Abstract
Preterm and young neonates are prone to inadequate surfactant production and are susceptible to respiratory distress syndrome characterized by alveolar damage and hyaline-membrane formation. Glucocorticoid therapy is commonly used in preterm and young infants to enhance lung maturation and surfactant synthesis. Recently, vascular endothelial growth factor (VEGF) was suggested to be a novel therapeutic agent for lung maturation that lacked adverse effects in mice. The purpose of this study was to assess the safety of incremental concentration (0.0005, 0.005, and 0.05 mg/ml) and duration (16, 24, and 32 hours) of recombinant human VEGF after bronchoscopic instillation (10 ml) in neonatal lambs. High-dose VEGF caused locally extensive plum-red consolidation that was microscopically characterized by interstitial and alveolar infiltrates of cells that were morphologically and phenotypically (CD68+) consistent with monocytes/macrophages. T cells (CD3+) and B cells (CD79+) were located primarily in bronchus/bronchiole-associated lymphoid tissue and were not consistently altered by treatment with VEGF. The dose of VEGF had significant effects on both gross lesions (P < .0047) and microscopic monocyte/macrophage recruitment scores (P < .0001). Thus, the VEGF dose instilled into the lung greatly influenced cellular recruitment and lesion development. The post-dosing interval of VEGF in this study had minor impact (no statistical significance) on cellular recruitment. This study showed that airway deposition of VEGF in the neonatal lamb induces monocyte/macrophage recruitment to the lung and high doses can cause severe lesions. The cellular recruitment suggests further research is needed to define dosages that are efficacious in enhancing lung maturation while minimizing potential adverse effects.
Keywords: Lung maturation, macrophage, monocyte, respiratory distress syndrome, sheep, vascular endothelial growth factor
Preterm and young infants are at increased risk for respiratory distress syndrome (RDS) associated with inadequate surfactant expression. A lack of surfactant causes increased alveolar surface tension, hypoventilation/hypoxemia, and progression to cellular injury with the characteristic fibrinonecrotic exudate in alveolar lumens.21 Surfactant replacement is recognized as an effective prophylactic therapy for RDS, and glucocorticoids are often used pre- and postnatally in infants to stimulate lung growth and surfactant development. 5,22 However, neonatal glucocorticoid administration has been associated with adverse effects, including the following: hypertension, infection, gastrointestinal bleeding or perforation, hypertrophic cardiomyopathy, and lung and neural developmental abnormalities.5,11,13,24,25,30 The potential for adverse effects has prompted efforts to identify novel agents to stimulate lung maturation while minimizing the potential for adverse effects.
Vascular endothelial growth factor (VEGF) is well recognized as an important regulator of vasculogenesis and angiogenesis.7,10 However, in the lung, VEGF has additional functions, including stimulation of airway epithelial-cell proliferation and enhancing surfactant protein (SP)-A and SP-C mRNA expression.6 Recently, VEGF was given to preterm mice as a novel therapy for RDS.8 Intratracheal VEGF treatment enhanced lung maturation and increased the percentage of preterm pup survival, whereas adverse effects, such as neovascularization or pulmonary edema, were not evident.
The lamb model is often used in the study of perinatal lung development and disease.12,23 The purpose of this study was to characterize the safety of variable VEGF dose and time after instillation in the neonatal lamb model for future extrapolation to the preterm lamb model.
Materials and Methods
Animals
Healthy neonatal lambs (2–4 days of age) were acquired through Iowa State University’s Laboratory Animal Resources (Table 1), housed indoors in a temperature-controlled environment and grouped together in a pen. All procedures were approved by the animal care and use committee. Body temperatures of lambs were taken to further ensure the health status of the lambs. Antibiotic (ceftiofur 2.2 mg/kg/day, intramuscular) was administered during the course of the experiment to minimize potential for complications with bacterial pneumonia. Each lamb was given xylazine (0.1 mg/kg) intravenously (IV) for sedation and was placed in right lateral recumbency. A sterile bronchoscope was used to instill 10 ml sterile saline solution, recombinant human VEGF (0.05, 0.005, or 0.0005 mg/ml; endotoxin <100 pg/µg in stock solution; diluted with sterile saline solution and titrated to pH of 7.0; Catalog #PR2951A; Invitrogen, Carlsbad, CA, USA) or bovine serum albumin (BSA, 0.1 mg/ml) into the right mainstem bronchus just past the tracheal bifurcation. The lamb remained in right lateral recumbency for another 10 minutes and was given tolazoline (3 mg/kg, IV slowly) to reverse the sedation. During the postinstillation period and before euthanasia, no evidence of respiratory distress or alteration was noted. At the appropriate time point after instillation (16, 24, or 32 hours), each lamb was euthanized with IV sodium pentobarbital.
Table 1.
Experimental design, gross lesion, and microscopic monocyte/macrophage recruitment scores in neonatal lambs after administration of VEGF.
| Lamb No. |
Treatments, hour* | Gross Lesion Score† |
Monocyte/ Macrophage Recruitment Score‡ |
|
|---|---|---|---|---|
| Saline solution | ||||
| 1 | 16 | − | 1 | |
| 2 | 24 | − | 1 | |
| 3 | 32 | − | 1 | |
| VEGF (0.0005 mg/ml) | ||||
| 4 | 16 | − | 2 | |
| 5 | 24 | − | 2 | |
| 6 | 32 | − | 2 | |
| VEGF (0.005 mg/ml) | ||||
| 7 | 16 | − | 2 | |
| 8 | 16 | + | 1.5 | |
| 9 | 24 | − | 2.5 | |
| 10 | 24 | − | 2 | |
| 11 | 24 | + | 2 | |
| 12 | 32 | + | 2.5 | |
| 13 | 32 | ++ | 3 | |
| VEGF (0.05 mg/ml) | ||||
| 14 | 16 | + | 3.5 | |
| 15 | 24 | + | 3 | |
| 16 | 32 | +++ | 4 | |
| BSA (0.1 mg/ml) | ||||
| 17 | 32 | − | 1 | |
| 18 | 32 | − | 1 | |
Lambs were given 10 ml of sterile saline solution, BSA, or VEGF for a duration of 16, 24, or 32 hours.
Lungs lesions were scored (see Materials and Methods section) at necropsy as follows: no lesions (−), mild (+), moderate (++), severe (+++); VEGF dose had a significant effect on gross lesion score (P < .0047).
Average monocyte/macrophage infiltration was scored as follows: normal limits (1), mild (2), moderate (3), severe (4); VEGF dose had a significant effect on monocyte/macrophage infiltration (P < .0001).
Tissues
Lungs were removed from the thoracic cavity, examined for lesions at site of bronchoscopic deposition, and given a lesion score of “−” (no lesion), “+” (mild, earliest detectable plum-red discoloration), “++” (moderate, distinct plum-red discoloration and more firm to touch than adjacent normal lung), or “+++” (severe, distinct raised, plum-red discoloration with consolidation). Tissue sections were collected bilaterally from the cranial, middle, and caudal lobes (1 slide per region), placed in 10% neutral-buffered formalin, and processed routinely for hematoxylin and eosin (HE) staining or immunohistochemistry.
Immunohistochemistry
Sections (6-µm thick) were deparaffinized through a series of ethanol baths and washed (Tris-buffered saline solution, pH 7.6, 0.1 M). These were immersed in a 1% trypsin solution for 40 minutes and then washed 3 times. Slides were then placed in a solution of 10% normal sheep serum per 10% normal goat serum in phosphate-buffered saline solution (PBS). The sections were then placed in primary antibody solutions (4°C for 72 hours) against either CD68 (1 : 300 mouse monoclonal anti-CD68, Dakocytomation Inc., Carpinteria, CA, USA), CD3 (1:100 mouse monoclonal anti-CD3; Dakocytomation), or CD79 (1:100 mouse monoclonal anti-CD79-alpha; Dakocytomation) and then washed multiple times. Endogenous peroxidase was blocked with 3% hydrogen peroxide in PBS (40 minutes) followed by multiple washes. The sections were then immersed in the biotinylated secondary antibody (1 : 300 goat polyclonal anti-mouse; Dakocytomation; 45 minutes) solution, washed (3 times), placed in streptavidin conjugated horseradish peroxidase solution (Vector Laboratories Inc., Burlingame, CA, USA; 45 minutes), washed (3 times) and exposed to chromogen (Vector red; Vector Laboratories). The slides were counter stained in Harris hematoxylin, dehydrated through a series of ethanol baths and cover slipped.
Morphometry
Sections of right cranial and middle lung lobes (sites of treatment deposition) were compared with the control (saline solution and BSA) lungs. A pathologist (blinded, without knowledge of treatment status) characterized the morphology of the infiltrating leukocyte populations and scored the cellular infiltrate in the treated areas as 1, within normal limits; 2, mild thickening of alveolar septa by monocyte infiltration, with infrequent macrophages in alveolar lumens; 3, thickening of alveolar septa by monocyte/macrophage infiltration, with increased numbers of macrophages in alveolar lumens; or 4, sheets of monocytes/macrophages fill alveolar lumens and interstitial spaces. Each section was scored twice and the average score recorded.
Statistics
The means for each group interaction (time and dose) were determined for both gross lesion scores and monocyte/macrophage recruitment scores and then were transformed to a logarithmic scale. Transformed mean scores were analyzed by an unreplicated 2-way analysis of variance. Significance was determined to be at P < .05.
Results
Sterile saline solution was used as a procedural control and BSA (at 10 times the VEGF concentration) was used as a nonspecific protein control. At necropsy, lungs, which had been infused with sterile saline solution and BSA, lacked gross and microscopic lesions. Lungs contralateral to the infusion site lacked gross and microscopic lesions in all cases. Gross lesions that consisted of mild multifocal to locally extensive plum-red congestion of the right cranial/middle lobes were seen in VEGF-treated lambs 8, 11, 12, 14, and 15 (Table 1). Lung of lamb No. 13 had increased plum-red coloration and was slightly firm to the touch compared with the adjacent normal lung. Lung of lamb No. 16 had a locally extensive, well-demarcated plum-red, slightly raised lesion in the right cranial/middle lobes and was firm to the touch. The VEGF dose had a significant effect (P < .0047) on the gross lesion scores.
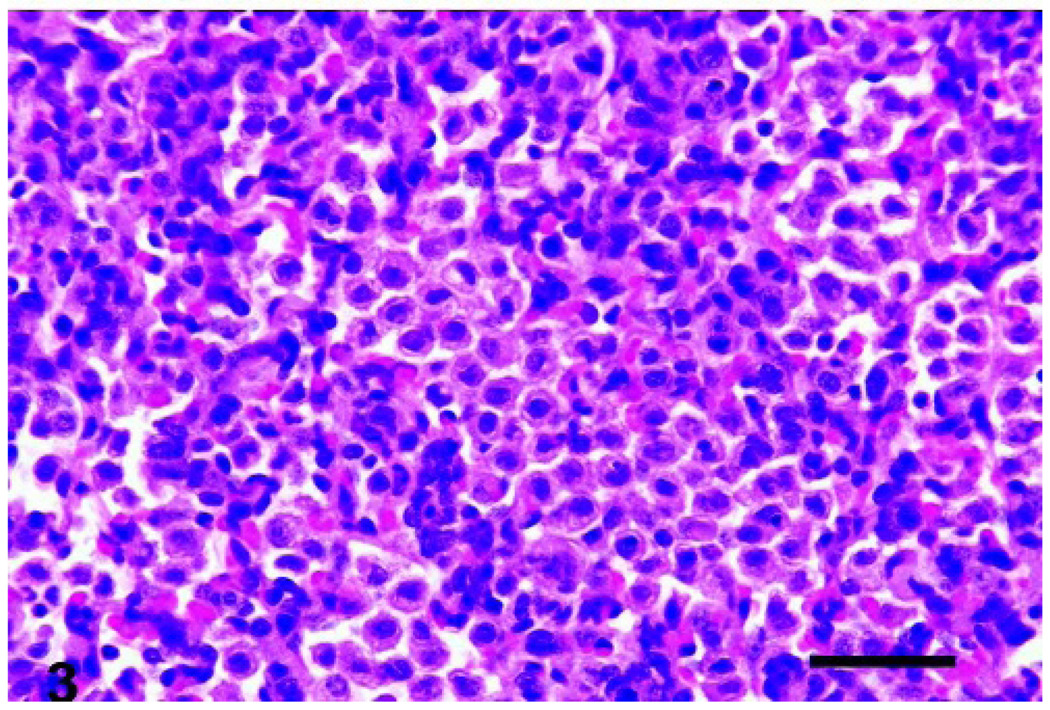
Microscopically, affected lungs had a cellular infiltrate in the alveolar lumen and septa that generally increased in severity according to the VEGF dosage. The cellular infiltrate was composed predominantly (~95%) of cells morphologically and phenotypically (CD68+) consistent with monocytes/macrophages (Fig. 1–3). T cells (CD3+) and B cells (CD79+) were localized primarily to aggregates surrounding airways, consistent with bronchus/bronchiole-associated lymphoid tissue, and the number of these cells was not consistently increased. Small numbers of neutrophils were seen in the alveolar septa. The microscopic cellular infiltration (monocytes/macrophages) score was significantly affected by the VEGF dose (P < .0001). The post-dosing interval of VEGF treatment produced minor (no statistical significance) alteration in the monocyte/macrophage recruitment.
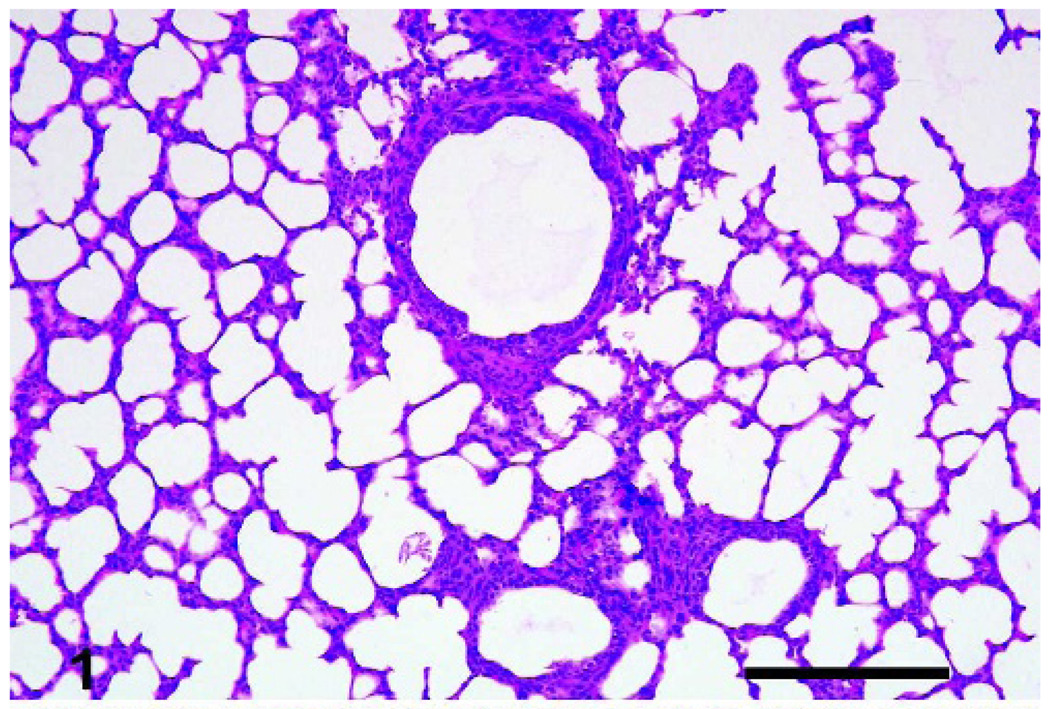
Fig. 1.
Lungs, lamb. Sections of control lungs lacked cellular infiltrate. Bar = 200 µm.
Fig. 3.
Lungs, lamb. The cellular infiltration in VEGF-treated lungs was composed primarily of monocytes/macrophages. Bar = 50 µm.
Discussion
The potential use of VEGF as a novel and safe perinatal therapy for RDS was recently suggested in preterm mice, and this created much interest for eventual use in pediatric patients.1,8,26 An appealing aspect of the VEGF therapy was the apparent lack of lesions and adverse effects, an important consideration when comparing VEGF with glucocorticoid therapy, which has documented short- and long-term adverse effects.5 In this study, bronchoscopic deposition of VEGF-induced monocyte/macrophage recruitment that progressively increased with the dose of VEGF. The high doses caused severe monocytic/macrophagic pneumonitis, with gross and microscopic lesions. The cellular infiltration was lacking in the sterile saline solution or BSA (nonspecific protein) group controls, as well as in all lamb lungs contralateral to the instillation site. The acute monocyte/macrophage recruitment was not consistent with cellular recruitment after antigen deposition in lung and was not consistent with any spontaneous natural respiratory disease of sheep.20,27 Furthermore, the cellular recruitment was not consistent with the predominantly neutrophilic inflammation that occurs hours after pulmonary lipopolysaccharide instillation in rats.15 In previous work, the administration of human VEGF either as a recombinant protein or by adenoviral-mediated gene transfer showed functional activity (e.g., angiogenesis or other vascular changes) in the lamb model.12,17 However, the recombinant human VEGF (rhVEGF) was administered by IV during gestation and not in the airways, whereas the instillation of adenovirus-mediated VEGF gene transfer was not morphologically evaluated for 3 to 4 weeks after administration. Thus, it is not feasible to directly compare the monocyte/macrophage recruitment or the lack of vascular alteration seen in this current study with those earlier experiments.
This experiment suggests that the monocytic recruitment seen in these lambs was a direct result of VEGF administration. Monocyte (macrophage) recruitment and activation by VEGF has been reported in the literature. For example, VEGF expression is associated with macrophage infiltration in human-breast carcinoma.19 Another study showed rhVEGF-induced monocyte adhesion and transmigration through human umbilical vein endothelial cells, and this was dependent on the interaction of monocyte β2-integrin with endothelial intercellular adhesion molecule-1.14 Recently, VEGF-mediated chemotaxis of monocytes/macrophages was shown to be critical for corneal neovascularization during inflammation.9 At least 2 of the VEGF receptors, VEGFR-1 (FLT-1) and VEGFR-2 (FLK-1), have been implicated in mediating this monocyte/macrophage activation and recruitment.7,29 Alternatively, the VEGF treatment may indirectly induce cellular recruitment through endothelial upregulation of chemokines, including monocyte chemotactic protein-1.28,31 Regardless of the mechanism, this study suggests that in the lamb model VEGF deposition in the lung can cause an acute dose-dependent recruitment of monocytes and macrophages.
The concentrations of VEGF deposited in this study ranged from 0.0005 to 0.05 mg/ml (× 10 ml) compared with the previous preterm mouse study, which used a concentration as high as 0.2 mg/ml (× 5 µl).8 The preterm mice had no monocyte/macrophage recruitment, even though a higher VEGF dose was used. This suggests that the total amount of VEGF administered may be more important than the solution concentration. Alternatively, this could merely reflect inherent physiologic differences in the animal models. While both the mouse and the lamb have been used for perinatal pulmonary research, there are distinct differences between the species regarding lung development. For instance, lambs and humans enter the alveolar stage of lung development late in gestation, whereas rodents begin alveolar development postnatally. 2,3,16 The stage of growth is relevant as developing alveolar epithelium is suggested to play an important role in regulating VEGF activity and alveolar capillary development.4 Although the duration of exposure did not appear to have significant impact in this study, recent work using transgenic mice with inducible VEGF expression showed that chronic VEGF expression can cause pulmonary hemorrhage, hemosiderosis, and increased perinatal mortality.18 Future efforts should further define the role of duration on cellular recruitment and lesions formation.
In conclusion, this study shows that airway deposition of VEGF in neonatal lambs causes pulmonary monocyte recruitment, with severe microscopic and gross lesions at high dosages. The VEGF-induced recruitment of monocytes/macrophages warrants caution and the need for further research in multiple species (rodent and nonrodent) before application to pediatric patients.
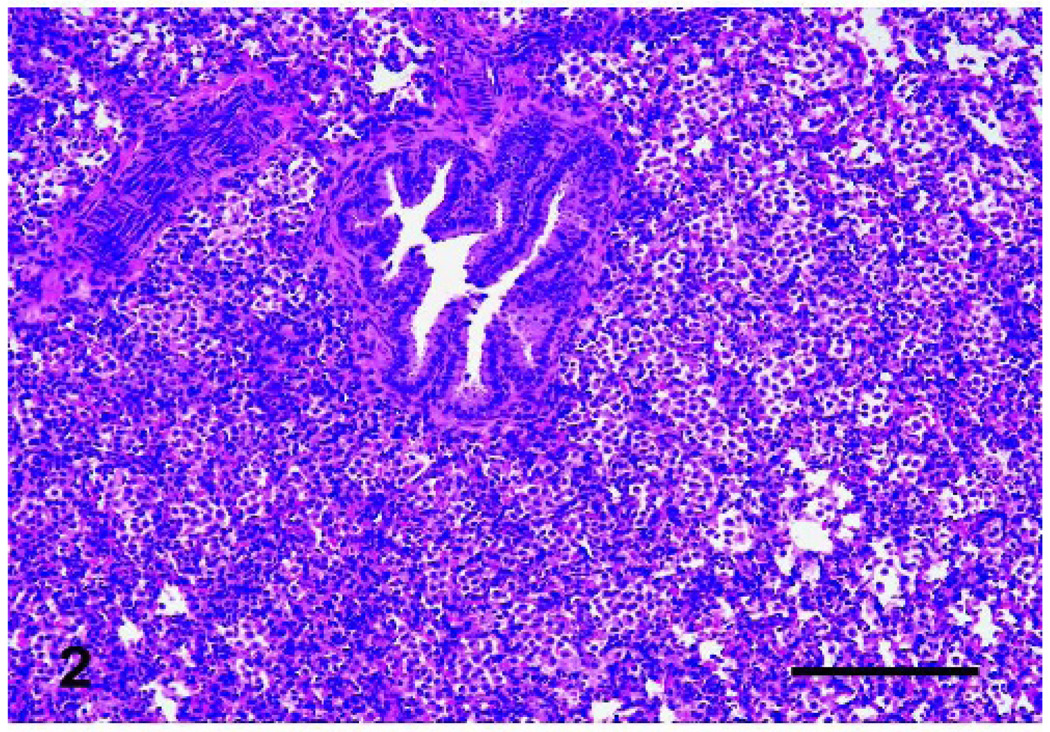
Fig. 2.
Lungs, lamb. Alveolar lumens and septa had severe cellular infiltration after high dose (0.05 mg/ml) VEGF treatment. Bar = 200 µm.
Acknowledgements
We would like to thank Dr. Jeanne M. Snyder (University of Iowa) for technical assistance. Funding for this work was provided in part by the NIH 5 K08 AI055499.
References
- 1.Abman S. Vascular endothelial growth factor: not only for vessels anymore. Pediatr Res. 2003;53:1. [Google Scholar]
- 2.Alcorn DG, Adamson TM, Maloney JE, Robinson PM. A morphologic and morphometric analysis of fetal lung development in the sheep. Anat Rec. 1981;201:655–667. doi: 10.1002/ar.1092010410. [DOI] [PubMed] [Google Scholar]
- 3.Amy RW, Bowes D, Burri PH, Haines J, Thurlbeck WM. Postnatal growth of the mouse lung. J Anat. 1977;124:131–151. [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatt AJ, Amin SB, Chess PR, Watkins RH, Maniscalco WM. Expression of vascular endothelial growth factor and Flk-1 in developing and glucocorticoid-treated mouse lung. Pediatr Res. 2000;47:606–613. doi: 10.1203/00006450-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Bolt RJ, Van Weissenbruch MM, Lafeber HN, Delemarre-van de Waal HA. Glucocorticoids and lung development in the fetus and preterm infant. Pediatr Pulmonol. 2001;32:76–91. doi: 10.1002/ppul.1092. [DOI] [PubMed] [Google Scholar]
- 6.Brown KR, England KM, Goss KL, Snyder JM, Acarregui MJ. VEGF induces airway epithelial cell proliferation in human fetal lung in vitro. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1001–L1010. doi: 10.1152/ajplung.2001.281.4.L1001. [DOI] [PubMed] [Google Scholar]
- 7.Clauss M. Functions of the VEGF receptor-1 (FLT-1) in the vasculature. Trends Cardiovasc Med. 1998;8:241–245. doi: 10.1016/s1050-1738(98)00015-2. [DOI] [PubMed] [Google Scholar]
- 8.Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, Nemery B, Dewerchin M, Van Veldhoven P, Plate K, Moons L, Collen D, Carmeliet P. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med. 2002;8:702–710. doi: 10.1038/nm721. [DOI] [PubMed] [Google Scholar]
- 9.Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D’Amore PA, Dana MR, Wiegand SJ, Streilein JW. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 11.George ME, Sharma V, Jacobson J, Simon S, Nopper AJ. Adverse effects of systemic glucocorticosteroid therapy in infants with hemangiomas. Arch Dermatol. 2004;140:963–969. doi: 10.1001/archderm.140.8.963. [DOI] [PubMed] [Google Scholar]
- 12.Grover TR, Parker TA, Markham NE, Abman SH. rhVEGF treatment preserves pulmonary vascular reactivity and structure in an experimental model of pulmonary hypertension in fetal sheep. Am J Physiol Lung Cell Mol Physiol. 2005;289:L315–L321. doi: 10.1152/ajplung.00038.2005. [DOI] [PubMed] [Google Scholar]
- 13.Halliday HL. The effect of postnatal steroids on growth and development. J Perinat Med. 2001;29:281–285. doi: 10.1515/JPM.2001.040. [DOI] [PubMed] [Google Scholar]
- 14.Heil M, Clauss M, Suzuki K, Buschmann IR, Willuweit A, Fischer S, Schaper W. Vascular endothelial growth factor (VEGF) stimulates monocyte migration through endothelial monolayers via increased integrin expression. Eur J Cell Biol. 2000;79:850–857. doi: 10.1078/0171-9335-00113. [DOI] [PubMed] [Google Scholar]
- 15.Herbein JF, Wright JR. Enhanced clearance of surfactant protein D during LPS-induced acute inflammation in rat lung. Am J Physiol Lung Cell Mol Physiol. 2001;281:L268–L277. doi: 10.1152/ajplung.2001.281.1.L268. [DOI] [PubMed] [Google Scholar]
- 16.Jobe AH, Ikegami M. Prevention of bronchopulmonary dysplasia. Curr Opin Pediatr. 2001;13:124–129. doi: 10.1097/00008480-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Lambert V, Michel R, Mazmanian GM, Dulmet EM, Capderou A, Herve P, Planche C, Serraf A. Induction of pulmonary angiogenesis by adenoviralmediated gene transfer of vascular endothelial growth factor. Ann Thorac Surg. 2004;77:458–463. doi: 10.1016/j.athoracsur.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Le Cras TD, Spitzmiller RE, Albertine KH, Greenberg JM, Whitsett JA, Akeson AL. VEGF causes pulmonary hemorrhage, hemosiderosis, and air space enlargement in neonatal mice. Am J Physiol Lung Cell Mol Physiol. 2004;287:L134–L142. doi: 10.1152/ajplung.00050.2004. [DOI] [PubMed] [Google Scholar]
- 19.Leek RD, Hunt NC, Landers RJ, Lewis CE, Royds JA, Harris AL. Macrophage infiltration is associated with VEGF and EGFR expression in breast cancer. J Pathol. 2000;190:430–436. doi: 10.1002/(SICI)1096-9896(200003)190:4<430::AID-PATH538>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Lopez A. Respiratory system, thoracic cavity and pleura. In: McGavin MD, Carlton WW, Zachary JF, editors. Thompson’s Special Veterinary Pathology. 3rd ed. Louis, MO: Mosby, St; 2001. pp. 125–195. [Google Scholar]
- 21.Maitra A, Kumar V. Diseases of infancy and childhood. In: Kumar V, Abbas AK, Fausto N, editors. Robbins and Cotran Pathologic Basis of Disease. 7th ed. Philadelphia, PA: Elsevier; 2005. pp. 469–508. [Google Scholar]
- 22.Mariencheck W, Crouch E. Modulation of surfactant protein D expression by glucocorticoids in fetal rat lung. Am J Respir Cell Mol Biol. 1994;10:419–429. doi: 10.1165/ajrcmb.10.4.8136157. [DOI] [PubMed] [Google Scholar]
- 23.Meyerholz DK, Grubor B, Fach SJ, Sacco RE, Lehmkuhl HD, Gallup JM, Ackermann MR. Reduced clearance of respiratory syncytial virus infection in a preterm lamb model. Microbes Infect. 2004;6:1312–1319. doi: 10.1016/j.micinf.2004.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Shea TM, Doyle LW. Perinatal glucocorticoid therapy and neurodevelopmental outcome: An epidemiologic perspective. Semin Neonatol. 2001;6:293–307. doi: 10.1053/siny.2001.0065. [DOI] [PubMed] [Google Scholar]
- 25.Pera A, Byun A, Gribar S, Schwartz R, Kumar D, Parimi P. Dexamethasone therapy and Candida sepsis in neonates less than 1250 grams. J Perinatol. 2002;22:204–208. doi: 10.1038/sj.jp.7210699. [DOI] [PubMed] [Google Scholar]
- 26.Rabinovitch M, Bland R. Novel notions on newborn lung disease. Nat Med. 2002;8:664–666. doi: 10.1038/nm0702-664. [DOI] [PubMed] [Google Scholar]
- 27.Rawn J, DeCamp MM, Jr, Swanson SJ, Warner A, Warren H, Mentzer SJ. Angiocentric recruitment of lymphocytes into the lung after the intrabronchial instillation of antigen. Exp Lung Res. 2000;26:89–103. doi: 10.1080/019021400269899. [DOI] [PubMed] [Google Scholar]
- 28.Yamada M, Kim S, Egashira K, Takeya M, Ikeda T, Mimura O, Iwao H. Molecular mechanism and role of endothelial monocyte chemoattractant protein-1 induction by vascular endothelial growth factor. Arterioscler Thromb Vasc Biol. 2003;23:1996–2001. doi: 10.1161/01.ATV.0000096208.80992.63. [DOI] [PubMed] [Google Scholar]
- 29.Yang ZF, Poon RT, Luo Y, Cheung CK, Ho DW, Lo CM, Fan ST. Up-regulation of vascular endothelial growth factor (VEGF) in small-for-size liver grafts enhances macrophage activities through VEGF receptor 2-dependent pathway. J Immunol. 2004;173:2507–2515. doi: 10.4049/jimmunol.173.4.2507. [DOI] [PubMed] [Google Scholar]
- 30.Yeh TF, Lin YJ, Lin HC, Huang CC, Hsieh WS, Lin CH, Tsai CH. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N Engl J Med. 2004;350:1304–1313. doi: 10.1056/NEJMoa032089. [DOI] [PubMed] [Google Scholar]
- 31.Zhao Q, Egashira K, Inoue S, Usui M, Kitamoto S, Ni W, Ishibashi M, Hiasa Ki K, Ichiki T, Shibuya M, Takeshita A. Vascular endothelial growth factor is necessary in the development of arteriosclerosis by recruiting/activating monocytes in a rat model of long-term inhibition of nitric oxide synthesis. Circulation. 2002;105:1110–1115. doi: 10.1161/hc0902.104718. [DOI] [PubMed] [Google Scholar]