Abstract
Preterm and young neonates have an increased predisposition to respiratory distress syndrome (RDS) associated with an immature development of lung surfactant. Glucocorticoids (GCs) are the major immunomodulatory agents used to increase lung development and reduce the mortality and morbidity of preterm infants with RDS. However, their safety remains uncertain, and the precise mechanisms by which they improve lung function are unclear. In previous studies, we found that vascular endothelial growth factor (VEGF) enhances the innate immune response by respiratory epithelial cells, causes a monocytic infiltration into the lung, and reduces the severity of infection by respiratory syncytial virus (RSV), a respiratory pathogen known to affect preterm infants at a high prevalence. The purpose of this study is to measure the effects of VEGF administration on local immune responses in neonatal lambs, as the ovine lung is well suited for comparison to the human lung, due to similarities in alveolar development, immune responses, and RSV susceptibility. We hypothesized that VEGF induces the expression of genes necessary for host immune responses. We analyzed global gene expression profiles in the lungs of neonate lambs treated with VEGF by real-time qPCR. We report that VEGF induced the expression of chemokines (IL-8, RANTES, MCP-1), cytokines (IFN-γ, IL-6, TNF-α, GMCSF), Toll-like receptor (TLR)-4, complement family members (C3, CFB, CFH) and collectins (SP-A, SP-D). These results suggest that VEGF can regulate local immune gene expression in vivo and should be further explored as a potential exogenous therapy for various lung diseases.
Keywords: Preterm infant, Neonate, Lung, Vascular endothelial growth factor, Immune response, Respiratory distress syndrome, Macrophage, Epithelial cell
1. Introduction
Respiratory distress syndrome (RDS) is a respiratory condition affecting 1% of newborn infants and is a significant cause of preterm infant mortality in the US [1,2]. The development of RDS is attributed to the developmental immaturity [3] and to the insufficiency of surfactant production of the neonatal lung. The incidence of RDS disease increases with low birth weight [4] but decreases with advanced gestational age [5]. An immature immune system, low birth weight, lung and airway immaturity, and impaired lung epithelial cell regeneration of neonates with RDS are all factors that may contribute to increased disease susceptibility to respiratory pathogens such as respiratory syncytial virus (RSV).
The study of human fetal-neonatal development requires the use of animal models with similar gestation to humans [6]. Although humans are phylogenetically closer to rodents than sheep [7], the ovine perinatal lung has more similarity to the human lung. In contrast to rodents, alveolar development in humans and lambs occurs prenatally [6,8]. Alveoli, distal bronchioles and respiratory epithelia are also similar in morphology and Clara cell numbers in humans and lambs, whereas mouse bronchioles have a large population of Clara cells. Also, human and ovine airways possess submucosal glands, which are not present in rodents and are critical for the innate immune response through the production of lactoperoxidase (LPO) and the NADPH oxidases (duox1 and duox2) [9–11].
Infants suffering from RDS often require mechanical ventilation and receive prophylactic and surfactant replacement therapy to reduce complications. Although surfactant and corticosteroids have decreased the mortality associated with respiratory problems in newborn infants [12,13], it has been suggested that both ventilation and treatment with surfactant can be risk factors for bronchopulmonary dysplasia (BPD) [14], while corticosteroids are associated with other adverse effects.
Glucocorticoids (GCs) are frequently used as prophylactic treatments to enhance the maturation of lungs in infants and minimize the adverse effects of preterm birth [12]. However, while repeated doses of prenatal betamethasone benefit lung function in animals, it also has adverse effects on neuronal myelination [15], hypothalamic–pituitary–adrenal function [16], and fetal growth [17]. In human infants, repeated corticosteroid administration was associated with neuronal [18] and developmental [19] problems as well as an increased risk for neonatal infection [19]. Also, the precise mechanisms by which GCs enhance lung function are unknown. As the efficacy and safety of GCs remain uncertain, the search for novel, safer surfactant replacement therapies is a priority.
Recent efforts in finding new therapeutic agents for lung maturation have pointed at vascular endothelial growth factor (VEGF) as a potential candidate. VEGF is an immunoregulator and a growth factor, responsible for mediating angiogenesis and vascular permeability [20,21]. VEGF is vital for lung development, and alterations in its expression are associated with chronic lung disease/bronchopulmonary dysplasia [22,23]. VEGF binds two receptors, VEGFR1 (also called Flt-1) and VEGFR2 (also called Flk-1 or KDR). VEGFR1 is induced by hypoxia and induces hematopoiesis, monocyte migration, recruitment of endothelial progenitors, and the induction of other growth factors. VEGFR2 induces endothelial cell proliferation, migration and survival. VEGF is a critical factor in embryonic and postnatal development of the vasculature, and recent evidence suggests that vascular development drives pulmonary alveolar development [22,24].
Previously, we have shown that although exogenous VEGF can induce leukocyte recruitment to the lung in a dose-dependent manner [25], in lower non-inflammatory doses, VEGF can reduce the severity of disease caused by RSV in the lungs of newborn lambs [26]. The precise mechanisms by which VEGF induces monocytic chemotaxis and inhibits viral replication are currently unknown. Because VEGF has been implicated in pivotal roles related to lung development, we reasoned that VEGF may enhance lung immunity through the induction of cytokines, chemokines and activation of growth factor pathways. We performed an in vivo quantitative global gene expression study of the effects of VEGF on genes involved in innate and adaptive immune responses central to RSV infection in the lungs of full-term lambs that did not have RDS. To support the role of VEGF in lung immune functions, we tested two hypotheses: (1) VEGF regulates numerous genes that play critical roles in innate and adaptive immune responses; and (2) VEGF is an immune-enhancing agent.
2. Materials and methods
2.1. Animals
Animal use and all experimental procedures were approved by Iowa State University’s Animal Care and Use Committee. The lambs in this study were the same animals used previously to characterize the bronchoscopic deposition of VEGF in the lungs of neonatal lambs [25]. The study was conducted in full-term lambs that did not have RDS. Briefly, healthy male and female neonatal lambs at 2–4 days of age (see [25]) were randomly assigned to three groups: a saline-inoculated group (n = 3), a VEGF-inoculated group (for low dose VEGF n = 1 at all time points; for medium dose VEGF n = 2 for 16 h, n = 3 for 24 h, n = 2 for 32 h; for high dose VEGF, n = 1 for all time points), and a bovine serum albumin (BSA)-inoculated group (n = 2). Lambs were sedated with xylazine (0.1 mg/kg) intravenously (i.v.), and a sterile bronchoscope was used to instill 10 ml of sterile saline, BSA, or recombinant human VEGF165 (0.05, 0.005, 0.0005 mg/ml; endotoxin <100 pg/μg in stock solution; diluted with sterile saline solution pH 7.0; Invitrogen, Carlsbad, CA) in the right mainstem bronchus. Human VEGF165 and ovine VEGF164 are 93.3 % identical at the amino acid level. Lambs were euthanized with sodium pentobarbital i.v. after 16, 24, or 32 h after inoculation. Lungs were removed, snap frozen in liquid nitrogen, and stored at −80 °C. Lung tissue from the inoculated side (right lung) was used for all experiments in this study.
2.2. RNA isolation
Total RNA was isolated from the inoculated right lung as follows: lungs were weighed and homogenized in 3 ml of TRIzol reagent (Invitrogen, Carslbad, CA) using an Omni TH homogenizer (Omni International, Marietta, GA). A portion of the homogenized lungs was further diluted in TRIzol to obtain a 0.0909 g of tissue/ml homogenate. The homogenate was vortexed, and nuclease-free chloroform was added to each sample, mixed, and spun at 12,000 × g for 10 min. The aqueous layers were transferred into microfuge tubes containing nuclease-free 2-propanol (Fisher Chemical, Fairlawn, NJ). The pellets obtained after centrifugation (12,000 × g for 10 min) were washed twice in ice-cold 75% nuclease-free ethanol, after which the supernatant was removed, and the pellets were allowed to air-dry. The pellets were resuspended in nuclease-free 0.1 mM EDTA, pH 7.0 and were heated at 65 °C for 5 min for better dissolution. RNA was diluted at 1:50, and concentrations and purity were determined by absorbance readings at 260 and 280 nm. The RNA samples were further subjected to DNase treatment using TURBO DNase (TURBO DNA-free kit, Ambion, Austin, TX). Eighty microliters of each RNA sample was recovered after DNase treatment and further diluted 1:10 with nuclease-free water (Ambion) and RNaseOUT (Invitrogen) for one-step qPCR use.
2.3. One-step real-time qPCR
Prior to final qPCR sample analyses, a test plate was run (using a mixture of RNA samples serially diluted from full-strength to 1:100,000 in-well) for each target of interest to identify (1) the specific RNA sample dilution range at which qPCR inhibition no longer existed; and (2) the extended dilution range of the standard curves within which all targets exhibited amplification efficiencies between 80 and 100%. Test plate set-up parameters and analysis were performed using PREXCEL-Q (P-Q) [27]. The threshold of sample-related inhibition was subsequently found to be 1:290 in-well, and all target standard curves were found to share the same optimal RNA dilution range of 1:400 to 1:8000 in-well. Five point standard curves were generated for each target in that range and the 1:10 RNA samples were diluted to their ideal concentration of 3.268 ng/μl that, when added to the final reaction mixtures for qPCR, arrives at the calculated ideal concentration for each RNA within the valid standard curve range for each target.
The optimally-diluted RNA samples were used as “RNA templates” in 20 μl fluorogenic one-step real-time qPCR reactions containing the RNA samples (ending up at 0.7844 ng total RNA/μl in all final qPCR reactions), 775 nM forward and reverse primers (see Table 1 for primer and probe sequences and [28]), 150 nM TaqMan probe (either 5′-6FAM, 3′-TAMRA-quenched or 5′-6FAM, 3′-MGBNFQ probes used), nuclease-free water, and the following reagents from the SuperScript™ III Platinum® One-Step quantitative RT-PCR System with ROX kit (Invitrogen, Carlsbad, CA): 5.5 mM MgSO4, SuperScript III RT/Platinum Taq mix, and one-step qPCR SuperMix containing 1 μM ROX. RNA samples, negative no-template controls, and standards were placed in duplicate wells of a 96-well qPCR reaction plate (Applied Biosystems, Forest City, CA) and were run in the GeneAmp 5700 Sequence Detection System (Applied Biosystems) for detection and relative quantification of targets at 15 min 55 °C, 2 min 95 °C, and 50 cycles of 15 s 95 °C, 30 s 58 °C.
Table 1.
List of primers/probes used for real-time qPCR.
| Primer name | Sequence (5′–3′) | GenBank Accession number or source |
|---|---|---|
| IL-8 forward | TTCCAAGCTGGCTGTTGCT | X78306 |
| IL-8 reverse | TTGACAGAACTGCAGCTTCACA | |
| IL-8 probe | FAM-CCGCTTTCCTGCTCTCTGCAGCTC-TAMRA | |
| RANTES forward | TGCTTCTGCCTCCCCATATG | Based on alignment of conserved sequences between human (NM_002985) and bovine (NM_175827) RANTES |
| RANTES reverse | GGGCGGGAGATATAGGCAAA | |
| RANTES probe | FAM-CACCACGCCCTGCT-MGBNFQ | |
| MCP-1 forward | GCTGTGATTTTCAAGACCATCCT | Based on conserved sequences reported in [28] |
| MCP-1 reverse | GGCGTCCTGGACCCATTT | |
| MCP-1 probe | FAM-AAAGAGTTTTGTGCAGACCCCAACC-TAMRA | |
| IFN-γ forward | TGGAGGACTTCAAAAGGCTGAT | NM_001009803 |
| IFN-γ reverse | GATGGCTTTGCGCTGGAT | |
| IFN-γ probe | FAM-CAAATTCCGGTGGATGATCTGC-TAMRA | |
| IL-6 forward | GCTGCTCCTGGTGATGACTTC | NM_001009392 |
| IL-6 reverse | GGTGGTGTCATTTTTGAAATCTTCT | |
| IL-6 probe | FAM-CTTTCCCTACCCCGGGTCCCCTG-MBGNFQ | |
| TNF-α forward | CAACCTGGGACACCCAGAAT | NM_001024860 |
| TNF-α reverse | TCTCAAGGAACGTTGCGAAGT | |
| TNF-α probe | FAM-CAAGGGCCAGGGTTCTTACCGGAA-TAMRA | |
| IFN-β forward | TGGTTCTCCTGCTGTGTTTCTC | NM_174350 |
| IFN-β reverse | CGTTGTTGGAATCGAAGCAA | |
| IFN-β probe | FAM-ACCACAGCTCTTTCCAGGAGCTACA-TAMRA | |
| GMCSF forward | CCCTGGCAGCTTGTGGAT | EU287484 |
| GMCSF reverse | GTGTCAGTGCTGTCGTTCAGAAG | |
| GMCSF probe | FAM-CCATCAAGGAGGCCCT-MGBNFQ | |
| C3 forward | GGACACCAGGGAGAAAGATGTG | AF038130 |
| C3 reverse | CGTGCAGCGCGATCAG | |
| C3 probe | FAM-CCCTTACAGCCTTTG-MGBNFQ | |
| CFB forward | GGCCCCCTGATTATTCACAA | EF446375 |
| CFB reverse | CAGCTGATCACGCCAACTTG | |
| CFB probe | FAM-AGGAGCCGCTTCAT-MGBNFQ | |
| CFH forward | CGGTGTCAGGCCTACTATGAACT | EU888587 |
| CFH reverse | GGTTCTGACCACTCTCCATTCC | |
| CFH probe | FAM-AGGGAAACAGAAACGTAGTAT-MGBNFQ | |
| TLR4 forward | CCATCGCCGCCAATATCA | DQ922636 |
| TLR4 reverse | TGGGACACCACGACAATAACC | |
| TLR4 probe | FAM-CCAGGAGGGTTTCCACAAAAGCCGT-TAMRA | |
| TGF-β forward | TGT GTT CGT CAG CTC TAC ATT GAC | NM_001009400 |
| TGF-β reverse | TAG CCC TTG GGT TCG TGA AT | |
| TGF-β probe | FAM-TCC AGC CCA GGT CCT TCC GGA-TAMRA | |
| IL-10 forward | GTCGGAAATGATCCAGTTTTACCT | Z29362 |
| IL-10 reverse | GTCAGGCCCATGGTTCTCA | |
| IL-10 probe | FAM-AGGAGGTGATGCCACAGG-MGBNFQ | |
| SP-A forward | TGACCCTTATGCTCCTCTGGAT | AF076633 |
| SP-A reverse | GGGCTTCCAAGACAAACTTCCT | |
| SP-A probe | FAM-TGGCTTCTGGCCTCGAGTGCG-TAMRA | |
| SP-D forward | ACGTTCTGCAGCTGAGAAT | AJ133002 |
| SP-D reverse | TCGGTCATGCTCAGGAAAGC | |
| SP-D probe | FAM-TTGACTCAGCTGGCCACAGCCCAGAACA-TAMRA | |
| SAA forward | GGGACGAGGTACGGAAGGAT | EU366476 |
| SAA reverse | TCCGGCCCCATTCGTT | |
| SAA probe | FAM-CGAAGGCCGACCAGT-MGBNFQ | |
| SMAP29 forward | GGCCCAACTGTTCTCCGAAT | L46854 |
| SMAP29 reverse | GCAGACCCTTAGGACTCTTTCCT | |
| SMAP29 probe | FAM-ATCAGAATAGCTGGGTGAATTGTGGGCC-TAMRA | |
| Duox1 forward | AGCCGGGCTGAGTTTGC | XM_587550 |
| Duox1 reverse | AACATGGACTGCACAAACATGTC | |
| Duox1 probe | FAM-AGTCCCTGGGCCTCAAGCCCC-TAMRA | |
| Duox2 forward | GCCCCAGGACATGTTTGTG | Based on conserved sequences between human (NM_014080) and sus scrofa (NM_213999) at the codon level |
| Duox2 reverse | GCCATTGCCATCCTTGTCA | |
| Duox2 probe | FAM-AGTCCATGTTCTCTCTG-MGBNFQ | |
| LPO forward | AAGCAGCGGGACTCTCTACAGA | NM_001009722 |
| LPO reverse | GACCTTCGTGATGTGGGTGTT | |
| LPO probe | FAM-CCTTCTCGCGCCTCA-MGBNFQ |
The resulting Ct values were gathered at an appropriate threshold for each different target using the GeneAmp 5700 SDS software, and relative quantitative analysis was performed using our own custom Excel files. Relative mRNA expression quantity for each qPCR target was calculated using the equation: relative quantity = 10(Ct−b)/m (or ). Final relative values normalized to control values were calculated using the efficiency-corrected comparative quantitative method ( method), which is identical to the 2−ΔCt or 2ΔCt (depending on the directionality of Ct subtraction) comparative quantitative method when target amplification efficiencies are assumed to be ~100% [29]. Relative quantity values for each target were thus calculated from each sample’s target mRNA Ct (generated at 0.7844 ng/μl in-well total RNA concentration) using the slope (m) and y-intercept (b) of the line generated for each target’s standard curve. qPCR amplification efficiency (E) and exponential amplification (EAMP) values were determined from the slope of the line (which is a plot of Ct versus log10 of 1/dilution factor). qPCR values E and EAMP were determined from the slope of the line using the relationships: E = 10−1/m − 1 and EAMP = E + 1 (or 10 −1/m), respectively. The slope of the line can also be expressed as: m = −1/log10(EAMP). Using the efficiency-corrected comparative quantitative expression: , averaged individual (relative) target values were normalized to their averaged group control (relative) target values. It is worth mentioning here that P-Q is a new Excel-based software program [27], which provides a careful approach to qPCR, and which has recently been endorsed by Dr. Stephen A. Bustin, an acknowledged leader in use of qPCR theory and methodology.
2.4. Statistical analysis
The results were analyzed by one-way ANOVA using SAS version 9.1 (SAS Institute, Cary, NC). Mean values of relative mRNA expression levels of VEGF-treated animals, saline-treated animals and BSA-treated animals were compared at *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001 and expressed as means ± SEM.
3. Results
3.1. Effect of VEGF on chemokine expression in the newborn lung
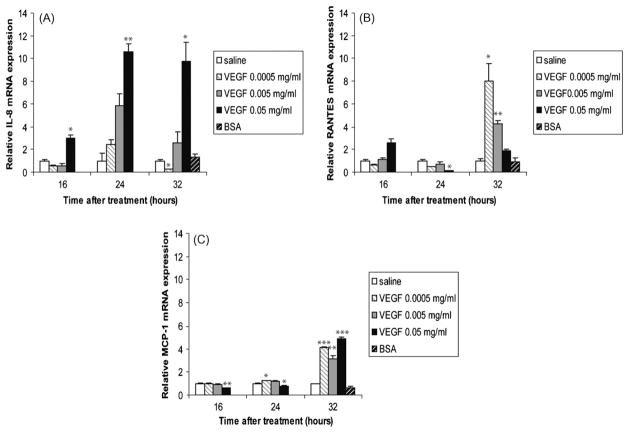
To address the effect of VEGF on local lung immunity, we first sought to investigate the effect of VEGF on the mRNA expression levels of chemokines. Fig. 1 shows that VEGF mediates the induction of IL-8, RANTES and MCP-1. In contrast to saline-treated animals, animals treated with the high dose VEGF (0.05 mg/ml) had an impressive increase in IL-8 mRNA at all time points studied (16, 24, and 32 h; Fig. 1A). RANTES mRNA showed a trend towards increased expression at 16 h, but was significantly decreased at 24 h with the high dose VEGF (Fig. 1B). However, at 32 h, the levels increased rapidly again, with an 8.02-fold increase in mRNA level seen in animals treated with the low dose VEGF (0.0005 mg/ml) and a 4.24-fold increase in animals treated with the medium dose VEGF (0.005 mg/ml). Because monocytes and macrophages were the main cells recruited to the lung following VEGF administration, we next looked at the expression levels of MCP-1, a chemotactic factor for these cells. The highest dose of VEGF decreased MCP-1 levels at 16 and 24 h (Fig. 1C). However, MCP-1 levels were higher than control animals at 32 h after treatment with all doses of VEGF, indicating that any dose of VEGF was sufficient to induce MCP-1 expression in the lung of treated animals. The expression level of the co-stimulatory molecule ICAM-1 was also measured but was not significantly affected by VEGF (data not shown). Animals treated with BSA had no statistically significant changes in gene expression at 32 h relative to saline-treated animals. Thus, VEGF is a potent stimulator of chemokines in the neonatal ovine lung.
Fig. 1.
VEGF induces chemokines gene expression. IL-8 (A), RANTES (B) and MCP-1 (C) mRNA levels in homogenized lungs of full-term lambs were determined by real-time qPCR. The chemokines mRNA expression levels were expressed as fold induction relative to saline-treated lambs. The data represent the mean ± SEM (n = 3 for saline; for low dose VEGF n = 1 at all time points; for medium dose VEGF n = 2 for 16 h, n = 3 for 24 h, n = 2 for 32 h; for high dose VEGF, n = 1 for all time points; for BSA, n = 2). *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001 by ANOVA when compared to saline-treated animals.
3.2. Effect of VEGF on inflammatory mediators in the lung
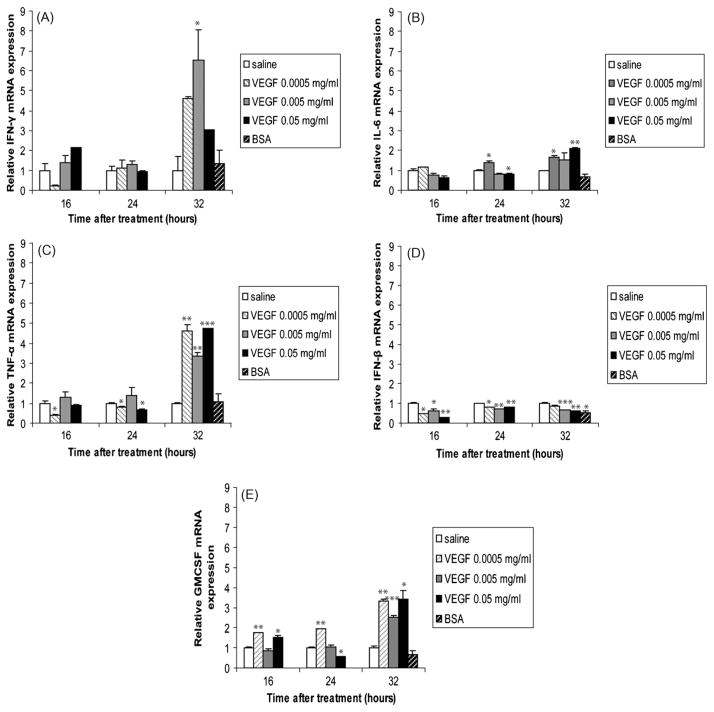
We next investigated the effects of VEGF administration on the expression levels of pro-inflammatory cytokines/mediators in the lung. Similarly to MCP-1 (Fig. 1C), IFN-γ mRNA levels were unaffected by VEGF at 16 and 24 h after treatment (Fig. 2A). However, in animals treated for 32 h with VEGF, IFN-γ levels increased significantly compared to saline-treated animals. The lowest dose of VEGF induced a 4.61-fold increase in IFN-γ mRNA. IL-6 is also a marker of a pro-inflammatory response. IL-6 mRNA levels were mostly increased in animals treated with VEGF for 32 h (Fig. 2B). Increases in TNF-α mRNA levels also coincided with the accumulation of macrophages to the lungs, at 32 h after stimulation with VEGF (Fig. 2C). The lowest dose of VEGF was sufficient to significantly induce TNF-α at this time point. Interestingly, TNF-α was significantly decreased by VEGF treatment at 16 and 24 h. As GCs were shown to inhibit the expression of many inflammatory molecules [30], including IFN-β and GMCSF in a human epithelial cell line [31], we also sought to examine the effects of VEGF on these inflammatory mediators. We found that VEGF significantly decreased the expression of IFN-β regardless of the dose or time (Fig. 2D), but not that of GMCSF (Fig. 2E). Animals treated with BSA had no statistically significant alterations in gene expression, expect when IFN-β was assessed, possibly due to animal to animal variations. Thus, the administration of VEGF in the lung environment has differential effects on the expression levels of inflammatory molecules.
Fig. 2.
Pro-inflammatory mediators are induced by VEGF. IFN-γ (A), IL-6 (B), TNF-α (C), IFN-β (D), and GMCSF (E) mRNA levels in homogenized lungs of full-term lambs were determined by real-time qPCR. The mRNA expression levels were expressed as fold induction relative to saline-treated lambs. The data represent the mean ± SEM (n = 3 for saline; for low dose VEGF n = 1 at all time points; for medium dose VEGF n = 2 for 16 h, n = 3 for 24 h, n = 2 for 32 h; for high dose VEGF, n = 1 for all time points; for BSA, n = 2). *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001 by ANOVA when compared to saline-treated animals.
3.3. Role of VEGF in the expression levels of complement factors
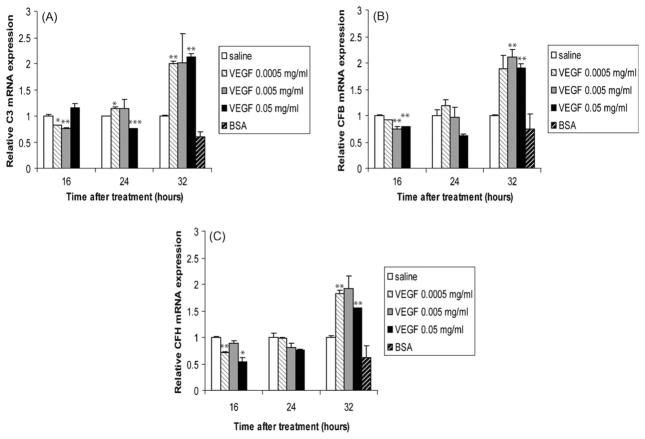
The activation of the complement system forms the first line of defense against invading pathogens and causes the release of complement factors. Previous studies have reported the ability of human bronchial epithelium [32], type II alveolar cells [33] and lung fibroblasts [34] to generate complement. We observed that in the lung, C3 (Fig. 3A), CFB (Fig. 3B), and CFH (Fig. 3C) were decreased by VEGF treatment during the early time points (16 and 24 h), but were all induced at 32 h in VEGF-treated animals, regardless of the dose used.
Fig. 3.
Complement is also induced in the lung of VEGF-treated lambs. C3 (A), CFB (B), and CFH (C) mRNA levels in homogenized lungs of full-term lambs were determined by real-time qPCR. The mRNA expression levels were expressed as fold induction relative to saline-treated lambs. The data represent the mean ± SEM (n = 3 for saline; for low dose VEGF n = 1 at all time points; for medium dose VEGF n = 2 for 16 h, n = 3 for 24 h, n = 2 for 32 h; for high dose VEGF, n = 1 for all time points; for BSA, n = 2). *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001 by ANOVA when compared to saline-treated animals.
3.4. VEGF induces the expression levels of TLR4
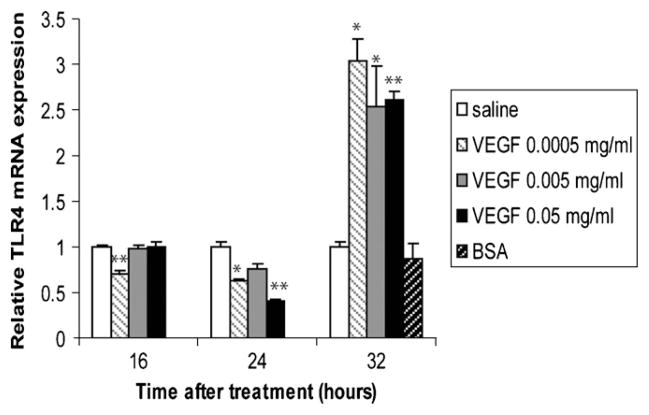
An invasion of microorganisms can be sensed in mammals by TLR family members, culminating in the activation of a range of host defense mechanisms. We found that TLR4 was inhibited in animals treated with VEGF for 16 and 24 h, but that it was significantly increased at 32 h with all doses of VEGF (Fig. 4).
Fig. 4.
Effects of VEGF treatment on TLR expression. TLR4 mRNA levels in homogenized lungs of full-term lambs were determined by real-time qPCR. The mRNA expression levels were expressed as fold induction relative to saline-treated lambs. The data represent the mean ± SEM (n = 3 for saline; for low dose VEGF n = 1 at all time points; for medium dose VEGF n = 2 for 16 h, n = 3 for 24 h, n = 2 for 32 h; for high dose VEGF, n = 1 for all time points; for BSA, n = 2). *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001 by ANOVA when compared to saline-treated animals.
3.5. The expression of anti-inflammatory cytokines is delayed in the lung
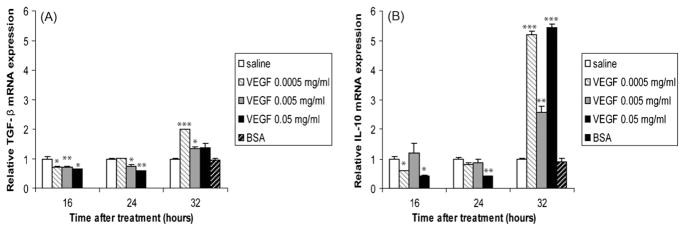
The net effect of an inflammatory response is determined by the balance between pro-inflammatory cytokines and anti-inflammatory cytokines. Cytokines such as TGF-β and IL-10 suppress the production of inflammatory mediators. We found that the expression of these cytokines was significantly inhibited by VEGF treatment at 16 and 24 h, but were significantly up-regulated by VEGF at 32 h (Fig. 5A and B).
Fig. 5.
Anti-inflammatory cytokines are also up-regulated by VEGF treatment. TGF-β (A) and IL-10 (B) mRNA levels mRNA levels in homogenized lungs of full-term lambs were determined by real-time qPCR. The mRNA expression levels were expressed as fold induction relative to saline-treated lambs. The data represent the mean ± SEM (n = 3 for saline; for low dose VEGF n = 1 at all time points; for medium dose VEGF n = 2 for 16 h, n = 3 for 24 h, n = 2 for 32 h; for high dose VEGF, n = 1 for all time points; for BSA, n = 2). *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001 by ANOVA when compared to saline-treated animals.
3.6. Collectins, the acute-phase reactant SAA, and the antimicrobial protein SMAP29 are differentially regulated by VEGF in the lung
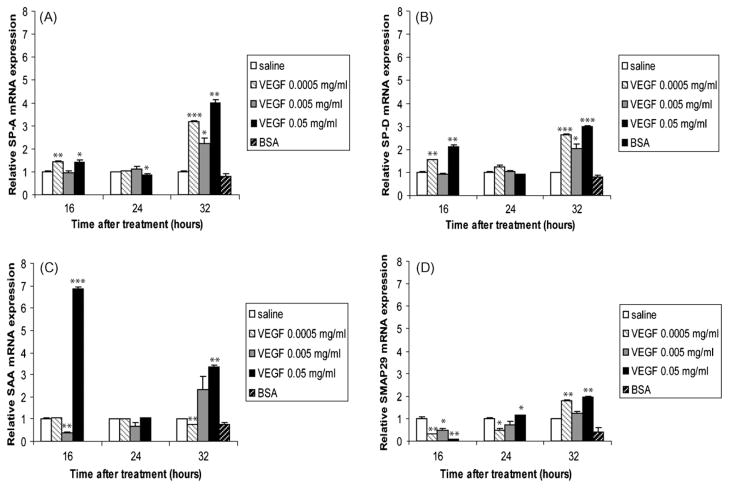
SP-A and SP-D are members of the collectin family of collagenous proteins with lectin activity and are produced by respiratory epithelia [35]. These lectins can enhance the clearance of respiratory pathogens such as RSV [36], and are able to activate macrophage functions [37,38]. We found that VEGF significantly enhanced SP-A (Fig. 6A) and SP-D (Fig. 6B) expression at 16 and 32 h after treatment. We also looked at the effects of VEGF administration on the expression levels of the acute-phase reactant SAA and on the antimicrobial protein SMAP29. Similarly to GCs [31], VEGF also enhanced the expression of SAA, with an impressive 6.84-fold increase in animals treated with the high dose VEGF for 16 h (Fig. 6C). SMAP29, an antimicrobial peptide belonging to the cathelicidin family, has documented biological activities against respiratory pathogens [39]. Compared to saline-treated animals, SMAP29 mRNA was decreased in animals treated with VEGF at 16 and 24 h. However, at 32 h, VEGF enhanced the levels of SMAP29 mRNA (Fig. 6D).
Fig. 6.
Collectins and the host defense molecules SAA and SMAP29 are induced by VEGF. SP-A (A), SP-D (B), SAA (C) and SMAP29 (D) mRNA levels in homogenized lungs of full-term lambs were determined by real-time qPCR. The mRNA expression levels were expressed as fold induction relative to saline-treated lambs. The data represent the mean ± SEM (n = 3 for saline; for low dose VEGF n = 1 at all time points; for medium dose VEGF n = 2 for 16 h, n = 3 for 24 h, n = 2 for 32 h; for high dose VEGF, n = 1 for all time points; for BSA, n = 2). *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001 by ANOVA when compared to saline-treated animals.
3.7. Effect of VEGF on the oxidative responses of lung epithelial cells
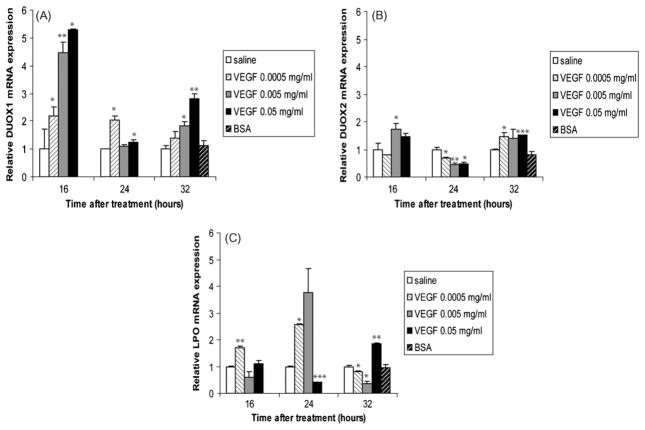
The airway epithelial proteins dual oxidase (duox)1 and 2 and the submucosal gland-produced protein lactoperoxidase (LPO) are important members of a recently described oxidative pulmonary epithelial host defense system against viruses and bacteria [9–11]. We investigated the effects of VEGF on these genes and found that duox1 mRNA was induced by VEGF in a dose-dependent manner at 16 and 32 h (Fig. 7A). Interestingly, the mRNA levels of duox1 were higher at 16 h than at 24 or 32 h. The expression profile of duox2 in response to VEGF was slightly different from that of duox1: duox2 mRNA levels were significantly decreased by all doses of VEGF at 24 h. Furthermore, although duox2 mRNA was increased in animals treated with VEGF at 16 and 32 h, the level of increase was much lower than that of duox1 at the same time points (Fig. 7B). Animals subjected to the low dose VEGF showed increased LPO mRNA expression at 16 and 24 h, but not at 32 h. LPO mRNA in animals given the medium dose of VEGF was undistinguishable from saline-treated animals at 16 and 24 h, but was significantly decreased at 32 h. Animals subjected to the high dose VEGF for 16 h showed no difference in LPO mRNA relative to saline-treated animals, while those treated for 24 h with the same dose VEGF showed significantly decreased LPO mRNA. Finally, those treated for 32 h showed a significantly increased LPO mRNA (Fig. 7C).
Fig. 7.
Effects of VEGF on epithelial cells oxidative responses. duox1 (A), duox2 (B), and LPO (C) mRNA levels in homogenized lungs of full-term lambs were determined by real-time qPCR. The mRNA expression levels were expressed as fold induction relative to saline-treated lambs. The data represent the mean ± SEM (n = 3 for saline; for low dose VEGF n = 1 at all time points; for medium dose VEGF n = 2 for 16 h, n = 3 for 24 h, n = 2 for 32 h; for high dose VEGF, n = 1 for all time points; for BSA, n = 2). *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001 by ANOVA when compared to saline-treated animals.
4. Discussion
RDS is a common and devastating disease affecting preterm infants and neonates at a high incidence [40]. Although symptoms of RDS commonly resolve completely after the acute phase, the disease may progress severely in some patients [41]. The search for novel agents that stimulate lung maturation during RDS has pointed at VEGF as a potential candidate [25,42] which has additional antiviral capabilities [26]. Thus, insights in the role and consequence of VEGF administration on the host immune response are critical.
In this study, we performed a global gene expression profile analysis in the neonatal ovine lung to quantitatively measure local in vivo immune responses due to VEGF administration. The key findings of our study are: (1) despite their immature immune system, newborn lambs express numerous host defense molecules, including chemokines, pro-inflammatory mediators, complement proteins, TLRs, anti-inflammatory molecules, antimicrobial proteins, surfactant proteins (SPs), and genes involved in the epithelial oxidative defense system; (2) administration of VEGF in the lung of these newborns can enhance the expression of some of those genes, depending on the dose and duration of VEGF treatment; and (3) VEGF has a complex mode of action: it can differentially regulate genes with similar functions, and it possesses some immnunosuppressive effects (enhanced TGF-β and IL-10 at 32 h, reduced IFN-β at all time points) along with its immunostimulatory functions. Our findings are partly in agreement with reports published on the enhancing effects of GCs on the expression of most epithelial cell genes involved in innate immunity, including TLRs [30,43], surfactant proteins [31,44], complement [31,45,46], and acute-phase proteins [31]. However, a fundamental difference between VEGF and GCs is the consistent inhibition of chemokines and inflammatory cytokines by GCs on airway epithelial cells in vivo and in vitro [31,47–50]. The reason for the differences can be attributed to the fact that GCs bind the glucocorticoid receptor and induce immunosuppression through inhibition of cytokine and chemokine gene expression and prostaglandin synthesis (reviewed in [51]). Although GCs are primarily used in preterm infants as inducers of surfactant synthesis and not as immunomodulatory agents, recent in vitro studies have focused on the effects of GCs on the expression of genes involved in the innate and adaptive immune responses in human peripheral blood mononuclear cells [30] and on epithelial cells [31]. In addition, recent in vivo studies have looked at the role of VEGF on lung injury [52] and maturation [52,53] in the mouse model. To our knowledge, our study is the first systematic attempt to examine the in vivo effects of VEGF on ovine neonatal lung immune responses.
The expression levels of chemokines, including IL-8, RANTES and MCP-1 mRNA were up-regulated in VEGF-treated animals compared to control lambs. IL-8 was immediately induced following the use of the high dose VEGF, suggesting that the up-regulation of IL-8 mRNA is a direct-effect of VEGF treatment. MCP-1 is a chemokine that can affect the immune response by recruiting monocytes and macrophages. MCP-1 however did not increase until 32 h after treatment. This late expression of MCP-1 is likely due to the increased accumulation of monocytes producing the chemokine, and thus would be an indirect (or secondary) effect of VEGF. RANTES followed a similar trend in expression as MCP-1. Although VEGF is inducing the expression of these chemokines, our study does not exclude the possibility that VEGF itself is acting as a chemotactic factor to recruit cells to the lung, as inflammatory cells such as monocytes and macrophages express VEGF receptor 1 [54,55].
Among the pro-inflammatory mediators studied, IFN-γ was the most induced cytokine at 32 h. The observed levels of IFN-γ suggest that (1) in addition to macrophages, T cells and/or NK cells are also recruited to the lung; and (2) VEGF can induce a strong pro-inflammatory response by activating the cells producing this cytokine. Our observation of elevated TNF-α, IL-6 and GMCSF levels at 32 h also suggests that these cytokines are produced during cellular infiltration and may not necessarily be a direct effect of VEGF. Although VEGF enhanced the expression of many pro-inflammatory mediators, we noticed that, similarly to GCs [30,31], the VEGF-mediated enhancement of the immune response was accompanied by the inhibition of the expression of the pro-inflammatory molecule IFN-β, regardless of the dose of VEGF or the time of treatment. VEGF also enhanced the levels of TGF-β and IL-10 mRNA levels (but only at 32 h), suggesting an attempt at balancing the effects of other pro-inflammatory cytokines.
C3, CFB and CFH are members of the complement family of proteins that are essential in immunity. Previous reports have shown that GCs can enhance the expression of C3 and CFB [46], as well as CFH [31,45]. Our results show that lambs treated with VEGF for 32 h had increased levels of these complement factors, further confirming that VEGF enhances immune responses after treatment.
TLRs are a class of single-membrane spanning receptors that play a key role in the innate immune system by recognizing specific pathogen associated molecular patterns (PAMPs) at the surface of invading pathogens and by activating host defense mechanisms. We show that similarly to GCs [30], VEGF can also induce the expression levels of TLR4. Recent articles have reported endotoxin effects on TLR4 [56] and on VEGF and VEGFR2 [57] expression. Those studies were performed using elevated amounts of endotoxin (10 mg). We do not attribute the changes in gene expression to endotoxin contamination, as the endotoxin content in the VEGF recombinant protein was very low (<100 pg/μg). Furthermore, the BSA used in control animals was not completely endotoxin-free and did not lead to significant changes in gene expression. Thus, the endotoxin-content of the VEGF recombinant protein has an independent effect on the mRNA expression of the genes studied.
Surfactant proteins are of particular relevance to RDS and to epithelial innate immune responses. These proteins are members of the collectin family, characterized by a collagen domain linked to a calcium-dependent lectin domain [58]. SP-A and SP-D play critical responses in the lung, including the prevention of alveolar collapse, and the use of opsonization and aggregation of pathogens such as RSV as a host defense mechanism. Brown et al. have shown that recombinant VEGF can increase the expression levels of SP-A mRNA in human fetal lung explants [59]. Other studies have shown that the glucocorticoid dexamethasone can induce SP-A in rat lung [44]. We observe that ovine SP-A and SP-D can also be induced in the lung following treatment with VEGF, and that this induction occurs rapidly (at 16 h), and that the low dose VEGF induces significant levels of SP-A and SP-D mRNA over saline-treated animals. This suggests that VEGF can act directly and immediately on epithelial cells to induce the expression of surfactant proteins. Our results are in accord with a recent report showing that in mice, VEGF induced surfactant production in the newborn and adult lungs, and alveolar development in the newborn lung [52]. Interestingly, there was also a positive correlation between VEGF levels in human amniotic fluid and surfactant phospholipids [52]. Studies performed in mice deficient in macrophage migration inhibitory factor (MIF), an upstream regulator of innate immune factors such as VEGF, have revealed deficiencies in the expression of VEGF and surfactant proteins [53].
In addition to collectins, SAA and SMAP29 have important roles in host defense. SAA is an acute-phase reactant that can be highly induced during the acute-phase response [60]. Of the 21 genes analyzed in this study, SAA was the most induced at 16 h following high dose VEGF treatment. Interestingly, at that same time point, the low dose VEGF had no effect on SAA levels, while the medium dose VEGF significantly inhibited SAA mRNA levels. The ovine cathelicidin SMAP29 showed a time-dependent increase in expression, with the highest levels observed at 32 h after stimulation. The low dose VEGF was sufficient to induce SMAP29 expression.
We next investigated the effects of VEGF on the NADPH oxidase isoforms duox1 and duox2, and on the submucosal gland-derived protein lactoperoxidase (LPO). Duox1 mRNA was rapidly induced by VEGF, and showed a dose-dependent increase at 16 h. Duox1 mRNA remained elevated (albeit at lower levels than at 16 h) at 24 and 32 h. Duox2 was induced at a lower level than duox1, and showed a different trend during the time course. The reasons behind the differences in mRNA levels between duox1 and duox2 are currently unknown, as the specific roles of these similar proteins are unclear. Interestingly, a recent study reported that although these two isoforms had 83% sequence similarities, the expression of duox2 mRNA in human fetal lung epithelial cells was lower than duox1 mRNA, even after treatment with a glucocorticoid mixture [61]. LPO mRNA was significantly enhanced by the low dose VEGF at 16 and 24 h, and by the high dose VEGF at 32 h. These studies suggest that VEGF may act directly on epithelial cells to activate the oxidative response in these cells.
The highest number of genes exhibiting increased mRNA levels was seen at 32 h post VEGF administration. This could be due to a cascading effect. VEGF receptors are present on several cell types in the lung, as are receptors for chemokines and cytokines that are triggered by VEGF. These chemokines and cytokines may trigger the expression of other genes. In addition, VEGF was administered intrabronchially in this study, and the inoculum delivered in this manner is distributed into some, but not all airways and alveoli. With time, there is likely some degree of VEGF absorption into the pulmonary lymphatic and alveolar capillaries, resulting in hematogenous distribution more uniformly to the lung parenchyma. Such distribution may increase the number of cells activated by VEGF and increase overall gene expression. Intrabronchial delivery was used in this study in order to mimick administration of VEGF via inhaler or nebulization and to avoid degradation in the blood. In future studies, the effects of VEGF administered intravenously will be tested. A limitation of the study is the lack of protein detection because specific antibodies for most targets are not available. Additional experiments will be performed in the future to understand the direct and indirect effects of VEGF administration.
GCs are immune suppressors, thus can increase the susceptibility of the host to opportunistic infections and/or intracellular infections. However, they can be beneficial to the host during a systemic inflammatory response. VEGF on the other hand acts as an immune-enhancing agent that also has the ability to inhibit RSV. It is important to keep in mind that the use of VEGF as a potential therapeutic agent has met concerns of potential adverse effects: transgenic mice that constitutively express VEGF in the respiratory epithelium had pulmonary lesions and chronic hemorrhage [62], VEGF caused pulmonary hemosiderosis in newborn and adult murine lungs by a nitric oxide-dependent mechanism [52], and deposition of high doses (in high volumes) of VEGF in the lung recruited monocytes and macrophages, and could cause gross and microscopic lesions [25]. However, the short-term use of lower VEGF doses have reported minimal clinical complications in sheep [25] and mice [42]. Our findings suggest that the immuno-enhancing activities of VEGF might have a therapeutic potential for lung maturation in infants at risk for RDS. Further studies on the effects of VEGF in preterm lambs as well as lambs suffering from RDS may identify yet additional effects on lung immunity.
Acknowledgments
This work was funded by NIH KO8 AI055499 (for the collection of the tissues during 2006) and by the current NIH RO1 AI062787. We thank Jia Liu and Dr. Weston Msikita for help with statistical analyses. We also thank Dr. Albert Van Geelen for critical reading of the manuscript.
Abbreviations
- RDS
respiratory distress syndrome
- GCs
glucocorticoids
- VEGF
vascular endothelial growth factor
- RSV
respiratory syncytial virus
- qPCR
quantitative polymerase chain reaction
- BPD
bronchopulmonary dysplasia
- SAA
serum amyloid A
- GMCSF
granulocyte macrophage colony stimulating factor
- SP
surfactant protein
- CFB
complement factor B
- CFH
complement factor H
- TLR
Toll-like receptor
- BSA
bovine serum albumin
References
- 1.Martin JA, Kochanek KD, Strobino DM, Guyer B, MacDorman MF. Annual summary of vital statistics—2003. Pediatrics. 2005;115:619–34. doi: 10.1542/peds.2004-2695. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez RJ, Martin RJ, Fanaroff A. A respiratory distress syndrome and its management. In: Fanaroff AA, Martin RJ, editors. Neonatal–perinatal medicine: diseases of the fetus and infant. St. Louis: Mosby; 2002. pp. 1001–11. [Google Scholar]
- 3.Bancalari EBM. Respiratory disorders of the newborn. In: Taussig LM, Landau LI, editors. Pediatric respiratory medicine. St. Louis: Mosby; 1999. pp. 464–8. [Google Scholar]
- 4.Koivisto M, Marttila R, Kurkinen-Raty M, Saarela T, Pokela ML, Jouppila P, et al. Changing incidence and outcome of infants with respiratory distress syndrome in the 1990s: a population-based survey. Acta Paediatr. 2004;93:177–84. doi: 10.1080/08035250410022864. [DOI] [PubMed] [Google Scholar]
- 5.Copper RL, Goldenberg RL, Creasy RK, DuBard MB, Davis RO, Entman SS, et al. A multicenter study of preterm birth weight and gestational age-specific neonatal mortality. Am J Obstet Gynecol. 1993;168:78–84. doi: 10.1016/s0002-9378(12)90889-3. [DOI] [PubMed] [Google Scholar]
- 6.Scheerlinck JP, Snibson KJ, Bowles VM, Sutton P. Biomedical applications of sheep models: from asthma to vaccines. Trends Biotechnol. 2008;26:259–66. doi: 10.1016/j.tibtech.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson-Smith MA, Trifonov V. Mammalian karyotype evolution. Nat Rev Genet. 2007;8:950–62. doi: 10.1038/nrg2199. [DOI] [PubMed] [Google Scholar]
- 8.Thurlbeck WM. Postnatal growth and development of the lung. Am Rev Respir Dis. 1975;111:803–44. doi: 10.1164/arrd.1975.111.6.803. [DOI] [PubMed] [Google Scholar]
- 9.Banfi B. A novel host defense system of airways is defective in cystic fibrosis: update. Am J Respir Crit Care Med. 2007;175:967. doi: 10.1164/ajrccm.175.9.967. [DOI] [PubMed] [Google Scholar]
- 10.Moskwa P, Lorentzen D, Excoffon KJ, Zabner J, McCray PB, Jr, Nauseef WM, et al. A novel host defense system of airways is defective in cystic fibrosis. Am J Respir Crit Care Med. 2007;175:174–83. doi: 10.1164/rccm.200607-1029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conner GE, Wijkstrom-Frei C, Randell SH, Fernandez VE, Salathe M. The lactoperoxidase system links anion transport to host defense in cystic fibrosis. FEBS Lett. 2007;581:271–8. doi: 10.1016/j.febslet.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crowley PA. Antenatal corticosteroid therapy: a meta-analysis of the randomized trials, 1972 to 1994. Am J Obstet Gynecol. 1995;173:322–35. doi: 10.1016/0002-9378(95)90222-8. [DOI] [PubMed] [Google Scholar]
- 13.Foix-L’helias L, Baud O, Lenclen R, Kaminski M, Lacaze-Masmonteil T. Benefit of antenatal glucocorticoids according to the cause of very premature birth. Arch Dis Child Fetal Neonatal Ed. 2005;90:F46–48. doi: 10.1136/adc.2003.042747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens TP, Harrinfton EW, Blennow M, Soll RF. Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database Syst Rev. 2007:CD003063. doi: 10.1002/14651858.CD003063.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunlop SA, Archer MA, Quinlivan JA, Beazley LD, Newnham JP. Repeated prenatal corticosteroids delay myelination in the ovine central nervous system. J Matern Fetal Med. 1997;6:309–13. doi: 10.1002/(SICI)1520-6661(199711/12)6:6<309::AID-MFM1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 16.Ikegami M, Jobe AH, Newnham J, Polk DH, Willet KE, Sly P. Repetitive prenatal glucocorticoids improve lung function and decrease growth in preterm lambs. Am J Respir Crit Care Med. 1997;156:178–84. doi: 10.1164/ajrccm.156.1.9612036. [DOI] [PubMed] [Google Scholar]
- 17.Jobe AH, Wada N, Berry LM, Ikegami M, Ervin MG. Single and repetitive maternal glucocorticoid exposures reduce fetal growth in sheep. Am J Obstet Gynecol. 1998;178:880–5. doi: 10.1016/s0002-9378(98)70518-6. [DOI] [PubMed] [Google Scholar]
- 18.Abbasi S, Hirsch D, Davis J, Tolosa J, Stouffer N, Debbs R, et al. Effect of single versus multiple courses of antenatal corticosteroids on maternal and neonatal outcome. Am J Obstet Gynecol. 2000;182:1243–9. doi: 10.1067/mob.2000.104789. [DOI] [PubMed] [Google Scholar]
- 19.French NP, Hagan R, Evans SF, Godfrey M, Newnham JP. Repeated antenatal corticosteroids: size at birth and subsequent development. Am J Obstet Gynecol. 1999;180:114–21. doi: 10.1016/s0002-9378(99)70160-2. [DOI] [PubMed] [Google Scholar]
- 20.McColley SA, Stellmach V, Boas SR, Jain M, Crawford SE. Serum vascular endothelial growth factor is elevated in cystic fibrosis and decreases with treatment of acute pulmonary exacerbation. Am J Respir Crit Care Med. 2000;161:1877–80. doi: 10.1164/ajrccm.161.6.9905022. [DOI] [PubMed] [Google Scholar]
- 21.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 22.Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, et al. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol. 2000;279:L600–7. doi: 10.1152/ajplung.2000.279.3.L600. [DOI] [PubMed] [Google Scholar]
- 23.Le Cras TD, Markham NE, Tuder RM, Voelkel NF, Abman SH. Treatment of newborn rats with a VEGF receptor inhibitor causes pulmonary hypertension and abnormal lung structure. Am J Physiol Lung Cell Mol Physiol. 2002;283:L555–62. doi: 10.1152/ajplung.00408.2001. [DOI] [PubMed] [Google Scholar]
- 24.Abman SH. Bronchopulmonary dysplasia: “a vascular hypothesis”. Am J Respir Crit Care Med. 2001;164:1755–6. doi: 10.1164/ajrccm.164.10.2109111c. [DOI] [PubMed] [Google Scholar]
- 25.Meyerholz DK, Grubor B, Lazic T, Gallup JM, de Macedo MM, McCray PB, Jr, et al. Monocytic/macrophagic pneumonitis after intrabronchial deposition of vascular endothelial growth factor in neonatal lambs. Vet Pathol. 2006;43:689–94. doi: 10.1354/vp.43-5-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyerholz DK, Gallup JM, Lazic T, de Macedo MM, Lehmkuhl HD, Ackermann MR. Pretreatment with recombinant human vascular endothelial growth factor reduces virus replication and inflammation in a perinatal lamb model of respiratory syncytial virus infection. Viral Immunol. 2007;20:188–96. doi: 10.1089/vim.2006.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallup JM, Ackermann MR. Addressing fluorogenic real-time qPCR inhibition using the novel custom Excel file system ‘FocusField2-6GallupqPCRSet-upTool-001’ to attain consistently high fidelity qPCR reactions. Biol Proced Online. 2006;8:87–152. doi: 10.1251/bpo122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunphy J, Horvath A, Barcham G, Balic A, Bischof R, Meeusen E. Isolation, characterisation and expression of mRNAs encoding the ovine CC chemokines, monocyte chemoattractant protein (MCP)-1alpha and -2. Vet Immunol Immunopathol. 2001;82:153–64. doi: 10.1016/s0165-2427(01)00356-7. [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Galon J, Franchimont D, Hiroi N, Frey G, Boettner A, Ehrhart-Bornstein M, et al. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J. 2002;16:61–71. doi: 10.1096/fj.01-0245com. [DOI] [PubMed] [Google Scholar]
- 31.Zhang N, Truong-Tran QA, Tancowny B, Harris KE, Schleimer RP. Glucocorticoids enhance or spare innate immunity: effects in airway epithelium are mediated by CCAAT/enhancer binding proteins. J Immunol. 2007;179:578–89. doi: 10.4049/jimmunol.179.1.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varsano S, Kaminsky M, Kaiser M, Rashkovsky L. Generation of complement C3 and expression of cell membrane complement inhibitory proteins by human bronchial epithelium cell line. Thorax. 2000;55:364–9. doi: 10.1136/thorax.55.5.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strunk RC, Eidlen DM, Mason RJ. Pulmonary alveolar type II epithelial cells synthesize and secrete proteins of the classical and alternative complement pathways. J Clin Invest. 1988;81:1419–26. doi: 10.1172/JCI113472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rothman BL, Merrow M, Despins A, Kennedy T, Kreutzer DL. Effect of lipopolysaccharide on C3 and C5 production by human lung cells. J Immunol. 1989;143:196–202. [PubMed] [Google Scholar]
- 35.McCormack FX, Whitsett JA. The pulmonary collectins, SP-A and SP-D, orchestrate innate immunity in the lung. J Clin Invest. 2002;109:707–12. doi: 10.1172/JCI15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LeVine AM, Elliott J, Whitsett JA, Srikiatkhachorn A, Crouch E, DeSilva N, et al. Surfactant protein-d enhances phagocytosis and pulmonary clearance of respiratory syncytial virus. Am J Respir Cell Mol Biol. 2004;31:193–9. doi: 10.1165/rcmb.2003-0107OC. [DOI] [PubMed] [Google Scholar]
- 37.Kuroki Y, Takahashi M, Nishitani C. Pulmonary collectins in innate immunity of the lung. Cell Microbiol. 2007;9:1871–9. doi: 10.1111/j.1462-5822.2007.00953.x. [DOI] [PubMed] [Google Scholar]
- 38.Henning LN, Azad AK, Parsa KV, Crowther JE, Tridandapani S, Schlesinger LS. Pulmonary surfactant protein A regulates TLR expression and activity in human macrophages. J Immunol. 2008;180:7847–58. doi: 10.4049/jimmunol.180.12.7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brogden KA, Kalfa VC, Ackermann MR, Palmquist DE, McCray PB, Jr, Tack BF. The ovine cathelicidin SMAP29 kills ovine respiratory pathogens in vitro and in an ovine model of pulmonary infection. Antimicrob Agents Chemother. 2001;45:331–4. doi: 10.1128/AAC.45.1.331-334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heron M. Deaths: leading causes for 2004. Natl Vital Stat Rep. 2007;56:1–95. [PubMed] [Google Scholar]
- 41.Bachofen M, Weibel ER. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med. 1982;3:35–56. [PubMed] [Google Scholar]
- 42.Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, et al. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med. 2002;8:702–10. doi: 10.1038/nm721. [DOI] [PubMed] [Google Scholar]
- 43.Imasato A, Desbois-Mouthon C, Han J, Kai H, Cato AC, Akira S, et al. Inhibition of p38 MAPK by glucocorticoids via induction of MAPK phosphatase-1 enhances nontypeable Haemophilus influenzae-induced expression of Toll-like receptor 2. J Biol Chem. 2002;277:47444–50. doi: 10.1074/jbc.M208140200. [DOI] [PubMed] [Google Scholar]
- 44.Floros J, Phelps DS, Harding HP, Church S, Ware J. Postnatal stimulation of rat surfactant protein A synthesis by dexamethasone. Am J Physiol. 1989;257:L137–43. doi: 10.1152/ajplung.1989.257.2.L137. [DOI] [PubMed] [Google Scholar]
- 45.Munoz-Canoves P, Tack BF, Vik DP. Analysis of complement factor H mRNA expression: dexamethasone and IFN-gamma increase the level of H in L cells. Biochemistry. 1989;28:9891–7. doi: 10.1021/bi00452a002. [DOI] [PubMed] [Google Scholar]
- 46.Coulpier M, Andreev S, Lemercier C, Dauchel H, Lees O, Fontaine M, et al. Activation of the endothelium by IL-1 alpha and glucocorticoids results in major increase of complement C3 and factor B production and generation of C3a. Clin Exp Immunol. 1995;101:142–9. doi: 10.1111/j.1365-2249.1995.tb02290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schleimer RP. Glucocorticoids suppress inflammation but spare innate immune responses in airway epithelium. Proc Am Thorac Soc. 2004;1:222–30. doi: 10.1513/pats.200402-018MS. [DOI] [PubMed] [Google Scholar]
- 48.John M, Oltmanns U, Binder C, Meiners S, Gellert K, Chung KF, et al. Inhibition of chemokine production from human airway smooth muscle cells by fluticasone, budesonide and beclomethasone. Pulm Pharmacol Ther. 2004;17:41–7. doi: 10.1016/j.pupt.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 49.Schwiebert LM, Stellato C, Schleimer RP. The epithelium as a target of glucocorticoid action in the treatment of asthma. Am J Respir Crit Care Med. 1996;154:S16–9. doi: 10.1164/ajrccm/154.2_Pt_2.S16. discussion S19–20. [DOI] [PubMed] [Google Scholar]
- 50.Zimmermann N, Hershey GK, Foster PS, Rothenberg ME. Chemokines in asthma: cooperative interaction between chemokines and IL-13. J Allergy Clin Immunol. 2003;111:227–42. doi: 10.1067/mai.2003.139. quiz 243. [DOI] [PubMed] [Google Scholar]
- 51.Stahn C, Lowenberg M, Hommes DW, Buttgereit F. Molecular mechanisms of glucocorticoid action and selective glucocorticoid receptor agonists. Mol Cell Endocrinol. 2007;275:71–8. doi: 10.1016/j.mce.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 52.Bhandari V, Choo-Wing R, Lee CG, Yusuf K, Nedrelow JH, Ambalavanan N, et al. Developmental regulation of NO-mediated VEGF-induced effects in the lung. Am J Respir Cell Mol Biol. 2008;39:420–30. doi: 10.1165/rcmb.2007-0024OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kevill KA, Bhandari V, Kettunen M, Leng L, Fan J, Mizue Y, et al. A role for macrophage migration inhibitory factor in the neonatal respiratory distress syndrome. J Immunol. 2008;180:601–8. doi: 10.4049/jimmunol.180.1.601. [DOI] [PubMed] [Google Scholar]
- 54.Clauss M, Weich H, Breier G, Knies U, Rockl W, Waltenberger J, et al. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J Biol Chem. 1996;271:17629–34. doi: 10.1074/jbc.271.30.17629. [DOI] [PubMed] [Google Scholar]
- 55.Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336–43. [PubMed] [Google Scholar]
- 56.Hillman NH, Moss TJ, Nitsos I, Kramer BW, Bachurski CJ, Ikegami M, et al. Toll-like receptors and agonist responses in the developing fetal sheep lung. Pediatr Res. 2008;63:388–93. doi: 10.1203/PDR.0b013e3181647b3a. [DOI] [PubMed] [Google Scholar]
- 57.Kallapur SG, Bachurski CJ, Le Cras TD, Joshi SN, Ikegami M, Jobe AH. Vascular changes after intra-amniotic endotoxin in preterm lamb lungs. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1178–85. doi: 10.1152/ajplung.00049.2004. [DOI] [PubMed] [Google Scholar]
- 58.Kishore U, Greenhough TJ, Waters P, Shrive AK, Ghai R, Kamran MF, et al. Surfactant proteins SP-A and SP-D: structure, function and receptors. Mol Immunol. 2006;43:1293–315. doi: 10.1016/j.molimm.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 59.Brown KR, England KM, Goss KL, Snyder JM, Acarregui MJ. VEGF induces airway epithelial cell proliferation in human fetal lung in vitro. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1001–10. doi: 10.1152/ajplung.2001.281.4.L1001. [DOI] [PubMed] [Google Scholar]
- 60.Steel DM, Whitehead AS. The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunol Today. 1994;15:81–8. doi: 10.1016/0167-5699(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 61.Fischer H, Gonzales LK, Kolla V, Schwarzer C, Miot F, Illek B, et al. Developmental regulation of DUOX1 expression and function in human fetal lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1506–14. doi: 10.1152/ajplung.00029.2007. [DOI] [PubMed] [Google Scholar]
- 62.Le Cras TD, Spitzmiller RE, Albertine KH, Greenberg JM, Whitsett JA, Akeson AL. VEGF causes pulmonary hemorrhage, hemosiderosis, and air space enlargement in neonatal mice. Am J Physiol Lung Cell Mol Physiol. 2004;287:L134–42. doi: 10.1152/ajplung.00050.2004. [DOI] [PubMed] [Google Scholar]