Abstract
Purpose
Toll-like receptors (TLRs) are pattern-recognition receptors that play an important role in innate and adaptive immune responses to microbial pathogens. Among TLRs, TLR4 recognizes lipopolysaccharides of Gram-negative bacteria. Genetic polymorphisms within the TLR4 gene have been reported to be associated with various inflammatory diseases; therefore, TLR4 appears to be a susceptibility gene for sarcoidosis. Although sarcoidosis has various clinical manifestations, its association with uveitis is more common in Japan than in other countries. The aim of this study was to investigate whether TLR4 polymorphisms were associated with sarcoidosis-related uveitis in a Japanese population.
Methods
Two hundred twenty-three patients with sarcoidosis and 206 healthy control subjects were recruited at seven sites in Japan. Eight single-nucleotide polymorphisms (SNPs) in TLR4 were genotyped with a TaqMan assay, and allelic and phenotypic diversity were assessed in affected and control subjects.
Results
We found no association with susceptibility to sarcoid-related uveitis for any of the SNPs analyzed. Strong linkage disequilibrium was observed among all the SNPs analyzed (D’≥0.78), which were located in one haplotype block.
Conclusion
TLR4 polymorphisms do not play an important role in the development of uveitis in Japanese patients with sarcoidosis.
Introduction
Sarcoidosis is a systemic inflammatory syndrome of unknown etiology characterized by the accumulation of immune effector cells in affected organs, such as lungs, lymph nodes, eyes, skin, and heart. The severity and mode of presentation are influenced by ethnicity. For instance, Löfgren’s syndrome, an acute form of sarcoidosis, is more common among Caucasians, and a more serious form of sarcoidosis has been observed in patients of African descent [1,2]. The incidence of ocular involvement is higher in Japan (50-93.5%) compared to that of other countries (25-60%) [2-8]. Clinically, ocular manifestations in sarcoidosis are considered bilateral, chronic, granulomatous uveitis [9]. The variety of clinical manifestations of sarcoidosis and the observed dependence on race and familial clustering suggest there may be a genetic predisposition for developing sarcoidosis [10-12]. Numerous studies have reported genetic associations between various major histocompatibility complex (MHC) genes and the susceptibility, severity, and presentation of sarcoidosis [13-17]. The first genome-wide search for genes that predispose for sarcoidosis in German families used microsatellite linkage analysis. These studies identified several chromosomal regions that contributed to the risk of sarcoidosis. The MHC genes displayed the most prominent signal in these regions. This supported the hypothesis that MHC genes were associated with the different manifestations of sarcoidosis [18]. Furthermore, another genome-wide association study that included >440,000 single-nucleotide polymorphisms (SNPs) in patients with sarcoidosis also reported a series of genetic associations [19].
It has been suggested that sarcoidosis results from exposure of genetically susceptible hosts to specific, but unidentified environmental agents [20]. The granuloma in sarcoidosis develops, in general, as a response to a persistent, weakly virulent antigen(s) that induces a local helper T cell type 1 (Th1) immune response [21].
Toll-like receptors (TLRs) are transmembrane proteins that function as pattern-recognition receptors (PRRs) in innate immunity. TLRs also provide a link to the activation of adaptive immunity by recognizing microbial components and inducing the production of cytokines, such as Interleukin 12 (IL12) and IL18 driving naïve T cells to differentiate into Th1 cells [22]. Among TLR family members, TLR4 is expressed in a wide variety of human cells, where it plays an important role in innate and adaptive immune responses to Gram-negative bacterial products, including lipopolysaccharides [22,23].
In recent studies, TLR4 polymorphisms have been reported to be associated with various inflammatory diseases, including atherosclerosis, Crohn’s disease, ulcerative colitis, rheumatoid arthritis, prostate cancer, and Alzheimer’s disease [23-28]. These findings suggest that genetic variants of TLR4 that have abnormal ligand recognition properties may contribute to the development of various diseases, including sarcoidosis.
Due to its various clinical manifestations, sarcoidosis is often considered to be a family of disorders with clinically and genetically different phenotypes, rather than a single entity [29]. This study investigated whether TLR4 polymorphisms were associated with susceptibility to sarcoidosis-related uveitis in Japanese patients.
Methods
Subjects
This study included 223 Japanese patients with sarcoidosis (52 men and 171 women) and 206 healthy Japanese controls (48 men and 158 women) from Yokohama City University, Hokkaido University, Fujita Health University, Tokyo University, Keio University, the Kumamoto City hospital, and the Yuasa Eye Clinic. All 223 patients had chronic sarcoidosis; 68 cases were clinically diagnosed and 155 cases were confirmed by biopsies. These 223 patients were divided into two groups: 196 (87.9%) with uveitis (the sarcoid-uveitis group) and 27 (12.1%) without uveitis (the sarcoid non-uveitis group). Clinical sarcoidosis was diagnosed according to the diagnostic criteria developed by the Japanese Society of Sarcoidosis and Other Granulomatous Disorders (JSSOG) in 1977 [9]. Uveitis with sarcoidosis was assessed based on the Guidelines for Diagnosis of Ocular Lesions in Sarcoidosis prepared by the JSSOG. The ocular features of sarcoidosis were defined as granulomatous uveitis plus two or more of the following: infiltration of the anterior chamber (mutton-fat keratic precipitates/iris nodules), trabecular meshwork nodules and/or tent-shaped peripheral anterior synechia, masses of vitreous opacities (snowball-like or string of pearls-like appearance), periphlebitis with perivascular nodules; multiple candle-wax type chorioretinal exudates and nodules, and/or laser photocoagulation spot-like chorioretinal atrophy [30].
All the control subjects were unrelated healthy volunteers that were ethnically similar to the patients. The study obtained the approval of the Ethics Committee of Yokohama City University School of Medicine, and was in compliance with the guidelines of the Declaration of Helsinki. Details of the study were explained to all patients and controls, and written informed consent was obtained for genetic screening.
Analysis of TLR4 polymorphisms
The QIAamp DNA Blood Maxi Kit (Qiagen, Tokyo, Japan) was used to extract genomic DNA from peripheral blood cells. Procedures were performed under standardized conditions to prevent variation in DNA quality.
The TLR4 gene is located on chromosome 9q32-q33. We evaluated eight single-nucleotide polymorphisms (SNPs), including: rs10759930, rs1927914, rs1927911, rs12377632, rs2149356, rs11536889, rs7037117, and rs7045953 within TLR4. These SNPs were selected based on previous reports [31-33] and information from public sources, including the NCBI dbSNP, ABI, and HapMap databases. The selected SNPs had minor allele frequencies that were >5% (Table 1). Each SNP was approximately 1 to 5 kb long, and the set of SNPs spanned TLR4, from approximately 5 kb of the predicted 5’-untranslated region (UTR) to approximately 6 kb of the predicted 3’ UTR. Genotyping of the SNPs was performed with the TaqMan 5’ exonuclease assay using primers supplied by ABI (Foster City, CA). The probe emitted a fluorescent signal and was incorporated during the TaqMan Assay for Real-Time PCR (7500 Real Time PCR System; Applied Biosystems), following the manufacturer’s instructions.
Table 1. Allele frequencies of SNPs in the TLR4 gene.
| dbSNP | Alleles | Position (bp) | Gene location | Minor allele frequency, n (%) | p (SUvs.SN) | p (SUvs.C) | p (SNvs.C) | ||
|---|---|---|---|---|---|---|---|---|---|
| Sarcoid uveitis n=196 | Sarcoid non-uveitis n=27 | Controls n=206 | |||||||
| rs10759930 | T>C | 119,501,442 | 5'-UTR | 142 (36.2) | 22 (40.7) | 157 (38.1) | 0.319 | 0.387 | 0.542 |
| rs1927914 | A>G | 119,504,546 | 5'-UTR | 141 (36.0) | 22 (40.7) | 158 (38.3) | 0.295 | 0.274 | 0.577 |
| rs1927911 | G>A | 119,509,875 | Intron | 137 (34.9) | 21 (38.9) | 159 (38.6) | 0.398 | 0.097 | 0.945 |
| rs12377632 | C>T | 119,512,551 | Intron | 139 (35.5) | 21 (38.9) | 157 (38.1) | 0.458 | 0.228 | 0.857 |
| rs2149356 | G>T | 119,514,020 | Intron | 137 (34.9) | 21 (38.9) | 158 (38.3) | 0.398 | 0.123 | 0.901 |
| rs11536889 | G>C | 119,517,952 | 3'-UTR | 93 (23.7) | 12 (22.2) | 108 (26.2) | 0.769 | 0.318 | 0.435 |
| rs7037117 | A>G | 119,523,484 | 3'-UTR | 87 (22.2) | 15 (27.8) | 89 (21.6) | 0.275 | 0.811 | 0.225 |
| rs7045953 | A>G | 119,525,616 | 3'-UTR | 31 ( 7.9) | 3 ( 5.6) | 39 (9.5) | 0.431* | 0.410 | 0.430* |
In the table, SNP represents single-nucleotide polymorphism, TLR4 represents toll-like receptor 4, and bp represents base pairs. Position is the distance from the short arm telomere. Gene location is the location of the SNP within the genomic TLR4 sequence. P values were calculated with the χ2 test using a 2×2 contingency table. In the table, SUvs.SN represents sarcoid uveitis versus sarcoid non-uveitis, SUvs.C represents sarcoid uveitis versus control, and SNvs.C represents sarcoid non-uveitis versus control. The asterisk indicates calculation using Fisher's Exact Test.
Statistical analysis
The Hardy-Weinberg equilibrium (HWE) was tested for each SNP in cases and controls. Differences in allele frequencies between cases and controls were assessed with the χ2 test or Fisher’s exact test. The Haploview 3.32 program was used to compute pairwise linkage disequilibrium (LD) statistics [34]. Standardized disequilibrium D’ and r2 were plotted. LD blocks were defined according to the criteria of Gabriel et al. [35]. Haplotype frequencies were estimated with an accelerated expectation-maximization algorithm, similar to the partition-ligation-expectation-maximization method described previously [36]. All p-values were derived from a 2-sided test, and p-values <0.05 were considered statistically significant.
Results
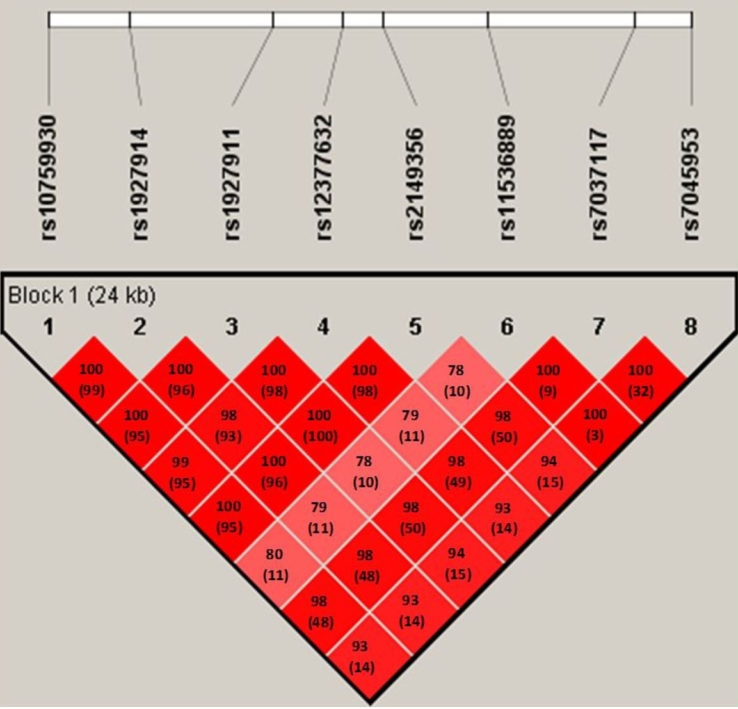
Eight SNPs in TLR4 were genotyped. All SNPs were in HWE among both cases and controls (data not shown). All eight SNPs were located in one haplotype block, and the magnitude of LD between each SNP was extremely high, with pair-wise D’ values that were ≥0.78 (Figure 1).
Figure 1.
Structure of linkage disequilibrium (LD) plotted for eight SNPs in the TLR4 gene. The D’ value and r2 value (in parentheses) that correspond to each SNP pair are expressed as a percentage and shown within the respective square. The gradient of D’ values (low to high values) is indicated by different shades of red (light to dark, respectively). The eight SNPs constitute a haplotype block that spans 24 kb of TLR4.
Table 1 shows the details of the eight SNPs, including their genomic locations and minor allele frequencies in cases and controls. No statistically significant association was observed for any of the SNPs between the sarcoid uveitis group and the sarcoid non-uveitis group, between the sarcoid uveitis group and the control subjects, or between the sarcoid non-uveitis group and the control subjects (p≥0.05; Table 1).
The genotypic frequencies of the eight SNPs are shown in Table 2, Table 3, and Table 4. There were no differences in the minor allele frequency of each SNP between the sarcoid uveitis group and the sarcoid non-uveitis group, between the sarcoid uveitis group and the control subjects, or between the sarcoid non-uveitis group and the control subjects (p≥0.05; Table 2, Table 3, and Table 4).
Table 2. Genotype frequencies of eight SNPs in TLR4. Patients with sarcoid uveitis compared to those with sarcoid non-uveitis.
| dbSNP | Alleles (Maj/Min) | Subjects | Genotype frequency n (%) | Minor allele dominance (Maj/Min + Min/Min) | ||||
|---|---|---|---|---|---|---|---|---|
| Maj/Maj | Maj/Min | Min/Min | p (SUvs.SN)* | p (SUvs.SN)** | OR (95%CI) | |||
| rs10759930 | T/C | SU | 77 (39.3) | 95 (48.5) | 24 (12.2) | 0.297 | 0.885 | 0.94 (0.41-2.14) |
| SN | 11 (40.7) | 10 (37.0) | 6 (22.2) | |||||
| rs1927914 | A/G | SU | 78 (39.8) | 94 (48.0) | 24 (12.2) | 0.306 | 0.925 | 0.96 (0.42-2.18) |
| SN | 11 (40.7) | 10 (37.0) | 6 (22.2) | |||||
| rs1927911 | G/A | SU | 81 (41.3) | 92 (46.9) | 23 (11.7) | 0.586 | 0.954 | 1.02 (0.45-2.32) |
| SN | 11 (40.7) | 11 (40.7) | 5 (18.5) | |||||
| rs12377632 | C/T | SU | 79 (40.3) | 94 (48.0) | 23 (11.7) | 0.567 | 0.966 | 0.98 (0.43-2.23) |
| SN | 11 (40.7) | 11 (40.7) | 5 (18.5) | |||||
| rs2149356 | G/T | SU | 81 (41.3) | 92 (46.9) | 23 (11.7) | 0.586 | 0.954 | 1.02 (0.45-2.32) |
| SN | 11 (40.7) | 11 (40.7) | 5 (18.5) | |||||
| rs11536889 | G/C | SU | 117 (59.7) | 66 (33.7) | 13 ( 6.6) | 0.812 | 0.966 | 1.02 (0.45-2.31) |
| SN | 16 (59.3) | 10 (37.0) | 1 (3.7) | |||||
| rs7037117 | A/G | SU | 115 (58.7) | 75 (38.3) | 6 (3.1) | 0.594 | 0.300 | 1.53 (0.68-3.43) |
| SN | 13 (48.1) | 13 (48.1) | 1 (3.7) | |||||
| rs7045953 | A/G | SU | 165 (84.2) | 29 (14.8) | 2 (1.0) | 0.755 | 0.598 | 0.67 (0.19-2.35) |
| SN | 24 (88.9) | 3 (11.1) | 0 (0.0) | |||||
In the table, SNP represents single-nucleotide polymorphism, TLR4 represents toll-like receptor 4, Maj represents major allele, Min represents minor allele, SU represents sarcoid uveitis, and SN represents sarcoid non-uveitis. The asterisk indicates the data was calculated with the χ2 test using a 3×2 contingency table. The double asterisk indicates the data was calculated with the χ2 test using a 2×2 contingency table.
Table 3. Genotype frequencies of eight SNPs in TLR4. Patients with sarcoid uveitis compared to control subjects.
| dbSNP | Alleles (Maj/Min) | Subjects | Genotype frequency n (%) | Minor allele dominance (Maj/Min + Min/Min) | ||||
|---|---|---|---|---|---|---|---|---|
| Maj/Maj | Maj/Min | Min/Min | p (SUvs.C)* | p (SUvs.C)** | OR (95%CI) | |||
| rs10759930 | T/C | SU | 77 (39.3) | 95 (48.5) | 24 (12.2) | 0.493 | 0.915 | 0.98 (0.66-1.46) |
| C | 82 (39.8) | 91 (44.2) | 33 (16.0) | |||||
| rs1927914 | A/G | SU | 78 (39.8) | 94 (48.0) | 24 (12.2) | 0.535 | 0.922 | 1.02 (0.72-2.44) |
| C | 81 (39.3) | 92 (44.7) | 33 (16.0) | |||||
| rs1927911 | G/A | SU | 81(41.3) | 92 (46.9) | 23 (11.7) | 0.318 | 0.756 | 1.07 (0.72-1.59) |
| C | 82 (39.8) | 89 (43.2) | 35 (16.7) | |||||
| rs12377632 | C/T | SU | 79 (40.3) | 94 (48.0) | 23 (11.7) | 0.440 | 0.919 | 1.02 (0.69-1.52) |
| C | 82 (39.8) | 91 (44.2) | 33 (16.0) | |||||
| rs2149356 | G/T | SU | 81 (41.3) | 92 (46.9) | 23 (11.7) | 0.464 | 0.682 | 1.09 (0.78-2.65) |
| C | 81 (39.3) | 92 (44.7) | 33 (16.0) | |||||
| rs11536889 | G/C | SU | 117 (59.7) | 66 (33.7) | 13 ( 6.6) | 0.617 | 0.327 | 1.22 (0.82-1.81) |
| C | 113 (54.9) | 78 (37.9) | 15 (7.3) | |||||
| rs7037117 | A/G | SU | 115 (58.7) | 75 (38.3) | 6 (3.1) | 0.066 | 0.312 | 0.81 (0.54-1.21) |
| C | 13 (63.6) | 61 (29.6) | 14 (6.8) | |||||
| rs7045953 | A/G | SU | 165 (84.2) | 29 (14.8) | 2 (1.0) | 0.582# | 0.485 | 1.20 (0.72-2.03) |
| C | 168 (81.6) | 37 (18.0) | 1 (0.5) | |||||
In the table, SNP represents single-nucleotide polymorphism, TLR4 represents toll-like receptor 4, Maj represents major allele, Min represents minor allele, SU represents sarcoid uveitis, and C represents control subjects. The asterisk indicates the data was calculated with the χ2 test using a 3×2 contingency table. The double asterisk indicates the data was calculated with the χ2 test using a 2×2 contingency table. The sharp (hash mark) indicates the data was calculated with Fisher's Exact Test.
Table 4. Genotype frequencies of eight SNPs in TLR4. Patients with Sarcoid non-uveitis compared to control subjects.
| dbSNP | Alleles (Maj/Min) | Subjects | Genotype frequency n (%) | Minor allele dominance (Maj/Min + Min/Min) | ||||
|---|---|---|---|---|---|---|---|---|
| Maj/Maj | Maj/Min | Min/Min | p (SNvs.C)* | p (SNvs.C)** | OR (95%CI) | |||
| rs10759930 | T/C | SN | 11 (40.7) | 10 (37.0) | 6 (22.2) | 0.659 | 0.926 | 1.04 (0.46-2.35) |
| C | 82 (39.8) | 91 (44.2) | 33 (16.0) | |||||
| rs1927914 | A/G | SN | 11 (40.7) | 10 (37.0) | 6 (22.2) | 0.645 | 0.887 | 1.06 (0.47-2.40) |
| C | 81 (39.3) | 92 (44.7) | 33 (16.0) | |||||
| rs1927911 | G/A | SN | 11 (40.7) | 11 (40.7) | 5 (18.5) | 0.965 | 0.926 | 1.04 (0.46-2.35) |
| C | 82 (39.8) | 89 (43.2) | 35 (16.7) | |||||
| rs12377632 | C/T | SN | 11 (40.7) | 11 (40.7) | 5 (18.5) | 0.923 | 0.926 | 1.04 (0.46-2.35) |
| C | 82 (39.8) | 91 (44.2) | 33 (16.0) | |||||
| rs2149356 | G/T | SN | 11 (40.7) | 11 (40.7) | 5 (18.5) | 0.911 | 0.887 | 1.06 (0.47-2.40) |
| C | 81 (39.3) | 92 (44.7) | 33 (16.0) | |||||
| rs11536889 | G/C | SN | 16 (59.3) | 10 (37.0) | 1 (3.7) | 0.766 | 0.665 | 1.20 (0.53-2.71) |
| C | 113 (54.9) | 78 (37.9) | 15 (7.3) | |||||
| rs7037117 | A/G | SN | 13 (48.1) | 13 (48.1) | 1 (3.7) | 0.145 | 0.120 | 0.53 (0.24-1.19) |
| C | 13 (63.6) | 61 (29.6) | 14 (6.8) | |||||
| rs7045953 | A/G | SN | 24 (88.9) | 3 (11.1) | 0 (0.0) | 0.625 | 0.347 | 1.81 (0.52-6.32) |
| C | 168 (81.6) | 37 (18.0) | 1 (0.5) | |||||
In the table, SNP represents single-nucleotide polymorphism, TLR4 represents toll-like receptor 4, Maj represents major allele, Min represents minor allele, SN represents sarcoid non-uveitis, and C represents control subjects. The asterisk indicates the data was calculated with the χ2 test using a 3×2 contingency table. The double asterisk indicates the data was calculated with the χ2 test using a 2×2 contingency table.
Furthermore, there were no significant differences in the haplotype frequencies of the eight SNPs between the sarcoid uveitis group and the sarcoid non-uveitis group, between the sarcoid uveitis group and the control subjects, or between the sarcoid non-uveitis group and the control subjects (p≥0.05; Table 5)
Table 5. Haplotype frequencies of SNPs in TLR4.
| Haplotype | Frequency, n (%) | p (SUvs.SN) | p (SUvs.C) | p (SNvs.C) | ||
|---|---|---|---|---|---|---|
| Sarcoid uveitis n=196 | Sarcoid non-uveitis n=27 | Controls n=206 | ||||
| TAGCGGAA | 80 (40.8) | 10 (37.0) | 75 (36.4) | 0.707 | 0.364 | 0.949 |
| TAGCGCAA | 43 (21.9) | 6 (22.2) | 50 (24.3) | 0.973 | 0.579 | 0.815 |
| CGATTGAA | 23 (11.7) | 3 (11.1) | 32 (15.5) | 1.000* | 0.268 | 0.593* |
| CGATTGGA | 27 (13.8) | 6 (22.2) | 25 (12.1) | 0.247 | 0.624 | 0.147 |
| CGATTGGG | 16 (8.2) | 2 (7.4) | 20 (9.7) | 1.000* | 0.588 | 0.757* |
| CGATTCAA | 3 (1.5) | 0 (0.0) | 2 (1.0) | 1.000* | 0.678* | 1.000* |
In the table, SNP represents single-nucleotide polymorphism and TLR4 represents toll-like receptor 4. Position is the distance from the short arm telomere. Haplotypes with frequency less than 1% are not listed. P values were calculated with the χ2 test using a 2×2 contingency table. In the table, SUvs.SN represents sarcoid uveitis versus sarcoid non-uveitis, SUvs.C represents sarcoid uveitis versus control, and SNvs.C represents sarcoid non-uveitis versus control. The asterisk indicates calculation using Fisher's Exact Test.
Discussion
In this study, we investigated whether there were any associations between TLR4 polymorphisms and uveitis with sarcoidosis. We found no associations for any of the SNPs analyzed. Several infectious organisms, including mycobacteria, mycoplasma, propionibacteria, and cytomegalovirus, have been proposed as potential causes of sarcoidosis [20,37,38]. The mycobacterium tuberculosis heat shock proteins (Mtb-HSPs) have also been suggested as causative agents of sarcoidosis based on the cross-reactivity observed between mycobacterial and human HSPs [39,40]. A SNP within the HSP-70/Hom gene was identified as a factor associated with susceptibility to sarcoidosis [41]. Others have also reported that SNPs in HSP-70/1 and HSP-70/Hom were associated with sarcoid-related uveitis [29]. TLR4 recognizes endogenous ligands, including heat shock proteins (HSP60, HSP70) and exogenous ligands; upon binding these ligands, TLR4 induces the production of proinflammatory cytokines [22,42,43]. Therefore, it was hypothesized that TLR4 and its ligands would be implicated in the development of sarcoidosis. The results of this study do not support that hypothesis.
The long arm of chromosome 9, which harbors TLR4, was identified as a region related to the susceptibility to sarcoidosis by genome-wide microsatellite linkage analysis in a German population [18]. This suggested that TLR4 polymorphisms were associated with sarcoidosis. Two different SNPs in TLR4, rs4986790 (Asp299Gly) and rs4986791 (Thr399Ile), affected the extracellular domain of the TLR4 receptor. These were reported to be associated with the development of chronic sarcoidosis in a German case control study [44]. However, the results could not be replicated in Dutch and Greek populations [45,46]. Previously, we analyzed the same SNPs in TLR4 that were examined in this study to determine whether they were associated with susceptibility to Behçet's disease (BD) and normal tension glaucoma [31,32]. In that study, we found that the SNP, rs7037117, located in the 3’-untranslated region, was significantly associated with BD and normal tension glaucoma. We also explored the same SNPs to determine associations to BD in a Korean population. We found the most frequent occurring haplotype was significantly increased in patients with BD that were positive for HLA-B*51 and in patients with BD and arthritis [33]. It was also reported that two non-synonymous TLR4 SNP sites, Asp299Gly (rs4986790) and Thr399Ile (rs4986791), were associated with elevated serum cytokines in a Caucasian population [26]. However, no studies have detected these non-synonymous mutations in Asian populations, including Koreans [47]. Thus, we did not examine those two non-synonymous SNPs because they were monomorphic in a Japanese population. Our results showed no differences in allelic diversity among patients that had sarcoidosis with or without uveitis and healthy control subjects. However, our results could not completely rule out the possibility that the TLR4 signaling pathway plays a role in the development of uveitis in patients with sarcoidosis. Although the discrepancy between our results and those of the German study on chronic sarcoidosis might be explained by racial differences, it will be necessary to confirm this negative association in future studies with larger cohorts.
In conclusion, the present study showed that TLR4 polymorphisms were not significantly associated to the development of uveitis with sarcoidosis in the Japanese population.
Acknowledgments
This study was supported by grants-in-aid from the Ministry of Education, Science, Sports, and Culture of Japan: a grant from the Ministry of Health, Labor, and Welfare, Japan.
References
- 1.Ohta H, Tazawa R, Nakamura A, Kimura Y, Maemondo M, Kikuchi T, Ebina M, Nukiwa T. Acute-onset sarcoidosis with erythema nodosum and polyarthralgia (Löfgren's syndrome) in Japan: a case report and a review of the literature. Intern Med. 2006;45:659–62. doi: 10.2169/internalmedicine.45.1452. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736–55. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 3.ACCESS Research Group Design of a case control etiologic study of sarcoidosis (ACCESS). J Clin Epidemiol. 1999;52:1173–86. doi: 10.1016/s0895-4356(99)00142-0. [DOI] [PubMed] [Google Scholar]
- 4.Rothova A. Ocular involvement in sarcoidosis. Br J Ophthalmol. 2000;84:110–6. doi: 10.1136/bjo.84.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silver MR, Messner LV. Sarcoidosis and its ocular manifestations. J Am Optom Assoc. 1994;65:321–7. [PubMed] [Google Scholar]
- 6.Obenauf CD, Shaw HE, Sydnor CF, Klintworth GK. Sarcoidosis and its ophthalmic manifestations. Am J Ophthalmol. 1978;86:648–55. doi: 10.1016/0002-9394(78)90184-8. [DOI] [PubMed] [Google Scholar]
- 7.Ohara K. Clinical manifestations of ocular sarcoidosis in Japanese patients. Nippon Rinsho. 1994;52:1577–81. [PubMed] [Google Scholar]
- 8.Matsuo T, Fujiwara N, Nakata Y. First presenting signs or symptoms of sarcoidosis in a Japanese population. Jpn J Ophthalmol. 2005;49:149–52. doi: 10.1007/s10384-004-0154-z. [DOI] [PubMed] [Google Scholar]
- 9.Kawaguchi T, Hanada A, Horie S, Sugamoto Y, Sugita S, Mochizuki M. Evaluation of characteristic ocular sign and systemic investigation in ocular sarcoidosis patients. Jpn J Ophthalmol. 2007;51:121–6. doi: 10.1007/s10384-006-0413-2. [DOI] [PubMed] [Google Scholar]
- 10.Iannuzzi MC, Iyengar SK, Gray-McGuire C, Elston RC, Baughman RP, Donohue JF, Hirst K, Judson MA, Kavuru MS, Maliarik MJ, Moller DR, Newman LS, Rabin DL, Rose CS, Rossman MD, Teirstein AS, Rybicki BA. Genome-wide search for sarcoidosis susceptibility genes in African Americans. Genes Immun. 2005;6:509–18. doi: 10.1038/sj.gene.6364235. [DOI] [PubMed] [Google Scholar]
- 11.Westney GE, Judson MA. Racial and ethnic disparities in sarcoidosis: from genetics to socioeconomics. Clin Chest Med. 2006;27:453–62. doi: 10.1016/j.ccm.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Rybicki BA, Major M, Popovich J, Jr, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234–41. doi: 10.1093/oxfordjournals.aje.a009096. [DOI] [PubMed] [Google Scholar]
- 13.Spagnolo P, Sato H, Grutters JC, Renzoni EA, Marshall SE, Ruven HJ, Wells AU, Tzouvelekis A, van Moorsel CH, van den Bosch JM, du Bois RM, Welsh KI. Analysis of BTNL2 genetic polymorphisms in British and Dutch patients with sarcoidosis. Tissue Antigens. 2007;70:219–27. doi: 10.1111/j.1399-0039.2007.00879.x. [DOI] [PubMed] [Google Scholar]
- 14.Rossman MD, Thompson B, Frederick M, Maliarik M, Iannuzzi MC, Rybicki BA, Pandey JP, Newman LS, Magira E, Beznik-Cizman B, Monos D. ACCESS Group. HLA-DRB1*1101: a significant risk factor for sarcoidosis in blacks and whites. Am J Hum Genet. 2003;73:720–35. doi: 10.1086/378097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foley PJ, McGrath DS, Puscinska E, Petrek M, Kolek V, Drabek J, Lympany PA, Pantelidis P, Welsh KI, Zielinski J, du Bois RM. Human leukocyte antigen-DRB1 position 11 residues are a common protective marker for sarcoidosis. Am J Respir Cell Mol Biol. 2001;25:272–7. doi: 10.1165/ajrcmb.25.3.4261. [DOI] [PubMed] [Google Scholar]
- 16.Berlin M, Fogdell-Hahn A, Olerup O, Eklund A, Grunewald J. HLA-DR predicts the prognosis in Scandinavian patients with pulmonary sarcoidosis. Am J Respir Crit Care Med. 1997;156:1601–5. doi: 10.1164/ajrccm.156.5.9704069. [DOI] [PubMed] [Google Scholar]
- 17.Ishihara M, Ohno S, Ishida T, Ando H, Naruse T, Nose Y, Inoko H. Molecular genetic studies of HLA class II alleles in sarcoidosis. Tissue Antigens. 1994;43:238–41. doi: 10.1111/j.1399-0039.1994.tb02331.x. [DOI] [PubMed] [Google Scholar]
- 18.Schürmann M, Reichel P, Müller-Myhsok B, Schlaak M, Müller-Quernheim J, Schwinger E. Results from a genome-wide search for predisposing genes in sarcoidosis. Am J Respir Crit Care Med. 2001;164:840–6. doi: 10.1164/ajrccm.164.5.2007056. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann S, Franke A, Fischer A, Jacobs G, Nothnagel M, Gaede KI, Schürmann M, Müller-Quernheim J, Krawczak M, Rosenstiel P, Schreiber S. Genome-wide association study identifies ANXA11 as a new susceptibility locus for sarcoidosis. Nat Genet. 2008;40:1103–6. doi: 10.1038/ng.198. [DOI] [PubMed] [Google Scholar]
- 20.Buck AA. Epidemiologic investigations of sarcoidosis. IV. Discussion and summary. Am J Hyg. 1961;74:189–202. doi: 10.1093/oxfordjournals.aje.a120210. [DOI] [PubMed] [Google Scholar]
- 21.Rosen Y. Pathology of sarcoidosis. Semin Respir Crit Care Med. 2007;28:36–52. doi: 10.1055/s-2007-970332. [DOI] [PubMed] [Google Scholar]
- 22.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 23.Zheng SL, Augustsson-Bälter K, Chang B, Hedelin M, Li L, Adami HO, Bensen J, Li G, Johnasson JE, Turner AR, Adams TS, Meyers DA, Isaacs WB, Xu J, Grönberg H. Sequence variants of toll like receptor 4 are associated with prostate cancer risk: results from the Cancer Prostate in Sweden study. Cancer Res. 2004;64:2918–22. doi: 10.1158/0008-5472.can-03-3280. [DOI] [PubMed] [Google Scholar]
- 24.Kiechl S, Lorenz E, Reindl M, Wiedermann CJ, Oberhollenzer F, Bonora E, Willeit J, Schwartz DA. Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med. 2002;347:185–92. doi: 10.1056/NEJMoa012673. [DOI] [PubMed] [Google Scholar]
- 25.Franchimont D, Vermeire S, El Housni H, Pierik M, Van Steen K, Gustot T, Quertinmont E, Abramowicz M, Van Gossum A, Devière J, Rutgeerts P. Deficient host-bacteria interactions in inflammatory bowel disease? The toll-like receptor (TLR)-4 Asp299gly polymorphism is associated with Crohn’s disease and ulcerative colitis. Gut. 2004;53:987–92. doi: 10.1136/gut.2003.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radstake TR, Franke B, Hanssen S, Netea MG, Welsing P, Barrera P, Joosten LA, van Riel PL, van den Berg WB. The toll-like receptor 4 Asp299Gly functional variant is associated with decreased rheumatoid arthritis disease susceptibility but does not influence disease severity and /or outcome. Arthritis Rheum. 2004;50:999–1001. doi: 10.1002/art.20114. [DOI] [PubMed] [Google Scholar]
- 27.Chen YC, Giovannucci E, Lazarus R, Kraft P, Ketkar S, Hunter DJ. Sequence variants of toll-like receptor 4 and susceptibility to prostate cancer. Cancer Res. 2005;65:11771–8. doi: 10.1158/0008-5472.CAN-05-2078. [DOI] [PubMed] [Google Scholar]
- 28.Balistreri CR, Grimaldi MP, Chiappelli M, Licastro F, Castiglia L, Listì F, Vasto S, Lio D, Caruso C, Candore G. Association between the polymorphisms of TLR4 and CD14 genes and Alzheimer's disease. Curr Pharm Des. 2008;14:2672–7. doi: 10.2174/138161208786264089. [DOI] [PubMed] [Google Scholar]
- 29.Spagnolo P, Sato H, Marshall SE, Antoniou KM, Ahmad T, Wells AU, Ahad MA, Lightman S, du Bois RM, Welsh KI. Association between heat shock protein 70/Hom genetic polymorphisms and uveitis in patients with sarcoidosis. Invest Ophthalmol Vis Sci. 2007;48:3019–25. doi: 10.1167/iovs.06-1485. [DOI] [PubMed] [Google Scholar]
- 30.Asukata Y, Ishihara M, Hasumi Y, Nakamura S, Hayashi K, Ohno S, Mizuki N. Guidelines for the diagnosis of ocular sarcoidosis. Ocul Immunol Inflamm. 2008;16:77–81. doi: 10.1080/09273940802051100. [DOI] [PubMed] [Google Scholar]
- 31.Meguro A, Ota M, Katsuyama Y, Oka A, Ohno S, Inoko H, Mizuki N. Association of the toll-like receptor 4 gene polymorphisms with Behcet's disease. Ann Rheum Dis. 2008;67:725–7. doi: 10.1136/ard.2007.079871. [DOI] [PubMed] [Google Scholar]
- 32.Shibuya E, Meguro A, Ota M, Kashiwagi K, Mabuchi F, Iijima H, Kawase K, Yamamoto T, Nakamura M, Negi A, Sagara T, Nishida T, Inatani M, Tanihara H, Aihara M, Araie M, Fukuchi T, Abe H, Higashide T, Sugiyama K, Kanamoto T, Kiuchi Y, Iwase A, Ohno S, Inoko H, Mizuki N. Association of Toll-like receptor 4 gene polymorphisms with normal tension glaucoma. Invest Ophthalmol Vis Sci. 2008;49:4453–7. doi: 10.1167/iovs.07-1575. [DOI] [PubMed] [Google Scholar]
- 33.Horie Y, Meguro A, Ota M, Kitaichi N, Katsuyama Y, Takemoto Y, Namba K, Yoshida K, Song YW, Park KS, Lee EB, Inoko H, Mizuki N, Ohno S. Association of TLR4 polymorphisms with Behcet's disease in a Korean population. Rheumatology (Oxford) 2009;48:638–42. doi: 10.1093/rheumatology/kep077. [DOI] [PubMed] [Google Scholar]
- 34.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 35.Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, Liu-Cordero SN, Rotimi C, Adeyemo A, Cooper R, Ward R, Lander ES, Daly MJ, Altshuler D. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–9. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 36.Qin ZS, Niu T, Liu J. Partition-ligation-expectation-maximization algorithm for haplotype inference with single-nucleotide polymorphisms. Am J Hum Genet. 2002;71:1242–7. doi: 10.1086/344207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta D, Agarwal R, Aggarwal AN, Jindal SK. Molecular evidence for the role mycobacteria in sarcoidosis: a meta-analysis. Eur Respir J. 2007;30:508–16. doi: 10.1183/09031936.00002607. [DOI] [PubMed] [Google Scholar]
- 38.Yasuhara T, Tada R, Nakano Y, Tei M, Mochida C, Kamei M, Kinoshita S. The presence of Propionibacterium spp. in the vitreous fluid of uveitis patients with sarcoidosis. Acta Ophthalmol Scand. 2005;83:364–9. doi: 10.1111/j.1600-0420.2005.00449.x. [DOI] [PubMed] [Google Scholar]
- 39.Dubaniewicz A, Kämpfer S, Singh M. Serum anti-mycobacterial heat shock proteins antibodies in sarcoidosis and tuberculosis. Tuberculosis (Edinb) 2006;86:60–7. doi: 10.1016/j.tube.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Dubaniewicz A, Trzonkowski P, Dubaniewicz-Wybieralska M, Dubaniewicz A, Singh M, Myśliwski A. Mycobacterial heat shock protein-induced blood T lymphocytes subsets and cytokine pattern:comparison of sarcoidosis with tuberculosis and healthy controls. Respirology. 2007;12:346–54. doi: 10.1111/j.1440-1843.2007.01076.x. [DOI] [PubMed] [Google Scholar]
- 41.Bogunia-Kubik K, Koscinska K, Suchnicki K, Lange A. HSP70-hom gene single nucleotide (+2763 G/A and +2437 C/T) polymorphisms in sarcoidosis. Int J Immunogenet. 2006;33:135–40. doi: 10.1111/j.1744-313X.2006.00584.x. [DOI] [PubMed] [Google Scholar]
- 42.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 43.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–45. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 44.Pabst S, Baumgarten G, Stremmel A, Lennarz M, Knüfermann P, Gillissen A, Vetter H, Grohé C. Toll-like receptor (TLR) 4 polymorphims are associated with a chronic course of sarcoidosis. Clin Exp Immunol. 2006;143:420–6. doi: 10.1111/j.1365-2249.2006.03008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veltkamp M, Grutters JC, van Moorsel CH, Ruven HJ, van den Bosch JM. Toll-like receptor (TLR) 4 polymorphism Asp299Gly is not associated with disease course in Dutch sarcoidosis patients. Clin Exp Immunol. 2006;145:215–8. doi: 10.1111/j.1365-2249.2006.03127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gazouli M, Koundourakis A, Ikonomopoulos J, Gialafos EJ, Rapti A, Gorgoulis VG, Kittas C. CARD15/NOD2, CD14, and toll-like receptor 4 gene polymorphisms in Greek patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2006;23:23–9. [PubMed] [Google Scholar]
- 47.Na KS, Kim TH, Rahman P, Peddle L, Choi CB, Inman RD. Analysis of single nucleotide polymorphisms in Toll-like receptor 4 shows no association with ankylosing spondylitis in a Korean population. Rheumatol Int. 2008;28:627–30. doi: 10.1007/s00296-007-0490-7. [DOI] [PubMed] [Google Scholar]