Abstract
Cellular prion protein (PrPc) undergoes a disintegrin-mediated physiological cleavage, generating a soluble amino-terminal fragment (N1), the function of which remained unknown. Recombinant N1 inhibits staurosporine-induced caspase-3 activation by modulating p53 transcription and activity, whereas the PrPc-derived pathological fragment (N2) remains biologically inert. Furthermore, N1 protects retinal ganglion cells from hypoxia-induced apoptosis, reduces the number of terminal deoxynucleotidyltransferase-mediated biotinylated UTP nick end labeling-positive and p53-immunoreactive neurons in a pressure-induced ischemia model of the rat retina and triggers a partial recovery of b-waves but not a-waves of rat electroretinograms. Our work is the first demonstration that the α-secretase-derived PrPc fragment N1, but not N2, displays in vivo and in vitro neuroprotective function by modulating p53 pathway. It further demonstrates that distinct N-terminal cleavage products of PrPc harbor different biological activities underlying the various phenotypes linking PrPc to cell survival.
Introduction
The investigation of the physiological function of PrPc has long been neglected due to the lack of an obvious phenotype in PrPc-deficient mice. Recently, several works have shed light on the putative implication of PrPc in cell adhesion, neurite outgrowth, synaptogenesis, and myelinization (for a review see Ref. 1). In addition, albeit debated, a role in the control of cell viability has also been proposed. Thus, some reports have suggested that PrPc might have a protective function against Bax-induced cell death, oxidative stress, and hypoxic injury (1). Conversely, in several experimental systems, overexpressed or endogenous PrPc both lead to exacerbated cellular responsiveness to apoptotic insults (2–7). In addition, cell degeneration in the nervous system of old transgenic mice harboring high copy number of the PrPc gene had been observed (8). Overall, these data suggest that PrPc could display both pro- and antiapoptotic functions, depending on the cell context and/or physiological situation.
A subset of plasma membrane-tethered PrPc molecules undergoes proteolytic processing events. PrPc is mainly endoproteolyzed at the 110/111 peptidyl bond to produce a 17-kDa C-terminal fragment, C1, which remains membrane-bound (9) and a 9-kDa soluble N-terminal counterpart, referred to as N1, released in the extracellular space (10–12). In brain from transmissible spongiform encephalopathy-affected individuals, the PrPc undergoes alternative proteolytic attack. Thus, a 21-kDa C-terminal fragment, C2, and its 7-kDa N2 N-terminal counterpart derive from an additional cleavage around the 90/91 residues (9).
It is noteworthy that transgenic mice expressing PrPc proteins lacking N-terminal residues 33–120 or 33–133 exhibit exacerbated neurodegeneration (13–15). Furthermore, we reported that the overexpression of the α-secretase-derived PrPc fragment C1 lacking the N-terminal domain was detrimental in vitro (16). Overall, these observations suggest that PrPc N-terminal moiety is crucial for its function, but the putative function of secreted N1 has never been delineated. Does proteolytic release of N1 correspond to an inactivating mechanism impairing PrPc biological function, or alternatively, does it represent a maturation process allowing N1 to trigger its own physiological function? Here we show that N1 but not N2 displays a protective phenotype by modulating the p53 pathway in vitro as well as in vivo in a pressure-induced ischemia model of rat retina.
MATERIALS AND METHODS
Animals
Mated wild type black 57 mice were purchased from Charles River (Charles River Breeding Laboratory, St. Aubain les Elbeuf, France). PrPc Zrch-1 knock-out mice were kindly provided by Charles Weissmann (Scripps Florida).
Adult male Brown Norway rats weighing 200 g (Charles River Laboratory) were housed in clear plastic cages in a room with controlled temperature (22 ± 1 °C) and fixed 50-1x fluorescent (Philips) lighting schedule (lights on from 08:00 to 20:00 h). Food and tap water were provided ad libitum. Rats were acclimated for 1 week before experiments. These studies were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Antibodies and Pharmacological Agents
SAF32 antibody directed against PrPc residues 79–92 and SAF61, which recognizes the C-terminal domain of PrPc (17), was generously provided by J. Grassi (Commissariat à l'Energie Atomique/Saclay, Gif sur Yvette, France). Carbachol and staurosporine were purchased from Sigma. Atropine was from ICN Biochemicals (Aurora, OH). LY294002 was obtained from Cayman (VWR, Fontenay sous Bois, France).
Construction of Glutathione S-Transferase (GST)5-N-terminal PrPc Fusion-expressing Vector and Purification of PrP N- terminal Recombinant Fragments
cDNA encoding various sequences of mouse PrPc were generated by PCR: amino acids 23–110 (N1), 23–89 (N2), 41–107 (NT), and 23–110 where the KKRPKPG N-terminal domain was replaced by KQHPSPG (NK). Amplicons were cloned into EcoRI and Xho sites of pGEX-KG (18). To produce and purify PrPc-derived N-terminal recombinant fragments, the pGEX-KG GST-N-terminal PrPc-expressing vectors were transformed into BL21 gold strain of Escherichia coli (Stratagene, Amsterdam Zuidoost, The Netherlands). E. coli were grown in Luria-broth medium and allowed to reach A600 = 0.6. Fusion proteins were induced with 0.5 mm isopropyl 1-thio-β-d-galactopyranoside (Sigma) for 4 h at 37 °C. Cells were pelleted at 5000 × g for 20 min at 4 °C and resuspended in phosphate-buffered saline (PBS; 50 μl per ml of original culture) supplemented with complete protease inhibitor mixture (Sigma), phenylmethylsulfonyl fluoride (Sigma), and lysozyme (150 μm/ml; Sigma) and incubated for 30 min on ice. Proteins were solubilized by the addition of Triton X-100 (1%), MgCl2 (10 mm), DNase I (5 μg/ml; Promega) and incubation on ice for 30 min. Debris were pelleted for 20 min at 10,000 × g. Glutathione-Sepharose beads (GE Healthcare) preswollen in PBS (70% slurry) were added to the crude lysate and swirled for 1 h at 4 °C. Beads were pelleted, washed five times with 10 volumes of PBS, and resuspended in 1 ml of PBS. Peptides were cleaved with thrombin (5 units/ml; GE Healthcare) for 1 h at room temperature. Thrombin was removed using Sepharose benzamidine beads (GE Healthcare). Control experiments were carried out with empty pGEX-KG-glutathione S-transferase, referred to as KG hereafter). The monisotopic mass of each peptide was checked by matrix-assisted laser desorption ionization time-of-flight mass spectrometry analysis performed after reverse phase, solid phase extraction with a C18 ZipTip (Millipore) and was in accordance with the theoretical mass calculated from the sequence data. Theoretical mass (TM) N1 10,172.96 Da, measured mass (MM) N1 10,172.29 Da, N2 TM 7833.36 Da, MM 7832.24 Da, NT TM 6844.22 Da, MM 6843.11 Da, NK TM 10153.83 Da, MM 10,152.9 Da. GST was produced and purified as described above, and then GST bound to Sepharose beads was subjected to thrombin digestion. Bead supernatant was used as negative control (KG).
Cell Systems and Transfections
Human embryonic cells (HEK293) stably expressing full-length 3F4MoPrP (3F4-tagged murine PrPc), C-terminal PrPc fragment (C1), and M1 muscarinic receptors were established and maintained as previously detailed (10, 16). p19arf-deficient and p19arf/p53 double knock-out fibroblasts were kindly provided by Dr. M. Roussel (19). ERK1-deficient cells were from Dr. G. Pages (Nice). The PrPc-deficient hippocampus-derived cell line HpL3-4 kindly supplied by Dr. T. Onodera was described previously (20). Primary cultured mouse cortical neurons were transfected using Amaxa® Nucleofector® kits for primary culture of mouse neurons (Lonza, Cologne, Germany) according to the manufacturer's specifications. Cells were maintained for 48 h at 37 °C under 5% CO2 before being challenged with staurosporine in the presence or absence of N1 fragment. Treatments and caspase-3 measurements were performed as described below.
Primary Cultured Cortical Neurons
Embryonic cortical neurons were prepared as previously detailed (21). Briefly, cells from cerebral hemispheres of E14 mice from PrP+/+ and PrP0/0 embryos were dissociated in Ham's F-12 (Invitrogen) supplemented with 0.6% glucose and 10% fetal calf serum. A total of 106 cells were seeded in 35-mm diameter dishes precoated with polylysine (10 μg/ml; Sigma) and kept for 4 days before being assayed for apoptosis.
Primary Cultured Rat Retinal Cells
Retina cell primary cultures were prepared from Brown Norway newborns. Retinas were removed and mechanically dissociated at room temperature in neurobasal medium containing 34 units/ml papain, 0.4 mg/ml l-cysteine, 0.4 mg/ml bovine serum albumin (all from Sigma), and 0.5 mg/ml DNase I (Promega). Cells washed in neurobasal medium were passed through a 40-mm mesh and seeded at 106 cells/ml on poly-d-lysine-coated dishes (Biocoat; BD Biosciences) or coverslips in neurobasal medium supplemented with 5% fetal bovine serum, 10 mg/ml basic fibroblast growth factor, 1% N2 supplement and kept at 37 °C under 5% CO2 for 7 days before being assayed for survival, caspase-3 activity, and terminal deoxynucleotidyltransferase-mediated biotinylated UTP nick end labeling (TUNEL) staining.
Western Blot Analyses
Recombinant N-terminal fragments were resolved on 16.5% Tris/Tricine gel and analyzed by standard immunoblotting techniques using SAF32 antibody. For analysis of PrPc immunoreactivity, cells were homogenized in lysis buffer (10 mm Tris, pH 7.5, 150 mm NaCl, 0.5% Triton X-100, 0.5% deoxycholate, 5 mm EDTA). Equal amounts of proteins were resolved on 12% SDS-polyacrylamide gel and analyzed by immunoblotting using and SAF32. Blots were developed using the ECL method according to the manufacturer's instructions (Roche Applied Science). Chemiluminescence was recorded using a luminescence image analyzer LAS-3000 (Raytest, Courbevoie, France), and quantification of captured images was performed using Aida Image Analyzer software (Raytest).
Immunoprecipitation and Analysis of N1 Fragments
Cells plated in 35-mm dishes were grown to confluence and incubated with required pharmacological agent for 8 h in Dulbecco's modified Eagle's medium. Media were collected, and N1 was immunoprecipitated as detailed elsewhere (10). Immunocaptured peptides were subjected to SDS-PAGE analysis on 16.5% Tris/Tricine gel as described above. For biochemical detection of internalized N1, cells were acid-rinsed, homogenized in the above lysis buffer, and then processed for immunoprecipitation as described previously (10).
Cell Viability Assays
Cells were grown in 96-well plates. At confluence, N1 (1 μm) or an equivalent volume of control supernatant produced after thrombin digestion of GST-Sepharose beads (KG) was added into fresh medium. After 4 h of incubation, N1 was added again to the cells in the presence or absence of staurosporine (STS; 2 μm), and cells were returned to 37 °C for 16 h. Fifty μl of 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) reactive was added to the cells, and absorbance was measured at 452 nm as previously detailed (22).
Membrane integrity was evaluated by measuring the lactate dehydrogenase activity in the culture medium using the cytoTox-ONETM kit (Promega). In oxygen glucose deprivation (OGD) experiments (see below), the assay was processed according to the manufacturer's recommendations for 40 μl of medium immediately after 2 h of hypoxia and 24 h after return to complete medium.
Caspase-3-like Activity Measurements
Cells were grown in 6-well plates and incubated with STS (2 μm for 16 h for HEK293 cells and primary cortical neurons; 1 μm for 2 h for fibroblasts) after they reached confluence. Samples were processed for a caspase-3-like activity assay as already detailed (22). Fluorimetry was recorded at 360 and 460 nm (excitation and emission wavelengths) by means of a microtiter plate reader (Labsystems, Fisher Bioblock Scientific (Illkirch, France)). Caspase-3-like activity was calculated from the linear part of fluorimetry recorded and expressed in units/h/mg of proteins (established by the Bio-Rad procedure). One unit corresponds to 4 nmol of 7-amino-4-methylcoumarin released. When blockade of internalization was required, cells were pretreated with 0.45 m sucrose for 30 min as previously detailed (23) or with 10 μm Dynasore. When necessary, LY294002 was added simultaneously to the peptide before staurosporine treatment. When stated, cells were treated with carbachol (100 μm) and/or atropine (10 μm) together with staurosporine (2 μm) and incubated for 16 h. Caspase-3 activity was then monitored as above.
TUNEL Analysis
The same number of cells were plated on glass coverslips until they reached confluence and then were preincubated with N1 or control supernatant (KG). After 4 h of incubation, cells were retreated with N1 or KG and with staurosporine (1 μm, 2 h). Cells were then fixed for 20 min in paraformaldehyde (4% in PBS), rinsed, and permeabilized overnight in 70% ethanol before being processed for labeling with the dUTP nick end labeling in situ cell death detection kit, POD (Roche Applied Science) as recommended by the supplier. For quantification, total cells were counterstained with erythrosine B. Images were captured on Olympus BX41 microscope (Olympus, Rungis, France) using Olympus DP12 software. Cells undergoing cell death (3,3′-diaminobenzidine-labeled nuclei) were counted on 10 independent optical fields for each experimental condition.
In situ analyses of apoptotic cell death in rat retina were performed 48 h postischemia, on 10-μm-thick frozen retina sections of ischemia-induced or sham control. TUNEL-positive nuclei were detected with the in situ cell death detection-fluorescein kit (Roche Applied Science) essentially as described previously (24). Briefly, sections were fixed in 4% paraformaldehyde, permeabilized in PBS, 0.1% Tween 20, washed in PBS, and incubated in PBS containing 1.5% H2O2 for 30 min before incubation for 1 h at 37 °C with terminal deoxynucleotidyltransferase to incorporate fluorescein nucleotides into DNA strand breaks. Sections were rinsed in PBS and mounted in Vectashield (Vector Laboratories, Burlingame, CA) containing 4′,6-diamino-2-phenylindole (DAPI). Images of retinal sections were acquired and photographed using a fluorescent Axioplan2 imaging microscope (Zeiss, Thornwood, NY).
p53 Transcriptional Activity and Promoter Transactivation
Reporter constructs p21waf-1-luciferase and PG13-luciferase (provided by Dr. B. Vogelstein (Baltimore, MD)) used to measure p53 transcriptional activity have been extensively described elsewhere (4, 16, 25). Briefly, cells grown in 12-well plates were co-transfected with a 4:1 ratio of p21waf-1 or PG13-luciferase reporter constructs and β-galactosidase expression vector (to normalize transfection efficiency). Luciferase and β-galactosidase activities were assayed 24 h after transfection according to already described procedures (25) using the luciferase assay system and β-galactosidase enzyme assay system (Promega). The p53 promoter-luciferase (pp53) construct (kindly provided by Dr. M. Oren (Rehovot, Israel)) described earlier (26) was used to determine p53 promoter transactivation as above. When required, cells were treated with carbachol (100 μm), atropine (10 μm), and/or LY294002 (10 μm) for 16 h.
Real-time Quantitative PCR
Total RNAs from cells were isolated using the RNeasy kit (Promega) following the instructions of the manufacturer. After DNase I treatment, 1 μg of total RNA was reverse-transcribed using oligo(dT) priming and avian myeloblastosis virus reverse transcriptase (Promega). Real-time PCR was performed in an ABI PRISM 5700 sequence detector system (Applied Biosystems, Courtaboeuf, France) using the SYBR Green detection protocol as outlined by the manufacturer. Human p53-specific primers were designed using Primer Express software (Applied Biosystems, Courtaboeuf, France): forward, 5′-GAA CCC TTG CTT GCA ATA GG-3′; reverse, 5′-GTG AGG TAG GTG CAA ATG CC-3′. The relative expression level of p53 gene is normalized for RNA concentrations with housekeeping gene (human glyceraldehyde-3-phosphate dehydrogenase) using the following primers: forward, 5′-TGG GCT ACA CTG AGC ACC AG-3′; reverse, 5′-CAG CGT CAA AGG TGG AGG AG-3′. mRNA values are expressed in arbitrary units.
Immunofluorescence
In situ detection of intracellular N1 fragments was performed by immunohistochemistry on HPL3-4 PrPc-deficient cells cultured on glass coverslips in 35-mm dishes. Cells were acid-rinsed and fixed with paraformaldehyde (1.5%) for 20 min and permeabilized with Triton X100 (0.1%; Sigma). Cells were washed three times in PBS and blocked with milk (1%) in PBS for 30 min, and the primary antibody (SAF32) was applied for 2 h. After three washes with PBS, cells were incubated for 1 h with goat anti-mouse secondary antibody conjugated to Alexa Fluor-594 (Interchim, Montluçon, France). Hoechst (Interchim) was added to the PBS during the first of the last three washes to stain the nuclei. Coverslips were then mounted in Vectashield mounting medium (Vector Laboratories, Inc., Burlingame, CA), and staining was visualized with an Axioplan 2 imaging microscope (Carl Zeiss, Sartrouville, France) with oil immersion objective ×63, coupled to a cooled CCD camera (Raper Scientist, Tucson, AZ).
Oxygen Glucose Deprivation
Retinal cells were subjected to oxygen glucose deprivation (OGD) as described previously (27). Cells were treated with an appropriate fragment (1 μm) or equivalent volume of KG for 4 h before being washed with deoxygenated Hanks' buffered saline solution medium. Cultures were retreated with fragment (1 μm) or KG in Hanks' buffered saline solution and placed in a modular incubator chamber (Billups-Rothenberg, Del Mar, CA) flushed with a 4.5% carbon dioxide, 94.5% nitrogen gas mixture for 10 min. The chamber was sealed and placed in a 37 °C incubator for 90 min. After 90 min of hypoxia, medium was washed and replaced with neurobasal medium supplemented with 5% fetal bovine serum, 10 mg/ml basic fibroblast growth factor, 1%, N2 supplement and returned to 37 °C under 5% CO2 atmosphere for 24 h. Control cells were similarly washed and treated with fragments in oxygenated Hanks' buffered saline solution for 6 h before being returned to complete neurobasal medium for 24 h.
Intravitreal Injection and Retinal Ischemia
To investigate the antiapoptotic effect of N1, the rats were treated intravitreally with N1, N2, NT, or KG. Intraocular injection was done under dim red light after general anesthesia with sodium pentobarbital (60 mg/kg intraperitoneally) and local anesthesia by topical application of oxybuprocaine (0.4%) hydrochloride in the right and left eye of adult Brown Norway rats. The estimated final vitreal concentration of 1 μm N1 and NT was calculated by assuming an average vitreous chamber volume of 30 μl. Peptide or control (3 μl) was injected in the vitreous at day 0 via a 30-gauge needle (Microlance, BD Biosciences) attached to a 10-μl Hamilton syringe under an operating microscope. The needle was inserted through the sclera 1 mm behind the nimbus on the upper pole of the left eye to avoid contact with the lens. Animals with eyes that showed any experimental trauma (opacification of the lens or retinal hemorrhage) when checked with an ophthalmoscope were excluded. Ninety min after intravitreal injection, the rats were maintained under anesthesia by boosts with 50% of the initial dose of anesthetic and placed in a stereotaxic frame. After topical instillation of a drop of oxybuprocaine (Laboratoire Chauvin, Montpellier, France), the anterior chamber of the right eye was cannulated with a 30-gauge needle connected to a reservoir containing Hanks' balanced salt solution. The left eye served as control. Retinal ischemia was induced by increasing the intraocular pressure to 130 mm Hg (28). The increased intraocular pressure was maintained for 45 min. At this intraocular pressure, systolic collapse of the central retinal artery was observed by direct ophthalmoscopy. The detailed preconditioning paradigm is diagrammed in Fig. 7a. During the experiment, the animals were kept normothermic with heated jackets (38 °C). Animals were killed at day 2 or 7. At least three animals were used in each group of experimental conditions. Sham-treated control right eyes underwent a similar procedure but without the elevation of the saline bag, so that the normal ocular tension was maintained.
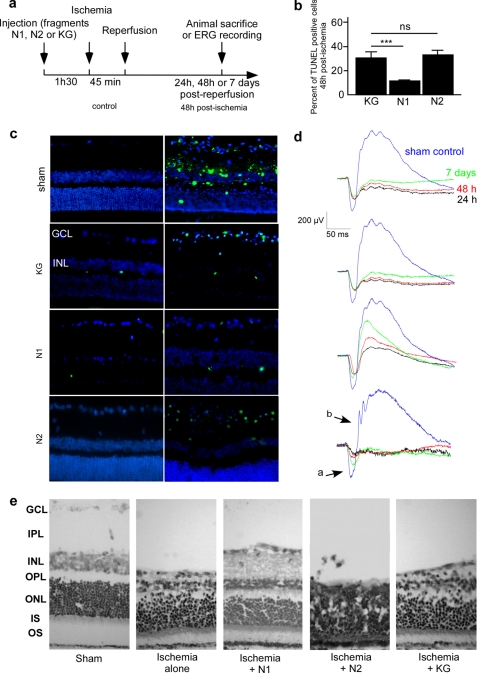
FIGURE 7.
N1 protects retina against morphological and functional alterations induced by ischemia. a, schematic representation of preconditioning and ischemia procedures. Rats were injected in the vitreous humor with N1, N2 (1 μm final concentration), or KG 90 min before induction of 45-min pressure-induced ischemia. b, quantification of TUNEL-positive cells in retinas 48 h after ischemia. Bars correspond to percentage of total DAPI-stained nuclei. Counts were performed on six retina sections of the same animal for two independent optical fields. For each condition, two animals were analyzed. *, p < 0.01; **, p < 0.001; ***, p < 0.0001. c, representative microphotographs showing overlay of fluorescein (green) and DAPI (blue) immunostained nuclei in retina slices. In sections, fluorescein stained the nuclei of apoptotic cells using the TUNEL labeling method. Labeled nuclei appear in ganglion cell and inner nuclear layers. d, ERG recording done as described under “Materials and Methods” after 24 h, 48 h, or 7 days of recovery for corresponding representative scotopic ERG traces recorded 24 h (dark line), 48 h (red line), and 7 days (green line) after the end of ischemia and compared with sham control (blue line) obtained for non-ischemic eye. e, histology of the ischemic untreated, N1-, N2-, or KG-treated retinas after 7 days of recovery compared with control retina, stained by 1% cresyl violet. Specimens were visualized in light microscopy. GCL, ganglion cell layer; IPL, inner plexiform; INL, inner nuclear; OPL, outer plexiform; ONL, outer nuclear; IS, inner segments of rods; OS, outer segments of rods and cones. *, p < 0.05; **, p < 0.001; ***, p < 0.0001; ns, not significant.
Preparation of Retinal Sections
On day 2 or 7, three rats of each group were dark-adapted for 4 h and were sacrificed with intraperitoneal injection of an overdose of sodium pentobarbital. Eyes were removed, punctured at the limbus, and perfused with ice-cold 4% paraformaldehyde in PBS. After 15 min of fixation, the cornea, the lens, and the vitreous were removed, and the eye cups were cryoprotected in sucrose (20%) in PBS for 1 h and then embedded into Tissue-Teck® (Sakura Finetek) under frozen isopentane. Frozen sections (10 μm) were next prepared and frozen at −80 °C until use. Morphological and histological analyses of the retinas were done by staining with cresyl violet (1%).
p53 Immunohistochemistry
The 2-day retinal sections were preincubated for 45 min in PBS containing 1:500 normal horse serum (Vector laboratories, Inc., Burlingame, CA). After washing three times in PBS, sections were then incubated overnight in PBS containing p53 rabbit polyclonal antibody (1:30; FL-393, Santa Cruz Biotechnology, Inc. (Santa Cruz, CA)) in a dark humidified chamber maintained at 4 °C. After rinsing, sections were incubated with fluorescein isothiocyanate-conjugated anti-rabbit IgG (1:10; Invitrogen) for 30 min in a dark humidified chamber at room temperature and then with fluorescent DAPI stain for 10 min (1:20,000) and rinsed twice in PBS. The slides were examined using a fluorescent Axioplan2 Imaging microscope (Zeiss).
Electroretinography
Full-field electroretinogram (ERG) responses were obtained after overnight dark adaptation as previously described (the rats were anesthetized with sodium pentobarbital (60 mg/kg intraperitoneally)) under dim red light (640 nm) and placed on a heating pad to maintain body temperature near 38 °C. Pupils were dilated with 2.5% Neosynephrine and 0.5% Mydriaticum, and corneas were kept moist with local application of 1% carboxymethylcellulose sodium (Celluvisc; Allergan (Irvine, CA)). The scotopic ERG responses were recorded with an ERG test system (UTAS 2000, LKC Technologies (Gaithersburg, MD)). Treated and control animals were submitted to 2.98 log candelas/s/m2 flash intensity produced by a Grass PS 22 xenon flash positioned 15 cm from the eye. The averaged responses represent the mean of seven flashes delivered 60 min apart. Electroretinograms were recorded before treatment and then at different times of recovery (1, 2, or 7 days). The amplitude of the a-wave was measured from the prestimulus base line to the apex negative peak of the a-wave. The b-wave amplitude was measured from the a-wave negative peak to the b-wave positive peak as described previously (29).
Statistical Analysis
Statistical analysis was performed with PRISM software (GraphPad Software, San Diego, CA) by using the Newmann-Keuls multiple comparison tests for one-way analysis of variance and t test.
RESULTS
Recombinant N1 Protects HEK293 Cells and Primary Cultured Neurons against Staurosporine-induced Apoptosis Independently of Endogenous PrPc
We have produced GST-N1 fusion protein and released N1 peptide (residues 23–110 of PrPc) by thrombin digestion. The molecular mass and integrity of the recovered peptide were confirmed by mass spectrometry (data not shown) and Western blotting using the SAF32 antibody recognizing residues 79–92 of the PrPc N-terminal domain (Fig. 1a). As expected, recombinant N1 fragment that harbors additional amino acid residues adjacent to the thrombin cleavage site on the pGEX-KG cloning vector (see Fig. 4a) migrates more slowly than control N1 secreted by PrPc-overexpressing cells (Fig. 1a). A first set of experiments examined the influence of recombinant N1 on STS-induced toxicity in HEK293 cells. Fig. 1b shows that STS triggers a 28.5% reduction of cell viability that was half-rescued by recombinant N1 (15.25 ± 1.90% (p < 0.05, n = 4) increase of N1-treated cell viability). Recombinant N1 triggered similar reductions of staurosporine-induced caspase-3 activation (27.22 ± 6.46%, p < 0.05, n = 6) (Fig. 1c) and TUNEL-positive nuclei (33.13 ± 7.68%, p < 0.05, n = 10) (Fig. 1d).
FIGURE 1.
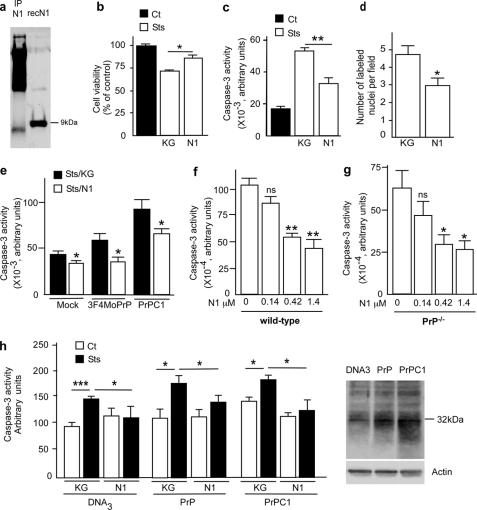
Recombinant N1 fragment protects cells against staurosporine-induced apoptotic cell death in HEK293 cells and mouse primary cultured cortical neurons. a, recombinant N1 fragment was produced in E. coli and purified as described under “Materials and Methods.” Secreted N1 was immunocaptured from HEK293 supernatant as described under “Materials and Methods,” analyzed by 16.5% Tris/Tricine SDS-PAGE, and revealed by immunoblotting with SAF32. b–d, 1 μm recombinant N1 or an equivalent volume of supernatant produced after thrombin digestion of GST-Sepharose beads (KG) was applied to HEK293 cells. After 4 h of incubation, cells were treated again with N1 or KG, before being challenged with staurosporine (2 μm, 16 h) and processed for XTT measurement (b), caspase-3 activity determination (c), or TUNEL labeling (d). Bars in b and c, means ± S.E. of 4–6 independent determinations with two technical replicas. Bars in d, means ± S.E. of the number of labeled nuclei in 10 independent optical fields. e, caspase-3 activity in mock-transfected (Mock) HEK293, 3F4MoPrP, or C1-expressing cells treated with N1 or KG as detailed above. f and g, primary cultured cortical neurons were obtained from the indicated embryonic day 14 mouse embryos as described under “Materials and Methods” and treated as detailed above with increasing concentrations of N1, prior to staurosporine challenge (2 μm, 16 h) and measurement of caspase-3 activity. Bars, means ± S.E. of 3–4 independent determinations carried out in duplicate. h, caspase-3 activity measured in primary cultured mouse cortical neurons transfected with the cDNA of full-length PrP (PrP), C1 (PrPC1), or parental plasmid (DNA3). Forty-eight h post-transfection, cells were incubated with N1 (1 μm, 4 h) or an equivalent volume of KG. Cells were treated again with N1, challenged with staurosporine (2 μm, 16 h), and processed for caspase-3 activity determination. Bars, means of three independent determinations carried out in duplicate. Right, a representative Western blot using SAF61 antibody of PrPc and C1 expression in cortical neurons after nucleofection. *, p < 0.05; **, p < 0.001; ***, p < 0.0001; ns, not significant; IP, immunoprecipitation.
FIGURE 4.
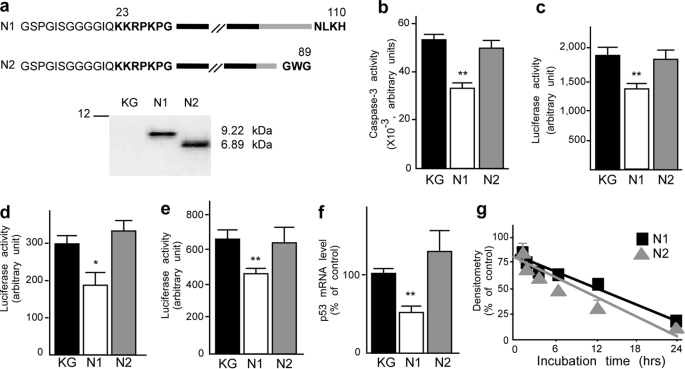
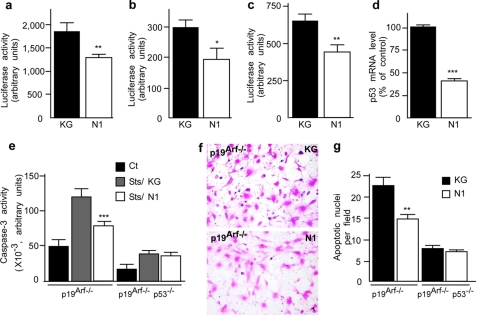
The pathological fragment N2 does not exhibit protective activity. a, schematic representation of the sequences of the various recombinant PrPc-derived N-terminal fragments and their analysis with SAF32 monoclonal antibody (see “Materials and Methods”). Residues shown in boldface type are present on endogenous fragments, whereas lightface residues come from the PGEX-KG constructs and are present only on recombinant peptides. These additional residues explain the shift in the migration of fragment N1 recombinant compared with endogenous N1 (see Fig 1a). b, HEK293 cells were treated for 4 h with recombinant N1 or N2 fragments (1 μm) or an equivalent volume of control supernatant (KG). Peptides were applied again to the cells before cell death induction with staurosporine (2 μm, 16 h). Cells were recovered, lysed, and processed for caspase-3 activity measurement as detailed under “Materials and Methods.” Bars, means ± S.E. of four independent experiments carried out in duplicate. PG13-luciferase (c), p21waf-1-luciferase (d), and pp53-luciferase (e) reporter constructs were transiently transfected in HEK293 cells together with β-galactosidase-expressing vector, as detailed under “Materials and Methods.” Twenty-four h after transfection, cells were treated for 16 h with the indicated recombinant fragments (1 μm) or KG. Luciferase and β-galactosidase activities were measured as detailed under “Materials and Methods.” The bars correspond to the ratio of luciferase/β-galactosidase and are the means of 3–6 independent experiments performed in triplicate. f, confluent HEK293 cells were treated for 8 h with N1, N2 (1 μm), or KG and then monitored for p53 mRNA levels as detailed under “Materials and Methods.” Bars, means of three independent experiments carried out in duplicate. g, recombinant N1 or N2 was added into HEK293 cell medium, and then fluids were collected at the indicated time points, and fragments were immunoprecipitated using SAF32 as detailed under “Materials and Methods.” Immunocomplexes were applied on a 16.5% Tris/Tricine SDS-polyacrylamide gel and probed with SAF32. Plots correspond to densitometric analysis of extracellular fragments and show the means ± S.E. of three independent experiments. *, p < 0.05; **, p < 0.001.
We and others have reported that cells overexpressing full-length PrPc (3, 5) or its C-terminal fragment C1 (16) display enhanced responsiveness to proapoptotic stimuli, including STS. We have therefore investigated the potential of recombinant N1 to protect these cells from STS-induced cell death. As expected, PrPc and C1 overexpressions both increase STS-induced caspase-3 activation (Fig. 1e, compare black bars). Interestingly, recombinant N1 significantly reduced STS-evoked caspase-3 activation in HEK293 cells (34.8 ± 12.8%, p < 0.05, n = 4 and 30.5 ± 3.5%, p < 0.05, n = 4 in 3F4MoPrP- and C1-expressing cells, respectively).
We examined the putative antiapoptotic function of N1 in primary cultured mouse cortical neurons. As we previously reported (4), wild type primary cultured embryonic cortical neurons exhibited an exacerbated sensitivity to staurosporine when compared with PrPc-deficient neurons (see controls in Fig. 1, f and g). Clearly, recombinant N1 significantly and dose-dependently lowered STS-induced caspase-3 activation in both wild type and PrPc-deficient neurons (Fig. 1, f and g). In addition, overexpression of PrPc or C1 fragment in primary cultured mouse cortical neurons augments STS-induced caspase-3 activity (Fig. 1h, compare control black bars). Here again, recombinant N1 reduces caspase-3 activity evoked by STS in DNA3, PrP, and C1-expressing neurons (36.27 ± 13.40%, p < 0.05, n = 3; 23.75 ± 6.85%, p < 0.05, n = 5; and 35.46 ± 10.32%, p < 0.05, n = 6, respectively). Overall, the above data suggest that N1 could behave as a soluble neuroprotective factor in various cell systems independently of endogenous PrPc.
N1 Protective Function Does Not Require N1 Internalization
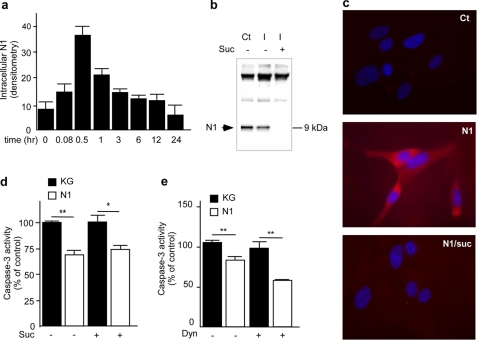
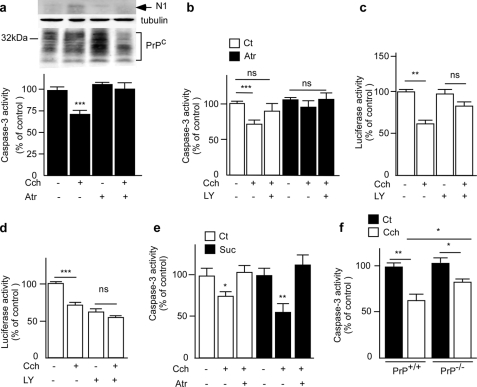
The N-terminal sequence of PrPc directs the internalization of the molecule (30). We therefore looked at N1 translocation into HEK293 cells and primary cultured neurons and investigated whether such putative internalization could underlie its neuroprotective function. Kinetic analysis of the fate of recombinant N1 added in the cell media indicates that N1 is rapidly detected inside the cells. N1 translocation into intracellular compartments peaked after 30 min and then returned to base line (Fig. 2a). This internalization was efficiently blocked by 0.45 m sucrose treatment as shown by immunoprecipitation (Fig. 2b) and in situ labeling (Fig. 2c) of N1 in HEK293 cells and HpL3–4 PrPc-deficient neurons, respectively. Blockade of N1 internalization with sucrose does not modify the N1-induced reduction of STS-stimulated caspase-3 activation (Fig. 2d). Furthermore, Dynasore, a pharmacological blocker of the dynamin-dependent internalization pathway (31), did not modify N1-dependent inhibition of STS-induced caspase-3 activation (Fig. 2e). Overall, the above results indicate that N1 can translocate into the cytosol, that N1 translocation does not require endogenous PrPc, and that N1-associated protective phenotype remains independent of its internalization.
FIGURE 2.
N1 translocation in intracellular compartment is not required for protective function. a, recombinant N1 was added to HEK293 cell medium, and cells were collected at each indicated time point. Internalized N1 was immunocaptured from cell lysate using SAF32, as detailed under “Materials and Methods,” applied on a 16.5% Tris/Tricine SDS-polyacrylamide gel, and probed with SAF32. Bars corresponding to densitometric analysis of intracellular N1 show the means ± S.E. of three independent experiments. b, HEK293 cells were treated (+) or not (−) for 20 min at 37 °C with 0.45 m sucrose to block internalization. N1 was added for 30 min into cell medium, and intracellular N1 (I) was immunocaptured from cell lysate. The first lane (Ct) corresponds to the amount of N1 recovered from the cell fluid when cells are maintained at 4 °C (note that upper bands show immunoglobulin used for immunoprecipitation). c, HpL3–4 PrP−/− neuronal cell line was seeded on glass coverslips and cultured for 24 h. Internalization processes were blocked as described above, and N1 was applied to the cells for 30 min. Coverslips were then processed for N1 immunolabeling using SAF32 as described under “Materials and Methods.” HEK293 cells were incubated or not with 0.45 m sucrose (Suc, d) or Dynasore (Dyn, e) for 20 min at 37 °C, and then recombinant N1 (1 μm) or an equivalent volume of KG was applied on cells, and dishes were returned to 37 °C for 30 min before being challenged with staurosporine (2 μm, 16 h). Caspase-3 activity was then determined as described under “Materials and Methods.” Bars, means ± S.E. of three independent experiments in triplicate (d) and duplicate (e). *, p < 0.05; **, p < 0.01.
N1 Down-regulates p53 Transcription
We examined whether N1 could down-regulate p53 activity, promoter transactivation, and mRNA levels. We first measured p53 transcriptional activity using a luciferase reporter construct (PG13) harboring the canonical sequence to which p53 binds in its target gene promoters. Fig. 3a shows that N1 lowers p53 activity in HEK293 cells (45.6 ± 9.3% (p < 0.001, n = 6) reduction compared with vehicle-treated cells). This result was confirmed by means of another luciferase reporter construct bearing the promoter of p21waf-1, a natural p53 target (32) (42.7 ± 14.0% reduction, p < 0.05, n = 4) (Fig. 3b). Importantly, both p53 promoter transactivation (Fig. 3c) and p53 mRNA levels (Fig. 3d) were also lowered by N1 (45.72 ± 8.76%, p < 0.001, n = 6 and 59.6 ± 3.3% p < 0.0001, n = 5 reduction for promoter transactivation and mRNA levels, respectively).
FIGURE 3.
N1 antiapoptotic activity is strictly p53-dependent. PG13-luciferase (a), p21waf-1-luciferase (b), or p53 promoter-luciferase (pp53) (c) reporter constructs were transiently transfected in HEK293 cells together with β-galactosidase-expressing vector, as detailed under “Materials and Methods.” Twenty-four h after transfection, cells were treated for 16 h with recombinant N1 (1 μm) or an equivalent volume of control supernatant (KG). The luciferase and β-galactosidase activities were measured as detailed under “Materials and Methods.” Bars correspond to the ratio of luciferase/β-galactosidase and show the means ± S.E. of 3–6 independent experiments performed in triplicate. d, cells treated with recombinant N1 (1 μm) or KG for 8 h were analyzed for levels of p53 mRNA by real time PCR as indicated under “Materials and Methods.” Bars correspond to p53 mRNA levels expressed as a percentage of control-treated cells and are the means ± S.E. of three independent experiments carried out in duplicate. e, recombinant N1 (1 μm) or equivalent volume of KG were applied to p19arf−/− and p19arf−/−p53−/− fibroblasts. After 4 h of incubation, cells were treated with N1 or KG before being challenged with staurosporine (1 μm, 2 h) and processed for caspase-3 activity measurement. Bars, means ± S.E. of five independent experiments performed in duplicate. p19arf−/− and p19arf−/−p53−/− cells (f), seeded on glass coverslips, were treated as in e and then processed for TUNEL labeling as described under “Materials and Methods.” f, representative pictures of TUNEL labeling of control (KG) and N1-treated p19arf−/− cells. g, TUNEL-positive nuclei were counted for 10 independent optical fields. Bars, means ± S.E. of labeled nuclei/field. *, p < 0.05; **, p < 0.001; ***, p < 0.0001.
In addition to its key role in apoptosis, p53 regulates senescence and cell cycle progression via the tumor suppressor p19arf. In the absence of p19arf, p53 apoptotic activity remains functional, whereas p53-mediated growth arrest is impaired. p19arf-deficient cells are therefore useful to investigate p53-dependent cell death activity without interference of cell cycle regulation (19). Interestingly, STS-induced caspase-3 activity measurements in p19arf−/− and p19arf−/−p53−/− fibroblasts indicate that both cell lines remain responsive to STS although to a lesser extent in double knock-out (Fig. 3e). N1 also protects p19arf−/− cells from STS insult, whereas the deletion of p53 fully abolishes the effect of N1 on STS-induced caspase-3 activation (Fig. 3e). Accordingly, the number of TUNEL-positive nuclei was significantly reduced by N1 treatment in p19arf−/− but not in p19arf−/−p53−/− fibroblasts (Fig. 3, f and g). Altogether, these results indicate that N1-associated protective function is fully dependent on p53.
N2 Does Not Display N1-associated Cytoprotective Function
Recombinant N2 was produced (Fig. 4a) and tested for its capacity to reduce STS-induced caspase-3 activity and impact on p53 activity and transcription. Interestingly, N2 proved unable to protect cells from STS-induced caspase-3 activation (Fig. 4b) and did not significantly modulate p53 activity (Fig. 4, c and d), promoter transactivation (Fig. 4e), and mRNA levels (Fig. 4f). Other fragments, NT that is deleted of the first N-terminal amino acids 23–40 of N1 and ending at 107 and NK, which is as long as N1 but harbors mutations in the N terminus (supplemental Fig. S1a), also appeared inert in these paradigms (supplemental Fig. S1, c–f).
To rule out a possible impact of C- and N-terminal deletions on fragment stability, recombinant N1, N2, NT, and NK were incubated on living cells for up to 24 h. Fig. 4g and supplemental Fig. S1b clearly show that the distinct phenotypes exhibited by N2, NT, and NK compared with N1 cannot be ascribed to distinct catabolic fates. The above data demonstrate that NT, NK, and transmissible spongiform encephalopathy-associated N2 fragments fail to elicit cytoprotection and therefore that N1 C- and N-terminal integrity is required for its biological function. In order to determine if N2 could block the binding and activity of N1, we carried out experiments where N2 was preincubated prior to N1 or co-incubated with N1 and therefore used as a putative competitor. Our data show that, in both cases, N2 did not interfere with the N1-associated protective phenotype (data not shown).
Carbachol-stimulated Release of Endogenous N1 Protects Cells against STS-induced Caspase-3 Activation
Physiological processing yielding endogenous N1 is increased upon carbachol activation of endogenous muscarinic receptors M1 and M3 (33). We examined whether endogenous N1 could mimic the protective p53-dependent phenotype elicited by recombinant N1. Fig. 5a confirms that carbachol treatment of HEK293 cells expressing M1 muscarinic receptors increases the recovery of endogenous N1 that was poorly detectable in basal conditions (Fig. 5a, top). Interestingly, carbachol treatment also triggers a decrease of caspase-3 activity (29.18 ± 4.90% (p < 0.0001, n = 6) reduction) (Fig. 5a, bottom) that was fully reversed by atropine (Fig. 5a). Three lines of data indicate that carbachol-associated protection is indeed due to endogenous N1 increase. First, in agreement with the results obtained with recombinant N1 (Fig. 2d), blockade of endocytosis with sucrose did not suppress carbachol-induced reduction of caspase-3 activity (Fig. 5e). Second, we demonstrated that N1 protective function involved the Akt pathway and was independent of ERK signaling (supplemental Fig. S2). Accordingly, the Akt inhibitor LY294002 fully prevents the carbachol effect on caspase-3 (Fig. 5b). Third, carbachol reduces p53 activity (Fig. 5c) and promoter transactivation (Fig. 5d) in a LY294002-sensitive manner.
FIGURE 5.
Carbachol-stimulated release of endogenous N1 protects cells against staurosporine-induced caspase-3 activation. a, HEK293 cells overexpressing muscarinic receptor M1 (HEK-M1) were treated with carbachol (100 μm) and/or atropine (10 μm) as indicated, simultaneously treated with staurosporine (2 μm), and incubated for 16 h before being processed for caspase-3 activity measurement. Bars show caspase-3 activity expressed as a percentage of control and show the means ± S.E. of six independent experiments carried out in duplicate. Inset, representative Western blot analyses of endogenous N1 immunoprecipitation from the cell medium and total PrPc expressed in the cells, detected with SAF32 antibody. Tubulin is shown as control of the amount of protein loaded. b, HEK-M1 cells were treated as in a in the absence (−) or in the presence (+) of LY294002 (10 μm). After 16 h of incubation at 37 °C, caspase-3 activity was measured (see “Materials and Methods”). Bars represent caspase-3 activity expressed as a percentage of staurosporine-treated cells and show the means ± S.E. of six independent experiments carried out in duplicate. HEK-M1 cells were transiently transfected with PG13-luciferase (c) or pp53-luciferase (d) reporter construct together with β-galactosidase-expressing vector as described under “Materials and Methods.” Twenty-four h post-transfection, cells were treated for 8 h with carbachol (100 μm) and LY294002 (10 μm) as indicated. Luciferase and β-galactosidase activities were measured. Bars correspond to the ratio of luciferase/β-galactosidase expressed as a percentage of untreated cells and show the means ± S.E. of 3–6 independent experiments performed in triplicate. e, cells were incubated with 0.45 m sucrose and processed as in a. Bars show the means of three independent experiments carried out in duplicate. f, wild type or PrP−/− primary cortical neurons were obtained from embryonic day 14 mouse embryos as described under “Materials and Methods.” Neurons were treated as detailed in a and then processed for caspase-3 activity measurement. Bars represent caspase-3 activity expressed as a percentage of staurosporine-treated cells and are the means ± S.E. of 3–4 independent experiments carried out in quadruplet. *, p < 0.05; **, p < 0.001; ***, p < 0.0001. ns, not significant.
It is important to consider that the activation of M1 muscarinic receptors also potentiates the liberation of neuroprotective soluble amyloid precursor protein α by stimulating the α-secretase cleavage of the β-amyloid precursor protein (34). To delineate the genuine contribution of endogenous N1 in carbachol-induced reduction of caspase-3 activation, we compared the effect of carbachol on wild type and PrPc-deficient primary cultured cortical neurons. Carbachol treatment triggered a significant reduction of caspase-3 activation in wild type neurons (39.71 ± 7.86%, p < 0.01, n = 4) (Fig. 5f), whereas the reduction was statistically smaller (25.8 ± 4.2%, p < 0.05, n = 4) (Fig. 5f) in PrPc-deficient neurons, indicating that about half of the carbachol-induced reduction of caspase-3 activation was indeed linked to the presence of PrPc and therefore probably attributable to M1-stimulated liberation of endogenous N1.
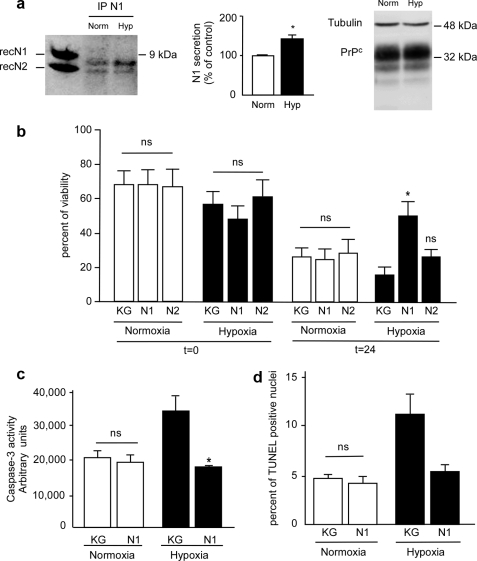
N1 Protects against OGD in Primary Cultured Retinal Ganglion Cells and Reduces Pressure-induced Ischemia Effects in the Rat Retina
We examined whether N1 could protect primary cultured rat retinal ganglion cells (RGC) against OGD, an in vitro model mimicking ischemia-associated apoptosis. First, N1 is recovered in the medium of RGC, and second, 2 h of OGD significantly increased the secretion of endogenous N1 (42.1 ± 11.2%, p < 0.05, n = 3), whereas the total level of PrPc remained unchanged (Fig. 6a). Twenty-four h post-OGD, recombinant N1 increased cell viability (Fig. 6b) and reduced hypoxia-induced caspase-3 activation (Fig. 6c) and the number of TUNEL-positive cells (Fig. 6d) (reductions of 29.92 ± 12.22% (p < 0.05, n = 3), 42.82 ± 2.82% (p < 0.05, n = 3), and 51.5 ± 4.6% (p < 0.05, n = 3), respectively).
FIGURE 6.
N1 protects rat RGC against OGD. a, RGC were subjected or not to OGD for 2 h (see “Materials and Methods”) and then returned to complete medium for 24 h. Medium was collected, and N1 was immunoprecipitated (IP) with the monoclonal antibody SAF32 and analyzed by 16.5% Tris/Tricine SDS-PAGE and Western blotting using SAF32. RecN1 and RecN2 correspond to the migration pattern of recombinant N1 and N2 (150 ng) analyzed under the same conditions. Bars correspond to the densitometric analysis of N1 and are expressed as a percentage of control N1 recovered under normoxic conditions. Values are the means ± S.E. of three independent experiments. b, 7 days after dissociation, RGC were treated with N1 (2 μm, 4 h) or an equivalent volume of KG, cells were then retreated and placed under normoxic or hypoxic conditions as in a, viability was determined just before return to complete medium or after 24 h by measurement of release of lactate dehydrogenase in the culture medium as described under “Materials and Methods.” c, caspase-3 activity was measured 24 h posthypoxia. d, cells plated on coverslips were treated as in a and processed for TUNEL staining. Bars represent the means ± S.E. of three independent determinations carried out in duplicate. *, p < 0.05.
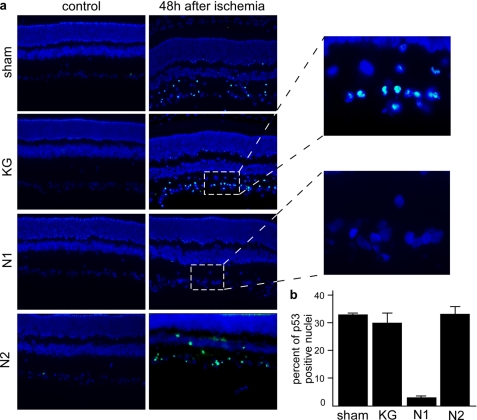
The use of the rat eye as a read out of putative biological function of N1 was based on five lines of considerations and data. First, the retina is an open window to the central nervous system; second, a previous study indicated that PrPc depletion increased the photoreceptors susceptibility to light damage; third, the retina's vulnerability to transient ischemia is associated with increased apoptotic neuronal stigmata; fourth, transient ischemia-induced apoptosis involves up-regulation of p53 expression; fifth, muscarinic receptors convey protective signals against the cytotoxicity triggered by intraocular pressure. Our demonstration that muscarinic receptor-mediated production of endogenous N1 could lower p53-dependent caspase-3 activation led us to envision that pressure-induced ischemia could represent a relevant model to establish N1 potential, in vivo. The protocol of injection, ischemia, and reperfusion is depicted in Fig. 7a. Fig. 7b clearly shows that N1 but not N2 reduced the number of apoptotic cells by about 65% 48 h after ischemia. Microphotographs showing fluorescein and DAPI overlays confirmed that most of the TUNEL-positive cells were located in the ganglion cell layer but also to a lesser extent in the inner nuclear layer (Fig. 7c) and that inactive fragments N2 (Fig. 7) and NT (supplemental Fig. S3a) did not reduce cell death. ERGs were monitored at 1, 2, and 7 days following ischemia. ERG traces show that an ischemic insult of 45 min decreased the amplitudes of both a- and b-waves of treated and non-treated rats at all time periods after reperfusion (Fig. 7d). Clearly, b-waves but not a-waves were partially rescued by N1 but not by N2 (Fig. 7d) and NT (supplemental Fig. S3b). Thus, at days 1 and 2 after reperfusion (Fig. 7d), the amplitudes of b-waves in the N1-treated group (32 ± 5 and 45 ± 7%, respectively) were significantly larger (p < 0.05; Mann-Whitney U test) than those of untreated (14.5 ± 6 and 19 ± 8%, respectively), vehicle-treated (11 ± 9 and 15 ± 6%, respectively), N2-treated (17 ± 12 and 20 ± 9%, respectively), and NT-treated (6 ± 7 and 15 ± 5%, respectively) groups (supplemental Fig. S3b). Seven days after recovery when the N1-associated effect on b-waves was maximal (Fig. 7d), retina slices were colored with cresyl violet to examine their architecture. As expected, ischemia induced a pronounced disorganization of the retina associated with a cell loss leading to a thinning of all retina layers (Fig. 7e). In N1-injected animals, the retina structure is conserved, and the thickness of the layers is partly rescued (Fig. 7e), whereas vehicle N2 (Fig. 7e) or NT (supplemental Fig. S3c) injections neither prevent the structural disorganization triggered by ischemia nor rescue retina thickness. Overall, biological data showing important recovery of retina structure and function agree well with the observed protective effect of N1 on ischemia-induced neuronal cell death. According to our previous data obtained in cellulo, one should predict that N1 could protect retinal neurons by down-regulating p53, in vivo. First, as expected from previous studies (35), pressure-induced ischemia drastically increased p53-like immunoreactivity in control conditions (Fig. 8, a and b). Of greatest interest, 48 h after ischemia, N1-treated but not vehicle N2- or NT-treated eyes exhibited drastically lowered p53 expression (Fig. 8, a and b, and supplemental Fig. S3, d and e). Overall, our data show that N1 contributed to reduce p53-dependent cell death triggered by pressure-induced ischemia and partly rescued retina function in vivo.
FIGURE 8.
Ischemia-induced increase of p53 expression is diminished by N1 pretreatment. a, representative microphotographs showing overlay of p53 immunoreactivity (green) and DAPI-immunostained nuclei (blue) in retina slices, 48 h after pressure-induced ischemia. Ischemic untreated sections; N1-, N2-, or KG-treated sections; and correspondent control sections are compared. b, quantification of p53-positive cells expressed as a percentage of those obtained in sham control conditions ± S.E. of 4–10 independent sections.
DISCUSSION
Although several functions have been proposed for PrPc, its genuine physiological roles remained elusive and often debated. Several studies suggested an implication in cell survival (for a review, see Ref. 1), whereas, apparently contradicting this view, others and we have reported that overexpressed and endogenous PrPc could sensitize cells to proapoptotic stimuli in several distinct experimental systems involving transformed cells and primary cultured neurons (2, 4–7). One important aspect neglected when considering the PrPc biology is the fact that PrPc undergoes a set of physiological proteolytic cleavages. Thus, we demonstrated that PrPc undergoes constitutive and protein kinase C-regulated hydrolysis between its residues 110 and 111 (10–12). This processing leads to a 9-kDa soluble fragment, referred to as N1, released in the extracellular environment, and its C-terminal domain counterpart (C1) that remains tethered to the plasma membrane and that was shown to be generated in vivo (9).
The putative influence of this proteolysis has remained poorly understood. Can it be considered as a degradation mechanism aimed at clearing full-length PrPc, thereby impairing its associated function, or can it be seen as a maturation process yielding biologically active metabolites? In the latter case, do these metabolites account for proposed PrPc-associated functions, or alternatively, do they harbor their own physiological function? If so, is this function unrelated to the one associated with full-length PrPc, or does it interfere with it?
Our first report showing that the overexpression of C1 potentiated staurosporine-induced caspase-3 activation through a p53-dependent mechanism (16), as we reported for full-length PrPc (2), suggested that some of the PrPc catabolites could harbor a function mimicking that of the parent protein. However, no data were yet available concerning the secreted N1 product, and evidence that PrPc catabolites could also exhibit their function in vivo still awaited demonstration.
To our knowledge, our study is the first direct demonstration of a biological function associated with the soluble PrPc-derived N1 fragment in cells. Thus, we show that recombinant N1 attenuates STS-evoked caspase-3 activation and reduces the number of TUNEL-positive nuclei in cell cultures by down-regulating the p53 pathway in an Akt-dependent but ERK-independent manner. It is interesting to note that the stimulation of muscarinic receptors by carbachol, which increases the recovery of endogenously secreted N1, also triggers Akt-dependent neuroprotection. Serum-deprived PC12 cells were protected from cell death by M1 stimulation (36), whereas M1 agonists protect from DNA damage, oxidative stress, or mitochondrial impairment in neurons (37). Apparently, M1-mediated neuroprotection involves Akt-dependent and extracellular signal-regulated kinase-independent cell survival pathways (38, 39). Altogether, the above studies suggest that carbachol-stimulated production of endogenous N1 triggers a neuroprotective phenotype that strikingly resembles the one obtained with recombinant N1, suggesting that carbachol-induced phenotype could be at least partly mediated by endogenous N1. This hypothesis is strongly supported by our demonstration that PrPc deficiency partly rescued carbachol-induced reduction of caspase-3 activity in neurons (Fig. 5f).
A question arises as to whether N1 could account for the protective phenotype elicited by PrPc in certain experimental conditions. Two lines of evidence suggest that PrPc protective function is transduced by the phosphatidylinositol 3-kinase/Akt signaling pathway. First, it has been shown that PrPc can recruit phosphatidylinositol 3-kinase/Akt, thereby contributing to cell survival (40). Second, it has recently been suggested that PrPc deletion in mice results in aggravation of neuronal injury after mild focal cerebral ischemia (41). PrPc deletion was later shown to impair the antiapoptotic phosphatidylinositol 3-kinase/Akt pathway, resulting in a reduced postischemic phospho-Akt expression and aggravation of neuronal injury (42). These results are in good agreement with our finding of a survival signal triggered by N1 through phosphatidylinositol 3-kinase signaling pathway and suggest that, in ischemic injury, the protective phenotype of PrPc could be due to the release of N1 after PrPc cleavage by disintegrins.
N1 also protects primary cultured retinal ganglion cells from OGD by modulating cell death. These data first show that the N1 protective phenotype is not restricted to STS-challenged stimulus. It was also very important to correlate cell biology approach and in vivo experiments. Thus, we examined the potential of N1 to protect rat retina from pressure-induced ischemia. It is well admitted that ischemia mainly results from oxygen deprivation and glucose reduction. Interestingly, we clearly established that N1 protects retina neurons from cell death associated with pressure-induced ischemia, as shown by the lowering of TUNEL-positive neurons, in situ. This protection was accompanied by a strong reduction in the number of p53-positive neurons and by a preservation of the retina architecture. Finally, of utmost importance was our observation that N1-induced protection indeed has a functional consequence because N1 partly rescued alterations observed in ERG traces upon pressure-induced ischemia. This is the very first demonstration of a biological function harbored by a PrPc processing product in vivo. Interestingly, OGD augments the recovery of secreted endogenous N1. It is therefore tempting to speculate on a compensatory mechanism aimed at reducing hypoxia-induced stress via the up-regulation of N1 production.
Our data could explain the phenotypes observed in several transgenic mice. Thus, PrPc deleted of residues 32–121 and 32–134 (collectively called ΔPrP) has been reported to cause progressive neurodegenerative illness (13). This phenotype is abolished when ΔPrP animals are backcrossed with wild type mice. On the other hand, the deletion of 23–88 N-terminal amino acids abrogates the ability of PrPc to rescue mice from ΔPrP-induced toxicity (43).
Another important aspect of our study lies with our observation that the N1-associated protective function requires the integrity of the N-terminal moiety, both in vitro and in vivo. Thus, NT and NK fragments (see supplemental Fig S1a) do not protect cells from staurosporine-induced cell death and fail to reduce pressure-induced ischemia in the retina of live animals. This agrees well with a recent report showing that residues 23–31 are essential for PrPc to protect cerebellar granule neurons against ΔPrP toxicity and Dpl-induced degeneration in mice (44). In the same line of reasoning, the examination of the sequence domain of PrPc important for cytoprotective activity in yeast revealed that the 23–31 sequence was crucial for protection against Bax-induced apoptosis (45). It is noteworthy that the 23–31 motif has been shown to be important for both endocytosis (23, 46, 47) and binding to transmembrane receptors (48, 49). However, our data showing that N1 could indeed be endocytosed but that its protective phenotype was not prevented by blockade of endocytosis (Figs. 2, d and e, and 5e) or mutations abolishing the intracellular translocation (data not shown) strongly suggest that N1-associated protective function was independent of its endocytosis. Alternatively, one could speculate that N1 acts as a soluble factor that would signal after interacting with a yet unknown binding protein. This hypothesis is supported by a recent report showing that the in vitro PrP-DA construct in which the N terminus is tethered to the membrane and consequently not released in the extracellular space fails to protect cells against paraquat-induced oxidative injury in neuronal cells (50). These results are in accordance with the need of N-terminal processing and subsequent release in the extracellular matrix for PrPc to exert its antiapoptotic activity.
We did not yet delineate the molecular intermediate involved in N1-protective function. However, since N1 function does not involve the ERK1/2 pathway (see above), we could already eliminate STSI1 (stress-inducible protein 1) as a candidate because a recent paper indicated that the PrPc-associated STI1-mediated pathway involved ERK1/2 signaling (51).
The fact that N2 lacking the 21 last C-terminal amino acids is inert in all of the tested paradigms suggests that the C-terminal part of N1 could also be of importance for its neuroprotective activity. However, NK that harbors a strictly identical C terminus is inactive. Therefore, one could consider that the deletion of the C-terminal end of N2 may have changed its conformation, thereby leading to the loss of neuroprotective function.
Our previous (2, 16) and present works demonstrated that full-length PrPc and its α-secretase-derived C1 metabolite exacerbated STS-induced caspase-3 activation and cell death by up-regulating the p53 pathway. Consequently, one should consider that two PrPc-derived catabolites (i.e. C1 and N1) produced by the same proteolytic attack could display opposite phenotypes and even cross-talk for such a function. Our results showing that recombinant N1 protects cells overexpressing C1 or PrPc full-length from apoptosis suggested that, once released in the extracellular space, N1 protective function predominates over the C1-associated proapoptotic phenotype. Indeed, when cleavage of endogenous PrPc yielding equimolar amounts of N1 and C1 was amplified by muscarinic receptor stimulation, the overall phenotype was that triggered by N1, indicating that in these conditions, N1-associated protective function was clearly dominant over the C1-associated toxic effect. It is tempting to speculate that when PrPc cleavage is exacerbated by various stimuli, including muscarinic receptor activation, N1 liberation accounts for apparent PrPc-associated antiapoptotic phenotype, whereas PrPc is biologically inert in basal conditions and even contributes to exacerbate cell death when cells are challenged by various proapoptotic stimuli.
Investigating the role of proteolytic processing and its consequences on the function of PrPc is of great importance because the cleavage is modified in pathological situations. Thus, in infected brains, N1 production is conserved, but an additional cleavage occurs to give rise to the shorter fragment N2 (9, 52). The question of transmissible spongiform encephalopathies being in part due to a loss of function or a gain of toxic function is still open. In this regard, our results showing that N2 does not exhibit any protective activity are of great interest and are in accordance with a study by Lee et al. (53) showing that fusion of PrP-(1–124) to Dpl (doppel) confers resistance to serum deprivation, whereas fusion construct PrP-(1–95)-Dpl that resembles N2 does not. This was confirmed in vivo. Thus, Baumann et al. (54) showed that a chimeric protein in which the amino-terminal 1–134 sequence of PrPc was grafted onto Dpl exerts a neurotrophic function. Importantly, Cronier et al. (55) have recently reported that PrPsc-infected primary neurons undergo apoptosis. Whether N1 but not N2 is able to protect infected cells from cell death and slow the degenerative process will have to be addressed. Moreover, our results suggest that modulation of desintegrin cleavage could be beneficial. This strategy has been tested in vivo with success for Alzheimer disease (56, 57).
Finally, our data show that even a rather small modulation of N1 production by muscarinic stimulation could prove useful to trigger neuroprotection. It is interesting to note that the stimulation of soluble amyloid precursor protein α production by genetic manipulation of the α-secretase ADAM10 lowers antibody load and rescues cognitive deficits in Alzheimer disease animal models (58). This study and our data open an avenue to experiments aimed at establishing whether the manipulation of N1 could also be beneficial in prion-infected mice. Another aspect is correlated to the striking parallel between PrPc/N1 and β-amyloid precursor protein/Aβ. Currently, work is in progress in our laboratory to establish a putative protective effect of N1 toward Aβ-induced toxicity, thereby reinforcing the strong network, suggesting an intimate cross-talk between PrPc and β-amyloid precursor protein (59).
Acknowledgments
We are grateful to Drs. M. Roussel (Memphis, TN), G. Pages (Nice, France), B. Slack (Boston, MA), and T. Onodera (Tokyo, Japan) for providing cells; Drs. M. Oren and B. Vogelstein for supplying PG13, p21, and p53 promoter reporter constructs; and Dr. E. Macia (Valbonne) for Dynasore. We sincerely thank Dr. M. Ettaiche for advice in in vivo studies.
This work was supported by CNRS, the Fédération pour la Recherche sur le Cerveau, and the Fondation pour la Recherche Médicale.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- GST
- glutathione S-transferase
- PBS
- phosphate-buffered saline
- TM
- theoretical mass
- MM
- measured mass
- TUNEL
- terminal deoxynucleotidyltransferase-mediated biotinylated UTP nick end labeling
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine
- STS
- staurosporine
- DAPI
- 4′,6-diamino-2-phenylindole
- OGD
- oxygen glucose deprivation
- RGC
- rat retinal ganglion cells
- ERK
- extracellular signal-regulated kinase.
REFERENCES
- 1.Linden R., Martins V. R., Prado M. A., Cammarota M., Izquierdo I., Brentani R. R. (2008) Physiol. Rev. 88, 673–728 [DOI] [PubMed] [Google Scholar]
- 2.Paitel E., Alves da Costa C., Vilette D., Grassi J., Checler F. (2002) J. Neurochem. 83, 1208–1214 [DOI] [PubMed] [Google Scholar]
- 3.Paitel E., Fahraeus R., Checler F. (2003) J. Biol. Chem. 278, 10061–10066 [DOI] [PubMed] [Google Scholar]
- 4.Paitel E., Sunyach C., Alves da Costa C., Bourdon J. C., Vincent B., Checler F. (2004) J. Biol. Chem. 279, 612–618 [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y., Qin K., Wang J., Hung T., Zhao R. Y. (2006) Biochem. Biophys. Res. Commun. 349, 759–768 [DOI] [PubMed] [Google Scholar]
- 6.Gougoumas D. D., Vizirianakis I. S., Triviai I. N., Tsiftsoglou A. S. (2007) J. Cell. Physiol. 211, 551–559 [DOI] [PubMed] [Google Scholar]
- 7.Nicolas O., Gavín R., Braun N., Ureña J. M., Fontana X., Soriano E., Aguzzi A., del Río J. A. (2007) FASEB J. 21, 3107–3117 [DOI] [PubMed] [Google Scholar]
- 8.Westaway D., DeArmond S. J., Cayetano-Canlas J., Groth D., Foster D., Yang S. L., Torchia M., Carlson G. A., Prusiner S. B. (1994) Cell 76, 117–129 [DOI] [PubMed] [Google Scholar]
- 9.Chen S. G., Teplow D. B., Parchi P., Teller J. K., Gambetti P., Autilio-Gambetti L. (1995) J. Biol. Chem. 270, 19173–19180 [DOI] [PubMed] [Google Scholar]
- 10.Vincent B., Paitel E., Frobert Y., Lehmann S., Grassi J., Checler F. (2000) J. Biol. Chem. 275, 35612–35616 [DOI] [PubMed] [Google Scholar]
- 11.Vincent B., Paitel E., Saftig P., Frobert Y., Hartmann D., De Strooper B., Grassi J., Lopez-Perez E., Checler F. (2001) J. Biol. Chem. 276, 37743–37746 [DOI] [PubMed] [Google Scholar]
- 12.Cissé M. A., Sunyach C., Lefranc-Jullien S., Postina R., Vincent B., Checler F. (2005) J. Biol. Chem. 280, 40624–40631 [DOI] [PubMed] [Google Scholar]
- 13.Shmerling D., Hegyi I., Fischer M., Blättler T., Brandner S., Götz J., Rülicke T., Flechsig E., Cozzio A., von Mering C., Hangartner C., Aguzzi A., Weissmann C. (1998) Cell 93, 203–214 [DOI] [PubMed] [Google Scholar]
- 14.Radovanovic I., Braun N., Giger O. T., Mertz K., Miele G., Prinz M., Navarro B., Aguzzi A. (2005) J. Neurosci. 25, 4879–4888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li A., Barmada S. J., Roth K. A., Harris D. A. (2007) J. Neurosci. 27, 852–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sunyach C., Cisse M. A., da Costa C. A., Vincent B., Checler F. (2007) J. Biol. Chem. 282, 1956–1963 [DOI] [PubMed] [Google Scholar]
- 17.Demart S., Fournier J. G., Creminon C., Frobert Y., Lamoury F., Marce D., Lasmézas C., Dormont D., Grassi J., Deslys J. P. (1999) Biochem. Biophys. Res. Commun. 265, 652–657 [DOI] [PubMed] [Google Scholar]
- 18.Guan K. L., Dixon J. E. (1991) J. Biol. Chem. 266, 17026–17030 [PubMed] [Google Scholar]
- 19.Kamijo T., Zindy F., Roussel M. F., Quelle D. E., Downing J. R., Ashmun R. A., Grosveld G., Sherr C. J. (1997) Cell 91, 649–659 [DOI] [PubMed] [Google Scholar]
- 20.Kuwahara C., Takeuchi A. M., Nishimura T., Haraguchi K., Kubosaki A., Matsumoto Y., Saeki K., Matsumoto Y., Yokoyama T., Itohara S., Onodera T. (1999) Nature 400, 225–226 [DOI] [PubMed] [Google Scholar]
- 21.Vincent B., Beaudet A., Dauch P., Vincent J. P., Checler F. (1996) J. Neurosci. 16, 5049–5059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.da Costa C. A., Ancolio K., Checler F. (2000) J. Biol. Chem. 275, 24065–24069 [DOI] [PubMed] [Google Scholar]
- 23.Sunyach C., Checler F. (2005) J. Neurochem. 92, 1399–1407 [DOI] [PubMed] [Google Scholar]
- 24.Ettaiche M., Heurteaux C., Blondeau N., Borsotto M., Tinel N., Lazdunski M. (2001) Brain Res. 890, 118–129 [DOI] [PubMed] [Google Scholar]
- 25.Alves da Costa C., Paitel E., Mattson M. P., Amson R., Telerman A., Ancolio K., Checler F. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 4043–4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Deiry W. S., Kern S. E., Pietenpol J. A., Kinzler K. W., Vogelstein B. (1992) Nat. Gen. 1, 45–49 [DOI] [PubMed] [Google Scholar]
- 27.Grabb M. C., Choi D. W. (1999) J. Neurosci. 19, 1657–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ettaiche M., Fillacier K., Widmann C., Heurteaux C., Lazdunski M. (1999) Invest. Ophthalmol. Vis. Sci. 40, 729–736 [PubMed] [Google Scholar]
- 29.Ettaiche M., Deval E., Cougnon M., Lazdunski M., Voilley N. (2006) J. Neurosci. 26, 5800–5809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris D. A., Huber M. T., van Dijken P., Shyng S. L., Chait B. T., Wang R. (1993) Biochemistry 32, 1009–1016 [DOI] [PubMed] [Google Scholar]
- 31.Macia E., Ehrlich M., Massol R., Boucrot E., Brunner C., Kirchhausen T. (2006) Dev. Cell 10, 839–850 [DOI] [PubMed] [Google Scholar]
- 32.el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. (1993) Cell 75, 817–825 [DOI] [PubMed] [Google Scholar]
- 33.Alfa Cissé M., Sunyach C., Slack B. E., Fisher A., Vincent B., Checler F. (2007) J. Neurosci. 27, 4083–4092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nitsch R. M., Slack B. E., Wurtman R. J., Growdon J. H. (1992) Science 258, 304–307 [DOI] [PubMed] [Google Scholar]
- 35.Rosenbaum D. M., Rosenbaum P. S., Gupta H., Singh M., Aggarwal A., Hall D. H., Roth S., Kessler J. A. (1998) Invest. Ophthalmol. Vis. Sci. 39, 2132–2139 [PubMed] [Google Scholar]
- 36.Lindenboim L., Pinkas-Kramarski R., Sokolovsky M., Stein R. (1995) J. Neurochem. 64, 2491–2499 [DOI] [PubMed] [Google Scholar]
- 37.De Sarno P., Shestopal S. A., King T. D., Zmijewska A., Song L., Jope R. S. (2003) J. Biol. Chem. 278, 11086–11093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cui Q. L., Fogle E., Almazan G. (2006) Neurochem. Int. 48, 383–393 [DOI] [PubMed] [Google Scholar]
- 39.Murga C., Laguinge L., Wetzker R., Cuadrado A., Gutkind J. S. (1998) J. Biol. Chem. 273, 19080–19085 [DOI] [PubMed] [Google Scholar]
- 40.Vassallo N., Herms J., Behrens C., Krebs B., Saeki K., Onodera T., Windl O., Kretzschmar H. A. (2005) Biochem. Biophys. Res. Commun. 332, 75–82 [DOI] [PubMed] [Google Scholar]
- 41.McLennan N. F., Brennan P. M., McNeill A., Davies I., Fotheringham A., Rennison K. A., Ritchie D., Brannan F., Head M. W., Ironside J. W., Williams A., Bell J. E. (2004) Am. J. Pathol. 165, 227–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weise J., Sandau R., Schwarting S., Crome O., Wrede A., Schulz-Schaeffer W., Zerr I., Bähr M. (2006) Stroke 37, 1296–1300 [DOI] [PubMed] [Google Scholar]
- 43.Atarashi R., Nishida N., Shigematsu K., Goto S., Kondo T., Sakaguchi S., Katamine S. (2003) The J. Biol. Chem. 278, 28944–28949 [DOI] [PubMed] [Google Scholar]
- 44.Drisaldi B., Coomaraswamy J., Mastrangelo P., Strome B., Yang J., Watts J. C., Chishti M. A., Marvi M., Windl O., Ahrens R., Major F., Sy M. S., Kretzschmar H., Fraser P. E., Mount H. T., Westaway D. (2004) J. Biol. Chem. 279, 55443–55454 [DOI] [PubMed] [Google Scholar]
- 45.Li A., Harris D. A. (2005) J. Biol. Chem. 280, 17430–17434 [DOI] [PubMed] [Google Scholar]
- 46.Sunyach C., Jen A., Deng J., Fitzgerald K. T., Frobert Y., Grassi J., McCaffrey M. W., Morris R. J. (2003) EMBO J. 22, 3591–3601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor D. R., Watt N. T., Perera W. S., Hooper N. M. (2005) J. Cell Sci. 118, 5141–5153 [DOI] [PubMed] [Google Scholar]
- 48.Parkyn C. J., Vermeulen E. G., Mootoosamy R. C., Sunyach C., Jacobsen C., Oxvig C., Moestrup S., Liu Q., Bu G., Jen A., Morris R. J. (2008) J. Cell Sci. 121, 773–783 [DOI] [PubMed] [Google Scholar]
- 49.Taylor D. R., Hooper N. M. (2007) Biochem. J. 402, 17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dupiereux I., Falisse-Poirrier N., Zorzi W., Watt N. T., Thellin O., Zorzi D., Pierard O., Hooper N. M., Heinen E., Elmoualij B. (2008) J. Neurosci. Res. 86, 653–659 [DOI] [PubMed] [Google Scholar]
- 51.Caetano F. A., Lopes M. H., Hajj G. N., Machado C. F., Pinto Arantes C., Magalhães A. C., Vieira Mde P., Américo T. A., Massensini A. R., Priola S. A., Vorberg I., Gomez M. V., Linden R., Prado V. F., Martins V. R., Prado M. A. (2008) J. Neurosci. 28, 6691–6702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiménez-Huete A., Lievens P. M., Vidal R., Piccardo P., Ghetti B., Tagliavini F., Frangione B., Prelli F. (1998) Am. J. Pathol. 153, 1561–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee D. C., Sakudo A., Kim C. K., Nishimura T., Saeki K., Matsumoto Y., Yokoyama T., Chen S. G., Itohara S., Onodera T. (2006) Microbiol. Immunol. 50, 203–209 [DOI] [PubMed] [Google Scholar]
- 54.Baumann F., Pahnke J., Radovanovic I., Rülicke T., Bremer J., Tolnay M., Aguzzi A. (2009) PLoS ONE 4, e6707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cronier S., Laude H., Peyrin J. M. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 12271–12276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Etcheberrigaray R., Tan M., Dewachter I., Kuipéri C., Van der Auwera I., Wera S., Qiao L., Bank B., Nelson T. J., Kozikowski A. P., Van Leuven F., Alkon D. L. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 11141–11146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caccamo A., Oddo S., Billings L. M., Green K. N., Martinez-Coria H., Fisher A., LaFerla F. M. (2006) Neuron 49, 671–682 [DOI] [PubMed] [Google Scholar]
- 58.Postina R., Schroeder A., Dewachter I., Bohl J., Schmitt U., Kojro E., Prinzen C., Endres K., Hiemke C., Blessing M., Flamez P., Dequenne A., Godaux E., van Leuven F., Fahrenholz F. (2004) J. Clin. Invest. 113, 1456–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Checler F., Vincent B. (2002) Trends Neurosci. 25, 616–620 [DOI] [PubMed] [Google Scholar]