Abstract
Aims
Nitrated fatty acids (NO2-FA) have been identified as endogenous anti-inflammatory signalling mediators generated by oxidative inflammatory reactions. Herein the in vivo generation of nitro-oleic acid (OA-NO2) and nitro-linoleic acid (LNO2) was measured in a murine model of myocardial ischaemia and reperfusion (I/R) and the effect of exogenous administration of OA-NO2 on I/R injury was evaluated.
Methods and results
In C57/BL6 mice subjected to 30 min of coronary artery ligation, endogenous OA-NO2 and LNO2 formation was observed after 30 min of reperfusion, whereas no NO2-FA were detected in sham-operated mice and mice with myocardial infarction without reperfusion. Exogenous administration of 20 nmol/g body weight OA-NO2 during the ischaemic episode induced profound protection against I/R injury with a 46% reduction in infarct size (normalized to area at risk) and a marked preservation of left ventricular function as assessed by transthoracic echocardiography, compared with vehicle-treated mice. Administration of OA-NO2 inhibited activation of the p65 subunit of nuclear factor κB (NFκB) in I/R tissue. Experiments using the NFκB inhibitor pyrrolidinedithiocarbamate also support that protection lent by OA-NO2 was in part mediated by inhibition of NFκB. OA-NO2 inhibition of NFκB activation was accompanied by suppression of downstream intercellular adhesion molecule 1 and monocyte chemotactic protein 1 expression, neutrophil infiltration, and myocyte apoptosis.
Conclusion
This study reveals the de novo generation of fatty acid nitration products in vivo and reveals the anti-inflammatory and potential therapeutic actions of OA-NO2 in myocardial I/R injury.
Keywords: Ischaemia/reperfusion, Nitric oxide, Nitro-fatty acids, Reactive oxygen species, Oxides of nitrogen, Lipid signalling
1. Introduction
Reperfusion injury is a major contributor to tissue damage during myocardial infarction, accounting for up to 50% of final infarct volume.1 Despite considerable insight into the mechanisms of cardiac ischaemia and reperfusion (I/R) injury, no effective therapeutic strategies have been introduced into clinical practice. Extensive experimental evidence reveals a role for reactive oxygen species (ROS) in mediating myocardial reperfusion injury.2 This concept was first introduced in 1973 as the ‘oxygen paradox’,3 based on the observation of reoxygenation-induced tissue injury in hypoxic rat hearts and was reinforced by interventional animal studies using antioxidant-based therapeutic strategies, ROS-generating systems,4–6 and finally, by direct measurement of ROS generation in vivo.7 Various sources of increased ROS generation during myocardial reperfusion yield products that readily undergo secondary reactions with radical and non-radical species, including nitric oxide (•NO) and its metabolites.8,9 This oxidative inflammatory milieu generated during reperfusion injury consists of a broad spectrum of oxidizing, nitrosating, and nitrating species and their products.10
Nitrated unsaturated fatty acids (NO2-FA) are endogenously occurring products of oxidant-induced nitration reactions. NO2-FA display predominantly anti-inflammatory signalling actions, which to date have only been exemplified in biochemical and cellular models of inflammation.11 Recently, potential cardioprotective actions of NO2-FA were suggested from studies of cultured cardiomyocytes subjected to simulated I/R injury, with these protective effects ascribed to mild mitochondrial uncoupling.12 In this study, nitration products of linoleic acid were endogenously generated during ischaemic preconditioning (IPC) of Langendorff-perfused rat hearts, suggesting a possible contribution of NO2-FA to the protection lent by IPC.
Herein, we translate these in vitro-based observations to an in vivo model of cardiovascular injury by first providing evidence for the in vivo formation of NO2-FA in a murine model of focal myocardial I/R. We then show that administration of nitro-oleic acid (OA-NO2) during ischaemia, at clinically relevant times, markedly reduces myocardial infarct size and preserves left ventricular function. These protective effects are in part mediated by inhibition of nuclear factor κB (NFκB) activation via post-translational modification of the p65 subunit by electrophilic NO2-FA, resulting in local and systemic down-regulation of downstream pro-inflammatory signalling. These studies reveal that tissue supplementation with an endogenous by-product of inflammatory reactions serves to inhibit myocardial I/R injury.
2. Methods
2.1. Animal studies
Male, 8- to 12-week-old C57/BL6 wild-type mice (Jackson Laboratory, Bar Harbor, ME, USA) were used for all studies. The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and was approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh (protocol number: 0710454).
2.2. I/R protocol
Mice were anaesthetized by intraperitoneal injection of 0.5 mg/g body weight of avertin (2-2-2-tribromoethanol, Sigma-Aldrich, USA). After lateral thoracotomy of the fourth intercostal space, a suture was placed around the left coronary artery after retraction of the left atrial appendage and the artery was ligated with a bow tie. After 30 min of ischaemia, the bow tie was released, the suture left in place for determination of infarct size (see below), and the chest cavity was closed; and 20 nmol/g bodyweight OA-NO2 in polyethylenglycol/ethanol (85:15, vol/vol) or 100 µL of vehicle (polyethylenglycol/ethanol, 85:15) were injected 15 min prior to reperfusion. Two further groups of animals were treated with OA-NO2 via Alzet osmotic mini-pumps over a period of 3 days prior to induction of ischaemia and intraperitoneal injection of 20 nmol/g body weight OA-NO2 at the time of reperfusion, respectively. A 6 mg/kg dose was delivered via a 7-day osmotic mini-pump. In some cases, the NFκB-inhibitor pyrrolidinedithiocarbamate (PDTC, 150 mg/kg, Sigma-Aldrich) was administered 30 min prior to surgery. OA-NO2 was synthesized as previously.13,14 Sham surgery animals underwent removal of the pericardium. Infarct size was determined using Evan's blue dye. Echocardiographic studies were performed before and 24 h after induction of myocardial ischaemia (see Scheme 1 for an illustration of the experimental protocol).
Scheme 1.
Experimental protocol for the assessment of OA-NO2 effects in the murine I/R model.
2.3. Detection and quantitative analysis of NO2-FA
For detection of endogenous NO2-FA levels, mice underwent surgery as described above. To assess the impact of duration of ischaemia on the formation of NO2-FA, ischaemic periods were varied, with times ranging from 15 min to 1 h. In one group, 20 nmol/g body weight OA-NO2 was administered 15 min prior to reperfusion. In addition to sham-operated mice, another group consisted of mice undergoing ligation of the left coronary artery without subsequent reperfusion. In these groups, reperfusion times were 30 min and then heart tissue was removed, lipid extraction was performed, and OA-NO2 and nitro-linoleic acid (LNO2) assessment was carried out by liquid chromatography/mass spectrometry (LC/MS) as before.14
2.4. Activation of NFκB p65 subunit
Hearts were harvested 1 h after reperfusion, homogenized and nuclear extraction was performed according to manufacturer recommendations using a commercially available nuclear extract kit (Active Motif, Carlsbad, CA, USA). Activation of the NFκB p65 subunit was detected and quantified via ELISA analysis (TransAM p65 chemikit, Active Motif).
2.5. Immunoprecipitation and assessment of p65 nitroalkylation
For immunoprecipitation of p65, homogenates from hearts harvested after 1 h of reperfusion were incubated with a rabbit polyclonal NFκB p65 antibody (abcam, 1:160) for 1 h at 4°C. Thereafter, the mixture was incubated with agarose (Protein A/G PLUS-Agarose, Santa Cruz Biotechnology, USA, 1:50). To control for non-specific binding of OA-NO2 to agarose beads, the NFκB p65 antibody was omitted in control samples. To release OA-NO2 covalently bound to p65, immunoprecipitates were incubated with 500 mM of β-mercaptoethanol (BME) for 2 h at 37°C. This exchange reaction reveals the reversible adduction of proteins by electrophiles in biological samples via the exchange reaction of protein-adducted species (e.g. OA-NO2) to BME and subsequent LC/MS analysis of BME-electrophile adduct formation.13,14 After BME incubation, samples were centrifuged at 1000 g, for 10 min at 4°C and were analyzed by LC/MS as above.
2.6. Tumour necrosis factor α and interleukin 6 serum levels
Blood was drawn from the inferior vena cava after 6 h of reperfusion and directly transferred into serum separator tubes (BD Microtainer, USA). After 2 h samples were centrifuged at room temperature at 6000 g for 10 min. Serum levels of tumour necrosis factor α (TNFα) and interleukin 6 (IL-6) were determined by ELISA according to manufacturer recommendations (Quantikine, R&D Systems, USA).
2.7. Myeloperoxidase tissue levels
For determination of tissue myeloperoxidase (MPO) levels, hearts were harvested 24 h after reperfusion and the left ventricle directly homogenized in lysis buffer [200 mM NaCl, 5 mM EDTA, 10 mM Tris, 10% glycerol, complete EDTA-free protease inhibitor cocktail (Roche Diagnostics, USA)]. Protein concentration was determined by BCA assay. MPO levels were assessed by ELISA (Hycult Biotechnology bv, The Netherlands).
2.8. Real-time quantitative polymerase chain reaction
Hearts were harvested after 24 h and RNA was isolated from the left ventricular free wall and quantitative mRNA expression assessed by real-time PCR using TaqMan gene expression assays for intercellular adhesion molecule 1 (ICAM-1, NM010493.2), monocyte chemotactic protein 1 (MCP-1, NM_011333), and vascular cell adhesion molecule (VCAM-1, NM_011693.2, Applied Biosystems, USA). All values were normalized to β2-microglobulin (NM_009735.3).
2.9. Immunofluorescence analysis of ICAM-1 and Ly-6-G
Six micrometre cross-sections were incubated for 1 h with a rat polyclonal antibody to ICAM-1 (abcam, USA, 1:25) and a rabbit polyclonal antibody to CD31 (abcam, 1:20) or a rat polyclonal antibody to Ly-6-G, specific to neutrophils (abcam, 1:20).
2.10. Assessment of apoptosis
End labelling of exposed DNA-fragments was performed with a TUNEL in situ cell death detection kit (Roche Diagnostics, Switzerland) as suggested by the manufacturer. In 10 random fields per slide, all nuclei and all TUNEL-positive nuclei were automatically counted using MetaMorph and the percentage of TUNEL-positive nuclei was calculated.
Caspase 3 activity was assessed in homogenized heart tissues harvested after 24 h of reperfusion according to manufacturer recommendations (BD Pharmingen™, BD Biosciences, USA).
2.11. Statistical analysis
Results are expressed as mean ± standard deviation. Continuous variables were tested for normal distribution using the Kolmogorov–Smirnov test. Statistical analysis was performed using one-way ANOVA followed by Bonferroni post hoc test or unpaired Student's t-test as appropriate. A value of P < 0.05 was considered statistically significant. All calculations were carried out using SPSS version 15.0.
3. Results
3.1. Endogenous formation of NO2-FA
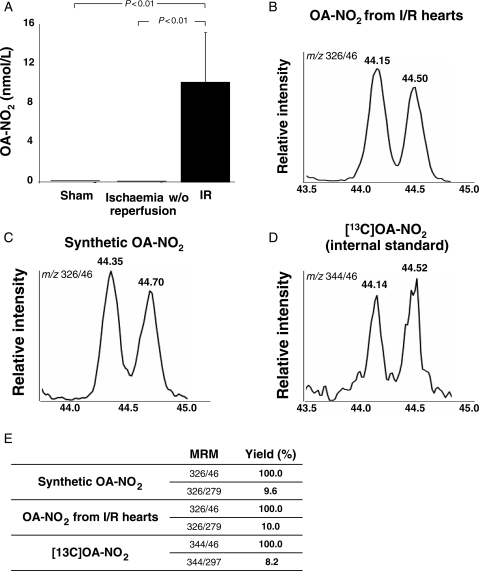
Analysis of myocardial tissue from sham-operated and mice following 30 min of ischaemia and 30 min of reperfusion revealed the generation of OA-NO2 in a concentration of 9.5 ± 4.7 nmol/L (n = 3) after I/R, with no detectable OA-NO2 in sham-operated animals and mice with myocardial infarction without reperfusion (Figure 1A). The HPLC-MS identification of OA-NO2 was based on (i) identical chromatographic retention times when comparing endogenously formed OA-NO2 with a synthetic [13C]-OA-NO2 internal standard (Figure 1B–D), (ii) similar ratios of ion intensity for synthetic, endogenously produced and 13C-labelled OA-NO2 when comparing the two main products of OA-NO2 collision induced dissociation m/z 326/46 and m/z 326/279 (Figure 1E), and (iii) the manifestation of an electrophilic reactivity via the formation of an adduct with BME (see Supplementary material online, Figure S1).13,14 In mice receiving intraperitoneal injection of synthetic OA-NO2 15 min prior to reperfusion, the myocardial tissue concentration of OA-NO2 after 30 min of reperfusion was 230 ± 4 nmol/L.
Figure 1.
Endogenous generation of OA-NO2 following ischaemia and reperfusion. (A) Tissue concentration of OA-NO2 in hearts from mice undergoing 30 min of ischaemia and 30 min of reperfusion, ischaemia without reperfusion or sham surgery. (B–D) Characterization of OA-NO2 in heart tissue from mice subjected to I/R injury. The chromatograms show co-elution of OA-NO2 extracted from heart tissue with synthetic OA-NO2 (C) and the internal standard (D). (E) The table shows the peak area ratios corresponding to the two main products of OA-NO2 collision induced dissociation: m/z 326/279 (neutral loss of HNO2) and m/z 326/46 (formation of NO2−), further confirming the identity of OA-NO2 extracted from the heart tissue.
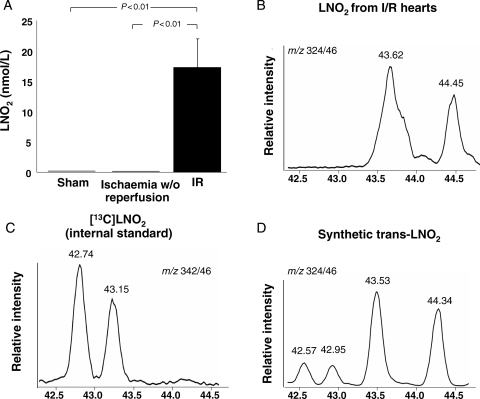
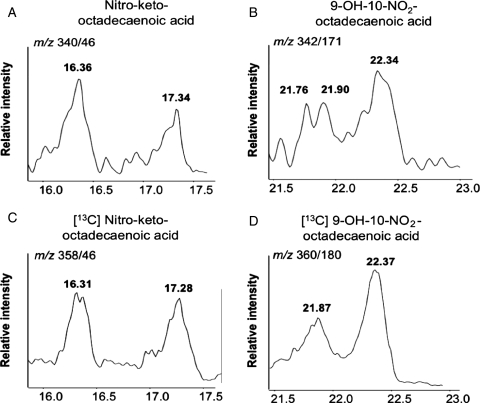
Following reperfusion a nitro-fatty acid with the mass transitions of LNO2 was formed that co-eluted with an LNO2 standard synthesized from trans- rather than cis-linoleic acid (Figure 2A–D). These data reveal that trans-LNO2 generation was favoured under these conditions, with a calculated concentration of 17.3 ± 4.7 nM. The specific position of the trans double bond and the location of the vinyl NO2-group remains to be determined. Identification of this product as the electrophilic nitroalkene derivative of LNO2 was affirmed by LC/MS analysis of the characteristic fragmentation pattern upon collision-induced dissociation, co-elution with a synthetic trans-LNO2 standard, and reactivity with BME. In addition to OA-NO2 and trans-LNO2 derivatives, additional hydroxyl and keto derivatives of LNO2 were detected by LC/MS analysis that also displayed electrophilic reactivity (Figure 3A–D). Variation of the ischaemic period from 30 to 15 min and 1 h, respectively, did not result in changes in tissue levels of oleic and linoleic acid nitration products (data not shown).
Figure 2.
Endogenous generation of LNO2 following ischaemia and reperfusion. (A) Tissue concentration of LNO2 after I/R, ischaemia without reperfusion or sham surgery. (B) Chromatogram of LNO2 extracted from mouse hearts subjected to ischaemia and reperfusion. LNO2 eluted later than the [13C]-labelled cis-LNO2 which was used as internal standard (C) and co-eluted with synthetic trans-LNO2 (D). The slight shift in retention times between chromatograms (B and D) is due to variations between separate chromatographic runs.
Figure 3.
LNO2 is further oxidized to keto (A) and hydroxyl (B) derivatives in murine heart tissue after I/R; and 3 µM [13C]LNO2 subjected to air oxidation overnight in Tris buffer (pH 8) was used as the precursor for oxidized 13C-labelled LNO2 derivatives. These species served as internal standards for identification of endogenously produced oxidized derivatives of LNO2 (C and D).
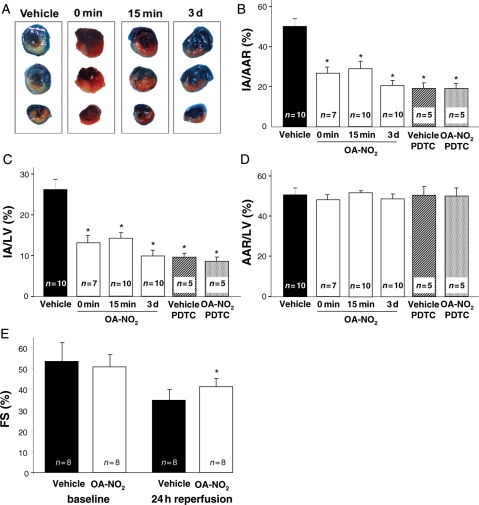
3.2. Nitro-oleate-induced reduction in myocardial infarct size
Following 30 min of myocardial ischaemia and 24 h of in vivo reperfusion, mice treated with OA-NO2 15 min prior to reperfusion exhibited a significant reduction of the IA/AAR (27.6 ± 7.9 vs. 50.1 ± 8.7%, P < 0.01) and IA/LV ratio (14.4 ± 3.9 vs. 25.8 ± 7.7%, P < 0.01) when compared with vehicle-treated animals (Figure 4A–C). Administration of OA-NO2 3 days prior to ischaemia or immediately before reperfusion resulted in IA/AAR ratios (19.6 ± 6.7 and 26.5 ± 7.0%) and IA/LV ratios (9.8 ± 4.2 and 12.9 ± 4.3%) that did not differ significantly from those observed when OA-NO2 was administered 15 min prior to ischaemia. There was a trend towards a more pronounced cardioprotection with the 3 days pretreatment that was not statistically significant (Figure 4B and C).
Figure 4.
Effect of OA-NO2 on infarct size and left ventricular function following ischaemia and reperfusion. (A) Representative images of hearts from vehicle- or OA-NO2-treated animals stained with Evan's blue dye. (B) OA-NO2 treatment at reperfusion, 15 min or 3 days prior to reperfusion lead to a significant reduction in infarct area/area at risk ratio when compared with vehicle-treated animals (*P < 0.01 vs. vehicle). Pretreatment with pyrrolidinedithiocarbamate (PDTC) abolished the effect of OA-NO2. (C) Infarct area to left ventricular (IA/LV) areas reflected the results observed for the IA/AAR ratio (*P < 0.01 vs. vehicle). (D) No difference in AAR/LV ratio was observed between the different treatment groups. (E) OA-NO2 treatment resulted in a significantly improved left ventricular function after I/R, as assessed by transthoracic M-mode echocardiography (*P < 0.01).
After pretreatment with PDTC, no difference in IA/AAR (18.2 ± 5.7 vs. 18.2 ± 5.8%, P = 1.00) and IA/LV ratio (8.4 ± 1.8 vs. 9.3 ± 3.8%, P = 1.00) was observed between OA-NO2 and vehicle-treated animals (Figure 4B and C). No statistically significant difference was observed between OA-NO2-treated animals and animals treated with PDTC and vehicle (P = 0.21) or animals treated with the combination of PDTC and OA-NO2 (P = 0.22, Figure 4B and C). All treatment groups showed similar AAR/LV ratios (Figure 4D). Mice did not exhibit any apparent discomfort or toxic effects of PDTC or OA-NO2 after 24 h of reperfusion.
3.3. Echocardiographic studies
After 24 h of reperfusion, transthoracic echocardiography revealed that mice treated with OA-NO2 displayed significantly better fractional shortening than vehicle-treated mice (41.1 ± 4.0 vs. 34.7 ± 5.1%, P < 0.01; Figure 4E). Mice from both groups did not exhibit increased LVEDd within 24 h of reperfusion and no differences in LVEDd were observed between groups before or after I/R (before I/R: 3.55 ± 0.07 mm vs. 3.53 ± 0.15 mm, P = 0.98; after I/R: 3.55 ± 0.20 mm vs. 3.51 ± 0.23 mm, P = 0.50). Heart rates did not differ significantly between groups during both measurements (before I/R: 420.0 ± 47.3 vs. 454.3 ± 56.7 min−1, P = 0.21; after I/R: 427.9 ± 80.5 vs. 441.9 ± 40.1 min−1, P = 0.67).
3.4. Activation of NFκB p65 subunit
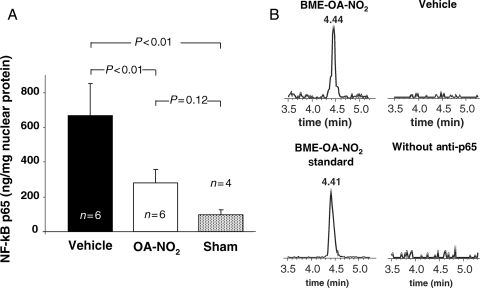
Mice treated with OA-NO2 showed markedly reduced NFκB p65 activation (Figure 5A). P65 activation in OA-NO2-treated mice was more pronounced than in sham-operated animals. Although differences between these two groups were not statistically significant, a trend towards residual p65 activity could be seen in OA-NO2-treated animals.
Figure 5.
Inhibition of NFκB activation by OA-NO2. (A) Reduction of NFκB p65 activity in myocardial tissue of OA-NO2 treated animals following I/R. (B) Assessment of nitroalkylation of p65 in myocardial tissue.
3.5. In vivo assessment of NFκB p65 nitroalkylation
Immunoprecipitation of NFκB p65 from heart tissue of OA-NO2-treated mice displayed covalently adducted OA-NO2. Affinity-purified NFκB p65 fractions were treated with BME and upon LC/MS analysis revealed exchange of the OA-NO2 with BME, yielding BME-OA-NO2 adducts (Figure 5B). No BME-OA-NO2 or LNO2 adduct formation was detected in vehicle-treated animals, presumably because of detection sensitivity. When the NFκB p65 antibody was omitted from immunoprecipitation protocols, BME-OA-NO2 adduct formation was not detectable, excluding the possibility of non-specific OA-NO2 binding to agarose beads.
3.6. Downstream mediators of NFκB
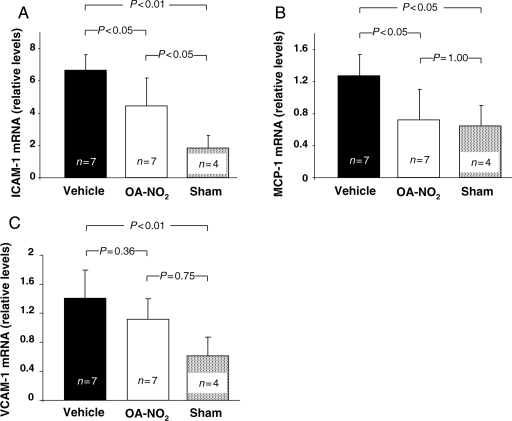
OA-NO2-treated animals showed significantly reduced mRNA expression for ICAM-1, MCP-1, and a reduction of mean VCAM-1 mRNA levels that was not statistically significant (Figure 6A–C). With the exception of MCP-1, sham-operated mice displayed mRNA levels lower than those of OA-NO2-treated mice. In the case of ICAM-1, these differences were significant.
Figure 6.
Effect of OA-NO2 on mRNA levels of adhesion molecules and MCP-1. (A) Reduced NFκB activation in OA-NO2-treated animals was accompanied by a significant reduction of ICAM-1 mRNA expression. (B) Significant suppression of MCP-1 mRNA levels in OA-NO2-treated animals. (C) VCAM-1 mRNA levels also showed a trend towards a reduction. All values are normalized to β2-microglobulin.
3.7. Leukocyte infiltration and systemic inflammation
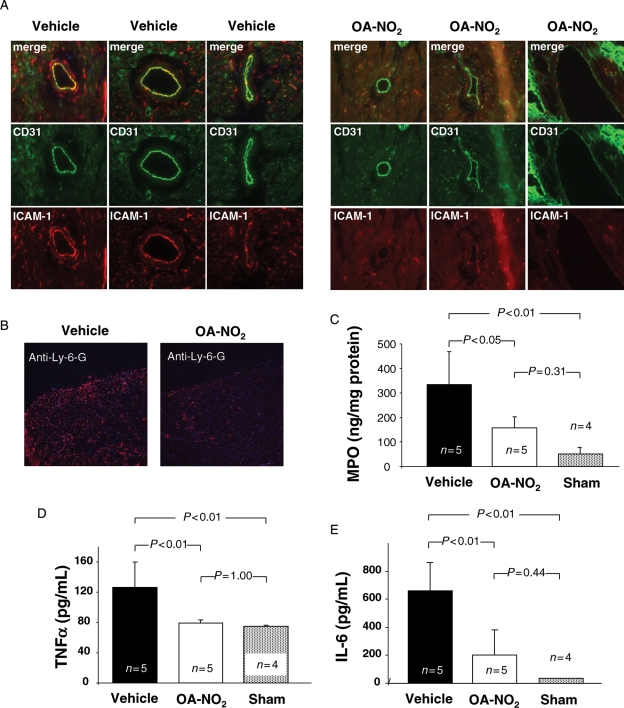
Immunofluorescence demonstrated a pronounced reduction of ICAM-1 protein expression in vessels of OA-NO2-treated mice in comparison to vehicle-treated animals (Figure 7A). Mice treated with OA-NO2 had markedly reduced neutrophil accumulation within the infarct zone compared with vehicle-treated mice as indicated by immunofluorescent staining for the neutrophil-specific GPI-anchored protein Ly-6-G (Figure 7B). Moreover, tissue levels of MPO, a marker of neutrophil-mediated inflammatory tissue damage, were also diminished in OA-NO2-treated animals (Figure 7C). Serum levels of TNFα and IL-6 were also significantly lower in OA-NO2-treated animals than in vehicle-treated animals (Figure 7D and E).
Figure 7.
Effect of OA-NO2 on leukocyte infiltration and systemic inflammation following ischaemia and reperfusion. (A) Immunofluorescent staining for ICAM-1 (red) and CD31 (green) in vessels of OA-NO2 (right panel) and vehicle-treated animals (left panel). OA-NO2-treated animals showed a marked reduction in ICAM-1 immunoreactivity. (B) Neutrophil accumulation in infarcted zone of myocardium. Immunofluorescent staining for neutrophil-specific Ly-6-G revealed increased neutrophil infiltration in vehicle-treated mice. (C) Determination of MPO tissue levels confirmed increased neutrophil infiltration in vehicle-treated animals. (D) Serum TNFα levels were significantly reduced in OA-NO2 treated animals 6 h after reperfusion. (E) Serum IL-6 levels underwent significant reduction in OA-NO2-treated mice.
3.8. Assessment of apoptosis
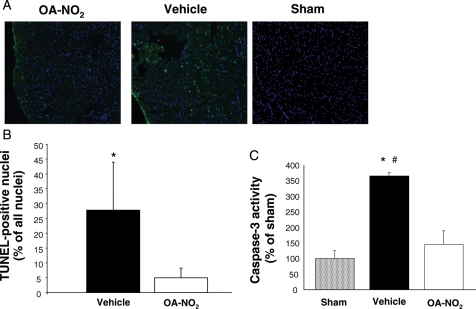
When compared with vehicle-treated animals, OA-NO2-treated mice consistently showed a significant reduction in the density and distribution of TUNEL-positive cells within the area at risk (Figure 8A and B). No TUNEL-positive apoptotic cells were observed in non-infarcted tissue. These data were confirmed by decreased caspase 3 activity in heart tissues from OA-NO2-treated animals when compared with vehicle-treated animals (Figure 8C).
Figure 8.
OA-NO2 reduced apoptosis in myocardial tissue after ischaemia and reperfusion. (A) Immunofluorescent staining for TUNEL-positive cells. (B) Quantification of TUNEL-positive cells; *P < 0.001. (C) Assessment of caspase 3 activity. *P < 0.05 for comparison with sham; #P < 0.05 for comparison with OA-NO2. No statistically significant difference was observed between OA-NO2 treated animals and sham animals.
4. Discussion
This clinically relevant in vivo murine model of focal ischaemic heart injury revealed the de novo generation of electrophilic NO2-FA derivatives in reperfused ischaemic heart tissue. Motivated by in vitro observations of the pluripotent anti-inflammatory signalling actions of NO2-FA, synthetic OA-NO2 was injected intraperitoneally 15 min before or at the time of reperfusion of mice subjected to 30 min of myocardial ischaemia induced by coronary artery ligation. This resulted in (i) a significant reduction in myocardial infarct volume, (ii) preservation of left ventricular function, (iii) inhibition of NFκB activation upon post-translational modification of the p65 subunit by OA-NO2, (iv) suppression of NFκB-dependent myocardial expression of the pro-inflammatory genes ICAM-1, MCP-1, and VCAM-1 and circulating TNFα and IL-6, (v) inhibition of myocardial neutrophil infiltration, and (vi) attenuation of myocyte apoptosis in the ischaemic zone.
The nitration of unsaturated fatty acids occurs endogenously and is stimulated by oxidative inflammatory conditions.15 Cholesteryl-nitrolinoleate is formed by LPS and cytokine-activated macrophages16 and LNO2, OA-NO2, and cholesteryl-nitrolinoleate have been detected in human plasma and lipoproteins.17–20 In cardiac tissue, nitrohydroxyeicosatetraenoic acid has been measured21 and following IPC of rat hearts on a Langendorff perfusion system, LNO2 formation was observed in mitochondria.12 Whereas these data confirm the presence of endogenous NO2-FA in vivo, the present study shows the enhanced de novo generation of fatty acid nitroalkene derivatives following an in vivo pathophysiological stimulus. Since increased generation of ROS, •NO-derived species, and protein tyrosine nitration products are a hallmark of myocardial ischaemia/reperfusion injury,2 the formation of NO2-FA in this setting is consistent with nitrating and oxidizing species being generated by multiple oxidative inflammatory reactions stemming from the precursors •NO and nitrite (NO2−).11,22
Multiple lines of in vitro evidence support that NO2-FA inhibit inflammatory signalling via •NO- and cGMP-independent mechanisms. For example, NO2-FA decrease neutrophil and platelet activation and suppress nitric oxide synthase 2 expression and related inflammatory responses.16,23 In vascular endothelium, NO2-FA inhibit VCAM-1-dependent transendothelial monocyte migration24 and potently induce HO-1 expression and activity.25 Finally, nanomolar concentrations of NO2-FA inhibit NFκB-dependent cytokine and adhesion molecule expression in cultured monocytes.16,24
The strongly electron-withdrawing nitro (NO2) group bonded to olefinic carbons of mono- or polyunsaturated fatty acids renders the vicinal olefinic carbon strongly electrophilic. This vinyl nitro (e.g. nitroalkene) configuration supports a kinetically rapid and reversible covalent adduction of nucleophilic amino acids, primarily cysteine, and to a lesser extent lysine and histidine residues.24,26,27 Current data support that many inflammatory-related transcription factors possess electrophile-reactive thiols that are functionally significant and thus contribute to the transduction of stress-related signalling reactions. This property facilitates the post-translational modification of various signalling mediators and transcription factors by NO2-FA, including the activation of PPARγ28,29 and Nrf2/Keap130 as well as the inhibition of NFκB by nitroalkylation of the NFκB p65 subunit.24
The myocardial protection lent by OA-NO2 administration in this model of I/R was mediated in part by inhibition of NFκB, although it is noted that there may have been some residual nuclear translocation of p65 in OA-NO2-treated animals when compared with sham animals. The precept that OA-NO2-induced NFκB inhibition is reinforced by the observation that inhibition of NFκB signalling by PDTC paralleled OA-NO2-mediated reduction in infarct size. PDTC, while not absolutely specific as a p65-directed reagent, has been used in similar studies as an inhibitor of NFκB.31–33 The slightly greater extent of protection lent by PDTC may be a consequence of a higher dose of PDTC being used and its administration at an earlier time point, 30 min prior to the surgery. Inhibition of NFκB signalling by NO2-FA is mediated by post-translational modification of the p65 subunit, an event contributing to reductions in ICAM-1, MCP-1, and VCAM-1 expression, neutrophil activation, and apoptosis. It remains possible that OA-NO2 also affects pro-inflammatory gene expression and cellular responses via mechanisms independent of NFκB regulation.
Administration of OA-NO2 reduced the extent of apoptosis in ischaemic myocardium, an effect that can also be a consequence of inhibition of NFκB activation. Whereas myocyte apoptosis plays a central role in the development of myocardial reperfusion injury,1 cellular necrosis may also significantly contribute to pathogenic events. It is also possible that apoptosis and necrosis are subject to common regulatory events such as changes in the mitochondrial permeability transition pore (mPTP).34 In this context, it is notable that NO2-FA covalently adduct the adenine nucleotide translocase, one of the components associated with the mPTP.12 Future studies can address whether NO2-FA inhibit both apoptosis and necrotic cell death via interference of mPTP assembly or function.
Current insight indicates that a complex array of events will occur when electrophilic species react with signalling mediators, thus the biological effects of OA-NO2 observed herein are not restricted to just one proposed linear mechanism of action. It is possible that, in addition to inhibition of NFκB activation,35–37 other events such as activation of Nrf2/Keap1 and subsequent upregulation of HO-1 expression, PPAR-γ activation, mitochondrial uncoupling, heat shock factor activation, and other actions of NO2-FA also can contribute to myocardial protection.
The formation of OA-NO2 and LNO2 during reperfusion, combined with the profound protective effects shown upon exogenous administration of OA-NO2 indicates that fatty acid nitration yields an endogenous mediator capable of limiting the damage induced by tissue reoxygenation. Its generation is a consequence of the same process central to reoxygenation-induced tissue damage, the formation and reactions of oxidative inflammatory mediators. In this context, it is important to note that NO2-FA generation was not observed when myocardial ischaemia occurred without reperfusion. Notably, no differences in extents of fatty acid nitration were observed with varying periods of ischaemia. Longer periods of ischaemia, and thus longer periods of reducing conditions in the tissue, might conceivably lead to increased formation of mediators of fatty acid nitration upon reintroduction of oxygen. A possible explanation for no differences in tissue NO2-FA content with varying ischaemia could be that the formation of precursors for fatty acid nitration reaches a steady-state concentration by 15 min and that thus upon reperfusion similar amounts of reactants are available for fatty acid nitration.
The profile of NO2-FA generation observed in vivo differed from that induced by IPC in the isolated rat heart.12 Thus, while OA-NO2 formation was observed in the blood-reperfused heart, no changes were detected following IPC. Furthermore, IPC resulted in the generation of cis-LNO2, whereas the present study revealed a predominant generation of trans-LNO2 in vivo. Several reasons could account for these differences. For example, activation of phospholipase A2 during IPC leads to preferential release of LNO2 rather than OA-NO2 from the membrane.38 In contrast, apoptotic and necrotic cell death observed during the prolonged ischaemia in the course of I/R might lead to a different profile of fatty acid liberation.39 Moreover, the absence of red cells, blood-borne inflammatory cells, plasma proteins, and other possible mediators results in a chemically different milieu in the isolated heart both during the ischaemic episode and during reperfusion. In particular, the predominant generation of trans-LNO2 by blood-perfused murine heart tissue, when compared with the cis-LNO2 noted in ischaemic preconditioned Langendorff hearts, might be explained by decreased levels of protein- and non-protein-thiols in Krebs–Henseleit buffer-perfused hearts. Thiol adduction confers a loss of the cis–trans configuration of NO2-FA, with preliminary observations indicating that upon the reverse reaction, de-alkylation favours NO2-FA release in the trans configuration. These reactions require further study to define potential roles determining cis–trans configuration.
Although the present data support a therapeutic response to acutely administered OA-NO2 within the first 24 h of reperfusion, the long-term effects upon treatment with OA-NO2 cannot be delineated from this study. With regard to clinical relevance, however, the timing and beneficial tissue response to OA-NO2 when administered even at the time of reperfusion is of relevance to a clinical setting, such as the presentation of a patient with an acute myocardial infarction before angioplasty.
In conclusion, this study demonstrates de novo fatty acid nitration in vivo following a pathophysiological stimulus. We further show that OA-NO2 supplementation exerts potent anti-inflammatory and tissue-preserving actions in the early phase of myocardial reperfusion injury. Overall, these data reveal that inflammatory-derived fatty acid nitration products mediate tissue-adaptive signalling reactions in vivo.
Supplementary material
Supplementary Material is available at Cardiovascular Research online.
Conflict of interest: B.A.F. acknowledges financial interest in Complexa, Inc.
Funding
This work was supported by the Deutsche Herzstiftung [to V.R.], the Deutsche Forschungsgemeinschaft [RU1472/1-1 to T.K.R.], the National Institutes of Health [R01 HL58115, R01 HL64937 to B.A.F.], the American Diabetes Association [7-08-JF-52 to F.J.S., 7-06-JF-06 to P.R.S.B.], and the American Heart Association [0665418U to F.J.S.].
Supplementary Material
References
- 1.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 2.Zweier JL, Talukder MA. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res. 2006;70:181–190. doi: 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 3.Hearse DJ, Humphrey SM, Chain EB. Abrupt reoxygenation of the anoxic potassium-arrested perfused rat heart: a study of myocardial enzyme release. J Mol Cell Cardiol. 1973;5:395–407. doi: 10.1016/0022-2828(73)90030-8. [DOI] [PubMed] [Google Scholar]
- 4.Granger DN, Rutili G, McCord JM. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981;81:22–29. [PubMed] [Google Scholar]
- 5.Burton KP, McCord JM, Ghai G. Myocardial alterations due to free-radical generation. Am J Physiol. 1984;246:H776–H783. doi: 10.1152/ajpheart.1984.246.6.H776. [DOI] [PubMed] [Google Scholar]
- 6.Ytrehus K, Myklebust R, Mjøs OD. Influence of oxygen radicals generated by xanthine oxidase in the isolated perfused rat heart. Cardiovasc Res. 1986;20:597–603. doi: 10.1093/cvr/20.8.597. [DOI] [PubMed] [Google Scholar]
- 7.Zweier JL, Flaherty JT, Weisfeldt ML. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci USA. 1987;84:1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eiserich JP, Baldus S, Brennan ML, Ma W, Zhang C, Tousson A, et al. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science. 2002;296:2391–2394. doi: 10.1126/science.1106830. [DOI] [PubMed] [Google Scholar]
- 9.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Donnell VB, Freeman BA. Interactions between nitric oxide and lipid oxidation pathways: implications for vascular disease. Circ Res. 2001;88:12–21. doi: 10.1161/01.res.88.1.12. [DOI] [PubMed] [Google Scholar]
- 11.Freeman BA, Baker PR, Schopfer FJ, Woodcock SR, Napolitano A, d'Ischia M. Nitro-fatty acid formation and signaling. J Biol Chem. 2008;283:15515–15519. doi: 10.1074/jbc.R800004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nadtochiy SM, Baker PR, Freeman BA, Brookes PS. Mitochondrial nitroalkene formation and mild uncoupling in ischaemic preconditioning: implications for cardioprotection. Cardiovasc Res. 2009;82:333–340. doi: 10.1093/cvr/cvn323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schopfer FJ, Batthyany C, Baker P, Bonacci G, Cole MP, Rudolph V, et al. Detection and quantification of protein adduction by electrophilic fatty acid nitration products: mitochondrial generation of fatty acid nitroalkene derivatives. Free Radic Biol Med. 2009;46:1250–1259. doi: 10.1016/j.freeradbiomed.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudolph V, Schopfer FJ, Khoo NK, Rudolph TK, Cole MP, Woodcock SR, et al. Nitro-fatty acid metabolome: saturation, desaturation, beta-oxidation, and protein adduction. J Biol Chem. 2009;284:1461–1473. doi: 10.1074/jbc.M802298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubbo H, Radi R, Trujillo M, Telleri R, Kalyanaraman B, Barnes S, et al. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem. 1994;269:26066–26075. [PubMed] [Google Scholar]
- 16.Ferreira AM, Ferrari M, Trostchansky A, Batthyány C, Souza JM, Alvarez MN, et al. Macrophage activation induces formation of the anti-inflammatory lipid cholesteryl-nitrolinoleate. Biochem J. 2008;417:223–234. doi: 10.1042/BJ20080701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsikas D, Zoerner A, Mitschke A, Homsi Y, Gutzki FM, Jordan J. Specific GC-MS/MS stable-isotope dilution methodology for free 9- and 10-nitro-oleic acid in human plasma challenges previous LC-MS/MS reports. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2895–2908. doi: 10.1016/j.jchromb.2008.12.062. [DOI] [PubMed] [Google Scholar]
- 18.Baker PR, Schopfer FJ, Sweeney S, Freeman BA. Red cell membrane and plasma linoleic acid nitration products: synthesis, clinical identification, and quantitation. Proc Natl Acad Sci USA. 2004;101:11577–11582. doi: 10.1073/pnas.0402587101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lima ES, Di Mascio P, Rubbo H, Abdalla DS. Characterization of linoleic acid nitration in human blood plasma by mass spectrometry. Biochemistry. 2002;41:10717–10722. doi: 10.1021/bi025504j. [DOI] [PubMed] [Google Scholar]
- 20.Lima ES, Di Mascio P, Abdalla DS. Cholesteryl nitrolinoleate, a nitrated lipid present in human blood plasma and lipoproteins. J Lipid Res. 2003;44:1660–1666. doi: 10.1194/jlr.M200467-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Balazy M, Iesaki T, Park JL, Jiang H, Kaminski PM, Wolin MS. Vicinal nitrohydroxyeicosatrienoic acids: vasodilator lipids formed by reaction of nitrogen dioxide with arachidonic acid. J Pharmacol Exp Ther. 2001;299:611–619. [PubMed] [Google Scholar]
- 22.O'Donnell VB, Eiserich JP, Chumley PH, Jablonsky MJ, Krishna NR, Kirk M, et al. Nitration of unsaturated fatty acids by nitric oxide-derived reactive nitrogen species peroxynitrite, nitrous acid, nitrogen dioxide, and nitronium ion. Chem Res Toxicol. 1999;12:83–92. doi: 10.1021/tx980207u. [DOI] [PubMed] [Google Scholar]
- 23.Coles B, Bloodsworth A, Clark SR, Lewis MJ, Cross AR, Freeman BA, et al. Nitrolinoleate inhibits superoxide generation, degranulation, and integrin expression by human neutrophils: novel antiinflammatory properties of nitric oxide-derived reactive species in vascular cells. Circ Res. 2002;91:375–381. doi: 10.1161/01.res.0000032114.68919.ef. [DOI] [PubMed] [Google Scholar]
- 24.Cui T, Schopfer FJ, Zhang J, Chen K, Ichikawa T, Baker PR, et al. Nitrated fatty acids: endogenous anti-inflammatory signaling mediators. J Biol Chem. 2006;281:35686–35698. doi: 10.1074/jbc.M603357200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright MM, Schopfer FJ, Baker PR, Vidyasagar V, Powell P, Chumley P, et al. Fatty acid transduction of nitric oxide signaling: nitrolinoleic acid potently activates endothelial heme oxygenase 1 expression. Proc Natl Acad Sci USA. 2006;103:4299–4304. doi: 10.1073/pnas.0506541103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker LM, Baker PR, Golin-Bisello F, Schopfer FJ, Fink M, Woodcock SR, et al. Nitro-fatty acid reaction with glutathione and cysteine. Kinetic analysis of thiol alkylation by a Michael addition reaction. J Biol Chem. 2007;282:31085–31093. doi: 10.1074/jbc.M704085200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batthyany C, Schopfer FJ, Baker PR, Durán R, Baker LM, Huang Y, et al. Reversible post-translational modification of proteins by nitrated fatty acids in vivo. J Biol Chem. 2006;281:20450–20463. doi: 10.1074/jbc.M602814200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schopfer FJ, Lin Y, Baker PR, Cui T, Garcia-Barrio M, Zhang J, et al. Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor gamma ligand. Proc Natl Acad Sci USA. 2005;102:2340–2345. doi: 10.1073/pnas.0408384102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Zhang J, Schopfer FJ, Martynowski D, Garcia-Barrio MT, Kovach A, et al. Molecular recognition of nitrated fatty acids by PPAR gamma. Nat Struct Mol Biol. 2008;15:865–867. doi: 10.1038/nsmb.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villacorta L, Zhang J, Garcia-Barrio MT, Chen XL, Freeman BA, Chen YE, et al. Nitro-linoleic acid inhibits vascular smooth muscle cell proliferation via the Keap1/Nrf2 signaling pathway. Am J Physiol Heart Circ Physiol. 2007;293:H770–H776. doi: 10.1152/ajpheart.00261.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schreck R. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992;175:1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyd JH, Mathur S, Wang Y, Bateman RM, Walley KR. Toll-like receptor stimulation in cardiomyoctes decreases contractility and initiates an NF-kappaB dependent inflammatory response. Cardiovasc Res. 2006;72:384–393. doi: 10.1016/j.cardiores.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 33.Zhao TC, Taher MM, Valerie KC, Kukreja RC. P38 triggers late preconditioning elicited by anisomycin in heart. Circ Res. 2001;89:915–922. doi: 10.1161/hh2201.099452. [DOI] [PubMed] [Google Scholar]
- 34.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia–reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall G, Hasday JD, Rogers TB. Regulating the regulator: NF-kappaB signaling in heart. J Mol Cell Cardiol. 2006;41:580–591. doi: 10.1016/j.yjmcc.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Valen G. Signal transduction through nuclear factor kappa B in ischemia–reperfusion and heart failure. Basic Res Cardiol. 2004;99:1–7. doi: 10.1007/s00395-003-0442-7. [DOI] [PubMed] [Google Scholar]
- 37.Morishita R, Sugimoto T, Aoki M, Kida I, Tomita N, Moriguchi A, et al. In vivo transfection of cis element ‘decoy’ against nuclear factor-kappaB binding site prevents myocardial infarction. Nat Med. 1997;3:894–899. doi: 10.1038/nm0897-894. [DOI] [PubMed] [Google Scholar]
- 38.Starkopf J, Andreasen TV, Bugge E, Ytrehus K. Lipid peroxidation, arachidonic acid and products of the lipoxygenase pathway in ischaemic preconditioning of rat heart. Cardiovasc Res. 1998;37:66–75. doi: 10.1016/s0008-6363(97)00240-x. [DOI] [PubMed] [Google Scholar]
- 39.Hegstad AC, Strand H, Ytrehus K. Phospholipid peroxidation after 60 min of global ischaemia and 10 min of reperfusion. A study in the isolated rat heart. J Mol Cell Cardiol. 1994;26:569–578. doi: 10.1006/jmcc.1994.1069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.