Abstract
The 26 S proteasome complex that comprises the 20 S core and 19 S regulatory (with six ATPases) particles is engaged in an ATP-dependent degradation of a variety of key regulatory proteins and, thus, controls important cellular processes. Interestingly, several recent studies have implicated the 19 S regulatory particle in controlling eukaryotic transcriptional initiation or activation independently of the 20 S core particle. However, the mechanism of action of the 19 S proteasome subcomplex in regulation of eukaryotic transcriptional activation is not clearly understood in vivo. Here, using a chromatin immunoprecipitation assay in conjunction with mutational and transcriptional analyses in Saccharomyces cerevisiae, we show that the 19 S proteasomal subcomplex establishes a specific protein interaction network at the upstream activating sequence of the promoter. Such an interaction network is essential for formation of the preinitiation complex at the core promoter to initiate transcription. Furthermore, we demonstrate that the formation of the transcription complex assembly at the promoter is dependent on 19 S ATPase activity. Intriguingly, 19 S ATPases appear to cross-talk for stimulation of the assembly of transcription factors at the promoter. Together, these results provide significant insights as to how the 19 S proteasome subcomplex regulates the formation of the active transcription complex assembly (and, hence, transcriptional initiation) at the promoter in vivo.
Introduction
In eukaryotes, transcription is mechanistically divided into different steps such as preinitiation complex (PIC)4 formation and initiation, elongation, and termination. Transcriptional initiation is an important regulatory step of gene expression, which is greatly stimulated by the gene-specific activators whose recognition sites are present at the upstream region of the promoter. A variety of studies (1–5) indicates that activator interacts directly with one or more components of the transcription machinery to stimulate the assembly of general transcription factors such as TFIIA, TFIIB, TFIID, TFIIE, TFIIF, and TFIIH as well as RNA polymerase II holoenzyme for formation of the PIC at the core promoter to initiate transcription. Thus, a large number of proteins must interact with each other and with the promoter DNA either directly or indirectly during transcriptional initiation.
A growing number of recent studies (6–28) have implicated the 26 S proteasome complex in controlling and orchestrating the interactions of the transcriptional initiation factors and their localization and abundance in regulating transcriptional initiation or activation. The 26 S proteasome is a highly versatile protein degradation machine with molecular chaperonin activity and consists of a 20 S proteolytic core particle (CP) and a 19 S regulatory particle (RP). The 20 S CP has a cylinder-like structure composed of a stack of two α and two β rings, whereas the 19 S RP comprises a “lid” of eight non-ATPases and a “base” of six ATPases (Rpt1-Rpt6) and three non-ATPases (29–35). 19 S ATPases provide the molecular chaperonin activity to the 19 S RP (36). Furthermore, 19 S ATPase activity is essential for the assembly of the 26 S proteasome complex (34) and participates in the degradation of the proteins marked by a chain of more than four Lys-48-linked ubiquitin molecules (37–39). The lid of the 26 S proteasome associates with the polyubiquitin chain, and subsequently the base unfolds the substrate protein in an ATP-dependent manner, finally translocating it to the central chamber of the 20 S CP for proteolysis (31, 32, 40–49). Through this mechanism the 26 S proteasome complex regulates the functions and fates of many transcription factors in a highly responsive and coordinated manner to control many important biological processes (50, 51). In fact, the temperature-sensitive (ts) inactivation of either the 19 S RP or 20 S CP in yeast alters the levels of ∼70% of the genomic transcript at least by 2-fold (52).
Interestingly, the genome-wide location analysis in yeast revealed that several hundred genes are associated with either the 19 S RP or 20 S CP (52, 53). Consistent with this observation, Sulahian et al. (20) have shown that the transcription of several stress-responsive genes such as HSP26, HSP104, and GAD1 is regulated by the 19 S RP independently of the proteolytic function of the 20 S CP. On the other hand, expression of the ribosomal protein genes is dependent on the 20 S CP (52, 54, 55). Thus, the 26 S proteasome plays distinct functions in regulating transcription at different sets of genes in proteolysis-dependent as well as proteolysis-independent manners. Although the mechanisms for the proteolytic role of the proteasome in transcription are relatively well established (28), how the 19 S RP regulates eukaryotic transcriptional activation independently of the 20 S CP is not clearly understood. Recent biochemical studies in yeast (13, 23) have implicated the non-proteolytic role of the 19 S RP in regulation of the activator-coactivator/promoter interactions (and hence transcriptional activation). However, the mechanism of action of the 19 S RP in regulation of eukaryotic transcriptional activation remains unknown in vivo. Here, using a formaldehyde-based in vivo cross-linking and chromatin immunoprecipitation (ChIP) assay in conjunction with mutational and transcriptional analyses, we show that the 19 S RP is essential in establishing a specific protein interaction network at the upstream activating sequence (UAS) of the active gene promoter in yeast (Saccharomyces cerevisiae). Such an interaction network is essential for formation of the PIC assembly at the core promoter to initiate transcription. Furthermore, our study reveals that the 19 S RP does not merely behave as a physical adaptor to establish the specific protein interaction network at the promoter. Rather, its ATPase activity is essential for formation of the transcriptional initiation complex. Interestingly, we also find that 19 S ATPases appear to cross-talk to facilitate the formation of the transcription complex assembly at the promoter in vivo. Collectively, our data demonstrate that the 19 S proteasomal ATPases function in a cooperative manner to establish a specific interaction network of the transcription factors at the promoter for efficient transcriptional initiation in vivo, thus significantly advancing our fundamental knowledge of the regulatory mechanisms of eukaryotic transcriptional activation by the 19 S RP in vivo.
EXPERIMENTAL PROCEDURES
Plasmids
The plasmid pFA6a-13Myc-KanMX6 (56) was used for genomic Myc epitope tagging of the proteins of interest. The plasmid pRS416 was used in the PCR-based gene disruption.
Yeast Strains and Media
Yeast (S. cerevisiae) strains harboring the ts mutation in SPT15 and its isogenic wild type equivalent were obtained from Cormack and Struhl, Harvard Medical School (57). The srb4-ts and wild type strains were obtained from Thompson and Young, MIT (58). The rpt1-K256R (DY98), rpt3-K219R (DY93), rpt4-297R (DY219), rpt5-K228R (DY155), and rpt6-K195R(DY100) point mutant strains were from Finley et al., Harvard Medical School (59). The FY67 and FY1097 strains were obtained from Roberts and Winston, Harvard Medical School (60). Multiple Myc epitope tags were added at the original chromosomal loci of RPT6, RPN9, PRS3, PRE6, and RPN12 in FY67 to generate NSY1 (Rpt6p-Myc), NSY5 (Rpn9p-Myc), NSY6 (Prs3p-Myc), NSY8 (Pre6p-Myc), and NSY7 (Rpn12p-Myc), respectively (56). Strains PSY17 (Rpt2p-Myc) and PSY18 (Rpt6p-Myc) were generated by adding multiple Myc epitope tags at the C termini of Rpt2p and Rpt6p, respectively, in SC599 (obtained from the laboratory of Stephen A. Johnston; UT Southwestern Medical Center). The endogenous GAL4 gene of NSY1 was disrupted using the PCR-based gene disruption method (61) to generate NSY9 (Rpt6p-Myc, Δgal4::TRP1). Multiple Myc epitope tags were added at the original chromosomal loci of RPT6 in FY1097 to generate NSY2 (Rpt6p-Myc, Δspt20). Strains PSY8 (Rpt6p-Myc in spt15-ts) and PSY7 (Rpt6p-Myc in wild type strain) were generated by insertion of multiple Myc epitope tags at the original chromosomal locus of RPT6 in spt15-ts and wild type equivalent, respectively. Similarly, Rpt6p was C-terminal-tagged with the Myc epitope in the srb4-ts strain (Z628) and its wild type equivalent (Z579) to generate PSY6 and PSY5, respectively. Strains SMY4 (Srb4p-Myc in rpt1-K256R), SMY7 (Srb4p-Myc in rpt5-K228R), SMY6 (Srb4p-Myc in rpt6-K195R), and PBY8 (Srb4p-Myc in the wild type strain) were generated by inserting multiple Myc epitope tags at the C-terminal tail of Srb4p in rpt1-K256R, rpt5-K228R, rpt6-K195R, and wild type strains, respectively.
For the ChIP studies at the GAL genes in the wild type and deletion and point mutant strains, yeast cells were first grown in YPR (yeast extract-peptone plus 2% raffinose) to an A600 of 0.9 and then transferred to YPG (yeast extract-peptone plus 2% galactose) for 90 min of induction at 30 °C before formaldehyde-based in vivo cross-linking. However, spt15-ts, srb4-ts, and isogenic wild type strains were grown in YPG at 23 °C to an A600 of 0.85 and then transferred to 37 °C for 1 h before cross-linking. Similar growth conditions were used for ChIP studies at the ACT1 promoter.
ChIP Assay
The ChIP assay was performed as described previously (62–65). Briefly, yeast cells were treated with 1% formaldehyde, collected, and resuspended in lysis buffer. After sonication, cell lysates (400 μl of lysate from 50 ml of yeast culture) were precleared by centrifugation, and then 100 μl of lysate was used for each immunoprecipitation. Immunoprecipitated protein-DNA complexes were treated with proteinase K, the cross-links were reversed, and then DNA was purified. Immunoprecipitated DNA was dissolved in 20 μl of TE 8.0 (10 mm Tris-HCl, pH 8.0, and 1 mm EDTA), and 1 μl of immunoprecipitated DNA was analyzed by PCR. PCR reactions contained [α-32P]dATP (2.5 μCi for each 25-μl reaction), and PCR products were detected by autoradiography after separation on a 6% polyacrylamide gel. As a control, “input” DNA was isolated from 5 μl of lysate without going through the immunoprecipitation steps and was suspended in 100 μl of TE 8.0. To compare the PCR signal arising from the immunoprecipitated DNA with that of the input DNA, 1 μl of input DNA was used in the PCR analysis. Serial dilutions of the input and immunoprecipitated (IP) DNAs were used to assess the linear range of PCR amplification as described previously (66). The PCR data presented in this article are within the linear range of PCR analysis.
For analysis of recruitment of the proteasome components, we modified the above ChIP protocol as follows (65). 800 μl of lysate was prepared from 100 ml of yeast culture. 400 μl of lysate was used for each immunoprecipitation (using 10 μl of anti-hemagglutinin or anti-Myc antibody and 100 μl of protein A/G plus agarose beads from Santa Cruz Biotechnology, Inc.), and immunoprecipitated DNA sample was dissolved in 10 μl of TE 8.0 of which 1 μl was used in PCR analysis. In parallel, the PCR for input DNA was performed using 1 μl of DNA that was prepared by dissolving purified DNA from 5 μl of lysate in 100 μl of TE 8.0.
Primer pairs used for PCR analysis were as follows: GAL1(UAS), 5′-CGCTTAACTGCTCATTGCTATATTG-3′ and 5′-TTGTTCGGAGCAGTGCGGCGC-3′; GAL1(Core), 5′-ATAGGATGATAATGCGATTAGTTTTTTAGCCTT-3′ and 5′-GAAAATGTTGAAAGTATTAGTTAAAGTGGTTATGCA-3′; GAL7(Core), 5′-CTATGTTCAGTTAGTTTGGCTAGC-3′ and 5′-TTGATGCTCTGCATAATAATGCCC-3′; GAL10(Core), 5′-GCTAAGATAATGGGGCTCTTTACAT-3′; 5′-TTTCACTTTGTAACTGAGCTGTCAT-3′; ACT1(Core), 5′-AACCGTTTTGAAACCAAACTCGCCT-3′ and 5′-TTCTTGGTTTGAGTAGAAAGGGGAA-3′. Autoradiograms were scanned and quantitated by the National Institutes of Health Image 1.62 program. IP DNA was quantitated as the ratio of IP to input.
Whole Cell Extract Preparation and Western Blot Analysis
For analysis of the global levels of proteins of interest such as TBP, Srb4p-Myc, histone H3, Rpb1p, TAF10p, and TAF12p in wild type and 19 S ATPase point mutant strains, the yeast cells were grown in YPR up to an A600 of 0.9 and then induced for 90 min in YPG. The harvested cells were used to prepare the whole cell extract with solubilized chromatin following the protocol as described previously for the ChIP assay. The whole cell extract was run on an SDS-polyacrylamide gel and then analyzed by Western blot. The anti-TBP (obtained from Michael R. Green, University of Massachusetts Medical School), anti-Myc (9E10; Santa Cruz Biotechnology), anti-histone H3 (Abcam, Inc.), anti-Rpb1p (8WG16; Covance), anti-TAF10p and anti-TAF12p (from Michael R. Green) antibodies against TBP, Myc-tagged Srb4p, histone H3, Rpb1p, TAF10p, and TAF12p, respectively, were used for Western blot analysis.
Primer Extension Analysis
Primer extension analysis was performed as described previously (64). The primers used for analysis of GAL1 and ACT1 mRNAs were as follows: GAL1, 5′-CCTTGACGTTACCTTGACGTTAAAGTATAGAGG-3′; ACT1, 5′-CGGCAAAACCGGCTTTACAC-3′.
RESULTS
The 19 S Base, but Not Lid or 20 S CP, Is Predominantly Recruited to the GAL1 UAS in a Transcription-dependent Manner
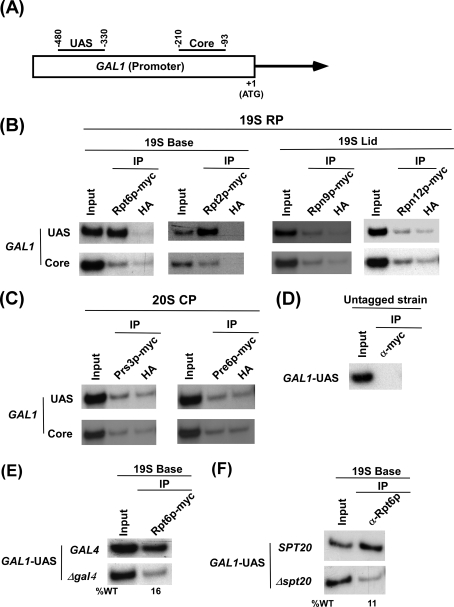
To analyze recruitment of various components (e.g. 19 S base and lid and 20 S CP) of the 26 S proteasome complex at the promoter (Fig. 1A) of a transcriptionally active gene, GAL1, we tagged the Rpt6p (19 S base), Rpt2p (19 S base), Rpn9p (19 S lid), Rpn12p (19 S lid), Prs3p (20 S CP), and Pre6p (20 S CP) components of the proteasome by Myc epitope in their endogenous chromosomal loci. Because these proteins are essential for cellular viability, functionalities of these Myc-tagged proteasome components were analyzed by the growth of the strains bearing these Myc-tagged proteins. The strains containing Myc-tagged components of the proteasome grew normally on solid growth medium (supplemental Fig. 1A). We also analyzed the growth of these epitope-tagged strains in liquid growth medium for 4 h after an A600 of 0.3. Within this 4-h time, the Myc-tagged strains (except the strain carrying Myc-tagged Rpt6p) grew relatively slowly as compared with the untagged strain (supplemental Figs. 1, B and C). Thus, Myc epitope seems to mildly alter the functions of Rpt2p, Rpn9p, Rpn12p, Prs3p, and Pre6p. However, such a mild loss of function would not significantly interfere with recruitment analysis of these components. Thus, using these strains we performed the ChIP assay at the GAL1 UAS and core promoter (Fig. 1A) as described previously (62–65). Fig. 1B shows that the 19 S base (Rpt6p and Rpt2p) was predominantly recruited to the UAS but not the core of the GAL1 promoter in galactose-containing growth medium (inducing conditions). However, the 19 S lid (Rpn9p and Rpn12p) or 20 S CP (Prs3p and Pre6p) was not recruited to the GAL1 promoter under inducing conditions (Figs. 1, B and C). As a control, we show the absence of ChIP signal in the strain that does not bear Myc-tagged proteasome component (Fig. 1D). Together, these results demonstrate that the 19 S base is specifically recruited to the GAL1 UAS independently of the 19 S lid or 20 S CP in galactose-containing growth medium. However, the 19 S base was not recruited to the GAL1 UAS under non-inducing conditions (raffinose-containing growth medium) (data not shown). Thus, the 19 S base is recruited to the GAL1 UAS in a transcription-dependent manner in vivo. Like the 19 S base, activator (Gal4p), co-activator (SAGA (Spt-Ada-Gcn5-acetyltransferase)), and SRB/Mediator are also recruited to the UAS, but not core promoter, of GAL1 (64). Thus, like Gal4p, SAGA, and Mediator, the 19 S base is an UAS-specific factor and, hence, might play a crucial role in regulating the protein interaction network at the GAL1 promoter during transcriptional activation in vivo. Consistent with our in vivo data, Sun et al. (67) have also biochemically characterized the 19 S base without lid or 20 S CP and named it as APIS (ATPases-independent of 20 S). Furthermore, a recent study also demonstrated the presence of the 19 S base independently of the 19 S lid or 20 S CP in nucleus (68).
FIGURE 1.
The 19 S base is predominantly recruited to the UAS, but not core promoter, of GAL1 dependently on Gal4p and SAGA. A, shown is a schematic diagram of the GAL1 promoter with the PCR amplification regions (UAS and Core) in the ChIP assay. B and C, analysis of recruitment of the 26 S proteasome to the GAL1 promoter is shown. The Rpt6p (Base), Rpt2p (Base), Rpn9p (Lid), Rpn12p (Lid), and Prs3p and Pre6p (20CP) components of the 26 S proteasome complex were tagged by Myc epitope at their chromosomal loci. The yeast strains expressing Myc-tagged proteasome components were grown at 30 °C in YPG (yeast extract, peptone plus 2% galactose) up to an A600 of 1.0 before formaldehyde-based in vivo cross-linking. The ChIP assay was performed as described previously (62–65). Primer pairs (“Experimental Procedures”) located in the UAS and core promoter regions of GAL1 were used for PCR analysis of the immunoprecipitated DNA samples. Immunoprecipitation was performed using a mouse monoclonal antibody against the c-Myc epitope tag (9E10; Santa Cruz Biotechnology). The anti-hemagglutinin (HA; Santa Cruz Biotechnology, Inc.) was used as a nonspecific antibody. D, shown is the ChIP analysis at the GAL1 UAS in the yeast strain that does not bear Myc-tagged proteins using an anti-Myc antibody. E, Gal4p is essential for recruitment of the 19 S base to the GAL1 UAS. The wild type (WT) and GAL4 deletion mutant strains expressing Myc-tagged Rpt6p were first grown in YPR at 30 °C to an A600 of 0.9 and then transferred to YPG at 30 °C for 90 min before formaldehyde-based cross-linking. The immunoprecipitation was performed as in panels B and C. F, SAGA is essential for recruitment of the 19 S base to the GAL1 UAS. The wild type and SPT20 deletion mutant strains were first grown in YPR at 30 °C to an A600 of 0.9 and then transferred to YPG at 30 °C for 90 min before formaldehyde-based cross-linking. The immunoprecipitation was performed using a polyclonal antibody against Rpt6p (obtained from Stephen A. Johnston and Thomas Kodadek).
Recruitment of the 19 S Base to the GAL1 UAS Is Dependent on Gal4p-SAGA Interaction
Because the 19 S base is recruited to the GAL1 UAS, we next asked whether activator is essential for recruitment of the 19 S base. To address this question, we analyzed recruitment of the 19 S base to the GAL1 UAS in the presence and absence of the activator, Gal4p. Fig. 1E shows that the 19 S base (Rpt6p) was not recruited to the GAL1 UAS in the absence of Gal4p. Thus, Gal4p targets recruitment of the 19 S base to the GAL1 UAS. However, our recent in vivo studies (64) have demonstrated that Gal4p also targets SAGA to the GAL1 UAS through its direct interaction with Tra1p (the largest subunit of SAGA). Thus, it seems likely that the 19 S base might not be directly targeted by Gal4p at the GAL1 UAS in vivo, and SAGA might play an important role in recruitment of the 19 S base. To test this hypothesis, we next analyzed recruitment of the 19 S base to the GAL1 UAS in the absence of Spt20p that maintains the structural integrity of SAGA (66, 69). Fig. 1F shows that recruitment of the 19 S base to the GAL1 UAS was impaired in Δspt20. Thus, Gal4p-SAGA interaction is essential for recruitment of the 19 S base to the GAL1 UAS in vivo.
ATPase Activity of the 19 S Base Enhances Gal4-SAGA Interaction at the GAL1 UAS
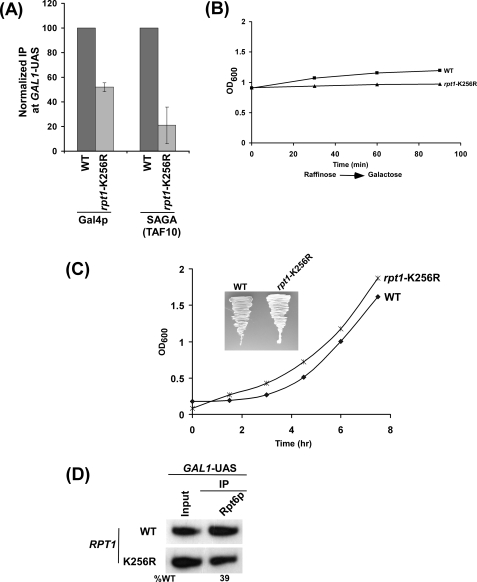
The data presented in Fig. 1 demonstrate that Gal4p-SAGA interaction is required for recruitment of the 19 S base to the GAL1 UAS. However, a recent biochemical study (13) has demonstrated that the 19 S RP enhances Gal4p-SAGA interaction. How does the 19 S base enhance the Gal4p-SAGA interaction, whereas its recruitment is dependent on Gal4p and SAGA? We hypothesized that Gal4p might interact weakly with SAGA in the absence of the 19 S base in vivo. Such a weak Gal4p-SAGA interaction recruits the 19 S base, which in turn enhances Gal4p-SAGA interaction in a positive feedback manner via its ATPase or molecular chaperonin activity. To test this hypothesis, we analyzed recruitment of Gal4p and SAGA (TAF10p) to the GAL1 UAS in the wild type and RPT1 ATPase point mutant (rpt1-K256R) strains. TAF10p is an integral component of both SAGA and TFIID (62, 70–72). SAGA is recruited to the UAS, whereas TFIID is recruited to the core promoter (62, 64, 72). Because GAL1 is a TFIID-independent but SAGA-dependent promoter, TAF10p is specifically recruited to the GAL1 UAS, but not to the core promoter, as a component of SAGA (62). Thus, TAF10p was chosen to monitor recruitment of SAGA to the GAL1 UAS.
The rpt1-K256R point mutant was generated by substituting the lysine (that interacts with ATP) to arginine in the Walker A box of the ATPase domain (59). Such a substitution maintains the positive charge and, thus, does not completely impair ATPase activity of Rpt1p (59). Using this point mutant, we performed the ChIP assay. We found that recruitment of SAGA (TAF10p) to the GAL1 UAS was significantly decreased (∼5-fold) in the rpt1-K256R point mutant (Fig. 2A). However, Gal4p recruitment was modestly reduced (∼2-fold) in this point mutant (Fig. 2A). Such a decrease in recruitment of Gal4p and SAGA could be due to slow growth of the point mutant during the 90-min induction time period in galactose-containing growth medium. To test this possibility, we analyzed the growth of the wild type and point mutant strains under inducing conditions. Fig. 2B shows that growth of the rpt1-K256R point mutant was not significantly changed as compared with the wild type strain during the 90-min induction time period. Furthermore, the point mutant grew normally in the solid (Fig. 2C, inset) as well as liquid (Fig. 2C) growth media containing dextrose. Thus, the decrease in recruitment of Gal4p and SAGA in the rpt1-K256R point mutant was not resulted from the growth defect of the mutant strain. Furthermore, our Western blot analysis revealed that the stability of TAF10p was not altered in the rpt1-K256R point mutant strain (supplemental Fig. 2B). Like TAF10p, recruitment of another SAGA component, TAF12p, to the GAL1 UAS was significantly reduced in the rpt1-K256R strain (supplemental Fig. 2A), and its stability was also not altered (supplemental Fig. 3B). TAF12p is also a component of TFIID complex, but it is not recruited to the core promoter of the TFIID-independent GAL1 gene (62). Rather, it is recruited to the GAL1 UAS as a component of the SAGA complex (62). Together, our data support the fact that ATPase activity of the 19 S base enhances recruitment of SAGA to the GAL1 UAS. However, recruitment of SAGA was significantly decreased (∼5-fold) as compared with that of Gal4p (∼2-fold). Thus, additional decrease of SAGA recruitment to the GAL1 UAS in the rpt1-K256R mutant was attributed to the alteration of Gal4p-SAGA interaction in vivo.
FIGURE 2.
ATPase activity of the 19 S base enhances the targeting of SAGA to the GAL1 UAS. A, shown is an analysis of Gal4p and SAGA (TAF10p) recruitment to the GAL1 UAS in the rpt1-K256R point mutant. Both the wild type (WT) and point mutant strains were first grown in YPR up to A600 of 0.9 and then transferred to YPG for 90 min before cross-linking. Immunoprecipitations were carried out using a mouse monoclonal antibody against the DNA binding domain of Gal4p (RK5C1; Santa Cruz Biotechnology) or a polyclonal antibody against TAF10p (obtained from Michael R. Green, University of Massachusetts Medical School). The ratio of immunoprecipitate over the input in the autoradiogram was measured. The ratio for the wild type strain was normalized to 100. The normalized ratio (represented as normalized IP) is presented in the form of a histogram. B, shown is growth analysis of the rpt1-K256R point mutant and wild type strains after induction in YPG. Both the wild type and mutant strains were grown in YPR up to an A600 of 0.9 and then transferred to YPG for induction. The A600 of both the wild type and mutant cells were measured at different induction time points. C, shown is growth analysis of the rpt1-K256R point mutant and wild type strains in YPD (yeast extract, peptone plus 2% dextrose). Both the wild type and mutant strains were grown in YPD up to an A600 of ∼0.2, and then the A600 of both the wild type and mutant cells was measured for the next 7 h. Inset, growth analysis of rpt1-K256R point mutant and wild type strains on solid YPD plate is shown. Plates were incubated at 30 °C for 60 h. D, analysis is shown of 19 S base recruitment to the GAL1 UAS in the rpt1-K256R point mutant. Both the wild type and mutant strains were grown and cross-linked as in panel A. Immunoprecipitation was performed as in Fig. 1F.
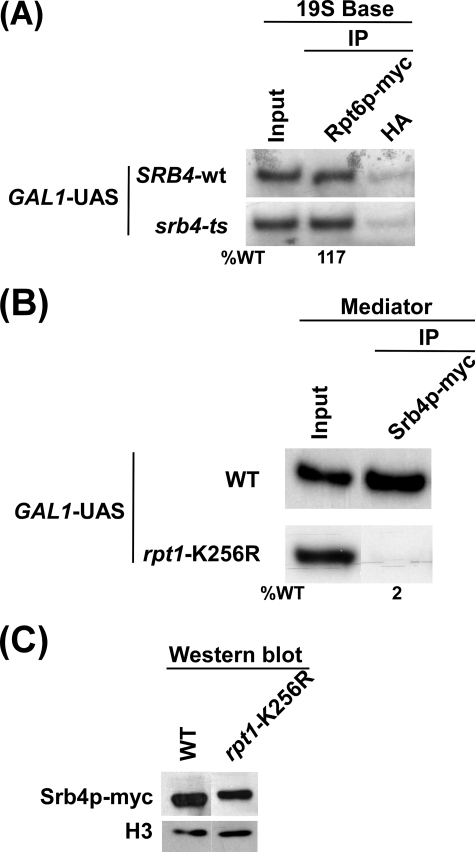
The Recruitment of the 19 S Base to the GAL1 UAS Is Not Dependent on the Mediator Complex
The Mediator complex has been implicated as the target of activator and plays an important role in formation of the transcription complex assembly at the core promoter. We have demonstrated previously that the Mediator complex is recruited to the GAL1 UAS in the presence of SAGA (64). Here, we show that SAGA is also essential for recruitment of the 19 S base to the GAL1 UAS (Fig. 1E). Thus, we next asked whether Mediator, like SAGA, is also essential for recruitment of the 19 S base. To address this question, we tagged the Rpt6p component of the 19 S base by Myc epitope in the wild type and ts mutant strains of Srb4p that maintains structural and functional integrity of the Mediator complex (64, 73, 74). Fig. 3A shows that recruitment of the 19 S base to the GAL1 UAS was not altered in the srb4-ts mutant strain when compared with wild type equivalent. Similarly, the Mediator complex does not alter recruitment of Gal4p and SAGA to the GAL1 UAS (64). Thus, these results indicate that the Mediator complex is associated with the GAL1 UAS after recruitment of SAGA and 19 S base.
FIGURE 3.
ATPase activity of the 19 S base is required for recruitment of Mediator to the GAL1 UAS. A, Mediator is dispensable for recruitment of the 19 S base to the GAL1 UAS. Both the wild type (WT) and srb4-ts mutant strains expressing Myc-tagged Rpt6p were grown in YPG at 23 °C up to an A600 of 0.85 and then transferred to 37 °C for 1 h before cross-linking. Immunoprecipitation was performed as in Figs. 1, B and C. B, ATPase activity of the 19 S base is essential for recruitment of the Mediator complex to the GAL1 UAS. Both the wild type and rpt1-K256R point mutant strains expressing Myc-tagged Srb4p were grown and cross-linked as in Fig. 2A. Immunoprecipitation was performed as in Figs. 1, B and C. C, shown is Western blot analysis. The wild type and rpt1-K256R mutant strains were grown as in panel B. The whole cell extracts from both the wild type and mutant strains were prepared as in the ChIP assay. The whole cell extracts were analyzed by Western blot assay using the anti-Myc and anti-histone H3 antibodies against Myc-tagged Srb4p and histone H3, respectively.
19 S ATPase Activity Is Essential to Recruit the Mediator Complex to the GAL1 UAS
The Mediator complex is dispensable for recruitment of the 19 S base to the GAL1 UAS (Fig. 3A). It is quite possible that ATPase activity of the 19 S base may be essential for recruitment of the Mediator complex. To test this possibility, we next analyzed recruitment of the Mediator complex to the GAL1 UAS in the rpt1-K256R point mutant and its wild type equivalent. Fig. 3B shows that recruitment of the Mediator complex (Srb4p) to the GAL1 UAS was almost lost in the rpt1-K256R point mutant strain. However, such an impaired recruitment of the Mediator complex in this point mutant could be due to the instability of Srb4p. To test this possibility, we analyzed the global level of Srb4p in the rpt1-K256R point mutant and wild type strains. We find that Srb4p is quite stable in this point mutant when compared with wild type equivalent (Fig. 3C), whereas its recruitment was almost lost in the point mutant (Fig. 3B). The level of histone H3 was monitored as a loading control (Fig. 3C). Furthermore, the dramatic impairment of the Mediator recruitment in the point mutant was not resulted from the growth defect (Fig. 2B). However, the loss of the Mediator recruitment to the GAL1 UAS in the rpt1-K256R point mutant strain could be due to disintegration of the 19 S base. To test this possibility, we analyzed recruitment of the 19 S base to the GAL1 UAS in the rpt1-K256R point mutant and wild type strains. Fig. 2D shows that recruitment of the 19 S base to the GAL1 UAS was modestly decreased in the rpt1-K256R point mutant strain. However, a significant amount of the 19 S base remained associated with the GAL1 UAS in this point mutant (Fig. 2D), which was unable to recruit the equivalent amount of the Mediator complex (Fig. 3B). Thus, recruitment of the Mediator complex to the GAL1 UAS is dependent on 19 S ATPase activity but not merely on its function as a physical adaptor.
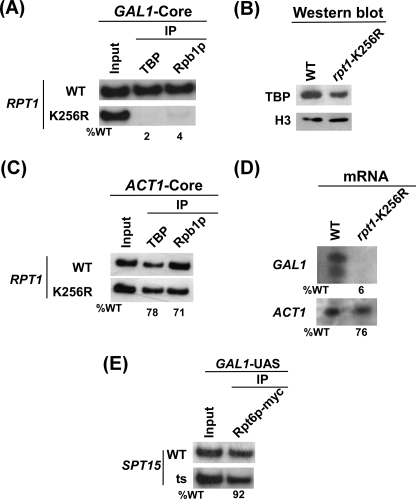
19 S ATPase Activity Is Essential for Formation of the PIC Assembly at the GAL1 Core Promoter
Our data demonstrate that 19 S ATPase activity is essential for recruitment of the Mediator complex. Furthermore, the Mediator complex is essential for recruitment of TBP and RNA polymerase II to the GAL1 core promoter for formation of the PIC assembly (64). Thus, formation of the PIC assembly at the GAL1 core promoter would require 19 S ATPase activity. Indeed, we find that recruitment of TBP to the GAL1 core promoter was almost lost in the rpt1-K256R point mutant strain when compared with wild type equivalent (Fig. 4A). However, TBP is quite stable in this point mutant (Fig. 4B). In addition, growth of this point mutant was not significantly changed when compared with wild type equivalent under the 90-min induction time period in galactose-containing growth medium (Fig. 2B). Thus, the dramatic loss of TBP recruitment to the GAL1 core promoter in this ATPase point mutant did not result from growth defect. Furthermore, we find that a significant amount of the 19 S base was present at the GAL1 UAS in this point mutant (Fig. 2D). Therefore, 19 S ATPase activity, but not merely its physical adaptor function, is required for recruitment of TBP to the GAL1 core promoter. Like TBP, RNA polymerase II (Rpb1p) was not recruited to the GAL1 core promoter in the rpt1-K256R point mutant strain (Fig. 4A and supplemental Fig. 2B). Thus, 19 S ATPase activity is essential for formation of the transcription complex assembly at the GAL1 core promoter.
FIGURE 4.
19 S ATPase activity is essential for formation of the PIC assembly at the GAL1 core promoter. A, shown is analysis of TBP and RNA polymerase II recruitment to the GAL1 core promoter in the wild type and rpt1-K256R point mutant strains. Both the wild type (WT) and point mutant strains were grown and cross-linked as in Fig. 2A. Immunoprecipitations were performed using polyclonal antibodies against TBP and the mouse monoclonal antibody 8WG16 (Covance) against the C-terminal domain of the RNA polymerase II large subunit (Rpb1p). B, shown is Western blot analysis. The wild type and rpt1-K256R mutant strains were grown as in panel A. The whole cell extracts from both the wild type and mutant strains were prepared as in the ChIP assay. The whole cell extracts were analyzed by Western blot assay using the anti-TBP and anti-histone H3 antibodies against TBP and histone H3, respectively. C, analysis is shown of TBP and RNA polymerase II recruitment to the ACT1 core promoter in the wild type and rpt1-K256R point mutant strains. The wild type and mutant strains were grown and cross-linked as in panel A. Immunoprecipitation was performed as in panel A. The primer pair located at the ACT1 core (“Experimental Procedures”) was used for PCR analysis of the immunoprecipitated DNA samples. D, transcription is shown. Total cellular RNA was prepared from the wild type or the mutant strain, and the mRNA levels from the GAL1 and ACT1 genes were quantitated as described previously (64). E, TBP is not required for recruitment of the 19 S base to the GAL1 UAS. Both the wild type and TBP (SPT15) ts mutant strains expressing Myc-tagged Rpt6p were grown, cross-linked as in Fig. 3A. Immunoprecipitation was carried out as in Fig. 3A.
The 19 S ATPase is essential for other cellular process. Thus, the 19 S ATPase point mutant, rpt1-K256R, with significantly impaired ATPase activity via conservative mutation of lysine to arginine in the Walker A box would affect other cellular process. Thus, the loss of the PIC formation at the GAL1 core promoter in the rpt1-K256R point mutant could be due to an indirect effect. To address this issue, we analyzed the formation of the PIC assembly at a proteasome-independent promoter, ACT1 (75). Fig. 4C shows a modest decrease of the PIC formation at the ACT1 core promoter in the rpt1-K256R point mutant strain when compared with wild type equivalent. However, the PIC formation was almost lost at the GAL1 core promoter in the 19 S ATPase point mutant within the 90-min induction time period (Fig. 4A). Thus, the modest decrease of the PIC formation at ACT1 was attributed to an indirect effect of the ATPase point mutant.
The PIC Is Dispensable for Recruitment of the 19 S Base at the GAL1 UAS
The 19 S base that is recruited to the UAS regulates formation of the PIC assembly at the core promoter. Next, we asked whether PIC has any role in regulating recruitment of the 19 S base at the UAS via reciprocal cooperativity. To address this question, we next analyzed recruitment of the 19 S base at the GAL1 UAS in the wild type and ts mutant of TBP, a central component of the PIC assembly. Fig. 4E shows that recruitment of the 19 S base to the GAL1 UAS was not dependent on TBP. Thus, the PIC does not regulate recruitment of the 19 S base to the GAL1 UAS.
The GAL1 Transcription Is Impaired in the Absence of 19 S ATPase Activity
Our data demonstrate that 19 S ATPase activity is essential for formation of the transcription complex assembly at the GAL1 promoter. Therefore, GAL1 transcription would be impaired in 19 S ATPases point mutant strain. To test this possibility, we analyzed GAL1 transcription in the wild type and 19 S ATPase point mutant strains using a primer extension assay. Fig. 4D shows that GAL1 transcription was almost lost in the rpt1-K256R ATPase mutant. This observation is consistent with the dramatic loss of the PIC formation at the GAL1 core promoter in the rpt1-K256R point mutant (Fig. 4A). As a control, we have analyzed the transcription of ACT1 whose expression is not dependent on the proteasome (75). The ACT1 transcription was not significantly altered in the rpt1-K256R point mutant as compared with that of GAL1. Thus, the severe loss of the GAL1 transcription was due to the impaired ATPase activity of the 19 S base.
19 S ATPases Appear to Cross-talk for Formation of the Transcriptional Initiation Complex Assembly
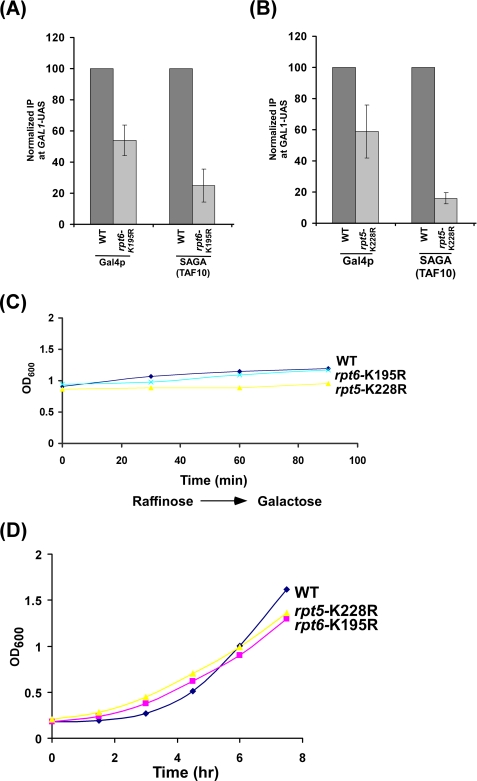
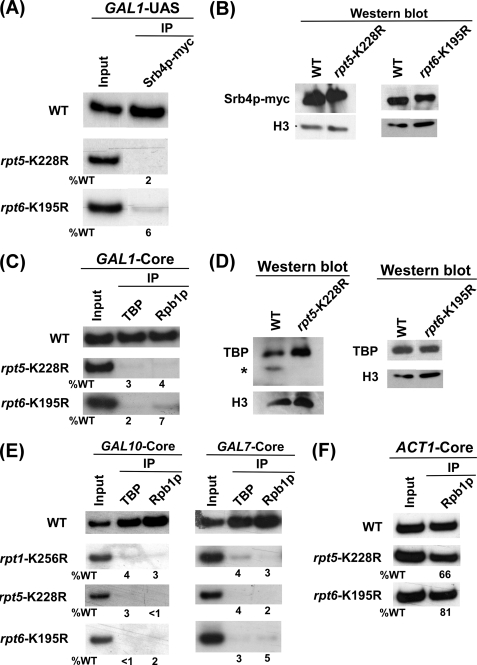
The 19 S base has six AAA (ATPases associated with diverse cellular activities) family ATPases forming a ring-shaped heterohexamer. The point mutation of Rpt1p ATPase does not alter the 19 S base structure but impairs ATPase activity (59). Intriguingly, the other five wild type 19 S ATPase subunits are unable to form the PIC at the promoter in the Rpt1p ATPase point mutant (Figs. 3B and 4A). Such an observation can be attributed to the specific requirement of the Rpt1p ATPase in formation of the transcriptional complex assembly at the promoter. Alternatively, it could be due to the fact that 19 S ATPases function through cross-talk, analogous to homohexameric AAA family ATPases (see, for example, Refs. 44, 59, 76, and 77). To test these possibilities, we next analyzed the formation of the transcription complex assembly at the GAL1 promoter in rpt5-K228R and rpt6-K195R ATPase point mutants. Consistent with rpt1-K256R point mutant, rpt5-K228R and rpt6-K195R point mutants altered the targeting of SAGA to the GAL1 UAS (Figs. 5, A–D). Furthermore, we find that Mediator, TBP, and RNA polymerase II were not recruited to the promoter in these point mutants (Figs. 6, A–D). Similarly, the transcription complex was not assembled at two other GAL genes, namely GAL7 and GAL10, in these point mutants (Fig. 6E). As a control, we analyzed recruitment of RNA polymerase II to the core promoter of the proteasome-independent gene, ACT1. Recruitment of RNA polymerase II was not significantly altered at the ACT1 promoter in these point mutants (Fig. 6F). Similarly, PIC formation at the GAL1 core promoter was almost impaired in other 19 S ATPase point mutants such as rpt3-K219R and rpt4-K297R (data not shown). Together, these results indicate that the 19 S AAA family ATPases with chaperonin activity appears to cross-talk for assembling the transcription factors at the promoter. Analogous to our observation, previous studies have implicated the presence of cross-talk or cooperativity in the ring-shaped homohexameric molecular chaperone formed by AAA family ATPases (see, for example, Refs. 44, 59, 76, and 77). However, the existence of cross-talk or cooperativity among ATPases within heterohexameric molecular chaperone was not known. Our ChIP data support the presence of cross-talk among ATPases in the ring-shaped heterohexameric chaperone, 19 S base.
FIGURE 5.
rpt5-K228R and rpt6-K195R point mutants alter SAGA targeting to the GAL1 UAS. A and B, shown is analysis of Gal4p and SAGA recruitment to the GAL1 UAS in rpt5-K228R and rpt6-K195R point mutants. The wild type (WT) and mutant strains were grown and cross-linked as in Fig. 2A. Immunoprecipitation was performed as in Fig. 2A. C, growth analysis is shown of rpt5-K228R and rpt6-K195R point mutants and wild type strains after induction in YPG. The wild type and mutant strains were grown in YPR up to an A600 of 0.9 and then transferred to YPG for induction. The A600 of both the wild type and mutant cells were measured at different induction time points. D, growth analysis is shown of rpt5-K228R, rpt6-K195R, and wild type strains in YPD. The wild type and mutant strains were grown in YPD up to an A600 of ∼0.2, and then the A600 of the wild type and mutant cells was measured for the next 7 h.
FIGURE 6.
19 S ATPases function in a cooperative manner for formation of the transcription complex assembly. A, recruitment of Mediator to the GAL1 UAS is impaired in rpt5-K228R and rpt6-K195R point mutant strains. The wild type (WT) and mutant strains expressing Myc epitope-tagged Srb4p were grown and cross-linked as in Fig. 3B. Immunoprecipitation was carried out as in Fig. 3B. B, Western blot analysis is shown. The wild type and mutant strains were grown as in panel A. The whole cell extracts from the wild type and mutant strains were prepared as in the ChIP assay. The whole cell extracts were analyzed by Western blot assay using the anti-Myc and anti-histone H3 antibodies against Myc-tagged Srb4p and histone H3, respectively. C, analysis is shown of TBP and RNA polymerase II recruitment to the GAL1 core promoter in rpt5-K228R, rpt6-K195R, and wild type strains. The wild type and point mutant strains were grown and cross-linked as in panel A. Immunoprecipitation was performed as in Fig. 4A. D, Western blot analysis is shown. rpt5-K228R and rpt6-K195R point mutant and wild type strains were grown as in panel A. The whole cell extracts from the wild type and mutant strains were prepared as in the ChIP assay. The whole cell extracts were analyzed by Western blot assay using the anti-TBP and anti-histone H3 antibodies against TBP and histone H3, respectively. E, 19 S ATPases are essential for the PIC formation at the GAL7 and GAL10 promoters. The wild type and 19 S ATPase point mutants were grown and cross-linked as in panel C. Immunoprecipitation was carried out as in panel C. The primer pairs located at the core promoters of the GAL7 and GAL10 genes were used for PCR analysis of the immunoprecipitated DNA samples. F, analysis is shown of RNA polymerase II recruitment to the ACT1 core promoter in the wild type and rpt5-K228R and rpt6-K195R point mutant strains. The wild type and mutant strains were grown and cross-linked as in panel C. Immunoprecipitation was carried out as in panel C.
DISCUSSION
The non-proteolytic function of the proteasome complex has recently been implicated in regulation of eukaryotic transcriptional activation (28). However, the mechanisms by which the proteasome regulates eukaryotic transcriptional activation in a proteolysis-independent manner remain largely unknown. Recent biochemical studies (13, 23) have demonstrated the non-proteolytic role of the proteasome in regulation of the activator-coactivator or activator-promoter interaction (and hence transcriptional activation). However, how the 19 S RP regulates transcriptional activation at the promoter is not yet clearly understood in vivo. Here, we show that the 19 S base, but not lid or 20 S CP, is predominantly recruited to the UAS of a transcriptionally active eukaryotic promoter in an activator/coactivator-dependent manner. Such a recruitment of the 19 S base enhances the activator-coactivator interaction to stimulate transcription in a positive feedback manner through its ATPase or molecular chaperonin activity. Furthermore, we demonstrate that the molecular chaperonin or ATPase activity, but not merely physical adaptor function, of the 19 S base is essential for downstream transcription complex assembly at the promoter. Intriguingly, 19 S ATPases function in a cooperative manner to form the transcription complex assembly at the promoter for transcriptional initiation. These results significantly advance our fundamental understanding of the non-proteolytic role of the proteasome in regulation of eukaryotic transcriptional activation in vivo.
Previous biochemical studies (13) have demonstrated that the 19 S RP enhances Gal4p-SAGA interaction and, hence, activates transcription of the GAL genes. However, it was not known whether similar enhancement of Gal4p-SAGA interaction occurs at the UASs of the GAL genes in vivo. In this study we show that the 19 S base is essential for enhancement of the Gal4p-SAGA interaction at the GAL1 UAS. We also demonstrate here that 19 S ATPase activity stimulates Gal4p-SAGA interaction. Consistent with our in vivo data, previous biochemical studies (13) have demonstrated the physical and genetic interactions between SAGA and the 19 S base. Interestingly, we find that Gal4p-SAGA interaction is essential for recruitment of the 19 S base at the GAL1 UAS. How does the 19 S base stimulate Gal4p-SAGA interaction? Our data support a model where Gal4p first recruits SAGA via weak Gal4p-SAGA interaction in the absence of 19 S ATPase activity, and subsequently, the 19 S base is recruited to the GAL1 UAS through its interaction with SAGA. The 19 S base thus recruited in turn enhances the interaction between Gal4p and SAGA in a positive feedback manner, possibly through proper folding or assembly of the SAGA components by its molecular chaperonin activity as demonstrated by Braun et al. (36).
In addition to its role in recruitment of the 19 S base, Gal4p-SAGA interaction is essential for association of the Mediator complex with the GAL1 UAS for formation of the PIC assembly (64). How does the 19 S base regulate recruitment of the Mediator complex to the GAL1 UAS in vivo? Our data demonstrate that Mediator is not essential for recruitment of the 19 S base. Interestingly, we find that recruitment of the Mediator complex is dependent on ATPase activity. We speculate that the 19 S base might help to fold or assemble the Mediator components at the GAL1 UAS through its molecular chaperonin activity. In support of this model, Sun et al. (67) have biochemically demonstrated the physical interaction between the 19 S base and Mediator complex. Thus, the Mediator complex is likely to be properly assembled at the GAL1 UAS through its interaction with the 19 S base.
The Mediator complex is essential for formation of the PIC assembly at the GAL1 core promoter (64, 78). Thus, the impaired recruitment of the Mediator complex to the GAL1 UAS in the absence of 19 S ATPase activity would lead to the loss of PIC formation at the core promoter. Indeed, our data show that TBP and RNA polymerase II components of the PIC assembly are not recruited to the GAL1 core promoter when 19 S ATPase activity was impaired by point mutation. However, it is not yet clear whether the loss of the PIC formation at the GAL1 core promoter in the 19 S ATPase point mutant is directly affected by the absence of 19 S ATPase activity or indirectly resulted from the impaired recruitment of the Mediator complex. Nonetheless, our data demonstrate that ATPase activity of the 19 S base is essential for the transcription complex assembly at the promoter for transcriptional initiation or activation in vivo. However, previous biochemical studies have demonstrated the interactions of the 19 S base with the components of PIC (79–81). Thus, the 19 S base might directly regulate the assembly of the PIC formation at the core promoter.
Although the 19 S base is essential for recruitment of the Mediator and PIC components, it is not known whether the Mediator complex or the PIC component regulates recruitment of the 19 S base to the GAL1 UAS via reciprocal cooperativity. Here, we show that neither the Mediator complex nor the PIC component regulates recruitment of the 19 S base to the GAL1 UAS. Only Gal4p-SAGA interaction is essential for recruitment of the 19 S base. These results support a specific interaction network of Gal4p-SAGA-19 S base-Mediator-PIC at the GAL1 promoter. Interestingly, we also find that 19 S ATPases function in a cooperative manner to facilitate formation of the transcription complex assembly at the promoter. The 19 S base has molecular chaperonin activity that is provided by the AAA family ATPases. The molecular chaperonin activity of the 19 S base seems to play an important role in formation of the transcription complex assembly and, hence, transcriptional initiation or activation. The fact that 19 S ATPases function in a cooperative manner indicates that these 19 S ATPases cross-talk among themselves in the ring-shaped structure. Similar cooperativity or cross-talk among ATPases in homohexameric ring-shaped molecular chaperon has been reported (see, for example, Refs. 44, 59, 76, and 77). Importantly, our data support the fact that different ATPases within heterohexameric ring-shaped 19 S base function through cross-talk. Our future challenge is to understand the structural basis for such cross-talk.
Acknowledgments
We thank Michael R. Green, Stephen A. Johnston, and Thomas Kodadek for antibodies, Daniel Finley, Stephen A. Johnston, Fred Winston, Kevin Struhl, and Richard A. Young for yeast strains, and Nadia Stanojevic, Pratibha Bajwa, Zhen Duan, and Thomas Shadle for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grant 1R15GM088798-01. This work was also supported by American Heart Association National Scientist Development Grant 0635008N, American Cancer Society Research Scholar Grant 06-52, a Mallinckrodt Foundation award, and several internal grants from Southern Illinois University.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
- PIC
- preinitiation complex
- CP
- core particle
- RP
- regulatory particle
- ts
- temperature-sensitive
- IP
- immunoprecipitate
- ChIP
- chromatin immunoprecipitation
- UAS
- upstream activating sequence
- TBP
- TATA box-binding protein.
REFERENCES
- 1.Orphanides G., Lagrange T., Reinberg D. (1996) Genes Dev. 10, 2657–2683 [DOI] [PubMed] [Google Scholar]
- 2.Roeder R. G. (1996) Trends Biochem. Sci. 21, 327–335 [PubMed] [Google Scholar]
- 3.Roeder R. G. (2005) FEBS Lett. 579, 909–915 [DOI] [PubMed] [Google Scholar]
- 4.Ptashne M., Gann A. (1997) Nature 386, 569–577 [DOI] [PubMed] [Google Scholar]
- 5.Lee T. I., Young R. A. (2000) Annu. Rev. Genet. 34, 77–137 [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez F., Delahodde A., Kodadek T., Johnston S. A. (2002) Science 296, 548–550 [DOI] [PubMed] [Google Scholar]
- 7.Reid G., Hübner M. R., Métivier R., Brand H., Denger S., Manu D., Beaudouin J., Ellenberg J., Gannon F. (2003) Mol. Cell 11, 695–707 [DOI] [PubMed] [Google Scholar]
- 8.Métivier R., Penot G., Hübner M. R., Reid G., Brand H., Kos M., Gannon F. (2003) Cell 115, 751–763 [DOI] [PubMed] [Google Scholar]
- 9.Morris M. C., Kaiser P., Rudyak S., Baskerville C., Watson M. H., Reed S. I. (2003) Nature 423, 1009–1013 [DOI] [PubMed] [Google Scholar]
- 10.Perissi V., Aggarwal A., Glass C. K., Rose D. W., Rosenfeld M. G. (2004) Cell 116, 511–526 [DOI] [PubMed] [Google Scholar]
- 11.Zhu Q., Yao J., Wani G., Chen J., Wang Q. E., Wani A. A. (2004) FEBS Lett. 556, 19–25 [DOI] [PubMed] [Google Scholar]
- 12.Zhu Q., Wani G., Yao J., Patnaik S., Wang Q. E., El-Mahdy M. A., Praetorius-Ibba M., Wani A. A. (2007) Oncogene 26, 4199–4208 [DOI] [PubMed] [Google Scholar]
- 13.Lee D., Ezhkova E., Li B., Pattenden S. G., Tansey W. P., Workman J. L. (2005) Cell 123, 423–436 [DOI] [PubMed] [Google Scholar]
- 14.Valley C. C., Métivier R., Solodin N. M., Fowler A. M., Mashek M. T., Hill L., Alarid E. T. (2005) Mol. Cell. Biol. 25, 5417–5428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Auld K. L., Silver P. A. (2006) Cell Cycle 5, 1503–1515 [DOI] [PubMed] [Google Scholar]
- 16.Collins G. A., Tansey W. P. (2006) Curr. Opin. Genet Dev. 16, 197–202 [DOI] [PubMed] [Google Scholar]
- 17.Kodadek T., Sikder D., Nalley K. (2006) Cell 127, 261–264 [DOI] [PubMed] [Google Scholar]
- 18.Pan Y., Bai C. B., Joyner A. L., Wang B. (2006) Mol. Cell. Biol. 26, 3365–3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasti M., Grand R. J., Yousef A. F., Shuen M., Mymryk J. S., Gallimore P. H., Turnell A. S. (2006) EMBO J. 25, 2710–2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sulahian R., Sikder D., Johnston S. A., Kodadek T. (2006) Nucleic Acids Res. 34, 1351–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szutorisz H., Georgiou A., Tora L., Dillon N. (2006) Cell 127, 1375–1388 [DOI] [PubMed] [Google Scholar]
- 22.Zhang Q., Zhang L., Wang B., Ou C. Y., Chien C. T., Jiang J. (2006) Dev. Cell 10, 719–729 [DOI] [PubMed] [Google Scholar]
- 23.Ferdous A., Sikder D., Gillette T., Nalley K., Kodadek T., Johnston S. A. (2007) Genes Dev. 21, 112–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinyamu H. K., Archer T. K. (2007) Mol. Cell. Biol. 27, 4891–4904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lassot I., Latreille D., Rousset E., Sourisseau M., Linares L. K., Chable-Bessia C., Coux O., Benkirane M., Kiernan R. E. (2007) Mol. Cell 25, 369–383 [DOI] [PubMed] [Google Scholar]
- 26.Yi P., Feng Q., Amazit L., Lonard D. M., Tsai S. Y., Tsai M. J., O'Malley B. W. (2008) Mol. Cell. 29, 465–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soond S. M., Townsend P. A., Barry S. P., Knight R. A., Latchman D. S., Stephanou A. (2008) J. Biol. Chem. 283, 16077–16083 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Bhaumik S. R., Malik S. (2008) Crit. Rev. Biochem. Mol. Biol. 43, 419–433 [DOI] [PubMed] [Google Scholar]
- 29.Glickman M. H., Rubin D. M., Fried V. A., Finley D. (1998) Mol. Cell. Biol. 18, 3149–3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dick T. P., Nussbaum A. K., Deeg M., Heinemeyer W., Groll M., Schirle M., Keilholz W., Stevanović S., Wolf D. H., Huber R., Rammensee H. G., Schild H. (1998) J. Biol. Chem. 273, 25637–26646 [DOI] [PubMed] [Google Scholar]
- 31.Voges D., Zwickl P., Baumeister W. (1999) Annu. Rev. Biochem. 68, 1015–1068 [DOI] [PubMed] [Google Scholar]
- 32.Coux O. (2002) Prog. Mol. Subcell. Biol. 29, 85–107 [DOI] [PubMed] [Google Scholar]
- 33.Groll M., Clausen T. (2003) Curr. Opin. Struct. Biol. 13, 665–673 [DOI] [PubMed] [Google Scholar]
- 34.Smith D. M., Chang S. C., Park S., Finley D., Cheng Y., Goldberg A. L. (2007) Mol. Cell 27, 731–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horwitz A. A., Navon A., Groll M., Smith D. M., Reis C., Goldberg A. L. (2007) J. Biol. Chem. 282, 22921–22929 [DOI] [PubMed] [Google Scholar]
- 36.Braun B. C., Glickman M., Kraft R., Dahlmann B., Kloetzel P. M., Finley D., Schmidt M. (1999) Nat. Cell Biol. 1, 221–226 [DOI] [PubMed] [Google Scholar]
- 37.Chau V., Tobias J. W., Bachmair A., Marriott D., Ecker D. J., Gonda D. K., Varshavsky A. (1989) Science 243, 1576–1583 [DOI] [PubMed] [Google Scholar]
- 38.Finley D., Sadis S., Monia B. P., Boucher P., Ecker D. J., Crooke S. T., Chau V. (1994) Mol. Cell. Biol. 14, 5501–5509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang M., Cheng D., Peng J., Pickart C. M. (2006) EMBO J. 25, 1710–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larsen C. N., Finley D. (1997) Cell 91, 431–434 [DOI] [PubMed] [Google Scholar]
- 41.Navon A., Goldberg A. L. (2001) Mol. Cell 8, 1339–1349 [DOI] [PubMed] [Google Scholar]
- 42.Verma R., Oania R., Graumann J., Deshaies R. J. (2004) Cell 118, 99–110 [DOI] [PubMed] [Google Scholar]
- 43.Guterman A., Glickman M. H. (2004) J. Biol. Chem. 279, 1729–1738 [DOI] [PubMed] [Google Scholar]
- 44.Smith D. M., Kafri G., Cheng Y., Ng D., Walz T., Goldberg A. L. (2005) Mol. Cell 20, 687–698 [DOI] [PubMed] [Google Scholar]
- 45.Hanna J., Hathaway N. A., Tone Y., Crosas B., Elsasser S., Kirkpatrick D. S., Leggett D. S., Gygi S. P., King R. W., Finley D. (2006) Cell 127, 99–111 [DOI] [PubMed] [Google Scholar]
- 46.Seong K. M., Baek J. H., Ahn B. Y., Yu M. H., Kim J. (2007) Mol. Cells. 24, 194–199 [PubMed] [Google Scholar]
- 47.Seong K. M., Baek J. H., Yu M. H., Kim J. (2007) FEBS Lett. 581, 2567–2673 [DOI] [PubMed] [Google Scholar]
- 48.Schreiner P., Chen X., Husnjak K., Randles L., Zhang N., Elsasser S., Finley D., Dikic I., Walters K. J., Groll M. (2008) Nature 453, 548–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Husnjak K., Elsasser S., Zhang N., Chen X., Randles L., Shi Y., Hofmann K., Walters K. J., Finley D., Dikic I. (2008) Nature 453, 481–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hilt W., Wolf D. H. (1996) Trends Biochem. Sci. 21, 96–102 [PubMed] [Google Scholar]
- 51.He H., Qi X. M., Grossmann J., Distelhorst C. W. (1998) J. Biol. Chem. 273, 25015–25019 [DOI] [PubMed] [Google Scholar]
- 52.Sikder D., Johnston S. A., Kodadek T. (2006) J. Biol. Chem. 281, 27346–27355 [DOI] [PubMed] [Google Scholar]
- 53.Auld K. L., Brown C. R., Casolari J. M., Komili S., Silver P. A. (2006) Mol. Cell 21, 861–871 [DOI] [PubMed] [Google Scholar]
- 54.Fleming J. A., Lightcap E. S., Sadis S., Thoroddsen V., Bulawa C. E., Blackman R. K. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 1461–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dembla-Rajpal N., Seipelt R., Wang Q., Rymond B. C. (2004) Biochim. Biophys. Acta 1680, 34–45 [DOI] [PubMed] [Google Scholar]
- 56.Longtine M. S., McKenzie A., 3rd, Demarini D. J., Shah N. G., Wach A., Brachat A., Philippsen P., Pringle J. R. (1998) Yeast 14, 953–961 [DOI] [PubMed] [Google Scholar]
- 57.Cormack B. P., Struhl K. (1992) Cell 69, 685–696 [DOI] [PubMed] [Google Scholar]
- 58.Thompson C. M., Young R. A. (1995) Proc. Natl. Acad. Sci. 92, 4587–4590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rubin D. M., Glickman M. H., Larsen C. N., Dhruvakumar S., Finley D. (1998) EMBO J. 17, 4909–4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roberts S. M., Winston F. (1996) Mol. Cell. Biol. 16, 3206–3213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brachmann C. B., Davies A., Cost G. J., Caputo E., Li J., Hieter P., Boeke J. D. (1998) Yeast 14, 115–132 [DOI] [PubMed] [Google Scholar]
- 62.Bhaumik S. R., Green M. R. (2001) Genes Dev. 15, 1935–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bhaumik S. R., Green M. R. (2003) Methods Enzymol. 370, 445–454 [DOI] [PubMed] [Google Scholar]
- 64.Bhaumik S. R., Raha T., Aiello D. P., Green M. R. (2004) Genes Dev. 18, 333–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shukla A., Stanojevic N., Duan Z., Sen P., Bhaumik S. R. (2006) Mol. Cell. Biol. 26, 3339–3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bhaumik S. R., Green M. R. (2002) Mol. Cell. Biol. 22, 7365–7371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun L., Johnston S. A., Kodadek T. (2002) Biochem. Biophys. Res. Commun. 296, 991–999 [DOI] [PubMed] [Google Scholar]
- 68.Isono E., Nishihara K., Saeki Y., Yashiroda H., Kamata N., Ge L., Ueda T., Kikuchi Y., Tanaka K., Nakano A., Toh-e A. (2007) Mol. Biol. Cell 18, 569–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sterner D. E., Grant P. A., Roberts S. M., Duggan L. J., Belotserkovskaya R., Pacella L. A., Winston F., Workman J. L., Berger S. L. (1999) Mol. Cell. Biol. 19, 86–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grant P. A., Schieltz D., Pray-Grant M. G., Steger D. J., Reese J. C., Yates J. R., 3rd, Workman J. L. (1998) Cell 94, 45–53 [DOI] [PubMed] [Google Scholar]
- 71.Green M. R. (2000) Trends Biochem. Sci. 25, 59–63 [DOI] [PubMed] [Google Scholar]
- 72.Li X. Y., Bhaumik S. R., Green M. R. (2000) Science 288, 1242–1244 [DOI] [PubMed] [Google Scholar]
- 73.Koh S. S., Ansari A. Z., Ptashne M., Young R. A. (1998) Mol. Cell 1, 895–904 [DOI] [PubMed] [Google Scholar]
- 74.Kang J. S., Kim S. H., Hwang M. S., Han S. J., Lee Y. C., Kim Y. J. (2001) J. Biol. Chem. 276, 42003–42010 [DOI] [PubMed] [Google Scholar]
- 75.Lipford J. R., Smith G. T., Chi Y., Deshaies R. J. (2005) Nature 438, 113–116 [DOI] [PubMed] [Google Scholar]
- 76.Zhang X., Shaw A., Bates P. A., Newman R. H., Gowen B., Orlova E., Gorman M. A., Kondo H., Dokurno P., Lally J., Leonard G., Meyer H., van Heel M., Freemont P. S. (2000) Mol Cell. 6, 1473–1484 [DOI] [PubMed] [Google Scholar]
- 77.Davies J. M., Brunger A. T., Weis W. I. (2008) Structure 16, 715–726 [DOI] [PubMed] [Google Scholar]
- 78.Kornberg R. D. (2005) Trends Biochem. Sci. 30, 235–239 [DOI] [PubMed] [Google Scholar]
- 79.Weeda G., Rossignol M., Fraser R. A., Winkler G. S., Vermeulen W., van't Veer L. J., Ma L., Hoeijmakers J. H., Egly J. M. (1997) Nucleic Acids Res. 25, 2274–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yanagi S., Shimbara N., Tamura T. A. (2000) Biochem. Biophys. Res. Commun. 279, 568–573 [DOI] [PubMed] [Google Scholar]
- 81.Makino Y., Yoshida T., Yogosawa S., Tanaka K., Muramatsu M., Tamura T. A. (1999) Genes Cells 4, 529–539 [DOI] [PubMed] [Google Scholar]