Abstract
Transglutaminase 2 (TG2) is a multifunctional protein that has been implicated in numerous pathologies including that of neurodegeneration and celiac disease, but the molecular interactions that mediate its diverse activities are largely unknown. Bcr and the closely related Abr negatively regulate the small G-protein Rac: loss of their combined function in vivo results in increased reactivity of innate immune cells. Bcr and Abr are GTPase-activating proteins that catalyze the hydrolysis of the GTP bound to Rac. However, how the Bcr and Abr GTPase-activating activity is regulated is not precisely understood. We here report a novel mechanism of regulation through direct protein-protein interaction with TG2. TG2 bound to the Rac-binding pocket in the GTPase-activating domains of Bcr and Abr, blocked Bcr activity and, through this mechanism, increased levels of active GTP-bound Rac and EGF-stimulated membrane ruffling. TG2 exists in at least two different conformations. Interestingly, experiments using TG2 mutants showed that Bcr exhibits preferential binding to the non-compacted conformation of TG2, in which its catalytic domain is exposed, but transamidation is not needed for the interaction. Thus, TG2 regulates levels of cellular GTP-bound Rac and actin cytoskeletal reorganization through a new mechanism involving direct inhibition of Bcr GTPase-activating activity.
Introduction
Transglutaminase 2 (TG2,2 also called tissue transglutaminase) is a member of the transglutaminase family that selectively catalyzes the Ca2+-dependent formation of covalent bonds between δ-carboxamide groups of glutamine residues and ϵ-amino groups of lysine residues or primary amines. Unlike other family members, TG2 is expressed in many tissues and cell types, also functions as a G protein in transmembrane signaling, and acts as a cell surface adhesion mediator (1–4). TG2 has been the focus of numerous studies that show it plays an important role in a variety of biological functions including differentiation, apoptosis, signaling, adhesion, migration, wound healing, inflammation, and phagocytosis of apoptotic cells. Although TG2 appears to have many functional domains, studies have mainly concentrated on its cross-linking activity, with little investigation into its non-enzymatic roles (3).
Bcr was originally identified through its involvement in chronic myeloid leukemia (5). Subsequent studies established that it contains a domain with GTPase-activating protein (GAP) activity for the Rho family of small GTPases that includes Rho, Rac and Cdc42 (6). Although the purified GAP domain of Bcr and of the highly related Abr are active toward both Rac and Cdc42 in vitro (7), they only act on Rac in vivo (8–10).
Rho family members are critical regulators of a variety of cellular functions including actin cytoskeleton rearrangement, growth, differentiation, and membrane trafficking (11–14). They act as molecular switches that cycle between an active, GTP-bound and an inactive, GDP-bound form. This cycle is tightly controlled by GAPs such as Bcr and Abr, by guanine nucleotide exchange factors (GEFs), and by guanine nucleotide dissociation inhibitors (GDIs). Although many studies have focused on activation of Rho GTPases, the deactivation by GAPs plays an equally important critical role in their regulation (15–17). For example, loss of the tumor suppressor DLC1, a RhoGAP, is associated with the development of hepatocellular carcinoma in man (18).
How the GAP activity of such proteins is regulated is not completely understood. The Bcr protein contains multiple domains that could be involved in regulation of the GAP activity. We recently identified a direct interaction with RhoGDI as one regulatory mechanism (19). However, it is likely that Bcr is regulated though multiple, different interactions. In an alternative approach to investigate how Bcr is regulated, we sought to identify Bcr-interacting proteins in a yeast two-hybrid screen, using the entire Bcr protein as bait, and isolated TG2. We here report that TG2 functions as a regulator of the BcrGAP activity, and, through it, controls levels of activated Rac. Furthermore, GTP-bound TG2 has reduced affinity for Bcr and reduced ability to inhibit the Bcr GAP activity.
EXPERIMENTAL PROCEDURES
Plasmids and Antibodies
The yeast two-hybrid screen has been previously described (20). Full-length human TG2 wild-type and C277S cDNAs in pcDNA3.1(+) were kindly provided by Gail Johnson (University of Rochester). Xpress-tagged wild type, ΔCT (residues 1–460), and NT (residues 1–139) TG2 were subcloned into pcDNA3.1/HisC vectors through the polymerase chain reaction (PCR) using pcDN3.1(+)-TG2 wild type as template. Xpress-tagged TG2 CT (residues 463–687) was subcloned into pcDNA3.1/HisB. To generate Bcr ΔGAP, a full-length human BCR cDNA clone in pSK flanked by EcoRI sites (B1/SK) was digested with EcoRI × HindIII, and the 2.8-kb fragment was subcloned into pSK digested with the same enzymes. The insert was removed by digestion with XbaI × KpnI and subcloned into pCDE digested with the same enzymes. The HindIII site in BCR is located in the GAP domain, and this construct lacks amino acid residues 1004–1271. BcrΔPK was constructed by isolating the N-terminal end of BCR as a 0.4-kb SalI-StuI fragment from B1/SK. This fragment includes the first 39 amino acid residues of the oligomerization domain. The 3′-end of BCR was purified as a NaeI × SalI fragment from B1/SK (with the EcoRI insert in a different orientation). The NaeI site is located at amino acid residue 434. Ligation of 5′ SalI-StuI + NaeI-SalI into pSK digested with SalI was followed by isolation of clones in the right orientation, to allow removal of the insert as 5′ XbaI-3′ KpnI fragment and ligation into pCDE × XbaI × KpnI. The Xpress-tagged BcrGAP, EGFP-Bcr, GST fusion BcrGAP, GST fusion AbrGAP, Xpress-tagged Bcr wild type, and Xpress-tagged BcrR1090A mutants have been described previously (8, 19). TG2 was also subcloned by PCR into pProEx/HTa for purification of recombinant His6-tagged TG2. Myc-tagged TG2 wild type, S171E, and R580L mutants were kindly provided by Richard Cerione (Cornell University). Bcr (C-20 and N-20) and GST antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against Xpress and Myc were from Invitrogen (Carlsbad, CA). Rac1 antibodies and TG2 antibodies were purchased from BD Biosciences (Franklin Lakes, NJ) and Lab Vision (Fremont, CA), respectively.
Cell Culture
COS-1 cells and Swiss3T3 cells were obtained from the American Type Culture Collection (Manassas, VA). All tissue culture media and supplements were from Invitrogen. COS-1 cells and Swiss3T3 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal calf serum, 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mm l-glutamine, and 1 mm sodium pyruvate. Human brain microvascular endothelial cells (HBMECs) (obtained from Monique Stins, The Johns Hopkins University) were cultured in RPMI 1640 medium containing 20% fetal calf serum, 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mm l-glutamine, 1 mm sodium pyruvate, and 5 units/ml heparin.
Transfection, Immunoprecipitation, and Western Blot Analysis
All procedures were performed as described previously (19) with some modifications. All transfections were to COS-1 cells unless otherwise indicated. Cells were transfected with Plus reagent and Lipofectamine (Invitrogen) according to the manufacturer's instructions and grown for 2 days prior to the assay. Because it has been reported that the expression levels of GTP binding-defective mutants of TG2 decrease over time and are lower compared with wild-type TG2 (21, 22), we only expressed proteins for 1 day after transfection and used 1 μg of wild-type TG2 DNA but 5 μg of TG2 S171E and TG2 R580L mutant DNAs in the same experiment. To detect the endogenous complex of Bcr and TG2, HBMECs were lysed in Nonidet P-40 lysis buffer (50 mm Tris, pH 7.5, 100 mm NaCl, 0.5% Nonidet P-40, 0.2 mm EDTA, 1 mm dithiothreitol, 25 mm NaF, 1 mm Na3VO4, 20 mm β-glycerophosphate, 1 mm phenylmethylsulfonyl fluoride (PMSF), 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 μg/ml pepstatin). Precleared cell lysates were incubated with Bcr (C-20) antibodies overnight at 4 °C followed by adding protein A conjugate-agarose beads and incubation for 1 h at 4 °C. Interactions were shown by Western blot with TG2 or Bcr (N-20) antibodies.
GST Pull-down Assay
All procedures were done as described previously (19) with minor modifications. Recombinant GST fusion proteins were purified from Escherichia coli as described previously (23). His6-tagged TG2 was purified described previously (22) but from E. coli Rosetta 2 (DE3)pLysS (EMD Chemicals, Gibbstown, NJ) and in a lysis buffer without added GDP. To test the interaction between TG2 and either BcrGAP or AbrGAP, in vitro binding experiments were carried out using purified recombinant GST, GST-BcrGAP, or GST-AbrGAP proteins and recombinant TG2 in binding buffer (Dulbecco's phosphate-buffered saline (PBS) containing 0.1% IgePal, 0.5 mm dithiothreitol, and 10% glycerol with 1 mm PMSF, 10 μg/ml aprotinin, and 10 μg/ml leupeptin) for 2 h at 4 °C, followed by incubation with glutathione-agarose beads for 1 h at 4 °C. GTP loading of Rac1 was done as described previously in the presence of excess EDTA over Mg2+ (19). Additional proteins in 450 μl of binding buffer were added and allowed to form a complex for 2 h at 4 °C, followed by incubation with glutathione-agarose beads for 1 h at 4 °C. For GTPγS or GDP loading of TG2 and in vitro binding assay, 25 pmol of TG2 was incubated in buffer (25 mm Tris, pH 7.4, 1 mm EGTA, 1 mm EDTA, 1 mm dithiothreitol, 2 mm MgCl2, 100 mm NaCl, 1% IgePal, 1 mm PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 10% glycerol, and 100 μm GTPγS or 1 mm GDP) for 20 min at 30 °C. 25 pmol of GST-BcrGAP was added and allowed to form a complex for 1 h at 4 °C, followed by incubation with glutathione-agarose beads for 1 h at 4 °C (24). Glutathione beads were washed in binding buffer, boiled in SDS sample buffer, and analyzed by SDS-PAGE/Western blot.
Immunofluorescence Microscopy
Swiss3T3 cells or COS-1 cells were grown on 18-mm coverslips (Fisher Scientific) and were transfected with cDNAs encoding EGFP-Bcr, TG2, or EGFP-Bcr plus TG2. For subcellular localization in Swiss3T3 cells, cells were washed twice with PBS 48 h after transfection and fixed in 4% paraformaldehyde (Electronic Scientific Co., Hatfield, PA) (15 min at room temperature), followed by permeabilization in 0.2% Triton X-100 (15 min, room temperature). Cells were blocked in 1% bovine serum albumin (BSA) in PBS and stained with TG2 antibodies (2 μg/ml; 2 h; 1% BSA), followed by incubation with Cy3-conjugated anti-mouse IgG (Jackson ImmunoResearch Laboratories, Inc, West Grove, PA). After mounting in Vectashield containing 4′,6′-diamidino-2-phenylindole (DAPI, Vector Laboratories, Burlingame, CA), cell images were acquired with a Leica TCS SP confocal microscope. For membrane ruffling in COS-1 cells, transfected cells were cultured for 24 h, starved in serum-free DMEM for 24 h, stimulated with 100 ng/ml epidermal growth factor (EGF) for 10 min, and fixed in 4% paraformaldehyde for 15 min. After permeabilization, blocking, and staining with TG2 antibodies as described above, cells were incubated with Alexa Fluor 350-conjugated anti-mouse IgG (Invitrogen Molecular Probes, Carlsbad, CA) and TRITC-conjugated phalloidin for 1 h. After mounting in Prolong gold antifade reagent (Molecular Probes), ∼100 transfected cells per coverslip were counted, and cell images were acquired with a Leica DM RA upright microscope.
Rac Activation Assay
The GST-Pak1 Rac binding domain was isolated and precoupled with glutathione-agarose beads as described elsewhere (25). Rac activation assays were performed as previously described (8). Affinity-precipitated proteins as well as 20 μl of the supernatant were separated by SDS-PAGE, followed by immunoblotting with Rac1 antibodies, TG2 antibodies or Bcr (N-20) antibodies. The resulting blots were scanned and analyzed with Un-Scan-It software (Silk Scientific, Orem, UT). The levels of activated Rac1 were normalized to Rac1 content in total cell lysates, and Rac1 activation is shown as the relative intensities of GTP-Rac1 bands compared with those of vector-expressing cells.
RESULTS
Bcr and TG2 Form a Complex in Vivo
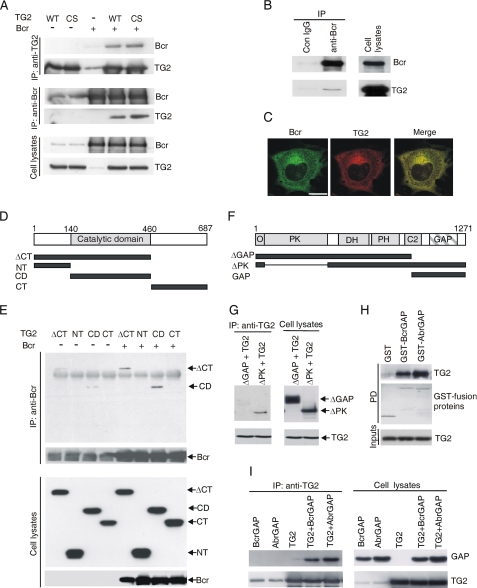
One of the yeast two-hybrid screen positive clones was identified by sequencing as TG2. To study whether Bcr binds to TG2 in mammalian cells, full-length wild-type (WT) or C277S (a transglutaminase activity-deficient mutant) TG2 was co-expressed with Bcr and immunoprecipitation (IP) using TG2 or Bcr antibodies was performed. Fig. 1A shows that both WT and C277S associate with Bcr. We also investigated if endogenous Bcr and TG2 form a complex. Lysates of HBMEC were prepared and immunoprecipitated using Bcr antibodies. We found that endogenous TG2 co-precipitated with endogenous Bcr, indicating the interaction between two proteins occurs when they are expressed at physiologically relevant levels (Fig. 1B). To further investigate the interaction between Bcr and TG2, we next performed subcellular localization analysis. Plasmids encoding EGFP-Bcr and TG2 were co-transfected into Swiss3T3 cells, and their cellular localization was compared using confocal microscopy. As shown in Fig. 1C, in non-simulated cells and in agreement with other results, TG2 and Bcr were mainly present in the cytosol (21, 26, 27), and the localization patterns of the two proteins were strikingly similar. These results show that Bcr physically interacts with TG2 in mammalian cells.
FIGURE 1.
Bcr interacts with TG2 via its GAP domain. A, lysates of Bcr transfected alone or together with TG2 WT or C277S mutant (CS) were subjected to IPs with TG2 or Bcr (N-20) antibodies and Western blotting as indicated. B, interaction of endogenous Bcr and TG2 in HBMEC detected by IP with Bcr (C-20) antibodies and Western blots with Bcr (N-20) or TG2 antibodies. C, localization of Bcr and TG2 in Swiss 3T3 cells detected by confocal microscopy after transfection of EGFP-Bcr and TG2. Bar, 20 μm. D, schematic view of TG2 constructs. E, lysates of cells transfected with Bcr and Xpress-tagged TG2 mutants; IP with Bcr (N-20) antibodies; Western blot with Bcr (N-20) or Xpress antibodies. F, schematic view of Bcr constructs. Domains include O, oligomerization; PK, protein serine/threonine kinase; DH, Dbl homology; PH, Pleckstrin homology; C2, calcium binding; GAP, GTPase-activating protein. G, after co-transfection of TG2 and Bcr mutants and IP with TG2 antibodies, interactions were analyzed by Bcr (N-20) or TG2 antibodies. H, purified GST, GST-BcrGAP, or GST-AbrGAP was incubated with recombinant His6-TG2 and the PD complex immunoblotted with TG2 antibodies. The GST fusion proteins were visualized by Coomassie Brilliant Blue staining of the membrane. I, Xpress-tagged AbrGAP or BcrGAP was transfected alone or together with TG2. IP with TG2 antibodies was followed by blotting with Xpress or TG2 antibodies.
Bcr Interacts with TG2 via the TG2 Catalytic Core Domain
TG2 is organized into four domains: an N-terminal β-sandwich domain, a catalytic core domain, and two C-terminal β-barrel domains (1). To assess which is or are important for the interaction with Bcr, we constructed Xpress-tagged deletion mutants of TG2 including ΔCT, lacking the two C-terminal β-barrel domains (including residues 1–460), NT including the N-terminal β-sandwich domain (residues 1–139), CD including the catalytic core domain (residues 140–460), and CT, which contains the two C-terminal β-barrel domains (residues 463–687) (Fig. 1D). Xpress-tagged TG2 mutants were transfected alone or together with Bcr, and IP was performed with Bcr antibodies. We found that Bcr interacted with ΔCT and CD equally well, but failed to bind to NT and CT (Fig. 1E), leading to the conclusion that the catalytic core domain of TG is essential for binding to Bcr.
TG2 Directly Interacts with the GAP Domains of both Abr and Bcr
We also investigated which region of Bcr binds to TG2. We expressed ΔGAP (lacking the C-terminal residues 1004–1271) or ΔPK (including residues 1–39 + 434–1271) Bcr deletion mutants with TG2 and performed IPs with TG2 antibodies (Fig. 1, F and G). As shown in Fig. 1G, ΔPK interacted with TG2, indicating that the Bcr serine/threonine kinase domain is not required for binding to TG2, whereas ΔGAP failed to co-immunoprecipitate with TG2. This result demonstrates that the GAP domain of Bcr is critical for the interaction. To examine if the Bcr GAP domain is sufficient to mediate the binding of Bcr to TG2, we performed in vitro protein-protein interaction assays with the isolated GAP domain as well as IPs in cell lysates using TG2 antibodies. Additionally, we also tested if the Abr GAP domain could interact with TG2, because this domain in Abr shares a high degree of homology with Bcr. We incubated GST, GST-Bcr GAP, or GST-Abr GAP with His6-tagged TG2 and examined the interaction by GST pull-down and immunoblotting with TG2 antibodies. As shown in Fig. 1H, TG2 specifically interacted with the Bcr GAP domain and not with GST alone. The GST-Abr GAP domain also bound with TG2. In addition, we confirmed this in vitro result in COS-1 cells, by transiently co-expressing Xpress tagged-Bcr GAP or Xpress-Abr GAP with TG2 and performing IPs using TG2 antibodies (Fig. 1I). We conclude that both Abr and Bcr directly associate with TG2, indicating that no other proteins are required for their complex formation and moreover, that the Bcr or Abr GAP domain is sufficient for the binding to TG2.
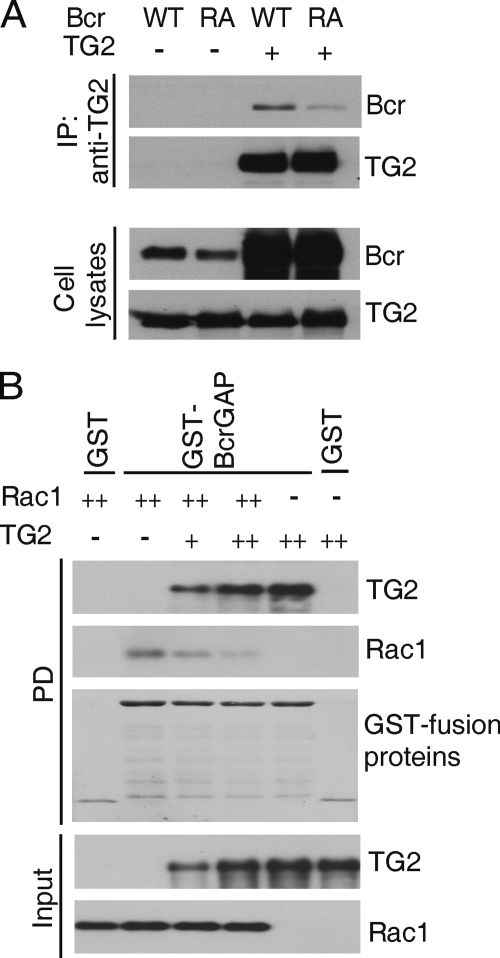
TG2 Blocks the Binding of Rac1 to Bcr
Bcr binds to activated Rac, its substrate, through its GAP domain (19). The Bcr GAP domain constructs used here span more than 250 residues. Because both TG2 and Rac1 interact with this region, we considered the possibility that they compete for a common binding site. We used a Bcr mutant (Bcr R1090A), which binds to and sequesters active Rac (GTP-Rac) but has no GAP activity (8). We co-transfected TG2 with wild-type Bcr or the Bcr R1090A mutant and performed IPs using TG2 antibodies. As shown in Fig. 2A, the interaction of TG2 with the Bcr R1090A mutant was significantly decreased compared to that with wild-type Bcr, suggesting that TG2 and Rac1 share a binding site in the Bcr GAP domain. This result prompted us to examine the interaction between Rac1 and BcrGAP in the absence or presence of TG2. As shown in Fig. 2B, complex formation between BcrGAP and GTP-loaded Rac1 was reduced with increasing amounts of TG2. Taken together, these results demonstrate that TG2 and Rac1 share a common binding region in the BcrGAP domain and that TG2 inhibits the binding of Rac1 to Bcr.
FIGURE 2.
TG2 blocks the binding of Rac1 to Bcr. A, Xpress-tagged Bcr WT or Bcr R1090A (RA) was co-transfected with TG2. After IP with TG2 antibodies, interactions were visualized with Xpress or TG2 antibodies. B, bacterially purified GST-BcrGAP or GST (25 pmol each) was incubated with GTP-loaded Rac1 (25 pmol) or His6-tagged TG2 (+, 12.5 pmol; ++, 25 pmol) as indicated and complex formation was measured by glutathione-agarose PD and Western blot analysis to detect Rac1 and TG2.
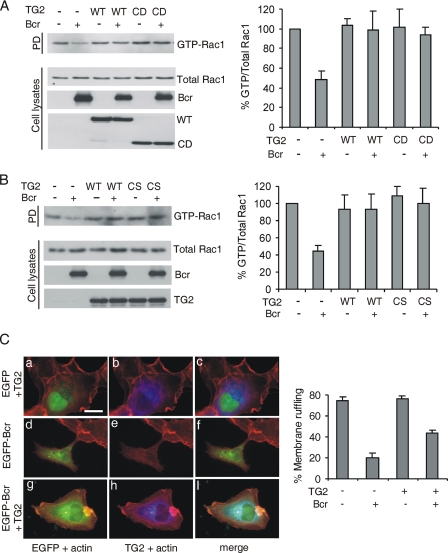
TG2 Blocks Bcr GAP Activity toward Rac1
Because Bcr and Abr act as GAPs for Rac, we investigated the possibility that TG2 could regulate this function of Bcr in cells. We co-transfected TG2 WT or TG2 CD with Bcr and performed assays for levels of activated endogenous Rac. Consistent with a previous report (8), Bcr reduced the levels of the active form of endogenous Rac1, compared with cells only expressing vector (Fig. 3A). TG2 alone had no significant effect on the levels of active Rac. Interestingly, the co-expression of wild-type TG2 restored the levels of active Rac1, which had been decreased by Bcr (Fig. 3A, left panel and quantitation, right panel). This result shows that TG2 inhibits the function of Bcr as a RacGAP. Interestingly, TG2 CD as well as TG2 full-length restored the levels of active Rac1 to that of controls not expressing Bcr. We also asked if the transamidation activity of TG2 might account for its inhibitory effect on the BcrGAP activity. However, as shown in Fig. 3B, a transamidation activity-defective mutant, TG2 C277S, also restored the levels of GTP-Rac1 to those restored by wild-type TG2. These results, in combination with the IP studies shown in Fig. 1 for the CS mutant, indicate that the TG2 catalytic activity is not necessary for either binding to Bcr or inhibition of the BcrGAP activity.
FIGURE 3.
TG2 blocks BcrGAP activity toward Rac1. A, left panel, after transfection of plasmids as indicated above the lanes, levels of endogenous active Rac1 were measured as described under “Experimental Procedures.” Expression levels of total Rac1, Bcr, or TG2 were determined with Rac1, Bcr (N-20), or Xpress antibodies. Right panel, the ratio of GTP-bound/total Rac1 compared with vector-expressing cells. Bars, mean ± S.D. of three independent experiments. B, left panel, levels of activated Rac after co-expression of Bcr and TG2 WT or CS. Total TG2 levels were measured using TG2 antibodies. Right panel, quantitation of results. C, left panel, cells transfected with the constructs indicated were serum-starved for 24 h and stimulated with EGF (100 ng/ml) for 10 min. TG2 or actin was visualized by immunofluorescence using TG2 antibodies (blue) or phalloidin (red). a, d, and g, EGFP and actin; b, e, and h, TG2 and actin; c, f, and i, merge. Scale bar, 30 μm. Right panel, quantitation of the number of transfected cells showing membrane ruffling of the total cell number. Error bars, standard deviation of duplicate coverslips. Results shown here represent one of three independent experiments.
Rac has many functions as a molecular switch. Ridley et al. (27) reported that microinjection of BcrGAP into serum-starved Swiss3T3 fibroblasts inhibits one important Rac-regulated activity, namely membrane ruffling stimulated by PDGF. We examined if TG2 affects this through an inhibitory effect on BcrGAP activity toward Rac1. As shown in Fig. 3C, when cells were stimulated with EGF, ∼75% exhibited ruffles, but only 20% of Bcr-expressing cells showed ruffles, consistent with a previous report (27). Expression of TG2 alone had no significant effect on membrane ruffling. Interestingly, in ∼45% of cells co-expressing Bcr and TG2, membrane ruffling was observed. These findings indicate that TG2 suppresses GAP activity of Bcr toward Rac in EGF-stimulated cells.
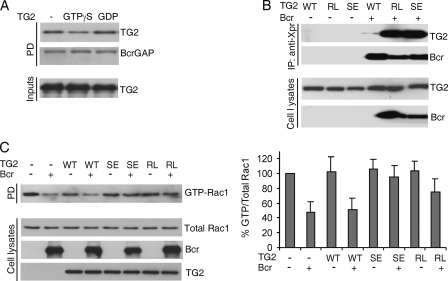
GTP Regulates the Interaction of TG2 with Bcr
TG2 is a multifunctional protein with GTP binding/hydrolyzing activity as well as an enzymatic Ca2+-dependent transamidation activity. We also examined if the binding of GTP to TG2 influences the interaction of TG2 with Bcr. We loaded recombinant TG2 with the non-hydrolyzable GTPγS or with GDP, then added GST-BcrGAP, and performed a GST pull-down assay. As shown in Fig. 4A, GTPγS binding to TG2 caused a significant reduction in the binding of TG2 to BcrGAP. To further test if guanine nucleotide binding could affect the interaction between TG2 and Bcr in cells, we performed co-transfections of Myc-tagged WT TG2 or Myc-tagged GTP binding-defective TG2 mutants S171E and R580L with Xpress-tagged Bcr. Interestingly, the two TG2 mutants immunoprecipitated much more efficiently with Bcr than WT TG2 (Fig. 4B).
FIGURE 4.
GTP inhibits the interaction of Bcr and TG2. A, GTPγS or GDP-loaded recombinant TG2 was incubated with GST-BcrGAP. The amount of TG2 bound to BcrGAP was analyzed by PD and immunoblotting with TG2 antibodies. GST-BcrGAP was visualized by Coomassie Brilliant Blue staining. B, the indicated combinations of plasmids including Xpress-tagged Bcr and Myc-tagged TG2 WT (1 μg), R580L (RL, 5 μg), or S171E (SE, 5 μg) were co-transfected into COS-1 cells and after 1 day of transfection, IPs were performed with Xpress antibodies, followed by Western blot with Xpress or Myc antibodies. C, left panel, the plasmids indicated were transfected as described in Fig. 4B. Rac activation assays were performed as described in the legend to Fig. 3A but after 1 day. Myc antibodies were used to determine TG2 mutant expression. Right panel, quantitation of results. Bars, mean ± S.D. of three independent experiments.
We therefore tested whether this large difference in interaction could influence the regulation of the BcrGAP activity by TG2, by measuring levels of active Rac1 in the presence of Bcr and WT or mutant TG2. Expression of the S171E and R580L mutants is less efficient than that of the WT TG2 and is also extinguished relatively soon after transfection (21, 22). Because of this, lysates were prepared 1 day after transfection, and amounts of WT TG2 used for transfection were lower than those used for the experiments in Fig. 3. As shown in Fig. 4C (left and right panels), under these suboptimal conditions, WT TG2 was not able to significantly block activity of the Bcr GAP domain. However, the GTP binding-deficient S171E and R580L mutants were very effective in blocking the GAP activity of Bcr and restoring GTP-Rac levels to that of cells not expressing Bcr. These results suggest that GTP binding-defective mutants inhibit BcrGAP activity more efficiently, because they can interact with Bcr more efficiently compared with WT TG2.
DISCUSSION
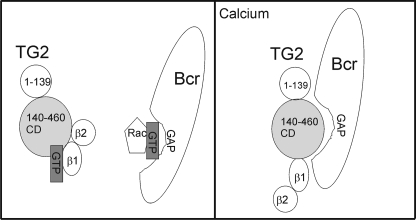
TG2 is a large multidomain protein with interesting but very complex activity and regulation in vivo and in vitro. A number of studies are consistent with a model in which TG2 exists in at least two distinct conformations, which may be dependent upon the physiological state of a cell. The so-called closed conformation is adopted when TG2 is guanine nucleotide-bound. In this form of TG2, the catalytic core domain is hidden, access to the domain is blocked, and the protein is inactive as transamidase. In contrast, the conformation of guanine nucleotide-free or Ca2+-activated TG2 is open (2, 28, 29). TG2 mutants such as R580A and S171E are unable to bind guanine nucleotides and are locked in this non-compacted conformation, exposing the catalytic core domain (22, 30). Our studies are consistent with a model in which the interaction between Bcr and TG2 is conformation dependent, taking place preferentially with the more open conformation of TG2 (Fig. 5). First, Bcr binds to the catalytic core domain of TG2, which is exposed in the open conformation. Second, the R580A and S171E mutants showed a very strong interaction with Bcr. Third, we found that the binding of TG2 to BcrGAP was increased in the presence of Ca2+ (data not shown). However, although Bcr bound to the TG2 catalytic core, the interaction was independent of the catalytic core domain being active as transamidase.
FIGURE 5.
Schematic diagram of regulation of Bcr by TG2. Left, in the presence of guanine nucleotides, TG2 is in a closed conformation with the catalytic core domain masked by the β1/β2 domains and unable to interact with Bcr, which is shown as in the process of binding GTP-bound Rac. Right, TG2 that is not bound to guanine nucleotides or is in the presence of calcium has an open conformation with the C-terminal β1 and β2 barrels not in contact with the catalytic core domain (28, 29). The catalytic core domain can bind to Bcr molecules that have hydrolyzed and released GDP-bound Rac.
The interaction between Bcr and TG2 was measured under normal physiological conditions, indicating that the more open conformation of TG2 is present in non-stressed cells. Many signal transduction pathways including those of EGF lead to transient increases in intracellular free Ca2+ that could locally induce the open conformation of TG2 (31). Indeed, Antonyak et al. (21) showed that HeLa cells stimulated with EGF contain both GTP-bound TG2 and transamidase-proficient TG2.
Interestingly, stimulation of the α1B-adrenergic receptor with epinephrine results in the activation of PLCδ1, the exchange of GDP for GTP on TG2 mediated by PLCδ1, and the release of calcium from intracellular stores (32). Thus, it is possible that TG2 undergoes a number of rapid conformational switches upon cell signaling, with an initially GTP-bound, effector binding state followed by a calcium-bound state that exposes the CD domain for interaction with proteins such as Bcr.
Our studies show that TG2 plays a role in the normal regulation of Bcr activity and through it, of Rac activation, and cytoskeletal organization. Although there have been a number of previous reports showing that TG2 can regulate Rho and Rho family-controlled processes, these studies either reported a different molecular mechanism or did not clearly identify one. One mechanism described involves the post-translational modification/transamidation activity of TG2, which may be relatively irreversible: when TG2 is activated by retinoic acid, it can directly modify and activate RhoA by transamidation, resulting in Rho-associated kinase-2 activation (33). TG2 can additionally activate Rac1 and RhoA by their transamidation to serotonin (34, 35). Cell surface TG2 was reported to regulate RhoA activity via a non-enzymatic mechanism involving integrin clustering (3). Also, Toth et al. (36) proposed that TG2 plays a role in Rac activation via integrin β3.
In addition, there have been several other studies showing that TG2 is involved in processes needing actin cytoskeletal reorganization (26, 36), a Rho family member-regulated process. Antonyak et al. (21) recently reported that TG2 is an essential component of EGF-stimulated migration. Interestingly, they showed that the TG2 R580L and C277V mutants, which bind to Bcr, both localize to the leading edge of cells. They speculate that the active site of TG2 may be involved in the direct binding to actin and act as a scaffold for the recruitment of other proteins that influence actin polymerization. This would be consistent with the results of our own studies, which show that TG2 binds to the Rac regulatory protein Bcr.
Thus, TG2 clearly is involved in the regulation of Rac, and it will be of interest to determine if the regulation is associated only with maintenance of normal cell homeostasis, or if it can be linked to apoptosis/cell death and/or pathologies such as celiac and neurodegenerative diseases. Our other studies3 show that TG2 can also use Bcr as a substrate and cross-link it. The combined data will allow us to start to dissect how TG2 regulates Rac under normal and pathological conditions.
Acknowledgments
We thank Leena Haataja, Anja Reichert, and Emmanuelle Faure for work on the yeast two-hybrid system; Vesa Kaartinen and Ron de Jong for designing, constructing, and testing BcrΔGAP and BcrΔPK, Gail V. W. Johnson (University of Rochester) for the generous gifts of TG2 wild-type and C277S clones; Richard A. Cerione (Cornell University) for the generous gifts of Myc-tagged TG2 wild-type and GTP-binding defective mutants constructs; and Kyunghwan Kim for careful review of the manuscript and helpful suggestions.
This work was supported, in whole or in part, by Public Health Service, National Institutes of Health, Grants HL071945 and HL060231.
N. Heisterkamp, manuscript in preparation.
- TG2
- transglutaminase 2
- EGF
- epidermal growth factor
- GAP
- GTPase-activating protein
- GDI
- guanine nucleotide dissociation inhibitor
- GEF
- guanine nucleotide exchange factor
- HBMEC
- human brain microvascular endothelial cells
- IP
- immunoprecipitation
- PBS
- phosphate-buffered saline
- PD
- pull-down
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate
- WT
- wild type.
REFERENCES
- 1.Fesus L., Piacentini M. (2002) Trends Biochem. Sci. 27, 534–539 [DOI] [PubMed] [Google Scholar]
- 2.Griffin M., Casadio R., Bergamini C. M. (2002) Biochem. J. 368, 377–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janiak A., Zemskov E. A., Belkin A. M. (2006) Mol. Biol. Cell 17, 1606–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zemskov E. A., Janiak A., Hang J., Waghray A., Belkin A. M. (2006) Front Biosci. 11, 1057–1076 [DOI] [PubMed] [Google Scholar]
- 5.Heisterkamp N., Groffen J. (2001) in Chronic Myeloid Leukemia: Biology and Treatment (Carella A. M., Daley G. Q., Eaves C. J., Goldman J. M., Hehlman R. eds) pp. 3–17, Martin Dunitz, Ltd., London [Google Scholar]
- 6.Diekmann D., Brill S., Garrett M. D., Totty N., Hsuan J., Monfries C., Hall C., Lim L., Hall A. (1991) Nature 351, 400–402 [DOI] [PubMed] [Google Scholar]
- 7.Chuang T. H., Xu X., Kaartinen V., Heisterkamp N., Groffen J., Bokoch G. M. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 10282–10286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho Y. J., Cunnick J. M., Yi S. J., Kaartinen V., Groffen J., Heisterkamp N. (2007) Mol. Cell. Biol. 27, 899–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunnick J. M., Schmidhuber S., Chen G., Yu M., Yi S. J., Cho Y. J., Kaartinen V., Minoo P., Warburton D., Groffen J., Heisterkamp N. (2009) Mol. Cell. Biol. 29, 5742–5750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaartinen V., Gonzalez-Gomez I., Voncken J. W., Haataja L., Faure E., Nagy A., Groffen J., Heisterkamp N. (2001) Development 128, 4217–4227 [DOI] [PubMed] [Google Scholar]
- 11.Bishop A. L., Hall A. (2000) Biochem. J. 348, 241–255 [PMC free article] [PubMed] [Google Scholar]
- 12.Bokoch G. M. (2005) Trends Cell Biol. 15, 163–171 [DOI] [PubMed] [Google Scholar]
- 13.Vega F. M., Ridley A. J. (2008) FEBS Lett. 582, 2093–2101 [DOI] [PubMed] [Google Scholar]
- 14.Heasman S. J., Ridley A. J. (2008) Nat. Rev. Mol. Cell Biol. 9, 690–701 [DOI] [PubMed] [Google Scholar]
- 15.Moon S. Y., Zheng Y. (2003) Trends Cell Biol. 13, 13–22 [DOI] [PubMed] [Google Scholar]
- 16.Bernards A., Settleman J. (2004) Trends Cell Biol. 14, 377–385 [DOI] [PubMed] [Google Scholar]
- 17.Bernards A., Settleman J. (2005) Growth Factors 23, 143–149 [DOI] [PubMed] [Google Scholar]
- 18.Lahoz A., Hall A. (2008) Genes Dev. 22, 1724–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kweon S. M., Cho Y. J., Minoo P., Groffen J., Heisterkamp N. (2008) J. Biol. Chem. 283, 3023–3030 [DOI] [PubMed] [Google Scholar]
- 20.Mishra S., Reichert A., Cunnick J., Senadheera D., Hemmeryckx B., Heisterkamp N., Groffen J. (2003) Oncogene 22, 8255–8262 [DOI] [PubMed] [Google Scholar]
- 21.Antonyak M. A., Li B., Regan A. D., Feng Q., Dusaban S. S., Cerione R. A. (2009) J. Biol. Chem. 284, 17914–17925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Datta S., Antonyak M. A., Cerione R. A. (2006) Biochemistry 45, 13163–13174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho Y. J., Hemmeryckx B., Groffen J., Heisterkamp N. (2005) Biochem. Biophys. Res. Commun. 333, 1276–1283 [DOI] [PubMed] [Google Scholar]
- 24.Feng J. F., Readon M., Yadav S. P., Im M. J. (1999) Biochemistry 38, 10743–10749 [DOI] [PubMed] [Google Scholar]
- 25.Benard V., Bokoch G. M. (2002) Methods Enzymol. 345, 349–359 [DOI] [PubMed] [Google Scholar]
- 26.Yi S. J., Choi H. J., Yoo J. O., Yuk J. S., Jung H. I., Lee S. H., Han J. A., Kim Y. M., Ha K. S. (2004) Biochem. Biophys. Res. Commun. 325, 819–826 [DOI] [PubMed] [Google Scholar]
- 27.Ridley A. J., Self A. J., Kasmi F., Paterson H. F., Hall A., Marshall C. J., Ellis C. (1993) EMBO J. 12, 5151–5160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinkas D. M., Strop P., Brunger A. T., Khosla C. (2007) PLoS Biol. 5, e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu S., Cerione R. A., Clardy J. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 2743–2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Begg G. E., Carrington L., Stokes P. H., Matthews J. M., Wouters M. A., Husain A., Lorand L., Iismaa S. E., Graham R. M. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 19683–19688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noh D. Y., Shin S. H., Rhee S. G. (1995) Biochim. Biophys. Acta 1242, 99–113 [DOI] [PubMed] [Google Scholar]
- 32.Baek K. J., Kang S. K., Damron D., Im M. (2001) J. Biol. Chem. 276, 5591–5597 [DOI] [PubMed] [Google Scholar]
- 33.Singh U. S., Kunar M. T., Kao Y. L., Baker K. M. (2001) EMBO J. 20, 2413–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dai Y., Dudek N. L., Patel T. B., Muma N. A. (2008) J. Pharmacol. Exp. Ther. 326, 153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guilluy C., Rolli-Derkinderen M., Tharaux P. L., Melino G., Pacaud P., Loirand G. (2007) J. Biol. Chem. 282, 2918–2928 [DOI] [PubMed] [Google Scholar]
- 36.Tóth B., Garabuczi E., Sarang Z., Vereb G., Vámosi G., Aeschlimann D., Blaskó B., Bécsi B., Erdõdi F., Lacy-Hulbert A., Zhang A., Falasca L., Birge R. B., Balajthy Z., Melino G., Fésüs L., Szondy Z. (2009) J. Immunol. 182, 2084–2092 [DOI] [PubMed] [Google Scholar]