Abstract
Vascular endothelial growth factor (VEGF) is increasingly recognized as a perinatal regulator of lung maturation and surfactant protein expression. Preterm and young infants are at increased risk for pulmonary immaturity characterized by insufficient surfactant production as well as increased risk for severe manifestations of respiratory syncytial virus (RSV) infection. Innate immune components including surfactant proteins A and D, and β-defensins have putative antimicrobial activity against pulmonary pathogens including RSV. Our hypothesis was that recombinant human VEGF (rhVEGF) pretreatment therapy would decrease RSV disease in the perinatal lamb RSV model. Newborn lambs were pretreated with rhVEGF, betamethasone, or saline and then inoculated with bovine RSV or sterile medium. Tissues were collected 5 d postinoculation, corresponding to the initiation of severe lesions and peak viral replication. In RSV-infected lambs, rhVEGF therapy increased the mean daily body temperature, decreased airway neutrophil exudate, and reduced RSV replication compared with betamethasone or saline pretreatment. Furthermore, rhVEGF therapy significantly mitigated the RSV-induced increase in surfactant protein A mRNA expression and decrease in surfactant protein D mRNA expression. In control (non-RSV-infected) lambs, pretreatment with rhVEGF increased sheep β-defensin-1 (SBD1) mRNA expression, but no alteration in surfactant proteins A and D was detected. This novel study demonstrates that rhVEGF pretreatment mitigates RSV disease and, in addition, rhVEGF regulation of innate immune genes is dependent on RSV infection status.
INTRODUCTION
RESPIRATORY SYNCYTIAL VIRUS (RSV) is an enveloped, negative sense, single-stranded RNA pneumovirus that is a significant cause of seasonal respiratory disease in humans (18). In healthy adults, RSV infection often results in a self-limiting respiratory disease. Yet, certain subpopulations including preterm and low birth weight infants are at increased risk for severe manifestations of RSV disease requiring hospitalization (24,32,40). RSV is the leading cause of bronchiolitis in infants and during the 1980s and into the mid-1990s annual RSV-associated hospitalization in the United States was estimated to be over 125,000 with nearly 500 deaths (29,35,36). Lesions frequently associated with severe RSV disease include necrotizing bronchiolitis (causing atelectasis, hyperinflation, and wheezing) and pneumonia (characterized radiographically as interstitial infiltrates, alveolar filling, and consolidation) (18). Bovine RSV infection in the perinatal lamb model is a viable model for study of the RSV disease and innate immune response (20,25).
Preterm and young infants are also predisposed to developing respiratory distress syndrome (RDS), which is associated with immature development of pulmonary surfactant (33). Glucocorticoid therapy, a promoter of lung maturation that was originally developed using sheep models, is conventionally used as a prophylactic treatment for premature birth (5,31,39). Unfortunately, glucocorticoid administration is also associated with potential short- to long-term adverse effects including alterations in neurological and cognitive development (21). The potential for adverse effects by glucocorticoids makes the search for novel and safer surfactant regulators a priority. Human airway epithelial explants treated with vascular endothelial growth factor (VEGF) exhibited proliferation with increased surfactant mRNA and protein expression (6) and surfactant proteins A and D have antiviral activity including opsonization and aggregation of RSV, and activation of macrophages (16). Furthermore, preterm mice treated with exogenous recombinant human VEGF (rhVEGF) had increased pulmonary maturation and survival (8). VEGF therapy has been constrained by concerns about potential adverse effects. For instance, transgenic mice with constitutive chronic expression of VEGF in respiratory epithelium had pulmonary lesions including chronic hemorrhage and alveolar remodeling (22). In a sheep model, intrabronchial deposition of exogenous rhVEGF induced a dose-dependent recruitment of monocyte/macrophages into the lung, causing gross and microscopic lesions (27). Although safety concerns regarding VEGF therapy are legitimate, studies of short-term and lower dose applications thus far have reported minimal clinical complications (8,27).
The prospect of rhVEGF therapy in perinatal patients at risk for RDS could have additional ramifications as these same patients are at elevated risk for severe RSV disease. Preterm infants have reduced surfactant protein expression including surfactant proteins A and D, which both have proven anti-RSV activity (15,17). Because VEGF is suggested to induce lung maturation and surfactant protein expression, this putative increase in (antiviral) surfactant proteins might prove useful as a therapy to prevent severe RSV disease (6,8,15). The hypothesis of this study was that rhVEGF pretreatment would diminish RSV disease in perinatal lambs. We compared this novel RDS therapy with traditional glucocorticoid therapy (betamethasone) and sham (sterile medium) treatment. In this study we characterize RSV infection through clinical signs, lesions, morphometry, and innate immune gene expression.
MATERIALS AND METHODS
Animals
Date-mated pregnant ewes were obtained from Laboratory Animal Resources (Iowa State University, Ames, IA), with all procedures approved by the Animal Care and Use Committee. After natural parturition, neonatal lambs (6–12 h old) were randomly pretreated with sterile saline (20 mL, intratracheal injection), sterile saline (20 mL, intratracheal) plus betamethasone (4.0 mg/kg, intramuscular), or recombinant human vascular endothelial growth factor (rhVEGF, 5 µg/mL × 20 mL, intratracheal; Invitrogen, Carlsbad, CA) (Table 1). After 30 min for acclimation, the lambs were further divided into two treatment groups receiving either sterile saline (20 mL, intratracheal) or bovine respiratory syncytial virus (bovine RSV strain 375, 103 to 104 TCID50 [50% tissue culture infective doses]/mL × 20 mL, intratracheal). Lambs were given daily antibiotic (ceftiofur, 2.2 mg/kg per day, intramuscular) to prevent bacterial complications (41). During the course of infection, lambs were monitored for clinical signs including body temperature. From our previous experience with this model of RSV disease, severe lesion development and peak viral replication occur on day 5 of infection (23,25). Lambs were killed with sodium pentobarbital on day 5 of infection, and lungs were examined for gross lesions. Tissue was collected bilaterally from the cranial and middle lobes. Tissues were either snap frozen on dry ice for quantitative polymerase chain reaction (qPCR) or positioned in cassettes and placed in 10% neutral-buffered formalin (24–48 h) for morphologic and immunohistochemical analysis.
TABLE 1.
Experimental design for assessing VEGF pretreatment in perinatal RSV diseasea
| Group | Pretreatmentb | Treatmentc |
|---|---|---|
| C/Media | Sterile saline | Sterile medium |
| V/Media | VEGF | Sterile medium |
| B/Media | Betamethasone | Sterile medium |
| C/RSV | Sterile saline | Bovine RSV |
| V/RSV | VEGF | Bovine RSV |
| B/RSV | Betamethasone | Bovine RSV |
Lambs were monitored during infection for clinical signs and tissues were collected on day 5 of infection to assess for lesions, morphometry, immunohistochemistry, and gene expression (RSV, SBD1, SP-A, and SP-D).
Intratracheal injection (20 mL, sterile saline or VEGF [5 µg/mL]) or intramuscular injection (betamethasone, 4 mg/kg) was administered approximately 30 min before treatment.
Intratracheal injection (20 mL) of sterile medium or RSV (bovine RSV strain 375, 103 to 104 TCID50/mL, intratracheal).
RNA isolation
Total tissue RNA was isolated from whole lung tissue (right middle lobe) for gene expression analysis by hydrolysis probe-based fluorogenic one-step real-time qPCR. Briefly, lung tissue samples were weighed, placed into 3 mL of TRIzol reagent (Invitrogen), and homogenized. The homogenate was vortexed and nuclease-free chloroform (200 µL) was added to each sample, mixed, and then microcentrifuged at 12,000 × g for 10 min. Top aqueous layers were transferred into 500 µL of nuclease-free 2-propanol (Fisher Chemical, Fairlawn, NJ), vortexed, allowed to sit and again microcentrifuged with subsequent removal of the top aqueous layer. The remaining pellet was washed (75% nuclease-free ethanol) and microcentrifuged, the final supernatant was removed, and the remaining samples were allowed to air dry under a fume hood. Each pellet was resuspended (nuclease-free 0.1 mM EDTA, pH 7.0), heated to 65°C for 5 min, and stored at 4°C. RNA isolates were assessed at 1:50 dilution for quantity and purity by spectrophotometry at 260 and 280 nm followed immediately by DNase treatment with TURBO DNase (TURBO DNA-free kit; Ambion, Austin, TX). For each sample, 80 µL of each supernatant RNA was recovered and diluted 1:10 with nuclease-free water (Ambion), resulting in 800 µL of each RNA isolate.
qPCR
qPCR was carried out as a one-step process as previously described in detail (14). Each of our final 25-µL one-step real-time qPCRs contained the following: 12.5 µL of one-step master mix (TaqMan one-step RT-PCR master mix reagents kit; Applied Biosystems, Foster City, CA), MultiScribe reverse transcriptase (RT, 0.25 U/µL), RNase inhibitor (0.4 U/µL), optimal forward and reverse primer and fluorogenic probe concentrations (Table 2), nuclease-free water, and 6.5 µL of each RNA sample/template (14,16). Thermocycling conditions for all qPCRs were as follows: 35 min at 48°C, 10 min at 95°C, and 50 cycles of 15 s at 95°C and 1 min at 58°C. All plates were run in duplicate on a GeneAmp 5700 sequence detection system (Applied Biosystems) and all output data were processed as custom Excel files (Microsoft, Redmond, WA). The Pfaffl equation (value = [(Etarget)ΔCt(control − treated)]/[(Ehousekeeper)ΔCt(control − treated)]) was used for relative quantitation based on target-specific fluorescent signals generated during qPCR of total RNA from the whole lung homogenates in this study (30). For each animal, RNA sample real-time target gene amplifications were normalized to the geometric mean of two housekeeping genes, hRibo18S and ovRPS15 (the latter sequence was kindly provided by S. Limesand, University of Colorado Health Sciences Center, Aurora, CO).
TABLE 2.
Primers and probes for ovine gene expression assessed by real-time qPCR
| Target gene |
Primer (concentration) |
Sequencea |
|---|---|---|
| SBD1 | Fwd (1000 nM) | 5′-CCATAGGAATAAAGGCGTCTGTG |
| Rev (1000 nM) | 5′-CGCGACAGGTGCCAATCT | |
| Probe (150 nM) | 5′-6FAM-CCGAGCAGGTGCCCTAGACACATGA-TAMRA | |
| SP-A | Fwd (500 nM) | 5′-TGACCCTTATGCTCCTCTGGAT |
| Rev (500 nM) | 5′-GGGCTTCCAAGACAAACTTCCT | |
| Probe (50 nM) | 5′-6FAM-TGGCTTCTGGCCTCGAGTGCG-TAMRA | |
| SP-D | Fwd (500 nM) | 5′-ACGTTCTGCAGCTGAGAAT |
| Rev (500 nM) | 5′-TCGGTCATGCTCAGGAAAGC | |
| Probe (100 nM) | 5′-6FAM-TTGACTCAGCTGGCCACAGCCCAGAACA-TAMRA | |
| Bovine RSV Ncap | Fwd (1000 nM) | 5′-CAGTCAAGAATATTATGCTTGGTCATG |
| Rev (1000 nM) | 5′-CCTAACTTTTGTGCATATTCATAGACTTC | |
| Probe (150 nM) | 5′-6FAM-CAACCTGTTCCATTTCTGCTTGTACGCTG-TAMRA | |
| ovRPS15 | Fwd (1000 nM) | 5′-CGAGATGGTGGGCAGCAT |
| Rev (1000 nM) | 5′-GCTTGATTTCCACCTGGTTGA | |
| Probe (150 nM) | 5′-VIC-CCGGCGTCTACAACGGCAAGACC-TAMRA | |
| hRibo18S | Fwd (50 nM) | 5′-CGGCTACCACATCCAAGGAA |
| Rev (50 nM) | 5′-GCTGGAATTACCGCGGCT | |
| Probe (200 nM) | 5′-VIC-TGCTGGCACCAGACTTGCCCTC-TAMRA |
6FAM or VIC, 5′ fluorescent reporter dye; TAMRA, fluorescent quencher dye.
Morphometry
Gross lesions were assessed on the basis of a scoring system in which multifocal to coalescing areas of plum-red consolidation were expressed as a percentage of lung area: 0, 0%; 1, <5%; 2, 5–20%; 3, 21–40%; 4, 41–60%; and 5, >60%.
Neutrophil infiltration into bronchioles was assessed by a pathologist (26). Briefly, low-magnification sites (n = 10) were randomly selected, a bronchiole from each site was targeted, and the number of neutrophils within the basement membrane and lumen were counted and values per section averaged.
Statistical analysis
In pretreatment of qPCR data, we found that the standard deviation of replicated measurements increased with its respective mean. Thus all qPCR measurements were log transformed in order to stabilize variance.
For each RNA sample, the arithmetic mean of log transformations of all measurements of its housekeeper genes (two genes) was used as a normalization factor. This normalization factor was then subtracted from each measurement of the target gene after being log transformed. The result is the relative gene expression of each target gene. This operation is equivalent to dividing raw measurements of each target gene by the geometric means of all measurements of its corresponding housekeeper genes.
We used a standard two-sample t test to compare expression of the same target gene under different treatment conditions. In the case of treatments with significantly different variances, we compared means through a Welch modified two-sample t test. Variances between different treatments were compared through an F statistic.
We used a balanced two-way analysis of variance (seven replications per treatment) to test for effect of treatments on body temperature, but grouping for possible variability due to day postinfection. Seven replications were randomly chosen from treatments with more than seven replications. Mean body temperature was calculated by taking the sum of daily body temperatures for each animal during the course of the infection and dividing by the days assessed. Significant differences in body temperature means of different treatments was established through the least significant difference (LSD) method.
We used two-way analysis of variance to test the treatments on scores for gross lesions and neutrophil infiltration. If significance was detected as a result of treatment then post-hoc tests were applied to scientifically relevant comparisons.
RESULTS
Clinical RSV infection
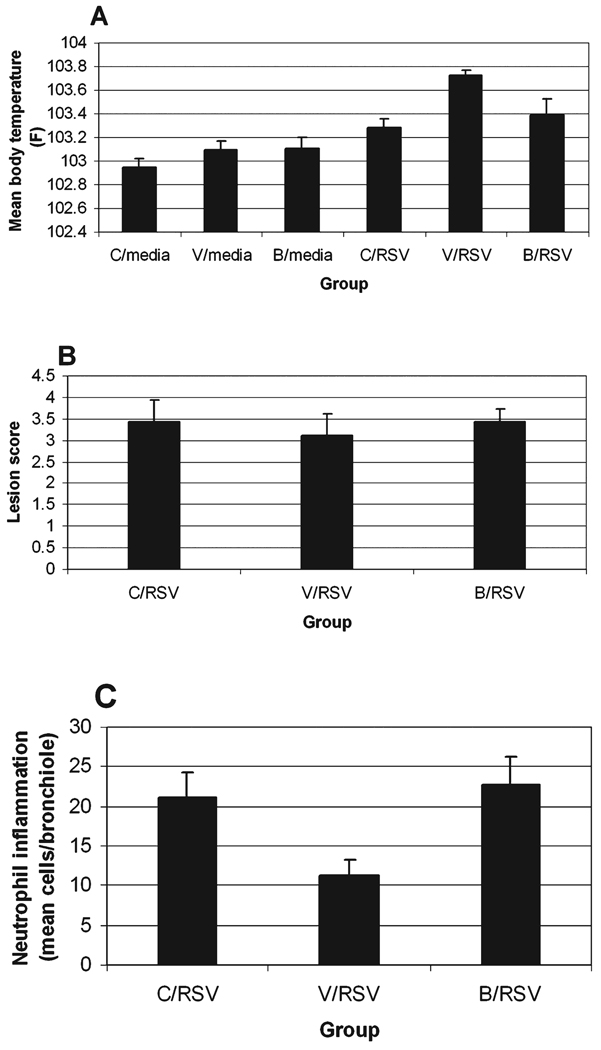
During the course of infection, all the RSV-infected animals exhibited mild to moderate clinical signs including increased body temperature, tachypnea, and cough, whereas the control groups lacked clinical signs. No significant difference between treatments was detected in degree of clinical signs such as tachypnea, cough, appetite, and clinical appearance (data not shown). RSV treatment caused a significant increase in mean body temperature during the course of infection compared with sham inoculation with sterile media (C/RSV > C/Media; p < 0.001) (Fig. 1A). Pretreatment of sham-inoculated groups caused no significant alteration in mean temperature; however, rhVEGF pretreatment of RSV infection increased mean body temperature compared with betamethasone pretreatment (V/RSV > B/RSV; p < 0.05), RSV infection alone (V/RSV > C/RSV; p < 0.05), and rhVEGF pretreatment alone (V/RSV > V/Media; p < 0.001). The increased body temperature induced by rhVEGF pretreatment in the RSV groups was not characterized by extremely high values on any given day, but rather by persistently elevated values throughout the course of infection.
FIG. 1.
Clinical comparison of sham- and RSV-infected neonatal lambs pretreated with saline, betamethasone, or rhVEGF. (A) Mean body temperature. RSV infection overall caused a significant increase in group mean body temperatures (p < 0.001). In RSV-infected lambs, rhVEGF pretreatment increased mean body temperature compared with RSV infection alone (C/RSV; p < 0.05), betamethasone (B/RSV; p < 0.05) pretreatment or compared with rhVEGF pretreatment of controls (7V/Media; p < 0.001). (B) Gross lesions. No significant alterations in gross lesions were detected. (C) Bronchiole inflammation. The number of neutrophils was reduced after rhVEGF pretreatment compared with saline (V/RSV < C/RSV; p < 0.07) or betamethasone (V/RSV < B/RSV; p < 0.05).
RSV lesions and replication
All RSV-infected groups exhibited the typical RSV-induced gross lesions of plum to red consolidation, whereas the group given media lacked lesions. Significant difference in distribution and severity of gross lesions between treatment groups was not detected (Fig. 1B). Microscopically, whereas all RSV-infected groups had typical RSV lesions (necrotizing bronchiolitis, epithelial syncytia, etc.), there was significant reduction in neutrophilic inflammation within the RSV-infected bronchioles of rhVEGF-pretreated versus saline (p < 0.07) and betamethasone groups (p < 0.05) (Fig. 1C).
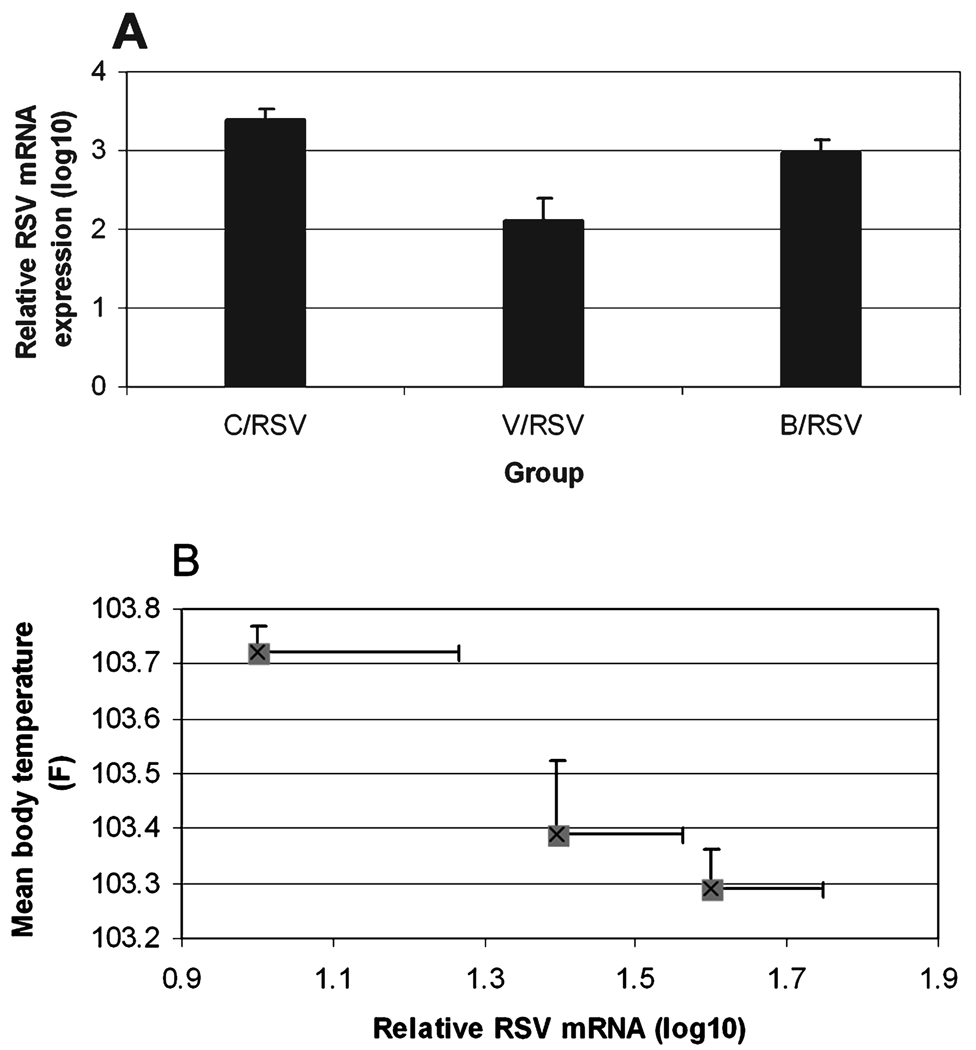
qPCR analysis of whole lung homogenates detected a significant reduction in RSV mRNA expression in the rhVEGF pretreatment group compared with saline (p < 0.00001) and betamethasone (p < 0.01) (Fig. 2A). Analysis between mean body temperature during the course of infection and levels of RSV replication by day 5 of infection demonstrated a strong correlation among individual animals (p < 0.06) and a group interaction (r= −0.88) can be seen in the group plot, demonstrating the V/RSV group as having the higher body temperature and lower viral replication than the other pretreatment groups (Fig. 2B).
FIG. 2.
RSV replication in neonatal lambs pretreated with saline, betamethasone, or rhVEGF. (A) RSV mRNA expression. RSV mRNA expression (log10) was reduced in the rhVEGF pretreatment group compared with saline (p < 0.00001) and betamethasone (p < 0.01) pretreatment. (B) Body temperature and RSV replication interaction. Among lambs there was a trend toward reduced viral replication with higher mean body temperature (p < 0.06). This correlation (r= −0.88) was distinct between the treatment groups V/RSV = 103.7; B/RSV = 103.4; C/RSV = 103.3).
Innate immune gene expression
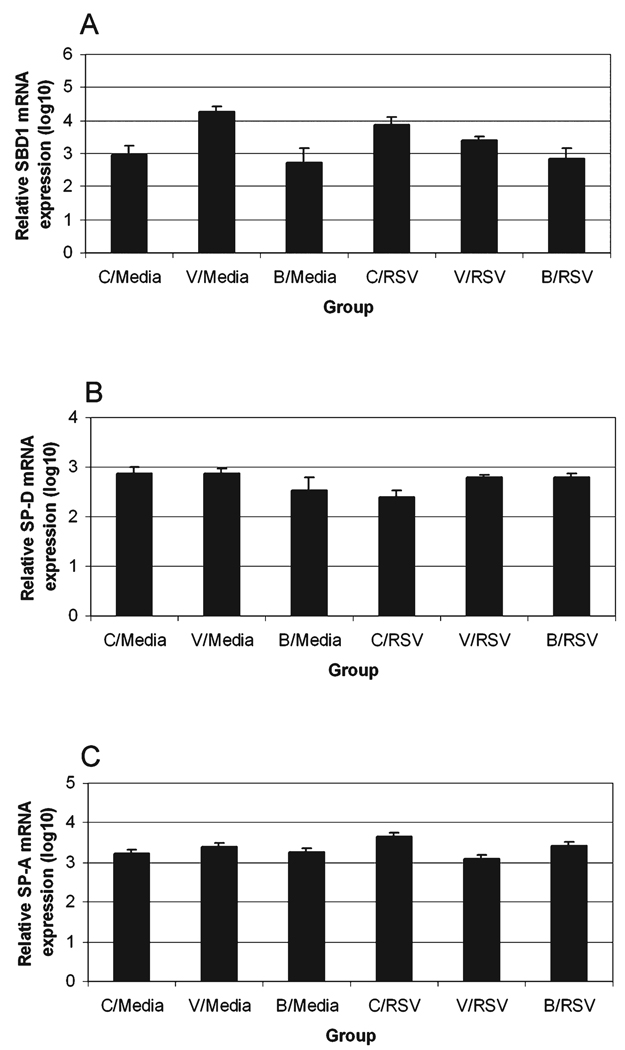
Sheep β-defensin-1 (SBD1) mRNA expression was increased in rhVEGF versus saline or betamethasone pretreated, sham-infected groups (p < 0.05) (Fig. 3A). During RSV infection, the saline pretreatment group had increased SBD1 expression (C/RSV > C/Media; p < 0.1); however, rhVEGF pretreatment of RSV infection suppressed SBD1 expression compared with either the rhVEGF or RSV group control (V/RSV < V/Media or C/RSV; p < 0.05).
FIG. 3.
Innate immune gene expression. (A) SBD1 mRNA expression (log10). SBD1 expression in sham inoculates was increased by rhVEGF relative to saline (p < 0.05) or betamethasone (p < 0.05) pretreatment, with similarly increased expression detected in RSV infection (C/RSV > C/Media; p < 0.1). Whereas rhVEGF pretreatment and RSV infection each increased SBD1 expression (V/Media > C/Media, p < 0.05; C/RSV > C/Media, p < 0.05), the combination of rhVEGF pretreatment and RSV infection suppressed SBD1 expression (V/Media vs. V/RSV, p < 0.05; C/RSV vs. V/RSV, p < 0.05). (B) SP-D mRNA expression (log10). SP-D expression was not significantly altered by pretreatment in sham-inoculated groups. RSV infection (C/Media > C/RSV; p < 0.05) reduced SP-D expression, whereas pretreatment with rhVEGF and betamethasone suppressed the RSV-induced alteration to near control levels (C/RSV < V/RSV or B/RSV; p < 0.05). (C) SPA mRNA expression (log10). SP-A expression was unaltered by pretreatment in sham-inoculated groups. RSV infection increased SP-A compared with sham-infected control (C/RSV > C/Media; p = 0.01) and this RSV-induced alteration was suppressed by rhVEGF and betamethasone pretreatment (C/RSV > V/RSV or B/RSV; p < 0.05).
Surfactant protein D (SP-D) mRNA expression was not significantly altered by pretreatments in the sterile media controls (Fig. 3B). Independently, RSV infection reduced SP-D mRNA expression (C/Media > C/RSV; p < 0.05). This reduction in SP-D mRNA expression by RSV infection was mitigated to near control levels by rhVEGF and betamethasone pretreatment (C/RSV < B/RSV or V/RSV; p < 0.05).
SP-A expression, similar to SP-D expression, was statistically unaltered by pretreatment in the control groups (Fig. 3C). RSV infection increased SP-A expression (C/RSV > C/Media; p < 0.01) but pretreatment with rhVEGF or betamethasone suppressed the RSV-induced alteration (C/RSV > V/RSV or B/RSV; p < 0.05)
DISCUSSION
The purpose of this study was to compare novel (rhVEGF), traditional (betamethasone), or sham (sterile medium) RDS therapy in a perinatal lamb model of RSV infection. The rationale for “pretreatment” of rhVEGF therapy was made on the presumption that rhVEGF could be used for RDS therapy (and increased antiviral surfactant proteins) and thereby would already be active in high-risk infants that become exposed to RSV. Indeed, rhVEGF pretreatment in this study mitigated several key parameters of RSV infection.
All RSV-infected lambs had mild to moderate clinical signs including increased body temperature, cough, and tachypnea consistent with previous work in our laboratory (23,25). Interestingly, clinical signs of RSV infection were not significantly mitigated except for body temperatures. In this particular animal model there is typically a transient, mild increase in body temperature on the first day because of inoculum (RSV antigen) delivery followed by another moderate increase on days 3–5 of infection corresponding to pulmonary viral replication and activation of the immune response. In this current study, rhVEGF pretreatment increased daily body temperature during the course of infection, resulting in an increase in mean body temperature. Interestingly, the rhVEGF pretreatment group also had decreased RSV viral mRNA expression on day 5 of the experiment, representing the time of maximum viral replication in this model (23,25). The increased mean body temperature in the rhVEGF pretreatment group likely affected RSV replication as they were inversely correlated. It is recognized that elevated body temperature is a by-product of the innate immune system, often resulting from cytokine (e.g., interleukin [IL]-1, IL-6, and tumor necrosis factor [TNF]-α) expression (10). Increased temperature can inhibit in vitro replication of some viruses including influenza and feline immunodeficiency virus and temperature regulation is in part the foundational precept of RSV temperature-sensitive vaccines (2,7,34). The correlation of increased body temperature during the course of disease to decreased RSV replication is a novel finding for this perinatal RSV model.
We speculate that at least three different mechanisms may be involved in the sustained elevated body temperature and reduced viral expression by rhVEGF pretreatment. rhVEGF can interact with the endothelial VEGF receptor and promote angiogenesis, a prominent component of this process being enhanced vascular permeability (11,12). This subtle (not detected clinically) vascular leakage could allow extravasation of antibodies or other innate immune components (e.g., mannose-binding protein) for antigen–antibody complex formation; an important source of pyrogenicity in viral infection (19). A second potential mechanism is related to the recruitment of monocytes and macrophages to sites of VEGF expression/administration (3,27). Monocytes/macrophages are important producers of pyrogenic cytokines after phagocytosis of antigen (38). rhVEGF recruitment of monocytes/macrophages to the lung could contribute to enhanced opportunity for interaction with virus from the inoculum (early infection) or infected cells (mid to late infection) for cytokine production. Last, although body temperature was correlated with reduced viral inhibition, we cannot exclude the premise that increased temperature and reduced RSV replication are both mediated as a direct result of some innate mediator such as cytokine or cellular effects, such as B cell proliferation seen in mice (13,28). Specifically, alterations to β-defensin and surfactant protein expression, which both have both antiviral and immunomodulatory capacity, may have contributed to the reduced RSV replication.
In this model of RSV disease, neutrophil inflammation in bronchioles was reduced by rhVEGF pretreatment. Neutrophilic exocytosis is a major cause of epithelial damage and airway obstruction contributing to ventilation compromise during RSV disease in children (37,42). VEGF interaction with endothelium can regulate adhesion molecule expression and even enhance neutrophil emigration; however, in this case the reduced neutrophil recruitment was likely due to diminished virus replication as this is a significant regulator of neutrophil emigration in RSV disease (4,22,43).
Concerning innate immune gene mRNA levels, we detected differential regulation of innate immune genes by rhVEGF pretreatment and RSV infection. SBD1 mRNA expression was upregulated by rhVEGF pretreatment but not significantly altered during RSV infection. This novel finding of rhVEGF-induced β-defensin mRNA expression is interesting and this relationship may be defined in part as β-defensins and VEGF have been reported to synergize in the recruitment of dendritic cells and in vasculogenesis of tumors (9). The lack of SBD1 mRNA alteration during RSV infection is consistent with investigation of laser capture microdissected bovine RSV-infected and noninfected epithelia in which SBD1 mRNA expression was not changed (20). Although SBD1 lacks NF-κB regulatory elements, the regulatory pathway of SBD1 is not yet fully defined (1).
In contrast to SBD1, surfactant proteins A and D were not altered by rhVEGF pretreatment in control (non-RSV-infected) lambs, but rhVEGF pretreatment (and betamethasone) did mitigate the magnitude of surfactant protein mRNA alteration seen during RSV disease. Betamethasone is a glucocorticoid therapeutically given to perinatal infants to increase surfactant expression for prevention of respiratory distress syndrome (5,38). VEGF has been proposed as a novel therapeutic for lung maturation and surfactant expression in place of glucocorticoids, which have potential adverse effects (8,21). Because rhVEGF and not betamethasone pretreatment diminished select parameters of RSV disease (e.g., viral replication and bronchiolar neutrophilic exudate), this suggests that the mitigation of surfactant protein mRNA alteration alone may not fully explain the RSV disease mitigation and that other factors are more fundamental.
In summary, this study demonstrates that rhVEGF pretreatment therapy can diminish select parameters of RSV disease. Furthermore, rhVEGF pretreatment interacts with RSV disease status to cause differential regulation of innate immune gene expression. The role of these innate immune gene alterations in relation to rhVEGF therapy of RSV infection is not fully known. These foundational results warrant further investigation into the kinetics of perinatal VEGF therapy and RSV disease.
ACKNOWLEDGMENTS
This work was funded in part by National Institutes of Health NIAID awards 05R01AI062787-02 and 5K08AI055499-03. The authors thank James DeGraaff and Dr. Kenji Kawashima for assistance.
REFERENCES
- 1.Ackermann MR, Gallup JM, Zabner J, et al. Differential expression of sheep β-defensin-1 and -2 and interleukin 8 during acute Mannheimia haemolytica pneumonia. Microb Pathog. 2004;37:21–27. doi: 10.1016/j.micpath.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Alix C, Martin JP, Braunwald J. Temperature sensitivity of two different steps in the viral life cycle of feline immunodeficiency virus. Virology. 1999;253:309–318. doi: 10.1006/viro.1998.9506. [DOI] [PubMed] [Google Scholar]
- 3.Barleon B, Sozzani S, Zhou D, et al. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor Flt-1. Blood. 1996;87:3336–3343. [PubMed] [Google Scholar]
- 4.Bataki EL, Evans GS, Everard ML. Respiratory syncytial virus and neutrophil activation. Clin Exp Immunol. 2005;140:470–477. doi: 10.1111/j.1365-2249.2005.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolt RJ, van Weissenbruch MM, Lafeber HN, Delemarre-van de Waal HA. Glucocorticoids and lung development in the fetus and preterm infant. Pediatr Pulmonol. 2001;32:76–91. doi: 10.1002/ppul.1092. [DOI] [PubMed] [Google Scholar]
- 6.Brown KR, England KM, Goss KL, et al. VEGF induces airway epithelial cell proliferation in human fetal lung in vitro. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1001–L1010. doi: 10.1152/ajplung.2001.281.4.L1001. [DOI] [PubMed] [Google Scholar]
- 7.Wright PF, Karron RA, Belshe RB, et al. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J Infect Dis. 2000;182:1331–1342. doi: 10.1086/315859. [DOI] [PubMed] [Google Scholar]
- 8.Compernolle V, Brusselmans K, Acker T, et al. Loss of HIF-2α and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med. 2002;8:702–710. doi: 10.1038/nm721. [DOI] [PubMed] [Google Scholar]
- 9.Conejo-Garcia JR, Benencia F, Courreges MC, et al. Tumor-infiltrating dendritic cell precursors recruited by a β- defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med. 2004;10:950–958. doi: 10.1038/nm1097. [DOI] [PubMed] [Google Scholar]
- 10.Conti B, Tabarean I, Andrei C, Bartfai T. Cytokines and fever. Front Biosci. 2004;9:1433–1449. doi: 10.2741/1341. [DOI] [PubMed] [Google Scholar]
- 11.Dvorak HF. How tumors make bad blood vessels and stroma. Am J Pathol. 2003;162:1747–1757. doi: 10.1016/s0002-9440(10)64309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 13.Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, Carbone DP. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150–4166. [PubMed] [Google Scholar]
- 14.Gallup JM, Kawashima K, Lucero G, Ackermann MR. New quick method for isolating RNA from laser captured cells stained by immunofluorescent immunohistochemistry; RNA suitable for direct use in fluorogenic TaqMan one-step real-time RT-PCR. Biol Proced Online. 2005;7:70–92. doi: 10.1251/bpo107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griese M. Respiratory syncytial virus and pulmonary surfactant. Viral Immunol. 2002;15:357–363. doi: 10.1089/08828240260066279. [DOI] [PubMed] [Google Scholar]
- 16.Grubor B, Gallup JM, Meyerholz DK, et al. Enhanced surfactant protein and defensin mRNA levels and reduced viral replication during parainfluenza virus type 3 pneumonia in neonatal lambs. Clin Diagn Lab Immunol. 2004;11:599–607. doi: 10.1128/CDLI.11.3.599-607.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grubor B, Meyerholz DK, Ackermann MR. Collectins and cationic antimicrobial peptides of the respiratory epithelia. Vet Pathol. 2006;43:595–612. doi: 10.1354/vp.43-5-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344:1917–1928. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- 19.Kato N. Pyrogenicity of human adenoviruses. J Gen Virol. 2000;81:2611–2616. doi: 10.1099/0022-1317-81-11-2611. [DOI] [PubMed] [Google Scholar]
- 20.Kawashima K, Meyerholz DK, Gallup JM, et al. Differential expression of ovine innate immune genes by preterm and neonatal lung epithelia infected with respiratory syncytial virus. Viral Immunol. 2006;19:316–323. doi: 10.1089/vim.2006.19.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kutschera J, Tomaselli J, Maurer U, et al. Minor neurological dysfunction, cognitive development, and somatic development at the age of 3 to 7 years after dexamethasone treatment in very-low birth-weight infants. Early Hum Dev. 2005;81:281–287. doi: 10.1016/j.earlhumdev.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Le Cras TD, Spitzmiller RE, Albertine KH, et al. VEGF causes pulmonary hemorrhage, hemosiderosis, and air space enlargement in neonatal mice. Am J Physiol Lung Cell Mol Physiol. 2004;287:L134–L142. doi: 10.1152/ajplung.00050.2004. [DOI] [PubMed] [Google Scholar]
- 23.Lehmkuhl HD, Cutlip RC. Experimentally induced respiratory syncytial viral infection in lambs. Am J Vet Res. 1979;40:512–544. [PubMed] [Google Scholar]
- 24.McNamara PS, Smyth RL. The pathogenesis of respiratory syncytial virus disease in childhood. Br Med Bull. 2002;61:13–28. doi: 10.1093/bmb/61.1.13. [DOI] [PubMed] [Google Scholar]
- 25.Meyerholz DK, Grubor B, Fach SJ, et al. Reduced clearance of respiratory syncytial virus infection in a preterm lamb model. Microbes Infect. 2004;6:1312–1319. doi: 10.1016/j.micinf.2004.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyerholz DK, Grubor B, Gallup JM, et al. Adenovirus-mediated gene therapy enhances parainfluenza virus 3 infection in neonatal lambs. J Clin Microbiol. 2004;42:4780–4787. doi: 10.1128/JCM.42.10.4780-4787.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyerholz DK, Grubor B, Lazic T, et al. Monocytic/macrophagic pneumonitis following intrabronchial deposition of vascular endothelial growth factor in neonatal lambs. Vet Pathol. 2006;43:689–694. doi: 10.1354/vp.43-5-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neuzil KM, Tang YW, Graham BS. Protective role of TNF-α in respiratory syncytial virus infection in vitro and in vivo. Am J Med Sci. 1996;311:201–204. doi: 10.1097/00000441-199605000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Panitch HB. Bronchiolitis in infants. Curr Opin Pediatr. 2001;13:256–260. doi: 10.1097/00008480-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Platzker AC, Kitterman JA, Mescher EJ, et al. Surfactant in the lung and tracheal fluid of the fetal lamb and acceleration of its appearance by dexamethasone. Pediatrics. 1975;56:554–561. [PubMed] [Google Scholar]
- 32.Resch B, Pasnocht A, Gusenleitner W, Muller W. Rehospitalisations for respiratory disease and respiratory syncytial virus infection in preterm infants of 29–36 weeks gestational age. J Infect. 2005;50:397–403. doi: 10.1016/j.jinf.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez RJ. Management of respiratory distress syndrome: An update. Respir Care. 2003;48:279–286. [PubMed] [Google Scholar]
- 34.Sakaguchi A, Hirayama E, Hiraki A, et al. Nuclear export of influenza viral ribonucleoprotein is temperature-dependently inhibited by dissociation of viral matrix protein. Virology. 2003;306:244–253. doi: 10.1016/s0042-6822(02)00013-2. [DOI] [PubMed] [Google Scholar]
- 35.Shay DK, Holman RC, Newman RD, et al. Bronchiolitis-associated hospitalizations among US children, 1980–1996. J Am Med Assoc. 1999;282:1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 36.Shay DK, Holman RC, Roosevelt GE, et al. Bronchiolitis-associated mortality and estimates of respiratory syncytial virus-associated deaths among US children, 1979–1997. J Infect Dis. 2001;183:16–22. doi: 10.1086/317655. [DOI] [PubMed] [Google Scholar]
- 37.Smith PK, Wang SZ, Dowling KD, Forsyth KD. Leucocyte populations in respiratory syncytial virus-induced bronchiolitis. J Paediatr Child Health. 2001;37:146–151. doi: 10.1046/j.1440-1754.2001.00618.x. [DOI] [PubMed] [Google Scholar]
- 38.Soukup JM, Becker S. Role of monocytes and eosinophils in human respiratory syncytial virus infection in vitro. Clin Immunol. 2003;107:178–185. doi: 10.1016/s1521-6616(03)00038-x. [DOI] [PubMed] [Google Scholar]
- 39.Tan RC, Ikegami M, Jobe AH, et al. Developmental and glucocorticoid regulation of surfactant protein mRNAs in preterm lambs. Am J Physiol. 1999;277:L1142–L1148. doi: 10.1152/ajplung.1999.277.6.L1142. [DOI] [PubMed] [Google Scholar]
- 40.Tripp RA. Pathogenesis of respiratory syncytial virus infection. Viral Immunol. 2004;17:165–181. doi: 10.1089/0882824041310513. [DOI] [PubMed] [Google Scholar]
- 41.Viuff, Tjornehoj BK, Larsen LE, et al. Replication and clearance of respiratory syncytial virus: apoptosis is an important pathway of virus clearance after experimental infection with bovine respiratory syncytial virus. Am J Pathol. 2002;161:2195–2207. doi: 10.1016/S0002-9440(10)64496-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang SZ, Xu H, Wraith A, et al. Neutrophils induce damage to respiratory epithelial cells infected with respiratory syncytial virus. Eur Respir J. 1998;12:612–618. doi: 10.1183/09031936.98.12030612. [DOI] [PubMed] [Google Scholar]
- 43.Zhang H, Issekutz AC. Growth factor regulation of neutrophil-endothelial cell interactions. Leukoc Biol. 2001;70:225–232. [PubMed] [Google Scholar]