Abstract
Activation of the innate immune system results in a rapid microbicidal response against microorganisms, which needs to be fine-tuned because uncontrolled immune responses can lead to infection and cancer, as well as conditions such as Crohn disease, atherosclerosis, and Alzheimer disease. Here we report that excessive activity of the conserved FOXO transcription factor DAF-16 enhances susceptibility to bacterial infections in Caenorhabditis elegans. We found that increased temperature activates not only DAF-16 nuclear import but also a control mechanism involved in DAF-16 nuclear export. The nuclear export of DAF-16 requires heat shock transcription factor HSF-1 and Hsp70/HSP-1. Furthermore, we show that increased expression of the water channel Aquoporin-1 is responsible for the deleterious consequences of excessive DAF-16-mediated immune response. These studies reveal a stress-inducible mechanism involved in the regulation of DAF-16 and indicate that uncontrolled DAF-16 activity and water homeostasis are a cause of the deleterious effects of excessive immune responses.
Introduction
The inflammatory response is a physiological process that takes place in the presence of a variety of noxious stimuli, including bacterial infections. A hallmark of inflammation is the increased tissue permeability, which allows components of the innate immune system to execute an inflammatory response at the site of infection. In addition to cellular immune effectors, humoral factors such as antimicrobial peptides, lysozymes, and reactive oxygen species accumulate at the site of infection to control invading microorganisms. Whereas this myriad of immune effectors plays a key role in the control of infections, they have the potential to damage host tissues. To provide insights into the mechanisms by which excessive immune responses may be deleterious to an infected host, we have taken advantage of the simple innate immune system of the genetically tractable nematode Caenorhabditis elegans.
Dauer formation abnormal (DAF)2-16 is a key FOXO transcription factor that controls innate immunity in C. elegans (1–3). DAF-16 is closely related to mammalian FOXO3a, which has been linked to inflammation in response to infection (4, 5). Like FOXO3a, the activity of DAF-16 is tightly regulated by a wide variety of external stimuli such as nutrients, oxidative stress, and heat stress (6–9). The activity of DAF-16 is tightly controlled at the level of subcellular localization and post-translational modifications of the protein, including phosphorylation (7, 8, 10, 11). The presence of such tight control suggests that there may be a stress-inducible mechanism that prevents the deleterious consequences of a hyperactive DAF-16.
Here we studied the effect of hyperactivation of DAF-16 in the context of C. elegans immunity. We found that while DAF-16 overexpression protects C. elegans from bacterial infections, its excessive activation has deleterious effects. Even though increased temperature promotes DAF-16 nuclear import, it also turns on a control mechanism that promotes DAF-16 nuclear export. The nuclear export of DAF-16 requires heat shock transcription factor HSF-1 and its transcriptional target Hsp70/HSP-1. Hyperactivation of DAF-16 by increased temperature or lack of nuclear export enhances C. elegans susceptibility to bacterial infections. Further studies indicate that increased expression of the water channel Aquaporin is responsible for the deleterious effects of excessive DAF-16-mediated immune response.
EXPERIMENTAL PROCEDURES
Bacterial and Nematode Strains
Escherichia coli OP50 (12), Pseudomonas aeruginosa-strain PA14 (13), Salmonella enterica strain 1344 (14) Yersinia pestis strain KIM5 (15), and Staphylococcus aureus MSSA476 (16) were used. C. elegans strains utilized were wild-type N2, TJ356-daf-16::gfp (zIs356 (pDAF-16::DAF-16-GFP;rol-6)), and daf-2(e1370). daf-2(e1370);daf-16::gfp animals were created by crossing daf-2 (e1370) males with zIs356 hermaphrodites.
C. elegans Survival Assay
C. elegans strains were maintained as hermaphrodites at 20 or 15 °C, grown on modified NG agar plates, and fed with E. coli strain OP50. P. aeruginosa, S. enterica, and S. aureus cultures were grown in Luria-Bertani (LB) at 37 °C overnight. Y. pestis cultures were grown in LB medium at room temperature overnight. Bacterial lawns used for C. elegans killing assays were prepared by spreading 20 μl of an overnight culture of the bacterial strains on modified NGM (nematode growth medium) agar plates (50 mm NaCl, and 0.35% peptone) in 3.5-cm diameter plates. Plates were incubated at 25 °C for 12 h before seeding them with young adult nematodes grown at 20 °C. The killing assays were performed at 20 °C unless otherwise indicated, and animals were scored and transferred once to twice a day to fresh plates. Animals were considered dead when they failed to respond to touch.
To test the effect of osmolarity on survival, modified NGM plates with 200 and 300 mm NaCl were prepared and used in survival assays. For cadmium survival assays, NGM plates containing 100 μm CdCl2 were prepared and used for seeding E. coli OP50. Worms were transferred to these plates after heat shock and scored for survival at 20 °C every day.
RNA Interference
We used the RNA interference technique to generate loss-of-function RNAi phenotypes by feeding worms with E. coli expressing double-stranded RNA that is homologous to a target gene. Briefly, E. coli strain HT115(DE3) harboring the appropriate vectors were grown in LB broth containing ampicillin (100 μg/ml) and tetracycline (10 μg/ml) at 37 °C overnight. Bacteria were plated onto NGM plates containing 100 μg/ml ampicillin and 5 mm isopropyl β-d-thiogalactoside and were allowed to grow overnight at 37 °C.
Gravid adults were allowed to lay eggs on RNAi expressing lawns of bacteria for 5 h. The eggs were allowed to develop into young adults on RNAi or vector control plates at 20 °C. Because hsp-1 RNAi causes larval arrest, worms were exposed to hsp-1 RNAi beginning from L4 stage for 2 days. Animals grown under these conditions were heat-shock treated or untreated (20 °C) and subsequently used for microscopy or transferred to pathogen plates for survival assays. Bacteria strains expressing double-stranded RNA to inactivate the C. elegans genes were obtained from Wellcome/CR and Open Biosystems. The identity of the clones was confirmed by sequencing.
Heat Shock Treatment
Animals on NGM plates were given heat shock at 35 °C in a water bath for 1 h and either used directly for protein preparation or allowed to recover at 20 °C for 4 h before exposure to pathogen. As a control, animals on NGM plates were maintained at 20 °C before exposure to pathogen or for microscopy.
Statistical Analyses
Animal survival was plotted as a non-linear regression curve using the PRISM (version 4.00) computer program. Survival curves are considered significantly different than the control when p values are <0.05. Prism uses the product limit or Kaplan-Meier method to calculate survival fractions and the logrank test, which is equivalent to the Mantel-Heanszel test, to compare survival curves.
Quantitative Real-time PCR
Gravid daf-16::gfp nematodes were lysed using a solution of sodium hydroxide and bleach, washed, and the eggs were synchronized for 22 h in S basal liquid medium at room temperature. Synchronized L1 animals were placed onto NGM plates seeded with E. coli OP50 and grown until young adult stage (3 days at 20 °C). The nematodes were untreated or subjected to heat shock at 35 °C for 1 h followed by recovery for 4 h at 20 °C and then harvested. The animals were harvested by washing the plates with M9 buffer, and RNA extracted using TRizol reagent. Genomic DNA was removed by treating the RNA samples with DNase using the DNA-free kit according to manufacturer's instruction (Ambion). qRT-PCR was conducted using the Applied Biosystems TaqMan One-Step Real-time PCR protocol using SYBR Green fluorescence (Applied Biosystems) on an Applied Biosystems 7900HT real-time PCR machine in 96-well plate format. Fifty nanograms of RNA were used for real-time PCR. Twenty microliter reactions were set-up in three replicates and performed as outlined by the manufacturer (Applied Biosystems). daf-16::gfp animals were heat shock treated and allowed to recover for 4 h. Total RNA was isolated from control and heat-shocked animals and subjected to RT-PCR analysis for aquaporin transcripts. Gene expression for three independent isolations of control nematodes were compared with three heat shock-treated nematodes using the comparative Ct method after normalization to act-1,-3,-4 (pan-actin) (17). Primer sequences are available upon request.
Nuclear Extract Preparation
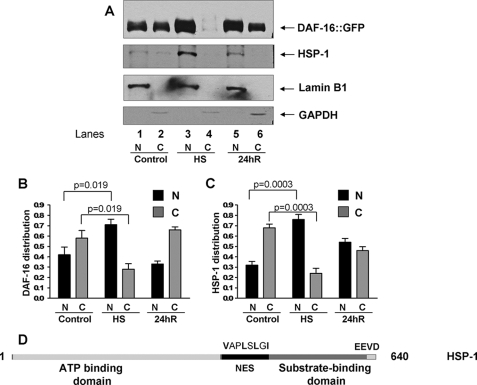
Synchronized daf-16::gfp young adult nematodes were collected from 16–20 10-cm NGM agar plates and washed in M9 buffer. The nuclear extract was prepared in a manner similar to the protocol described in Ref. 18. After washing, the animals with extract buffer (10 mm HEPES, pH 7.1; 5 mm MgCl2, 2 mm dithiothreitol, 10% glycerol, and protease inhibitors), they were resuspended in 0.5 volumes of extract buffer. The suspension was dripped into liquid N2, and the resulting balls were ground in a mortar. The powder was thawed on ice and sheared using a Dounce homogenizer (30 strokes, pistol B). Crude extracts were subsequently centrifuged for 5 min at 1,000 × g to obtain a soluble fraction and a pellet. The pellet or crude nuclear fraction was washed twice with extract buffer and solubilized in 200 μl of 2× Laemmli buffer. Protein in the supernatant or crude cytosol were precipitated using ice-cold acetone and solubilized in 600 μl of 2× Laemmli buffer. 20 μl of nuclear (N) and cytosolic (C) fractions were subjected to SDS-PAGE and Western blotting. Lamin B was used as marker for nuclear fraction and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) for cytosol. Bio-Rad Quantity One software was used for densitometry scan and analysis of Western blots. The optical density values for cytosolic fraction were multiplied by 3 to normalize the values for equivalent loading in nuclear and cytosolic fraction (Fig. 4, B and C).
FIGURE 4.
DAF-16 and HSP-1 are present in the nuclear fraction after heat shock. A, Western blot of nuclear and cytosolic subcellular fractions of daf-16::gfp adult animals before heat shock, after heat shock, and after 24 h of recovery. Nuclear and cytosolic fractions were solubilized in 200 μl and 600 μl of Laemmli buffer, respectively, and 20 μl of each sample were loaded. Lamin B1 is a nuclear fraction marker, and GAPDH is a cytosolic marker. Shown is a representative assay of four independent experiments. B, densitometric analysis of the amount of DAF-16 present in nuclear (N) or cytosolic (C) fractions. C, densitometric analysis of the amount of HSP-1 present in nuclear (N) or cytosolic (C) fractions. The optical density values for cytosolic fractions were multiplied by 3 to give corrected values. p values correspond to Student's t test from four independent experiments. N, nuclear extract; C, cytosolic extract. D, domain organization of HSP-1/F26D10.3. Predicted NES starting at amino acid position 397 is shown.
Immunological Detection of Proteins
Whole worm lysates were prepared in presence of protease inhibitors. Antibody to Hsp70/HSP-1 was obtained from Stressgen Biotechnology (Cat. spa 810). Actin was detected using a polyclonal antibody from Sigma. Antibodies to GAPDH, DAF-16, and lamin B were obtained from Cell Signaling, Santa Cruz Biotechnology, and AbCam, respectively.
RESULTS
Hyperactivation of DAF-16 by Heat Shock Makes C. elegans Susceptible to Pathogen Infection
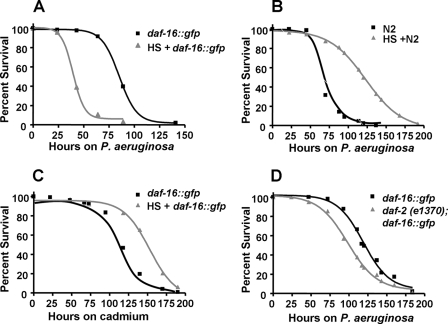
Increased temperature is an ancient immune mechanism used by metazoans in response to microbial infections. Whereas only homeothermic animals are capable of internally increasing the body temperature in response to infections, both homeotherms and poikilotherms develop behavioral fever in response to infection-like conditions such as those stimulated by injections of LPS (19). Using the nematode C. elegans, it has been demonstrated that heat-shock boosts innate immunity against bacteria through the activation of HSF-1 (2). In addition, DAF-16-overexpressing animals (8), which carry additional daf-16::gfp gene copies, were found to be resistant to pathogen infection (2). To study the effect on immune response of combined DAF-16 overexpression and heat shock in C. elegans, wild-type and daf-16::gfp animals were either left untreated or heat shocked at 35 °C for 1 h, they then recovered at 20 °C for 4 h, and were afterward exposed to the human opportunistic pathogen P. aeruginosa strain PA14. As shown in Fig. 1A, heat shock-treated daf-16::gfp animals were more susceptible to P. aeruginosa-mediated killing than non-heat-treated animals. As previously shown (2), heat shock alone enhanced the resistance to P. aeruginosa-mediated killing of wild-type animals (Fig. 1B).
FIGURE 1.
Heat shock treatment of daf-16::gfp animals increases their susceptibility to P. aeruginosa. A, heat-shocked and non-treated daf-16::gfp animals were exposed to P. aeruginosa (p < 0.0001). B, heat-shocked and non-treated wild-type N2 animals were exposed to P. aeruginosa (p < 0.0001). C, heat-shocked and non-treated daf-16::gfp animals were seeded on agar plates containing 100 μm CdCl2 and E. coli (p < 0.0001). D, daf-16::gfp, and daf-2 (e1370);daf-16::gfp animals were exposed to P. aeruginosa (p = 0.0042). Survival assays were performed as described under “Experimental Procedures.” The logrank test, which is equivalent to the Mantel-Heanszel test, was used to compare survival curves. Shown are representative assays of more than six independent experiments (A and B) or two independent experiments (C and D). n = 100 adult nematodes per strain.
Heat shock treatment of daf-16::gfp animals also increased their susceptibility to Gram-negative pathogens Y. pestis, S. enterica and Gram-positive pathogen S. aureus (supplemental Fig. S1, A–C), indicating that overexpression of DAF-16 together with heat shock has deleterious effects on C. elegans resistance to infections by pathogens in general. To address whether combined DAF-16 overexpression and heat shock affects responses not only to pathogen infection but also to general environmental insults, we used cadmium as a heavy metal general stressor. Fig. 1C shows that heat-shock treatment did not affect the susceptibility to cadmium of daf-16::gfp animals. Interestingly, while the combination of higher DAF-16 activity and heat shock has a deleterious effect on immune response to pathogen infection, it has a beneficial effect on response to cadmium exposure (Fig. 1C). These results indicate that higher DAF-16 activity combined with heat shock does not make the animals sickly and susceptible to any environmental insult.
Because heat shock enhances susceptibility to pathogen-mediated killing of daf-16::gfp animals, we hypothesized that other conditions that hyperactivate DAF-16 may also enhance susceptibility to P. aeruginosa. Insulin-like growth factor receptor, DAF-2, has been shown to suppress activation of DAF-16 through AKT pathway (8). It has also been shown that daf- 2 mutation causes activation of DAF-16 and nuclear accumulation of DAF-16 (7). As shown in Fig. 1D, daf-2(e1370);daf-16::gfp animals were more susceptible to P. aeruginosa than daf-16::gfp animals alone, providing further support to the idea that continued nuclear accumulation of DAF-16 has deleterious effects on immune responses against pathogen infections. These results suggest that extended nuclear accumulation of DAF-16 by either heat-shock or daf-2 mutation enhances C. elegans susceptibility to pathogen-mediated killing and that prompt nuclear export is an important mechanism to control the activity of DAF-16.
RNAi Inhibition of hsf-1 Delays Nuclear Export of DAF-16 after Heat Shock
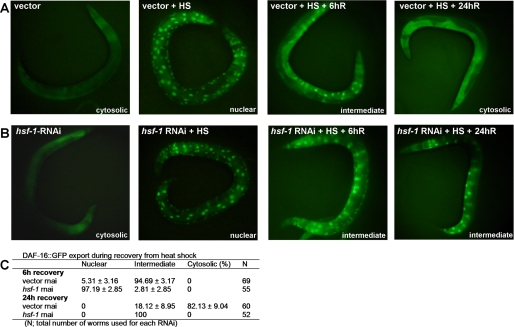
We have previously shown that the enhanced resistance to P. aeruginosa of daf-16::gfp animals is suppressed by RNAi-mediated inhibition of hsf-1 (2). Inhibition of hsf-1 results in the suppression of a system of chaperones required for C. elegans innate immunity (2). It is also possible that RNAi-mediated inhibition of hsf-1 causes heat-shock like conditions that result in an extended nuclear accumulation of DAF-16 and subsequent susceptibility to pathogen-mediated killing. To address whether HSF-1 plays a role in the activation and nuclear accumulation of DAF-16, the nuclear export of DAF-16 in hsf-1 RNAi animals was studied.
DAF-16::GFP is distributed throughout the nucleus and the cytoplasm in all tissues. As previously reported (8), when the animals are subjected to heat shock at 35 °C for 1 h, almost complete nuclear translocation of DAF-16 is observed (Fig. 2A). The animals were monitored during recovery, and the status of DAF-16 localization was categorized as cytosolic, intermediate, or nuclear (20), as indicated in Fig. 2C. As shown in Fig. 2B, hsf-1 RNAi delayed the nuclear export of DAF-16 during recovery from heat shock. hsf-1 RNAi animals displayed more nuclear or intermediate DAF-16 phenotype at 6 and 24 h of recovery, relative to vector control (Fig. 2C). These results show that the nuclear export of DAF-16 is delayed in the absence of HSF-1 and that an HSF-1-mediated mechanism might promote the nuclear export of DAF-16. These results also suggest that the suppression of the enhanced resistance to P. aeruginosa of daf-16::gfp animals by RNAi-mediated inhibition of HSF-1 may be due, at least in part, to the toxic effect of hyperactivation of DAF-16.
FIGURE 2.
HSF-1 is required for efficient nuclear export of DAF-16 after heat shock. A, DAF-16::GFP distribution was observed in daf-16::gfp animals grown on vector RNAi at 20 °C, immediately after heat shock at 35 °C for 1 h (HS), after recovery for 6 h (6hR), and after recovery for 24h (24hR). B, DAF-16::GFP distribution was observed in daf-16::gfp animals grown on hsf-1 RNAi at 20 °C, immediately after heat shock at 35 °C for 1 h (HS), after recovery for 6 h (6hR), and after recovery for 24 h (24hR). C, table showing the percentage of vector or hsf-1 RNAi adult animals with nuclear, intermediate, or cytosolic DAF-16::GFP after recovery for 6 and 24 h. Shown is a representative assay of more than six independent experiments. n = 50–70 nematodes per condition.
Hsp70-encoding Gene hsp-1 Is Required for Nuclear Export of DAF-16
Because HSF-1 is responsible for transcriptional up-regulation of HSPs during heat shock and other stresses, we examined the potential involvement of HSPs in the nuclear export of DAF-16. RNAi was used to inhibit the expression of cytosolic HSPs corresponding to Hsp70, Hsp90, Hsp110, and four small Hsps. Hsp90/DAF-21, Hsp110, or small heat shock proteins were not found to play a significant effect on DAF-16 export (Table 1).
TABLE 1.
Effect of HSP RNAi inhibition on DAF-16 nuclear export
| C. elegans strain | Cosmid no. | Gene | Cytosolic (%)a | Nb |
|---|---|---|---|---|
| TJ356 | Vector | 87.4 | 119 | |
| Y53C10A.12 | hsf-1 0 | 25 | ||
| C47E8.5 | daf-21/hsp90 | 96 | 24 | |
| C12C8.1 | hsp70 | 97.7 | 44 | |
| F26D10.3 | hsp-1 | 0 | 30 | |
| F44E5.4 | hsp70 | 96.4 | 56 | |
| F44E5.5 | hsp70 | 82.7 | 58 | |
| C30C11.4 | hsp110 | 96 | 74 | |
| F08H9.3 | hsp16 | 96 | 25 | |
| F08H9.4 | hsp16 | 84 | 25 | |
| F43D9.4 | sip-1 | 100 | 64 | |
| C09B8.6 | hsp25 | 98.4 | 62 |
a Percent of animals that displayed cytosolic DAF-16:GFP at 24 h of recovery from heat shock.
b N, total number of worms used for each RNAi.
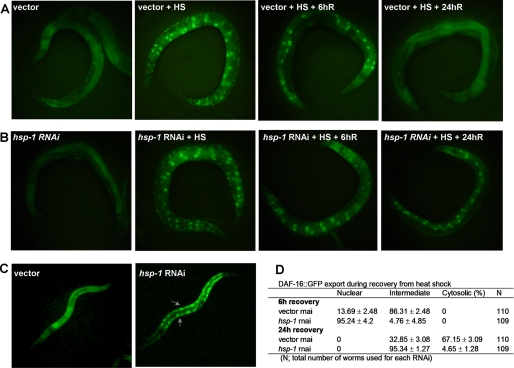
C. elegans has six full-length cytosolic Hsp70-encoding genes (21). Out of four cytosolic Hsp70-encoding genes that were inhibited by RNAi using available clones (22), only hsp-1 was found to be required for export of DAF-16 (Table 1). RNAi knockdown of other three cytosolic Hsp70-encoding genes (F44E5.4, F44E5.5, and C12C8.1) had little or no effect on DAF-16 export. We verified the specificity of RNAi clones by sequencing. BLAT analysis on Hsp70 RNAi clones showed no significant similarity, ruling out the possibility of cross RNAi.
The effect of hsp-1 RNAi knockdown on DAF-16 export was comparable to that of hsf-1 RNAi knockdown (Figs. 3 B and 2B). hsp-1 RNAi animals also exhibit delayed export of DAF-16 at 6 and 24 h of recovery from heat shock (Fig. 3D). We also found that hsp-1 RNAi knockdown caused intermediate level of nuclear accumulation of DAF-16 during larval development compared with vector control (Fig. 3C). Taken together, these results indicate that HSP-1 may be required for DAF-16 export during normal physiological conditions, such as larval development, as well as during recovery from heat stress.
FIGURE 3.
RNAi inhibition of hsp-1 delays nuclear export of DAF16::GFP after heat shock. A, DAF-16::GFP distribution was observed in daf-16::gfp animals grown on vector RNAi at 20 °C, immediately after heat shock at 35 °C for 1 h (HS), after recovery for 6 h (6hR), and after recovery for 24 h (24hR). B, DAF-16::GFP distribution was observed in daf-16::gfp animals grown on hsp-1 RNAi at 20 °C, immediately after heat shock at 35 °C for 1 h (HS), after recovery for 6 h (6hR), and after recovery for 24 h (24hR). C, DAF-16::GFP distribution was observed in daf-16::gfp L3 larvae grown on vector or hsp-1 RNAi at 20 °C. D, table showing the percentage of vector or hsp-1 RNAi adult animals with cytosolic, intermediate, or nuclear DAF-16::GFP after recovery for 6 and 24 h. Shown is a representative assay of more than three independent experiments. n = 100–110 nematodes per condition.
HSP-1 has a putative NES signal starting at amino acid position 397 and an EEVD motif at extreme C terminus, which is a hallmark for cytosolic HSP-70 proteins (Fig. 4B). However, it lacks an apparent nuclear localization signal. Thus, it is unknown whether HSP-1 is either present in the nuclei under control conditions or that it translocates to the nucleus during heat shock to play its role in DAF-16 nuclear export. To distinguish between these two possibilities, we analyzed the subcellular localization of HSP-1 before heat shock, at the end of heat shock, and after recovery. Using a subcellular fractionation method, we prepared nuclear and cytosolic fractions from daf-16::gfp animals and performed Western blots. Consistent with the imaging data shown in Fig. 2, most of DAF-16 was present in the nuclear fraction at the end of heat shock (Fig. 4A, lanes 3 and 4) and became redistributed to the cytosol during recovery (Fig. 4A, lanes 5 and 6 and B). Consistent with its role in DAF-16 nuclear export, HSP-1 was found to be present in both nuclear and cytosolic fractions before heat shock (Fig. 4A, lanes 1 and 2). In addition, HSP-1 was induced and found to be predominantly nuclear after heat shock (Fig. 4A, lanes 3 and 4; and C). Because no direct interaction between HSP-1 and DAF-16 could be detected by immunoprecipitation (not shown), currently it is not known whether HSP-1 interacts directly or indirectly with DAF-16 during export.
Aquaporin-1 Is Responsible for Susceptibility to P. aeruginosa-mediated Killing Caused by DAF-16 Hyperactivation
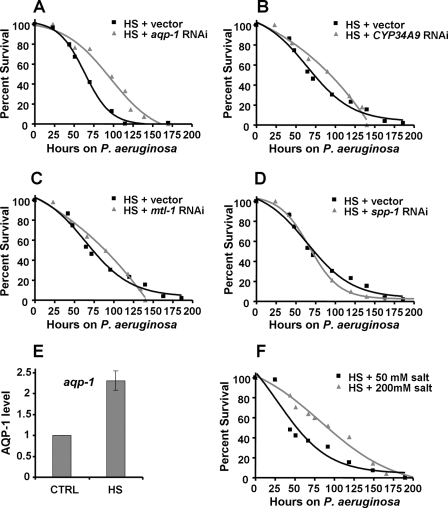
DAF-16 is involved in the transcriptional regulation of a large number of genes that play a role in longevity, stress response, and innate immunity (23, 24). Because activity of DAF-16 is tightly controlled, it is conceivable that hyperactivation of DAF-16 may lead to build-up of some of its transcriptional targets, which could cause deleterious effects on animals exposed to pathogens. To study whether DAF-16-regulated genes are responsible for the enhanced susceptibility to P. aeruginosa-mediated killing caused by DAF-16 hyperactivation, we used RNAi to inhibit DAF-16-up-regulated genes that are also known to be responsive to P. aeruginosa infection (25, 26). As shown in Fig. 5A, aqp-1 RNAi rescues the enhanced susceptibility to P. aeruginosa-mediated killing of heat-shocked daf-16::gfp animals. RNAi-mediated inhibition of other three DAF-16-dependent genes also involved in response to P. aeruginosa (mtl-1, spp-1 and cyp34A9) had no effect on the survival of heat-shocked daf-16::gfp animals (Fig. 5, B–D). None of these genes have an effect on the susceptibility to P. aeruginosa-mediated killing of daf-16::gfp or wild-type animals under control (non-heat-shocked) conditions (data not shown).
FIGURE 5.
aqp-1 RNAi rescues the susceptibility to P. aeruginosa-mediated killing caused by DAF-16 hyperactivation. A, heat-shocked daf-16::gfp animals, grown on vector or aqp-1 RNAi, were exposed to P. aeruginosa (p < 0.0001). B, heat-shocked daf-16::gfp animals, grown on vector or CYP34A9 RNAi, were exposed to P. aeruginosa (p = 0.2554). C, heat-shocked daf-16::gfp animals, grown on vector or mtl-1 RNAi, were exposed to P. aeruginosa (p = 0.7101). D, heat-shocked daf-16::gfp animals, grown on vector or spp-1 RNAi, were exposed to P. aeruginosa (p = 0.2924). E, quantitative real time-PCR analysis of aqp-1 in control versus heat shocked daf-16::gfp animals. Data were analyzed by normalization to pan-actin (act-1,-3,-4) and relative quantification using the comparative cycle threshold method. Bar graphs correspond to mean ± S.D. (p = 0.0015; n = 4). F, heat-shocked daf-16::gfp animals were exposed to P. aeruginosa on agar plates containing 50 mm NaCl or 200 mm NaCl (p = 0.0004). Survival assays were performed as described under “Experimental Procedures.” The logrank test, which is equivalent to the Mantel-Heanszel test, was used to compare survival curves. Shown are representative assays of three independent experiments (A–D), four independent experiments (E), and two independent experiments (F). n = 80–100 adult nematodes per strain.
Aquaporins are tetrameric membrane channel proteins expressed predominantly on plasma membranes, which are permeable to water and other solutes like urea and glycerol (27). They are present in all cellular organisms and regulate tissue water homeostasis in the kidney, brain, lungs, intestine in mammals (27). AQP-1 is expressed in the intestinal cells of C. elegans, it belongs to the aquaglyceroporin subfamily, and it shows significant permeability to glycerol and water (28). Decreased aqp-1 expression during P. aeruginosa infection (25, 26) suggests a role for AQP-1 in water homeostasis in C. elegans response to infections. The lack of suppression of the deleterious effect of hyperactivation of DAF-16 by aqp-1 RNAi in uninfected animals (supplemental Fig. S2), further supports the idea that water homeostasis in the intestinal cells is crucial for an appropriate immune response. The intestine of C. elegans is a primary interface between the immune system and potential bacterial pathogens. In addition, several known effectors of the C. elegans immune system are expressed in the intestinal cells (25, 26, 29–32).
To test the hypothesis that aqp-1 up-regulation is a cause of the deleterious effects of DAF-16 hyperactivity, we used quantitative real-time polymerase chain reaction (qRT-PCR) to compare the expression levels of aqp-1 of daf-16::gfp animals to that of heat-shocked daf-16::gfp animals. Fig. 5E shows that aqp-1 mRNA was significantly up-regulated 4 h after heat shock relative to control animals. The level of aqp-1 up-regulation by increased temperature is within the range of transcriptional up-regulation of aquaporin genes (1.5–4.5-fold) by treatment with high NaCl, ionomycin, and interleukin-1β in mammalian cell lines (33, 34).
Up-regulation of aqp-1 by heat shock suggests that animals might be under an osmotic imbalance because of the presence of a larger number of water channels on the intestinal cells. Indeed, expression of AQP-1 in heterologous Xenopus oocytes increases water uptake and swelling (28). We reasoned that water homeostasis imbalance caused by the up-regulation of water channels could be corrected by changing the osmolarity or ionic strength of exogenous medium. As shown in Fig. 5F, the use of modified agar plates with high NaCl concentrations to increase osmolarity rescues the enhanced susceptibility to P. aeruginosa-mediated killing because of hyperactivation of DAF-16 by heat shock. However, increased salt concentration does not protect wild-type animals from P. aeruginosa-mediated killing (supplemental Fig. S3). This indicates that a high concentration of salt does not rescue the enhanced susceptibility to P. aeruginosa-mediated killing of daf-16::gfp animals by reducing bacterial virulence.
DISCUSSION
Innate immunity comprises a variety of defense mechanisms against infections. C. elegans possesses a number of antibacterial factors and lectin-like proteins, which have the potential to act in recognition and clearance of microbes (29, 35, 36). In addition, C. elegans produces ROS species and lysozymes against invading microbes (29, 37). These immune effectors have also been shown to be controlled by conserved mechanisms including p38 MAPK, heat-shock factor, GATA and FOXO transcription factors (1, 2, 25, 26, 30, 38–40).
DAF-16 is a key FOXO transcription factor that controls innate immunity in C. elegans (1–3). DAF-16 activity is tightly regulated through the activity of different proteins, including AKT/PKB kinases, JNK MAPK, and histone deacetylase (7, 11, 20). Transient activation of endogenous DAF-16 by heat shock or DAF-16 overexpression appears to increase resistance to pathogens (2). To study the effect of excessive DAF-16 activity in the context of C. elegans immunity, we used different conditions that hyperactivate DAF-16. The hyperactivation of DAF-16 was achieved by overexpression of DAF-16 combined with its activation through either heat shock or daf-2 mutation. Even though DAF-16 overexpression is not physiological, it allows us to study the effects of excessive DAF-16 in the context of an infected animal. The results show that hyperactivation of DAF-16 by either heat shock or lack of DAF-2-mediated inhibition results in an increased susceptibility to pathogens, suggesting that continued DAF-16-mediated immune response has deleterious effects on an infected host. The lack of toxicity to cadmium caused by DAF-16 hyperactivity indicates that the activation of overexpressed DAF-16 does not cause general toxicity and that it specifically affects responses against bacterial infections.
The increased susceptibility to bacterial infections caused by DAF-16 hyperactivation is at least partly mediated through up-regulation of AQP-1, a water channel expressed in the intestinal cells of C. elegans. The intestine is the primary site of pathogen accumulation, secretion of antimicrobial factors, and likely also fluid uptake. C. elegans possesses 12 genes that encode aquaporins. Based on the permeability properties and tissue distribution, it is possible that in addition to AQP-1, other aquaporins also play a role in water homeostasis and pathogen susceptibility. C. elegans AQP-1, AQP-2, AQP-3, AQP-7, and AQP-8 belong to the aquaglyceroporin subfamily and show significant permeability to glycerol and water. Interestingly, at least two of the aquaporins, AQP-1 and AQP-3, are expressed in the C. elegans intestine, which is likely involved in water absorption and homeostasis. It would be very interesting to examine whether other aquaglyceroporins, especially intestinally expressed AQP-3, are either regulated by DAF-16 or whether their RNAi-mediated inhibition could rescue the enhanced susceptibility to pathogens caused by DAF-16 hyperactivity. The contribution of water deregulation on enhanced susceptibility to pathogens is supported by the protective effect of hypertonic medium. It is possible that the protection by hypertonic medium is mediated by increased production of osmoprotectants such as trehalose, which is induced under hypertonic conditions (41). The C. elegans gene encoding trehalose synthase is DAF-16-dependent and is responsible for the increased resistance to osmotic stress of PI3K mutants (41). Increase in osmoprotectants may compensate for increased levels of aquaporins and rescue the enhanced susceptibility to pathogens caused by DAF-16 hyperactivity. It is also possible that the hypertonic medium activates other pathways that may decrease DAF-16 activity.
This study suggests that deregulated water homeostasis is partly responsible for enhanced susceptibility. At this point, we cannot rule out the possibility that some of the deleterious effects of DAF-16 hyperactivity are a consequence of excessive ROS and/or other unidentified immune mediators capable of causing tissue damage. It is also possible that cells are damaged by deregulated water homeostasis or are otherwise unable to mount an effective immune response against bacterial infections. Interestingly, our results indicate that nematodes have devised their own control mechanism involving HSF-1 and Hsp70/HSP-1 to control DAF-16 activity by promoting its nuclear export after a transient activation.
In summary, our findings reveal an important role for HSF-1 and its transcriptional target Hsp70/HSP-1 in the regulation of the nuclear activity of DAF-16. The demonstration that increased temperature activates not only DAF-16 but also the expression of Hsp70/HSP-1, which controls DAF-16 activity by promoting its nuclear export, provides insights into a mechanism involved in the control of FOXO transcription factors. Finally, the results indicating that excessive DAF-16 activity may increase susceptibility to bacterial infections by altering water homeostasis caused by up-regulation of aqp-1 provide a molecular explanation for the deleterious effects of inflammation.
Acknowledgment
We thank the Caenorhabditis Genetics Center (University of Minnesota) for strains used in this study.
This work was supported, in whole or in part, by National Institutes of Health Grant GM070977 and The Whitehead Scholars Program (to A. A.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- DAF
- dauer formation abnormal
- FOXO
- forkhead box class O
- HSF
- heat shock factor
- HSP
- heat shock protein
- NGM
- nematode growth medium
- qRT-PCR
- quantitative real-time PCR
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- BLAT
- BLAST-like Alignment Tool
- WT
- wild type
- NES
- nuclear export signal
- AQP
- aquaporin
- MTL
- metallothionein
- SPP
- saposin
- CYP
- cytochrome P450
- MAPK
- mitogen-activated protein kinase
- GFP
- green fluorescent protein
- ROS
- reactive oxygen species.
REFERENCES
- 1.Garsin D. A., Villanueva J. M., Begun J., Kim D. H., Sifri C. D., Calderwood S. B., Ruvkun G., Ausubel F. M. (2003) Science 300, 1921. [DOI] [PubMed] [Google Scholar]
- 2.Singh V., Aballay A. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 13092–13097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyata S., Begun J., Troemel E. R., Ausubel F. M. (2008) Genetics 178, 903–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin L., Hron J. D., Peng S. L. (2004) Immunity 21, 203–213 [DOI] [PubMed] [Google Scholar]
- 5.Snoeks L., Weber C. R., Turner J. R., Bhattacharyya M., Wasland K., Savkovic S. D. (2008) Infect. Immun. 76, 4677–4685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunet A., Sweeney L. B., Sturgill J. F., Chua K. F., Greer P. L., Lin Y., Tran H., Ross S. E., Mostoslavsky R., Cohen H. Y., Hu L. S., Cheng H. L., Jedrychowski M. P., Gygi S. P., Sinclair D. A., Alt F. W., Greenberg M. E. (2004) Science 303, 2011–2015 [DOI] [PubMed] [Google Scholar]
- 7.Lin K., Hsin H., Libina N., Kenyon C. (2001) Nat. Genet. 28, 139–145 [DOI] [PubMed] [Google Scholar]
- 8.Henderson S. T., Johnson T. E. (2001) Curr. Biol. 11, 1975–1980 [DOI] [PubMed] [Google Scholar]
- 9.Iser W. B., Wolkow C. A. (2007) PLoS One 2, e1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee R. Y., Hench J., Ruvkun G. (2001) Curr. Biol. 11, 1950–1957 [DOI] [PubMed] [Google Scholar]
- 11.Berdichevsky A., Viswanathan M., Horvitz H. R., Guarente L. (2006) Cell 125, 1165–1177 [DOI] [PubMed] [Google Scholar]
- 12.Brenner S. (1974) Genetics 77, 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan M. W., Mahajan-Miklos S., Ausubel F. M. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 715–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wray C., Sojka W. J. (1978) Res. Vet. Sci. 25, 139–143 [PubMed] [Google Scholar]
- 15.Une T., Brubaker R. R. (1984) Infect. Immun. 43, 895–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enright M. C., Day N. P., Davies C. E., Peacock S. J., Spratt B. G. (2000) J. Clin. Microbiol. 38, 1008–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Styer K. L., Singh V., Macosko E., Steele S. E., Bargmann C. I., Aballay A. (2008) Science 322, 460–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tops B. B., Tabara H., Sijen T., Simmer F., Mello C. C., Plasterk R. H., Ketting R. F. (2005) Nucleic Acids Res. 33, 347–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bicego K. C., Steiner A. A., Antunes-Rodrigues J., Branco L. G. (2002) J. Appl. Physiol. 93, 512–516 [DOI] [PubMed] [Google Scholar]
- 20.Oh S. W., Mukhopadhyay A., Svrzikapa N., Jiang F., Davis R. J., Tissenbaum H. A. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 4494–4499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikolaidis N., Nei M. (2004) Mol. Biol. Evol. 21, 498–505 [DOI] [PubMed] [Google Scholar]
- 22.Kamath R. S., Fraser A. G., Dong Y., Poulin G., Durbin R., Gotta M., Kanapin A., Le Bot N., Moreno S., Sohrmann M., Welchman D. P., Zipperlen P., Ahringer J. (2003) Nature 421, 231–237 [DOI] [PubMed] [Google Scholar]
- 23.Murphy C. T., McCarroll S. A., Bargmann C. I., Fraser A., Kamath R. S., Ahringer J., Li H., Kenyon C. (2003) Nature 424, 277–283 [DOI] [PubMed] [Google Scholar]
- 24.Alper S., McBride S. J., Lackford B., Freedman J. H., Schwartz D. A. (2007) Mol. Cell. Biol. 27, 5544–5553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Troemel E. R., Chu S. W., Reinke V., Lee S. S., Ausubel F. M., Kim D. H. (2006) PLoS Genet. 2, e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shapira M., Hamlin B. J., Rong J., Chen K., Ronen M., Tan M. W. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 14086–14091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King L. S., Kozono D., Agre P. (2004) Nat. Rev. Mol. Cell Biol. 5, 687–698 [DOI] [PubMed] [Google Scholar]
- 28.Huang C. G., Lamitina T., Agre P., Strange K. (2007) Am. J. Physiol. Cell Physiol. 292, C1867–C1873 [DOI] [PubMed] [Google Scholar]
- 29.Mallo G. V., Kurz C. L., Couillault C., Pujol N., Granjeaud S., Kohara Y., Ewbank J. J. (2002) Curr. Biol. 12, 1209–1214 [DOI] [PubMed] [Google Scholar]
- 30.Kerry S., TeKippe M., Gaddis N. C., Aballay A. (2006) PLoS One 1, e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong D., Bazopoulou D., Pujol N., Tavernarakis N., Ewbank J. J. (2007) Genome Biol. 8, R194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Rourke D., Baban D., Demidova M., Mott R., Hodgkin J. (2006) Genome Res. 16, 1005–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li S. Z., McDill B. W., Kovach P. A., Ding L., Go W. Y., Ho S. N., Chen F. (2007) Am. J. Physiol. Cell Physiol. 292, C1606–C1616 [DOI] [PubMed] [Google Scholar]
- 34.Ito H., Yamamoto N., Arima H., Hirate H., Morishima T., Umenishi F., Tada T., Asai K., Katsuya H., Sobue K. (2006) J. Neurochem. 99, 107–118 [DOI] [PubMed] [Google Scholar]
- 35.Couillault C., Pujol N., Reboul J., Sabatier L., Guichou J. F., Kohara Y., Ewbank J. J. (2004) Nat. Immunol. 5, 488–494 [DOI] [PubMed] [Google Scholar]
- 36.Zugasti O., Ewbank J. J. (2009) Nat. Immunol. 10, 249–256 [DOI] [PubMed] [Google Scholar]
- 37.Chávez V., Mohri-Shiomi A., Maadani A., Vega L. A., Garsin D. A. (2007) Genetics 176, 1567–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim D. H., Feinbaum R., Alloing G., Emerson F. E., Garsin D. A., Inoue H., Tanaka-Hino M., Hisamoto N., Matsumoto K., Tan M. W., Ausubel F. M. (2002) Science 297, 623–626 [DOI] [PubMed] [Google Scholar]
- 39.Aballay A., Drenkard E., Hilbun L. R., Ausubel F. M. (2003) Curr. Biol. 13, 47–52 [DOI] [PubMed] [Google Scholar]
- 40.Huffman D. L., Abrami L., Sasik R., Corbeil J., van der Goot F. G., Aroian R. V. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 10995–11000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamitina S. T., Strange K. (2005) Am. J. Physiol. Cell Physiol. 288, C467–C474 [DOI] [PubMed] [Google Scholar]