Welsh M. J. (1995) EMBO J. 14, 876–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cotten J. F., Welsh M. J. (1997) J. Biol. Chem. 272, 25617–25622 [DOI] [PubMed] [Google Scholar]
- 54.Barrière H., Poujeol C., Tauc M., Blasi J. M., Counillon L., Poujeol P. (2001) Am. J. Physiol. 281, C810–C824 [DOI] [PubMed] [Google Scholar]
- 55.Di A., Brown M. E., Deriy L. V., Li C., Szeto F. L., Chen Y., Huang P., Tong J., Naren A. P., Bindokas V., Palfrey H. C., Nelson D. J. (2006) Nat. Cell Biol. 8, 933–944 [DOI] [PubMed] [Google Scholar]