Abstract
Aims
Given the importance of IgG Fc receptors in immune regulation, we hypothesized that Fcg receptor type III (FcgRIII, CD16) plays an important role in atherogenesis. We therefore analysed the formation of arterial lesions in LDL receptor-deficient (LDLR−/−) and FcgRIII−/−×LDLR−/− double knockout mice at three different points up to 24 weeks of exposure to a high-fat diet.
Methods and results
Analysis of Oil Red-O-stained sections revealed no difference in lesion formation between strains after 6 weeks of a high-fat diet, and a modest decrease after 14 weeks in double knockouts relative to LDLR−/− controls. After 24 weeks, lesion formation was decreased in the aortic root (30%) and innominate artery (50%) in FcgRIII double knockouts relative to LDLR−/− controls. Analysis of peripheral CD4+ T-cells by intracellular flow cytometry from double knockouts after 24 weeks of a high-fat diet revealed statistically significant increases in the percentages of cells producing interferon-γ, interleukin (IL)-10, and IL-4 relative to controls, differences that were also observed by analyses of whole aortas for cytokine mRNA levels. As determined by flow cytometry, FcgRIII deficiency resulted in an expansion of CD4+ cells and an increase in the CD4 to CD8 ratio. Analysis of plasma anti-oxidized LDL (OxLDL) antibodies by chemiluminescent assay revealed that IgG1 and IgG2c titers to OxLDL were increased in FcgRIII −/−×LDLR−/− double knockouts relative to LDLR−/− controls, while total IgG levels were similar.
Conclusion
These results reveal altered immunity in FcgRIII−/−×LDLR−/− mice and a reduction in lesion formation associated with increased production of IL-10 by an expansion of CD4+ T-cells. The reduction in lesion formation was manifest well after evidence of an immune response to OxLDL, suggesting that FcgRIII contributes to lesion progression in murine atherosclerosis.
Keywords: Fc receptors, CD16, Murine atherosclerosis, T-cells, Oxidized LDL, Lesions, IL-10, Interferon, Lymphoid follicle
1. Introduction
Atherosclerosis is a complex disease with many contributing factors, including important roles for many elements of both innate and adaptive immunity.1–10 Several studies have highlighted the importance of T-cells in the atherogenic process11–15 and in addition there is now a well-documented humoral response to the various oxidation-specific neoepitopes triggered by LDL oxidation. For example, in hypercholesterolaemic murine models, a protective effect was associated with expansion of naturally occurring E06/T15 idiotypic IgM, which has the capacity to block uptake of oxidized LDL (OxLDL) by scavenger receptors.16,17 In the case of anti-OxLDL IgG, although the role of such antibodies remains to be clarified, many studies have demonstrated their presence in plasma or atherosclerotic lesions.18–22 Because the formation of arterial lesions was reduced in Fc receptor gamma chain knockout mice, it can be inferred that the triggering of activating-type FcγRs by IgG immune complexes is proatherogenic.23 However, gamma chain is also associated with the T-cell/CD3 receptor complex and the glycoprotein VI (GPVI) complex and as such plays an important role in T-cell signalling and platelet activation.24–27 This suggests that there may be multiple mechanisms by which the absence of gamma chain impacts lesion formation, some of which may function independently of activation by immune complexes. We therefore focused on murine FcγRIII (CD16), an activating-type FcγR expressed on macrophages, dendritic cells, and NK T-cells that binds immune complexes containing IgG1, IgG2c, and IgG2b.28–30 Depending on the model, signalling associated with FcγRIII can enhance either T helper cell 1 (Th1) or Th2 biased responses.31,32 FcγRIII is also unique among other activating-type FcγRs (FcγRI and FcγRIV) in that it may influence thymic B and T-cell development through interactions with non-immunoglobulin (Ig) ligands.33,34 In the present study, we tested the hypothesis that the formation of arterial lesions would be decreased in mice deficient in FcγRIII. We found that relative to LDLR−/− controls, arterial lesion formation was dramatically decreased in FcγRIII-deficient mice on the LDLR receptor negative background, which was most apparent at a relatively later stage of atherogenesis. These data support that FcγRIII is important for lesion progression. In addition, analyses of cytokine production suggest that the decrease in arterial lesion formation in FcγRIII-deficient mice involves IL-10 produced by an expanded population of CD4+ T-cells. Signalling pathways associated with FcγRIII may represent targets for modulating the formation of atherosclerotic lesions.
2. Methods
2.1. Mice and study protocol
Expanded methods are provided as Supplemental material online. Male and female LDLR−/− and LDLR−/−×FcγRIII−/− mice, 4–7 weeks of age, were placed on high-fat western diet (Harlan-Teklad no. 88137) and analysed as indicated after 6, 14, or 24 weeks. All procedures and manipulations were approved by the local Institutional Animal Care and Use Committee (IACUC) and conform to guidelines listed in the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
2.2. Analysis of lesions
Oil Red-O-stained sections of the aortic root and innominate artery were analysed according to Plump et al.35 For immunohistochemical analyses for macrophages, acetone-fixed cryosections were stained with anti-CD68. Antibody binding was detected with goat anti-rat Fab'2 conjugated to alkaline phosphatase. To detect the presence of T-cells, serial transverse 10 µm sections of the aortic root, aortic arch, or innominate artery were stained with APC-anti-CD3 (or APC-anti-CD3 plus FITC-conjugated anti-B220) after 14 weeks (5 LDLR−/− and 5 double knockouts) or 24 weeks of high-fat diet (4 LDLR−/− and 4 double knockouts) and analysed with a Nikon Eclipse 80i immunofluorescence microscope with NIS Elements software.
2.3. Analyses of plasma for lipids and antibody levels
Total plasma cholesterol and triglycerides were determined enzymatically (Raichem, Columbia, MD, USA). Antibody titers to OxLDLs were determined by chemiluminescent immunoassay.36
2.4. Flow cytometry
To determine the CD4-CD8 ratio, splenocytes were counted by haemacytometer then stained with FITC-anti-mouse CD4 plus PE-anti-mouse CD8 and analysed by flow cytometry. For intracellular staining of cytokines, peripheral mononuclear cells or purified CD4+ splenocytes were restimulated for 6 h at 37° with a leukocyte activation cocktail (eBiosciences). Cells were stained first with PE-Cy5-anti-mouse CD4 followed by fixation in methanol-free paraformaldehyde. The cells were then washed in a permeabilization buffer containing saponin then stained with FITC-anti-mouse IL-10 (rat IgG2b), FITC-anti-mouse IL-4 (rat IgG1), or PE-anti-mouse interferon-γ (rat IgG1) prepared in permeabilization buffer (eBiosciences). Isotype-matched controls were included to determine background fluorescence in each experiment and cells were analysed with a FacScan or Facs Canto flow cytometer.
2.5. Real-time RT–PCR
For the analysis of mRNA levels, aortas were homogenized on ice in Trizol and total RNA immediately isolated and stored at −80°. Total RNA was subsequently reverse transcribed and the cDNA was used to measure IL-10 and interferon-γ (IFN-γ). The data were normalized to GAPDH and are presented as fold-increase relative to mRNA levels obtained from an aorta from a chow-fed C57BL/6 control.
2.6. Statistical methods
All data were analysed using Prism software. Unpaired Student's t-test was used to compare groups of data that were normally distributed and of similar variance; otherwise the non-parametric Mann–Whitney test was used. In each case, P < 0.05 was taken to indicate statistical significance.
3. Results
3.1. Effects of FcγR deficiency on lesion formation
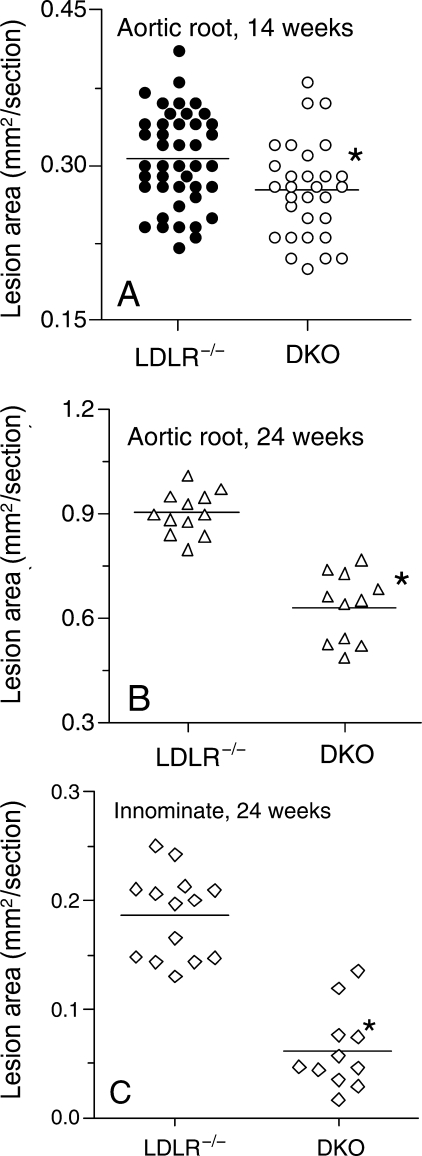
Lesion area in LDLR−/− and LDLR−/−×FcγRIII−/− mice was analysed in the aortic root after 6, 14, or 24 weeks of high-fat diet. By Oil Red-O staining, no differences in lesion formation were found between LDLR−/− and LDLR−/−×FcγRIII−/− mice after 6 weeks (not shown). After 14 weeks, FcγRIII deficiency was associated with a modest but statistically significant decrease in lesion area in the aortic root for males and females relative to LDLR−/− controls (Figure 1A). After 24 weeks of high-fat diet (Figures 1B and C, and 2), lesion formation relative to LDLR−/− controls had decreased 30% in the aortic root and 50% in the innominate artery (P < 0.0001 and 0.0008, respectively). No effect of gender was evident at any time point (not shown).
Figure 1.
Decreased lesion formation in the aortic root or innominate artery in FcγRIII−/− × LDLR−/− double knockouts. (A) Lesion formation in the aortic root determined by Oil Red-O staining at 14 weeks (for LDLR−/− n = 20 males and 23 females; for FcγRIII−/− × LDLR−/− n = 12 males and 18 females, P = 0.007). (B) Lesion formation in the aortic root after 24 weeks of high-fat diet (for LDLR−/− n = 6 males and 5 females; for FcγRIII−/− × LDLR−/− n = 6 males and 5 females, P < 0.0001). (C) Lesion formation in the innominate artery after 24 weeks of high-fat diet for the same mice as in (B) (P < 0.0008).
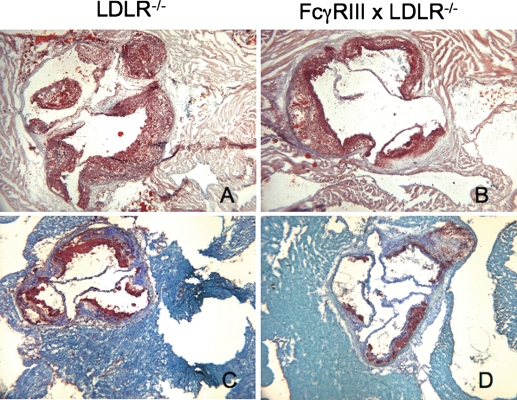
Figure 2.
Decreased lesion formation in FcγRIII−/− × LDLR−/− double knockout mice. Representative illustrations of the distribution of neutral lipid (A and B) or of CD68+ macrophages (C and D) in the aortic root of the indicated strains after 24 weeks of high-fat diet. Sections taken through the valve leaflets are shown in each case. All of the material extending into the lumen stained with Oil Red-O, but gaps indicative of cholesterol clefts are present in regions largely devoid of cells in each case.
As shown in Figure 2, the smaller lesions of FcγRIII−/− double knockouts delineated by Oil Red-O staining contained correspondingly less CD68+ macrophages. Analyses of CD3 immunofluorescence established the presence of numerous clusters of T-cells in the aortic root, aortic arch, and innominate artery from each strain of mice after 14 weeks of high-fat diet (data not shown). Results were similar after 24 weeks of high-fat diet. Representative examples of clusters of T-cells in the aortic root of an FcγRIII double knockout after 24 weeks of high-fat diet are shown in Figure 3. A difference between the controls and double knockouts was the presence of areas adjacent to the aorta in the double knockouts that stained densely for both CD3+ T-cells and B-cells after 24 weeks of high-fat diet (Figure 3). These regions resembled adventitial ectopic lymphoid follicles that were reported for the apolipoprotein E knockout mouse where clusters of T-cells and clusters of B-cells appeared together after a year or more in mice on standard chow.37 The implications of this are addressed below but the important point is that these data demonstrate the presence of significant numbers of T-cells in the double knockouts at a point (24 weeks of high-fat diet) when differences in cytokine mRNA levels in whole aortas were noted (see what follows).
Figure 3.
T-cell content of arterial lesions in the aortic root of FcγRIII double knockouts after 6 months of high-fat diet. Sections were fixed in acetone and stained with APC-anti-CD3 (A) or APC-anti-CD3 plus FITC-anti-B220 (B) as described in Methods. (A) A typical example of clusters of T-cells (red) is shown in the plaque from a section taken through the aortic root; (B) an ectopic follicle with a core of T-cells surrounded by numerous B-cells (green) in a section adjacent to the aortic root. The upper arrow denotes the aorta while the lower arrow is opposite a layer of heart muscle.
3.2. Effects of FcγR deficiency on plasma lipids
FcγRIII−/− deficiency was associated with increased total plasma cholesterol for both males and females (Tables 1 and 2) and the differences were statistically significant after 24 weeks of high-fat diet. Plasma triglyceride levels in FcγRIII−/− double knockouts also tended to be greater relative to LDLR−/− controls and the differences were statistically significant for females after 14 weeks of high-fat diet. FcγRIII−/−-deficient mice appeared to thrive on high-fat diet and at the time of sacrifice exhibited increased body weight relative to controls (Tables 1 and 2). These data suggest that the reduction in lesion formation in FcγRIII−/− double knockouts was not due to changes in plasma lipid levels.
Table 1.
Selected endpoints from LDLR−/− and FcγRIII−/− double knockouts after 14 weeks of high-fat diet
| Strain | N | TC | TG | BW | |
|---|---|---|---|---|---|
| Males | LDLR−/− | 25 | 742 ± 37 | 679 ± 48 | 39.2 ± 5.0 |
| DKO | 14 | 812 ± 34 | 785 ± 59 | 42.7 ± 1.9† | |
| Females | LDLR−/− | 21 | 578 ± 23 | 281 ± 18 | 24.2 ± 3.7 |
| DKO | 18 | 682 ± 51 | 373 ± 30‡ | 32.2 ± 4.7* |
TC, total plasma cholesterol; TG, plasma triglycerides, each in mg/dL; DKO, double knockout; BW, body weight, in grams.
For comparisons between strains: †, P = 0.03; ‡, P = 0.009; *, P > 0.0001.
Table 2.
Selected endpoints from LDLR−/− and FcγRIII−/− double knockouts after 24 weeks of high-fat diet
| Strain | N | TC | TG | BW | |
|---|---|---|---|---|---|
| Males | LDLR−/− | 6 | 788 ± 96 | 424 ± 75 | 37.4 ± 7.1 |
| DKO | 5 | 934 ± 86† | 620 ± 229 | 42.2 ± 7.2 | |
| Females | LDLR−/− | 6 | 660 ± 145 | 449 ± 186 | 26.3 ± 4.0 |
| DKO | 5 | 867 ± 134‡ | 550 ± 62 | 29.7 ± 5.1 |
TC, total plasma cholesterol; TG, plasma triglycerides, each in mg/dL; DKO, double knockout; BW, body weight, in grams.
For comparisons between strains: †P = 0.02, ‡P = 0.047.
3.3. Effects of FcγR deficiency on plasma anti-OxLDL antibodies
Analysis of plasma anti-OxLDL antibodies revealed increased IgG1 and IgG2c titers in FcγRIII−/− double knockouts relative to LDLR−/− controls. Statistically significant increases were obtained for each isotype at 14 and 24 weeks of high-fat diet when malondialdehyde-LDL was used for antigen (see Supplementary material online, Figure S1), and the difference was greatest in the case of IgG2c. Similar results were obtained with copper OxLDL (see Supplementary material online, Figure S1). Total plasma IgG was also not different at any point between LDLR−/− mice and FcγRIII−/− double knockouts (data not shown). Surprisingly, the levels of anti-OxLDL IgG titers for both strains, which were greatest at 14 weeks of high-fat diet, had fallen by 24 weeks, while anti-OxLDL IgM titers were greatest at 24 weeks. Similar to IgG1 and IgG2c titers, IgM titers in FcγRIII−/− double knockouts were significantly increased relative to LDLR−/− controls (see Supplementary material online, Figure S2). These data suggested that there was a dysregulation of antibody production to OxLDLs raising the possibility of altered cytokine production in FcγRIII−/− double knockouts after weeks of high-fat diet.
3.4. Analyses of cytokine production
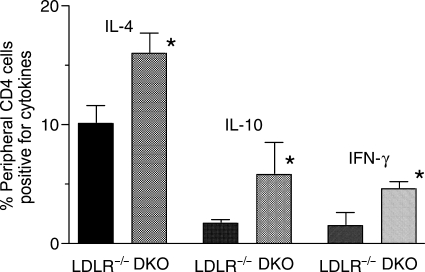
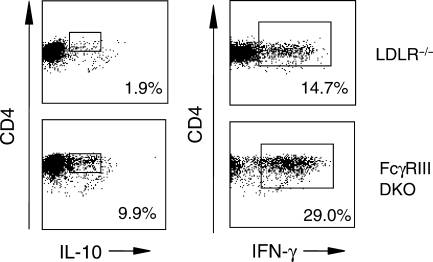
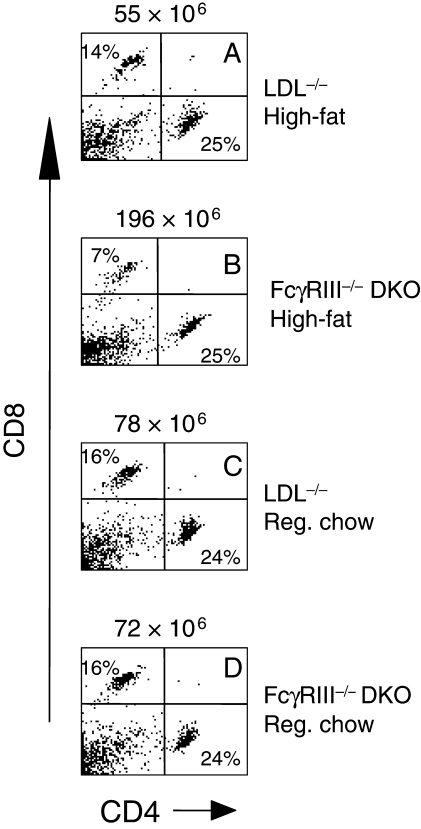
To address potential mechanisms by which the absence of FcγRIII resulted in decreased lesion formation, CD4+ T-cells isolated from peripheral blood (Figure 4), and spleen (Figure 5), were analysed for the presence of cytokines by intracellular flow cytometry after 24 weeks of high-fat diet. The results indicated similar increases in the numbers of CD4+ T-cells expressing interleukin-4 (IL-4), IL-10, or IFN-γ obtained from FcγRIII−/− double knockouts relative to LDLR−/− controls, each difference being statistically significant (Figure 4). Cytokine expression was barely detectable in cells obtained from age-matched controls of each strain on chow diet (data not shown), consistent with a response to the inflammatory effects of high-fat diet. More dramatic results were obtained with CD4+ T-cells purified from spleens. In the example shown in Figure 5, there was a 5.2-fold increase in the number of CD4+ T-cells expressing IL-10 in FcγRIII−/− double knockouts after 24 weeks of high-fat diet and a two-fold increase in the number of cells expressing IFN-γ. These data suggest an alteration in T-cell development and/or function in FcγRIII−/− double knockouts dependent in part on high-fat diet. This was supported further by measuring the number of CD4+ and CD8+ T-cells in spleens taken from each strain after 24 weeks of high-fat or chow diet. FcγRIII−/− double knockouts on high-fat diet exhibited a dramatic increase in the total number of splenocytes (Figure 6B), a significant increase in the total number of CD4+ cells, and as a result, a significant increase in the CD4 to CD8 ratio (for 14 double knockouts and 10 LDLR−/− controls, the mean CD4 to CD8 ratios were 3.0 ± 0.3 vs. 1.7 ± 0.1, respectively, P = 0.003). Thus, relative to the situation for controls, there was an expansion of the numbers of CD4+ T-cells in FcγRIII−/− double knockouts that was dependent on high-fat diet.
Figure 4.
Increased cytokine expression in peripheral blood CD4+ T-cells from FcγRIII−/− × LDLR−/− double knockout mice. CD4+ T-cells were obtained from four LDLR−/− mice and four FcγRIII−/− double knockouts after 24-weeks of high-fat diet. Levels of the indicated cytokines were determined by intracellular staining and flow cytometry. The differences in cytokine levels between LDLR−/− mice and FcγRIII−/− double knockouts were statistically significant in each case. For IL-4, P = 0.002; for IL-10, P = 0.02, and for IFN-γ, P = 0.009. Shown are the means ± SD.
Figure 5.
Increased cytokine expression in CD4+ splenocytes from FcγRIII−/−×LDLR−/− double knockout mice. Representative example of scatter profiles of the increase in the number of cells positive for IL-10 or IFN-γ (rectangular gates) in CD4+ T-cells purified from spleens from LDLR−/− mice and FcγRIII−/− double knockouts after 24 weeks of high-fat. A linear region of background fluorescence (less than 1% of the gated population) is present to the right of the positive cells as determined by reactivity of isotype controls (data not shown).
Figure 6.
Expansion of numbers of CD4+ T-cells with increased CD4/CD8 ratio in FcγRIII−/− × LDLR−/− double knockout mice. Representative example of scatter profiles showing the expansion of CD4+ T-cells in FcγRIII−/− double knockouts after 24 weeks of high-fat. Shown at the top of each figure is the total number of lymphocytes. The percentages of total lymphocytes that expressed either CD4 or CD8 are indicated within each figure. In all, 14 FcγRIII−/− double knockouts and 10 LDLR−/− mice were analysed. The mean CD4 to CD8 ratios were 3.0 ± 0.3 and 1.7 ± 0.1, means ± SD respectively, P = 0.003.
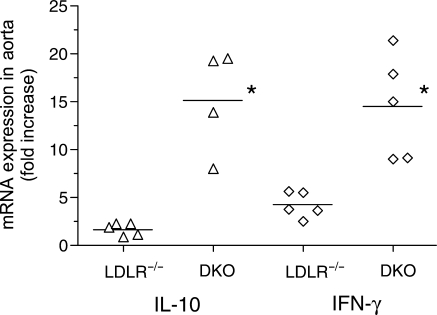
Data supporting that the changes in cytokine production noted above may have contributed to the reduction in lesion formation in the absence of FcγRIII was obtained by analysing mRNA levels of IL-10 and IFN-γ in aortas taken after 24 weeks of high-fat diet. Using an aorta from a chow-fed C57BL/6 mouse for baseline, we determined that the levels of mRNA for each cytokine in LDLR−/− mice were increased above baseline, the fold-increase being greatest for IFN-γ (P = 0.005) (Figure 7). Consistent with the changes in cytokine production determined by intracellular staining, mRNA levels for each cytokine in FcγRIII−/− double knockouts were significantly greater relative to LDLR−/− controls, the difference being greatest for IL-10.
Figure 7.
Increased cytokine mRNA levels in FcγRIII−/−×LDLR−/− double knockout mice. mRNA levels from aortas of LDLR−/− mice and FcγRIII−/− double knockouts obtained after 24 weeks of high-fat diet were determined by real-time RT–PCR for IL-10 and IFN-γ. The differences between LDLR−/− and FcγRIII−/− double knockouts were statistically significant at P = 0.015 for IL-10 and P = 0.008 for IFN-γ.
4. Discussion
There are two important findings of this study. The first is a significant reduction in the formation of arterial lesions in FcγRIII-deficient mice after 24 weeks of high-fat diet but not earlier, suggesting that FcγRIII plays an important role in lesion progression (Figure 1). The second is that this reduction was associated with the development of an expanded population of CD4+ T-cells producing the anti-atherogenic cytokine IL-1038–43 (Figures 4–6). The presence of T-cells in lesions from double knockouts together with decreased numbers of macrophages (Figures 2 and 3) suggests that T-cells may have been an important source of cytokines in the lesions (Figure 7). Contributions from macrophages or dendritic cells, however, cannot be excluded. While an exhaustive analysis of leukocyte content in lesions was not undertaken, the data suggest that the absence of FcγRIII was associated with differences in leukocyte content after 6 months of high-fat diet. It was interesting and surprising to note the presence of numerous clusters of T-cells and B-cells that resembled adventitial ectopic lymphoid follicles first reported for atherosclerosis in the apolipoprotein E knockout mouse.37 In the present study, we noted these structures at the level of the aortic root (Figure 3) and ascending arch (not shown) in the double knockouts, but not LDLR−/− controls. The role that these structures serve has not been defined, but it is interesting to speculate that the absence of FcγRIII signalling, which was associated with increased numbers of peripheral T-cells (Figures 5 and 6), may have played a role in enhancing the formation of these ectopic lymphoid structures.
The decreased accumulation of lipids and macrophages seen in lesions of FcγRIII−/− double knockouts was also observed in studies of IL-10 hyperexpression in murine atherosclerosis.41 Remarkably, the reduction in lesion formation was lower in the absence of FcγRIII despite the fact that total plasma cholesterol levels in FcγRIII−/− double knockouts tended to be greater relative to LDLR−/− controls (Tables 1 and 2). That the reduction in lesion formation was more evident after 24 weeks of high-fat diet rather than 14 weeks suggests that FcγRIII activity is important for lesion progression. These results contrast with the study of Hernandez-Vargas et al.23 who found that lesion formation was significantly decreased in gamma chain-deficient mice on the apoE-deficient background by 16 weeks of high-fat diet. Although results of later time points were not reported in that study, the differences between their findings at 16 weeks and ours at a similar time point suggest that the mechanisms underlying lesion formation are unique in each model. This is not surprising given the differences noted between the two models. As stated earlier, reports suggest that both T-cell signalling and platelet activation mediated by GPVI are altered by the absence of the Fc receptor gamma chain.24,25
Of the three activating-type FcγRs in mice, FcγRIII is unique in its IgG ligand binding properties and in its ability to bind non-Ig ligands, each of which could have played a role in the present study. The increase in both anti-OxLDL IgG2c (Th1 dependent) and IgG1 (Th2 dependent) in the absence of FcγRIII is consistent with increases in both Th1-dependent (IFN-γ) and Th2-dependent cytokines (IL-10) seen in this study (see Supplementary material online, Figure S1) and may have occurred as a result of the effects on B-cells of altered cytokine production. These results add to the complexity of FcγRIII activity in the regulation of immune phenomena in these models. Indeed, in combination with TLR-4 ligands such as lipopolysaccharide (LPS), FcγRIII cross-linking on dendritic cells regulates Th2 cytokine production independently of its ability to process immune complexes,31 a characteristic that was not shared by the high-affinity FcγRI.31 In mice, FcγRIII mediated signalling is important for thymic T-cell development and includes the involvement of non-immunoglobulin ligand(s).33,34,44 The effects of FcγRIII activity on lesion formation could therefore be dependent on the interaction with immune complexes and/or with non-immunoglobulin ligands. In support of this, a recent report demonstrated that FcγRIII is a receptor for E. coli.45
The finding of increased production of both IL-10 and IFN-γ was surprising in that IFN-γ is a Th1 cytokine with well-documented pro-atherogenic activity.46–50 On the other hand, IL-10 is a Th2-like cytokine with a well-documented anti-atherogenic role.38–43,51 Similar to the present study, hyperexpression of IL-10 in LDLR−/− mice resulted in a 50% reduction in aortic lesion formation after 20 weeks of high-fat diet,41 but in contrast, production of IFN-γ by T-cells was reduced and the ratio of anti-OxLDL IgG1 to IgG2c was increased. The increase in production of IFN-γ in the present study may have been at least in part responsible for the lack of a change in the ratio of anti-OxLDL IgG2c to IgG1 (see Supplementary material online, Figure S1). IFN-γ is important for priming T-cells for production of Th2 cytokines such as IL-452 and could have played such a role in the absence of FcγRIII. These findings are consistent with the changes in cytokines and the decrease in arterial lesion formation seen in the present study. Thus, while decreased lesion formation observed in the present study is consistent with the inhibitory role of IL-10, it does not exclude the importance of IFN-γ.
In summary, the data support that the activity of FcγRIII, a structurally and functionally unique activating-type FcγR, contributes to arterial lesion progression in LDL receptor-deficient mice and that IL-10 may play an important role in this phenomenon. In the absence of FcγRIII, there was an expansion of CD4+ T-cells producing IL-10 and IFN-γ and a reduction in the size and lipid content of arterial lesions that was more apparent after 24 weeks of high-fat diet. Signalling pathways regulating these phenomena may represent targets that could be exploited therapeutically to regulate the progression of atherosclerotic diseases.
Supplementary material
Supplementary Material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by funding from the Hitchcock Foundation, the US Department of Veterans Affairs (Merit Review Awards to P.M., J.K., and R.F.), and by the National Institutes of Health (HL086559 and HL088093 to J.W.).
Supplementary Material
References
- 1.Binder CJ, Chang MK, Shaw PX, Miller YI, Hartvigsen K, Dewan A, et al. Innate and acquired immunity in atherogenesis. Nat Med. 2002;8:1218–1226. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- 2.Caligiuri G, Nicoletti A, Poirier B, Hansson GK. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J Clin Invest. 2002;109:745–753. doi: 10.1172/JCI07272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 4.Major AS, Fazio S, Linton MF. B-lymphocyte deficiency increases atherosclerosis in LDL receptor-null mice. Arterioscler Thromb Vasc Biol. 2002;22:1892–1898. doi: 10.1161/01.atv.0000039169.47943.ee. [DOI] [PubMed] [Google Scholar]
- 5.Osterud B, Bjorklid E. Role of monocytes in atherogenesis. Physiol Rev. 2003;83:1069–1112. doi: 10.1152/physrev.00005.2003. [DOI] [PubMed] [Google Scholar]
- 6.Packard RR, Maganto-Garcia E, Gotsman I, Tabas I, Libby P, et al. CD11c+ Dendritic cells maintain antigen processing, presentation capabilities, and CD4+ T-cell priming efficacy under hypercholesterolemic conditions associated with atherosclerosis. Circ Res. 2008;103:965–973. doi: 10.1161/CIRCRESAHA.108.185793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun J, Sukhova GK, Wolters PJ, Yang M, Kitamoto S, Libby P, et al. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007;13:719–724. doi: 10.1038/nm1601. [DOI] [PubMed] [Google Scholar]
- 8.Swirski FK, Pittet MJ, Kircher MF, Aikawa E, Jaffer FA, Libby P, et al. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc Natl Acad Sci USA. 2006;103:10340–10345. doi: 10.1073/pnas.0604260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8:802–815. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- 10.Whitman SC, Rateri DL, Szilvassy SJ, Yokoyama W, Daugherty A. Depletion of natural killer cell function decreases atherosclerosis in low-density lipoprotein receptor null mice. Arterioscler Thromb Vasc Biol. 2004;24:1049–1054. doi: 10.1161/01.ATV.0000124923.95545.2c. [DOI] [PubMed] [Google Scholar]
- 11.Zhou X, Stemme S, Hansson GK. Evidence for a local immune response in atherosclerosis. CD4+ T cells infiltrate lesions of apolipoprotein-E-deficient mice. Am J Pathol. 1996;149:359–366. [PMC free article] [PubMed] [Google Scholar]
- 12.Huber SA, Sakkinen P, David C, Newell MK, Tracy RP. T helper-cell phenotype regulates atherosclerosis in mice under conditions of mild hypercholesterolemia. Circulation. 2001;103:2610–2616. doi: 10.1161/01.cir.103.21.2610. [DOI] [PubMed] [Google Scholar]
- 13.Zhou X, Robertson AK, Hjerpe C, Hansson GK. Adoptive transfer of CD4+ T cells reactive to modified low-density lipoprotein aggravates atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:864–870. doi: 10.1161/01.ATV.0000206122.61591.ff. [DOI] [PubMed] [Google Scholar]
- 14.Gotsman I, Grabie N, Gupta R, Dacosta R, MacConmara M, Lederer J, et al. Impaired regulatory T-cell response and enhanced atherosclerosis in the absence of inducible costimulatory molecule. Circulation. 2006;114:2047–2055. doi: 10.1161/CIRCULATIONAHA.106.633263. [DOI] [PubMed] [Google Scholar]
- 15.Laurat E, Poirier B, Tupin E, Caligiuri G, Hansson GK, Bariety J, et al. In vivo downregulation of T helper cell 1 immune responses reduces atherogenesis in apolipoprotein E-knockout mice. Circulation. 2001;104:197–202. doi: 10.1161/01.cir.104.2.197. [DOI] [PubMed] [Google Scholar]
- 16.Binder CJ, Horkko S, Dewan A, Chang MK, Kieu EP, Goodyear CS, et al. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med. 2003;9:736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 17.Hartvigsen K, Chou MY, Hansen LF, Shaw PX, Tsimikas S, Binder CJ, et al. The role of innate immunity in atherogenesis. J Lipid Res. 2009;50:s388–393. doi: 10.1194/jlr.R800100-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Binder CJ, Hartvigsen K, Chang MK, Miller M, Broide D, Palinski W, et al. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J Clin Invest. 2004;114:427–437. doi: 10.1172/JCI20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mironova M, Virella G, Lopes-Virella MF. Isolation and characterization of human antioxidized LDL autoantibodies. Arterioscler Thromb Vasc Biol. 1996;6:222–229. doi: 10.1161/01.atv.16.2.222. [DOI] [PubMed] [Google Scholar]
- 20.Palinski W, Miller E, Witztum JL. Immunization of low density lipoprotein (LDL) receptor-deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc Natl Acad Sci USA. 1995;92:821–825. doi: 10.1073/pnas.92.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palinski W, Ord VA, Plump AS, Breslow JL, Steinberg D, Witztum JL. ApoE-deficient mice are a model of lipoprotein oxidation in atherogenesis. Demonstration of oxidation-specific epitopes in lesions and high titers of autoantibodies to malondialdehyde-lysine in serum. Arterioscler Thromb. 1994;14:605–616. doi: 10.1161/01.atv.14.4.605. [DOI] [PubMed] [Google Scholar]
- 22.Yla-Herttuala S, Palinski W, Butler SW, Picard S, Steinberg D, Witztum JL. Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler Thromb. 1994;14:32–40. doi: 10.1161/01.atv.14.1.32. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez-Vargas P, Ortiz-Munoz G, Lopez-Franco O, Suzuki Y, Gallego-Delgado J, Sanjuan G, et al. Fcgamma receptor deficiency confers protection against atherosclerosis in apolipoprotein E knockout mice. Circ Res. 2006;99:188–1196. doi: 10.1161/01.RES.0000250556.07796.6c. [DOI] [PubMed] [Google Scholar]
- 24.Poole A, Gibbins JM, Turner M, van Vugt MJ, van de Winkel JG, Saito T, et al. The Fc receptor gamma-chain and the tyrosine kinase Syk are essential for activation of mouse platelets by collagen. EMBO J. 1997;16:2333–2341. doi: 10.1093/emboj/16.9.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qian D, Sperling AI, Lancki DW, Tatsumi Y, Barrett TA, Bluestone JA, et al. The gamma chain of the high-affinity receptor for IgE is a major functional subunit of the T-cell antigen receptor complex in gamma delta T lymphocytes. Proc Natl Acad Sci USA. 1993;90:11875–11879. doi: 10.1073/pnas.90.24.11875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodewald HR, Arulanandam AR, Koyasu S, Reinherz EL. The high affinity Fc epsilon receptor gamma subunit (Fc epsilon RI gamma) facilitates T cell receptor expression and antigen/major histocompatibility complex-driven signaling in the absence of CD3 zeta and CD3 eta. J Biol Chem. 1991;266:15974–15978. [PubMed] [Google Scholar]
- 27.Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 28.Hirano M, Davis RS, Fine WD, Nakamura S, Shimizu K, Yagi H, et al. IgEb immune complexes activate macrophages through FcgammaRIV binding. Nat Immunol. 2007;8:762–771. doi: 10.1038/ni1477. [DOI] [PubMed] [Google Scholar]
- 29.Hulett MD, Hogarth PM. Molecular basis of Fc receptor function. Adv Immunol. 1994;57:1–127. doi: 10.1016/s0065-2776(08)60671-9. [DOI] [PubMed] [Google Scholar]
- 30.Hazenbos WL, Heijnen IA, Meyer D, Hofhuis FM, Renardel de Lavalette CR, Schmidt RE, et al. Murine IgG1 complexes trigger immune effector functions predominantly via Fc gamma RIII (CD16) J Immunol. 1998;161:3026–3032. [PubMed] [Google Scholar]
- 31.Bandukwala HS, Clay BS, Tong J, Mody PD, Cannon JL, Shilling RA, et al. Signaling through Fc{gamma}RIII is required for optimal T helper type (Th)2 responses and Th2-mediated airway inflammation. J Exp Med. 2007;284:1875–1889. doi: 10.1084/jem.20061134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hazenbos WL, Gessner JE, Hofhuis FM, Kuipers H, Meyer D, Heijnen IA, et al. Impaired IgG-dependent anaphylaxis and Arthus reaction in FcgRIII (CD16) deficient mice. Immunity. 1996;5:181–188. doi: 10.1016/s1074-7613(00)80494-x. [DOI] [PubMed] [Google Scholar]
- 33.Lynch RG, Hagen M, Mueller A, Sandor M. Potential role of Fc gamma R in early development of murine lymphoid cells: evidence for functional interaction between Fc gamma R on pre-thymocytes and an alternative, non-Ig ligand on thymic stromal cells. Immunol Lett. 1995;44:105–109. doi: 10.1016/0165-2478(95)00200-o. [DOI] [PubMed] [Google Scholar]
- 34.Sandor M, Galon J, Takacs L, Tatsumi Y, Mueller AL, Sautes C, et al. An alternative Fc gamma-receptor ligand: potential role in T-cell development. Proc Natl Acad Sci USA. 1994;91:12857–12861. doi: 10.1073/pnas.91.26.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plump AS, Masucci-Magoulas L, Bruce C, Bisgaier CL, Breslow JL, Tall AR. Increased atherosclerosis in ApoE and LDL receptor gene knock-out mice as a result of human cholesteryl ester transfer protein transgene expression. Arterioscler Thromb Vasc Biol. 1999;19:1105–1110. doi: 10.1161/01.atv.19.4.1105. [DOI] [PubMed] [Google Scholar]
- 36.Bird DA, Gillotte KL, Horkko S, Friedman P, Dennis EA, Witztum JL, et al. Receptors for oxidized low-density lipoprotein on elicited mouse peritoneal macrophages can recognize both the modified lipid moieties and the modified protein moieties: implications with respect to macrophage recognition of apoptotic cells. Proc Natl Acad Sci USA. 1999;96:6347–6352. doi: 10.1073/pnas.96.11.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moos MP, John N, Grabner R, Nossmann S, Gunther B, Vollandt R, et al. The lamina adventitia is the major site of immune cell accumulation in standard chow-fed apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:2386–2391. doi: 10.1161/01.ATV.0000187470.31662.fe. [DOI] [PubMed] [Google Scholar]
- 38.Uyemura K, Demer LL, Castle SC, Jullien D, Berliner JA, Gately MK, et al. Cross-regulatory roles of interleukin (IL)-12 and IL-10 in atherosclerosis. J Clin Invest. 1996;97:2130–2138. doi: 10.1172/JCI118650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinderski Oslund LJ, Hedrick CC, Olvera T, Hagenbaugh A, Territo M, Berliner JA, et al. Interleukin-10 blocks atherosclerotic events in vitro and in vivo. Arterioscler Thromb Vasc Biol. 1999;19:2847–2863. doi: 10.1161/01.atv.19.12.2847. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Li D, Chen J, Xie J, Bandyopadhyay S, Zhang D, et al. Inhibition of atherogenesis in LDLR knockout mice by systemic delivery of adeno-associated virus type 2-hIL-10. Atherosclerosis. 2006;188:19–27. doi: 10.1016/j.atherosclerosis.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 41.Pinderski LJ, Fischbein MP, Subbanagounder G, Fishbein MC, Kubo N, Cheroutre H, et al. Overexpression of interleukin-10 by activated T lymphocytes inhibits atherosclerosis in LDL receptor-deficient mice by altering lymphocyte and macrophage phenotypes. Circ Res. 2002;90:1064–1071. doi: 10.1161/01.res.0000018941.10726.fa. [DOI] [PubMed] [Google Scholar]
- 42.Mallat Z, Besnard S, Duriez M, Deleuze V, Emmanuel F, Bureau MF, et al. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999;85:e17–e24. doi: 10.1161/01.res.85.8.e17. [DOI] [PubMed] [Google Scholar]
- 43.Caligiuri G, Rudling M, Ollivier V, Jacob MP, Michel JB, Hansson GK, et al. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol Med. 2003;9:10–17. [PMC free article] [PubMed] [Google Scholar]
- 44.Flamand V, Shores EW, Tran T, Huang K, Lee E, Grinberg A, et al. Delayed maturation of CD4- CD8- Fc gamma RII/III+ T and natural killer cell precursors in Fc epsilon RI gamma transgenic mice. J Exp Med. 1996;184:1725–1735. doi: 10.1084/jem.184.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinheiro da Silva F, Aloulou M, Skurnik D, Benhamou M, Andremont A, Velasco IT, et al. CD16 promotes Escherichia coli sepsis through an FcR gamma inhibitory pathway that prevents phagocytosis and facilitates inflammation. Nat Med. 2007;13:1368–1374. doi: 10.1038/nm1665. [DOI] [PubMed] [Google Scholar]
- 46.Gupta S, Pablo AM, Jiang X, Wang N, Tall AR, Schindler C. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J Clin Invest. 1997;99:2752–2761. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tellides G, Tereb DA, Kirkiles-Smith NC, Kim RW, Wilson JH, Schechner JS, et al. Interferon-gamma elicits arteriosclerosis in the absence of leukocytes. Nature. 2000;403:207–211. doi: 10.1038/35003221. [DOI] [PubMed] [Google Scholar]
- 48.Whitman SC, Ravisankar P, Daugherty A. IFN-gamma deficiency exerts gender-specific effects on atherogenesis in apolipoprotein E-/- mice. J Interferon Cytokine Res. 2002;22:661–670. doi: 10.1089/10799900260100141. [DOI] [PubMed] [Google Scholar]
- 49.Whitman SC, Ravisankar P, Elam H, Daugherty A. Exogenous interferon-gamma enhances atherosclerosis in apolipoprotein E-/- mice. Am J Pathol. 2000;157:1819–1824. doi: 10.1016/s0002-9440(10)64820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buono C, Come CE, Stavrakis G, Maguire GF, Connelly PW, Lichtman AH. Influence of interferon-gamma on the extent and phenotype of diet-induced atherosclerosis in the LDLR-deficient mouse. Arterioscler Thromb Vasc Biol. 2003;23:454–460. doi: 10.1161/01.ATV.0000059419.11002.6E. [DOI] [PubMed] [Google Scholar]
- 51.Potteaux S, Esposito B, van Oostrom O, Brun V, Ardouin P, Groux H, et al. Leukocyte-derived interleukin 10 is required for protection against atherosclerosis in low-density lipoprotein receptor knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:1474–1478. doi: 10.1161/01.ATV.0000134378.86443.cd. [DOI] [PubMed] [Google Scholar]
- 52.Bocek P, Jr, Foucras G, Paul WE. Interferon gamma enhances both in vitro and in vivo priming of CD4+ T cells for IL-4 production. J Exp Med. 2004;199:1619–1630. doi: 10.1084/jem.20032014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.