Abstract
Preterm infants have increased susceptibility to severe manifestations of respiratory syncytial virus (RSV) infection. The cause(s) for this age-dependent vulnerability is/are not well-defined, but alterations in innate immune products have been implicated. In sheep, RSV disease severity has similar age-dependent characteristics and sheep have several related innate molecules for study during pulmonary infection including surfactant protein A (SP-A), surfactant protein D (SP-D), sheep beta defensin 1 (SBD1), monocyte chemotactic protein 1 (MCP1), and Toll-like receptor 4 (TLR4). However, the in vivo cellular gene expression as a response to RSV infection is poorly understood. In this study, the effect of RSV infection on expression of these innate immune genes was determined for bovine RSV-infected (bRSV + fluorescence) epithelial cells, adjacent cells lacking bRSV antigen (adjoining cells lacking fluorescence), and control cells from non-infected lung using laser capture microdissection (LCM) and real-time RT-PCR. Control lambs had increased expression of innate immune molecules in full term (term) compared to preterm epithelia with statistical significance in SBD1, SP-D, and TLR4 mRNA. Infected cells (bRSV + fluorescent cells) had consistently higher mRNA levels of SP-A (preterm and term), MCP1 (preterm and term), and SP-D (preterm). Interestingly, bRSV − cells of infected term lambs had significantly reduced SP-D mRNA expression compared to bRSV + and control epithelia, suggesting that RSV infected cells may regulate the adjacent epithelial SP-D expression. This study defines specific innate immune components (e.g., SBD1, SP-D, and TLR4) that have differential age-dependent expression in the airway epithelia. Furthermore, cellular bRSV infection enhanced certain innate immune components while suppressing adjacent cellular SP-D expression in term animals. These in vivo gene expression results provide a framework for future studies on age-dependent susceptibility to RSV and RSV pathogenesis.
INTRODUCTION
Respiratory syncytial virus (RSV), an enveloped single-strand RNA pneumovirus, is a globally important respiratory pathogen. RSV typically causes upper respiratory tract disease in most people; however, it is of significant concern in children, especially preterm and young infants who have an increased risk for severe disease including bronchiolitis, pneumonia, and rarely death (3, 7). Premature and young infants have impaired host defense elements that include: reduced monocyte and neutrophil function (19, 24), limited complement activity (33), altered cytokine expression (20), and immature epithelial cell phenotypes (9). Pulmonary epithelial cells are a major source of antimicrobial peptides and proteins (AMPs) such as β defensins and surfactant proteins A and D (SP-A and SP-D, respectively). Furthermore, respiratory epithelia can express signaling molecules (e.g., Toll-like receptor 4 [TLR4]) or chemotactic mediators (e.g., monocyte chemotactic protein 1α [MCP1]), which regulate the immune response to RSV infection and these innate immune components are being increasingly appreciated for their direct and indirect activities against viral infections (11, 22). While these epithelial derived factors are implicated in host defense against RSV, their cell-specific expression during early clearance of natural RSV infection is not well defined. Recently, levels of ovine SP-A, SP-D and sheep β defensin 1 (SBD1) mRNA were increased during clearance of parainfluenza virus 3 (PIV3) infections in newborn lambs, indicative of a role in resolution of viral infection (13).
Investigation of the mechanisms of RSV infection requires a documented animal model with comparable disease manifestations. Late term fetal and preterm lambs have proved valuable as pulmonary models for the study of surfactant gene ontogeny (4, 17) and for study of RSV infection (5, 21). Sheep have consistency with human RSV disease including similar age-dependent susceptibility, lesions, and expression of innate immunity genes. Recent work has demonstrated enhanced microscopic lesions with reduced RSV antigen clearance in preterm versus neonatal lambs, similar to RSV disease in infants (28).
The purpose of this work was to determine the expression of innate immune genes by pulmonary epithelia with or without bovine RSV (bRSV) infection in preterm and full term (term) lambs. We examined mRNA expression levels from RSV antigen + and − epithelial cells retrieved by laser capture microdissection (LCM) in preterm and term neonatal lambs.
MATERIALS AND METHODS
Lambs and bRSV
Use of lambs was approved by Iowa State University’s Animal Care and Use Committee. The surgical delivery of preterm lambs, viral inoculation and incubation time was performed as previously described to reproduce a standard animal RSV model for impaired clearance of RSV infection and assess cellular innate immune gene expression (28). Briefly, preterm lambs were derived from healthy date-mated adult commercial ewes via surgical uterotomy (day 138 of gestation, term = 147) or by natural birth for term lambs. Within the first hour, fresh colostrum (~200 ml) was given via a stomach tube and ceftiofur (2.2 mg/kg/day, intramuscular) was given to prevent potential bacterial complications (32). Lambs were acclimated one day and then inoculated with bovine respiratory syncytial virus [strain 375, 103–4 TCID50/ml] or sterile media (sham control) by means of intratracheal deposition (20 ml). At 6 days post-inoculation, the lambs were euthanatized with sodium pentobarbital. Lung tissues were collected from preterm lambs with bRSV (n = 6) or sterile media (n = 6) inoculation; and term lambs (147 days) with bRSV (n = 4) or sterile media (n = 4) inoculation.
Laser capture microdissection (LCM)
Lung tissues were embedded in OCT media, snap frozen and tissue sections cut on a cryostat (6 μm). The bRSV-infected cells (bRSV +) were identified by immunofluorescence using a monoclonal anti–bRSV-4 antibody (kindly provided by Dr. Kenneth Platt, Iowa State University) and a biotinylated goat anti-mouse secondary IgG (Kirkegaard and Perry, Gaithersburg, MD). After appropriate washes, tissues were exposed to a Cy3-streptavidin reagent (Rockland, Inc., Gilbertsville, PA). Stained cells were retrieved by LCM (PixCell II, Arcturus, Mountain View, CA) and approximately 100–300 fluorescent cells (containing bRSV antigen), as identified during LCM by IF-IHC and morphology, were laser captured onto HS LCM caps (Arcturus). Then using a different HS LCM cap, the nonstaining cells (adjacent epithelium lacking bRSV antigen) were collected. These cells were immediately adjacent to the previously detached bRSV + cells. Last, on yet another different HS LCM cap, lung of sterile media (sham control) inoculated lambs was collected for epithelial cells. Effort was made to retrieve similar proportions of bronchiolar and alveolar epithelium between the bRSV and sterile media lungs during LCM. After all cells of interest were harvested onto HS LCM caps, total RNA was isolated.
Quantiative PCR (qPCR)
Total RNA was isolated, processed for real-time RT-PCR (one-step), and assessed for mRNA levels of SP-A, SP-D, SBD-1, TLR4, MCP-1a, and bRSV, as described previously (10). Briefly, RNA was isolated using components of a commercial kit (Invitrogen, Carlsbad, CA). RNA samples were spun down, and 6 μl of each was used as “RNA template” in 30-μl fluorogenic one-step real-time qPCR reactions as carried out in 96-well PCR reaction plates (ABI, Forest City, CA) using a GeneAmp 5700 Sequence Detection System for detection and relative quantification of mRNA targets (Table 1). Each plate contained both target and endogenous reference reactions for each sample, and negative no-template control (nuclease-free water) for each target and endogenous reference were also included. All samples were represented in duplicate. Each target signal mRNA level was normalized to its respective ovRPS15 signal using a recently described mathematical model, value = [(Etarget)Δ Ct (control-treated)]/[(Eref)Δ Ct (control-treated)] (30).
Table 1.
Primers (forward and reverse) and probes for ovine gene expression assessed by real-time RT-PCR
| SBD1 | Fwd: 5′-CCATAGGAATAAAGGCGTCTGTG |
| Rev: 5′-CGCGACAGGTGCCAATCT | |
| Probe: 5′-6FAM-CCGAGCAGGTGCCCTAGACACATGA-TAMRA | |
| SP-A | Fwd: 5′-TGACCCTTATGCTCCTCTGGAT |
| Rev: 5′-GGGCTTCCAAGACAAACTTCCT | |
| Probe: 5′-6FAM-TGGCTTCTGGCCTCGAGTGCG-TAMRA | |
| SP-D | Fwd: 5′-ACGTCTGCAGCTGAGAAT |
| Rev: 5′-TCGGTCATGCTCAGGAAAGC | |
| Probe: 5′-6FAM-TTGACTCAGCTGGCCACAGCCCAGAACA-TAMRA | |
| MCP1 | Fwd: 5′-GCTGTGATTTTCAAGACCATCCT |
| Rev: 5′-GGCGTCCTGGACCCATT | |
| Probe: 5′-6FAM-AAAGAGTTTTGTGCAGACCCCAACC-TAMRA | |
| TLR4 | Fwd: 5′-GAGAAGACTCAGAAAAGCCTTGCT |
| Rev: 5′-GCGGGTTGGTTTCTGCAT | |
| Probe: 5′-6FAM-TAAACCCCAGAGTCCAGAAGGAACAGCA-TAMRA | |
| bRSV Nucleocapsid | Fwd: 5′-CAGTCAAGAATATTATGCTTGGTCATG |
| Rev: 5′-CCTAACTTTTGTGCATATTCATAGACTTC | |
| Probe: 5′-6FAM-CAACCTGTTCCATTTCTGCTTGTACGCTG-TAMRA | |
| Ovine Ribo S15 | Fwd: 5′-CGAGATGGTGGGCAGCAT |
| Rev: 5′-GCTTGATTTCCACCTGGTTGA | |
| Probe: 5′-VIC-CCGGCGTCTACAACGGCAAGACC-TAMRA |
6FAM or VIC = 5′ Fluorescent reporter dye, TAMRA = Fluorescent quencher dye.
Statistical analysis
First, summary statistics (means and standard errors of the mean) were calculated for each group to describe the data. Next, a two-way ANOVA was used to assess for group effects. Where significant group differences were detected, post-hoc tests (with Bonferroni correction) were performed on groups that had scientific relevance. Unless otherwise noted, statistical significance was set at p < 0.05.
RESULTS
On the final day of the infection, lambs inoculated with bRSV had clinical signs (e.g., elevated body temperatures, increased respiration rate, etc.) consistent with previous work (28). This study applied LCM and real-time RT-PCR techniques to assess innate immune gene expression of bRSV antigen positive and adjacent bRSV antigen negative epithelial cells in pre- and term infected lambs. This work demonstrates, on the cellular level, differential innate immune expression by age and infection status.
SP-A
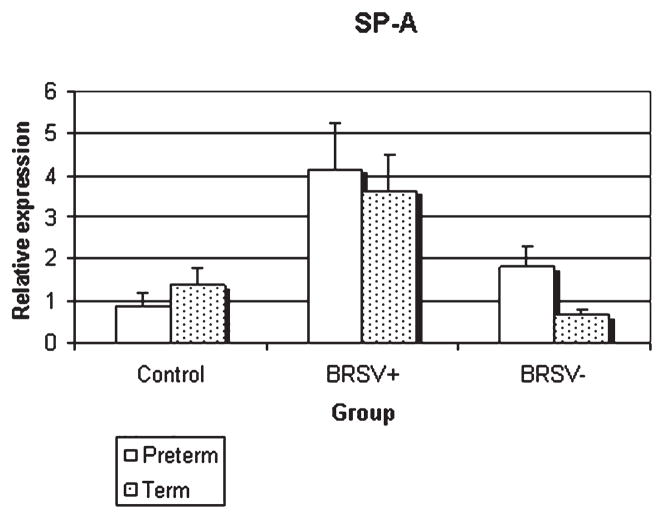
In preterm lamb lungs, epithelia containing bRSV antigen had elevated SP-A mRNA compared to adjacent epithelia lacking bRSV antigen (p < 0.05) and control epithelia (p < 0.01) (Fig. 1). In full term lamb lungs, the SP-A mRNA expression in epithelia with bRSV antigen was significantly elevated compared to adjacent cells lacking bRSV antigen (p < 0.05). Similar changes (close to statistical significance, p < 0.06) were seen compared to epithelia of term control lambs.
FIG. 1.
Relative SP-A mRNA expression in control and bRSV infected (bRSV antigen [+] and bRSV antigen [−] cells) pulmonary epithelia of preterm and term lambs. bRSV + cells had enhanced expression over either control (preterm, p < 0.01; term, p < 0.06) or bRSV − cells (preterm, p < 0.05; term, p < 0.05)
SP-D
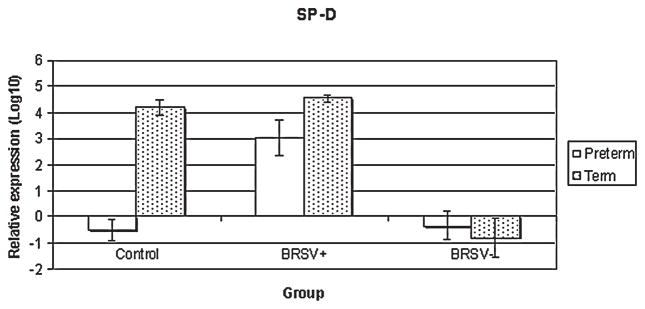
In preterm lambs, SP-D mRNA expression was elevated in bRSV antigen positive cells compared to bRSV antigen negative cells (p < 0.01) or control lamb epithelia (p < 0.01) (Fig. 2). In full term lambs, the bRSV antigen negative cells had reduced expression compared to either control (p < 0.01) or bRSV antigen positive cells (p < 0.01). Furthermore, the control term lambs had significantly elevated SP-D mRNA compared to preterm lambs (p < 0.00001).
FIG. 2.
Relative SP-D mRNA expression in control and bRSV infected (bRSV antigen [+] and bRSV antigen [−] cells) pulmonary epithelia of preterm and term lambs. In control epithelia, term lambs had enhanced SP-D mRNA expression compared to preterm animals (p < 0.00001). In preterm epithelia, bRSV + cells had enhanced SP-D expression over control (p < 0.01) or bRSV − epithelia (p < 0.01). In term epithelia, bRSV− cells of infected lambs had reduced expression compared to control (p < 0.01) or bRSV + epithelia (p < 0.01).
SBD1
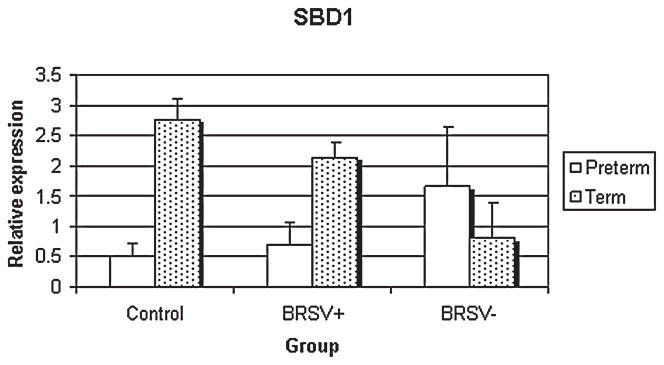
SBD1 mRNA had significantly enhanced expression with age (preterm versus full term) in the control (p < 0.05) (Fig. 3). No other scientifically relevant comparisons were statistically significant.
FIG. 3.
Relative SBD1 mRNA expression in control and bRSV infected (BRSV antigen [+] and bRSV antigen [−] cells) pulmonary epithelia of preterm and term lambs. Term lambs had greater SBD1 mRNA expression compared to preterm lambs in the control group (p < 0.05).
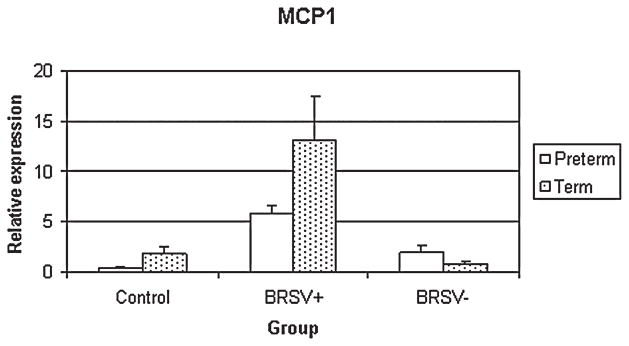
MCP1
In preterm lambs, MCP1 mRNA expression of the bRSV + epithelia was significantly increased compared to control (p < 0.05) epithelia (Fig. 4). The bRSV + epithelia of term lambs had increased expression over control (p < 0.01), bRSV− (p < 0.01) and preterm bRSV + epithelia (p < 0.05).
FIG. 4.
Relative MCP1 mRNA expression in control and bRSV infected (bRSV antigen [+] and bRSV antigen [−] cells) pulmonary epithelia of preterm and term lambs. The bRSV + preterm epithelia had enhanced MCP1 mRNA expression over control (p < 0.05). The bRSV + epithelia of term lambs had increased expression over control (p < 0.01), bRSV − (p < 0.01) and Preterm bRSV + epithelia (p < 0.05).
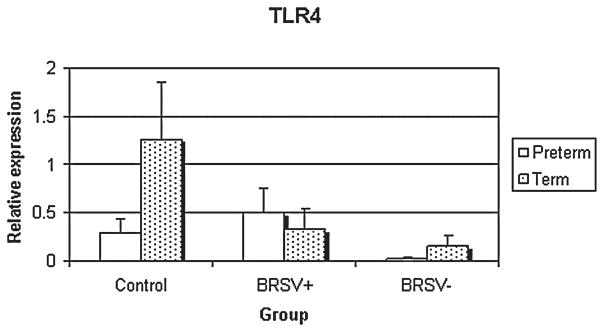
TLR4
Epithelia from the control lambs had significantly reduced TLR4 mRNA expression in preterm compared to term lambs (p < 0.05) (Fig. 5). In term lambs, TLR4 mRNA expression was significantly reduced in bRSV antigen negative cells compared to control epithelia (p < 0.05).
FIG. 5.
Relative TLR4 mRNA expression in control and bRSV infected (bRSV antigen [+] and bRSV antigen [−] cells) pulmonary epithelia of preterm and term lambs. The control term epithelia had enhanced expression compared to control preterm (p < 0.05) and bRSV − term epithelia (p < 0.05).
DISCUSSION
In this study, the influence of developmental age and bRSV infection status on SP-A, SP-D, SBD1, MCP1, and TLR4 mRNA expression were determined using laser capture microdissection (LCM) retrieved cells and real time quantitative polymerase chain reaction (qPCR).
Concerning surfactant proteins, SP-A mRNA expression generally increased in bRSV infected cells compared to adjacent non bRSV infected cells or cells from control lambs. This is similar to recent in vitro work with cultured human type II cells where SP-A mRNA was significantly increased in RSV infected cells (2). In that same study, SP-A secretion was reduced compared to controls and the authors suggested this disparity could be due to inefficient SP-A translation or increased protease activity by RSV infected cells. Assessment of translational products of these innate immune genes was not attempted in the current study. However, the neonatal lamb model of PIV3 infection suggests that whole-lung SP-A mRNA expression increases during clearance of PIV3 virus while whole-lung SP-A protein levels remains unchanged—similar to the in vitro study (2,13). RSV infected cells have increased activity of many initiator and effector caspases, which have functional protease activity and thus may, in part, explain reduced SP-A translational product (18). These caspases also participate in the mechanism of apoptosis, a proposed method in the eventual clearance of RSV infected cells (32). The increase in SP-A mRNA of bRSV infected cells of both preterm and term lambs suggests it is a fundamental cellular innate response to bRSV infection, but does not, however, explain age-dependent RSV disease severity.
SP-D expression in preterm lambs was similar to the expression pattern generally detected for SP-A with enhanced mRNA expression in bRSV infected cells. Interestingly, there was a distinct age-dependent difference of SP-D expression in the control group. SP-D plays an important role in RSV defense as SP-D knockout mice have reduced RSV clearance and increased inflammatory lesions compared to wild type mice (23). Similarly, the observation of reduced SP-D expression in preterm lambs compared to term lambs in the current study corresponds to the reduced clearance of bRSV previously described in preterm versus term lambs (28). This suggests that insufficient SP-D expression in preterm neonates may be a potential factor in the development of RSV disease.
In full term lambs, SP-D mRNA expression did not differ between control or bRSV infected epithelia; however, cells lacking bRSV antigen had reduced SP-D mRNA expression. Recent in vitro work on type II pneumocytes suggests SP-D regulation is more adapted for rapid alterations in synthesis, metabolism and secretion compared to SP-A (6). Furthermore, recent evidence suggests that the upstream promotor of the SP-D gene can be regulated by certain AP-1 elements including c-Jun and c-Fos as well as cytokines such as IL-4 (6, 16). Accordingly, adjacent inflammatory products may, in part, regulate SP-D expression in adjacent non bRSV infected cells of term lambs. The decreased SP-D mRNA expression of cells adjacent to bRSV infected cells is a novel finding and warrants further investigation.
Expression of SBD1 was developmentally regulated in control epithelia of lambs. The developmental regulation of SBD1 seen in this study is similar to sheep β defensin 2 (SBD2), another β defensin of sheep that is preferentially expressed in the gastrointestinal tract (27). bRSV infection of epithelial cells did not cause significant alteration of SBD1 mRNA expression when compared to control epithelia or cells lacking bRSV antigen. Recently, whole lung homogenates from lambs with acute Mannheimia hemolytica pneumonia were shown to have reduced SBD1 expression compared to uninfected controls (1). This contrasted another study in which PIV3 infected lambs had increased SBD1 expression late in the progression of the disease (13). The collective evidence on SBD1 regulation suggests that acute inflammation does not promote mRNA expression; however subacute to chronic processes such as epithelial regeneration/proliferation and differentiation may be more realistically associated with the increases in SBD1 mRNA expression (1, 13, 26). While this study did not detect statistically significant alteration of SBD1 expression in relation to bRSV status, the developmental regulation of SBD1 expression suggests that it cannot be ruled out as a participant in severe preterm RSV disease. Future work should focus on the later stages of bRSV infection/resolution to see if SBD1 expression is increased at later time periods.
MCP1 is a chemokine implicated in the pathogenesis of severe RSV infection of young infants and the secreted G-protein by RSV virus is thought to be responsible for significant upregulation of MCP1 expression (12, 25, 29). Enhanced pulmonary MCP1 expression can affect immune responses by leukocyte recruitment, mast cell degranulation and can influence the TH1/TH2 response (31). In the current study, full term lambs had increased MCP1 expression in bRSV infected epithelia over epithelial from adjacent non bRSV infected cells or control epithelia, and similar tendencies were detected in preterm lambs. The similar response by both age groups suggests that MCP1 expression may be involved in the typical bRSV clearance. Interestingly, the level of MCP1 expression by bRSV infected epithelia was significantly higher in term than in preterm lambs. This was unexpected since lambs/infants with increased maturity typically have reduced disease severity and MCP1 in human infants is often associated with severe RSV disease (12). One speculation may be that enhanced MCP1 mRNA expression by term over preterm lambs represents a protective rather than detrimental effect. Further efforts are needed to confirm and better define this discovery.
TLR4 is an important pattern recognition receptor of the innate immune system and is thought to play an important role in the innate immune response against RSV (15). In the present study, the significant developmental regulation of TLR4 expression in control epithelia is consistent with TLR4 ontogeny in the mouse lung (14). While there was significant developmental regulation in control epithelia, the only significant effect of bRSV infection status was decreased TLR4 expression in non–bRSV infected epithelia when compared to control epithelia. Recently, work in mice has suggested that the role of TLR4 in RSV clearance by different species may not be as universal as previously thought (8). The role of TLR4 in bRSV infection of lambs needs to be further addressed.
In conclusion, this novel study demonstrates the in vivo regulation of innate immune gene expression by developmental age as well as bRSV infection status. This study reaffirms age dependent regulation of innate immune genes that coincides with preterm RSV disease susceptibility, suggestive of interaction. Furthermore, this study shows that bRSV infection can alter not only infected epithelia, but also adjacent cellular innate immune gene expression.
Acknowledgments
This work was funded in part by National Institutes of Health NAIAD Awards 05R01AI062787-02 and 5K08AI055499-03, USDA/CSREES/NRI/CGP 2003-35204-13492, JG Salsbury Endowment Funds, and the Iowa Healthy Livestock Initiative.
References
- 1.Ackermann MR, Gallup JM, Zabner J, et al. Differential expression of sheep beta-defensin-1 and -2 and interleukin 8 during acute Mannheimia haemolytica pneumonia. Microb Pathogenesis. 2004;37:21–27. doi: 10.1016/j.micpath.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Alcorn JL, Stark JM, Chiappetta CL, et al. Effects of RSV infection on pulmonary surfactant protein SPA in cultured human type II cells: contrasting consequences on SP-A mRNA and protein. Am J Physiol Lung Cell Mol Physiol. 2005;289:L1113–L1122. doi: 10.1152/ajplung.00436.2004. [DOI] [PubMed] [Google Scholar]
- 3.Aujard Y, Fauroux B. Risk factors for severe respiratory syncytial virus infection in infants. Resp Med. 2002;96(suppl):S9–S14. [PubMed] [Google Scholar]
- 4.Bachurski CJ, Ross GF, Ikegami M, et al. Intra-amniotic endotoxin increases pulmonary surfactant proteins and induces SP-B processing in fetal sheep. Am J Physiol Lung Cell Mol Physiol. 2001;280:L279–285. doi: 10.1152/ajplung.2001.280.2.L279. [DOI] [PubMed] [Google Scholar]
- 5.Belknap EB, Ciszewski DK, Baker JC. Experimental respiratory syncytial virus infection in calves and lambs. J Vet Diagn Invest. 1995;7:285–298. doi: 10.1177/104063879500700226. [DOI] [PubMed] [Google Scholar]
- 6.Cao Y, Tao JQ, Bates SR, et al. IL-4 induces production of the lung collectin surfactant protein-D. J Allergy Clin Immunol. 2004;113:439–44. doi: 10.1016/j.jaci.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham CK, McMillan JA, Gross SJ. Rehospitalization for respiratory illness in infants of less than 32 weeks gestation. Pediatrics. 1991;88:527–532. [PubMed] [Google Scholar]
- 8.Ehl S, Bischoff R, Ostler T, et al. The role of Toll-like receptor 4 versus interleukin-12 in immunity to respiratory syncytial virus. Eur J Immunol. 2004;34:1146–1153. doi: 10.1002/eji.200324449. [DOI] [PubMed] [Google Scholar]
- 9.Flecknoe SL, Wallace MJ, Cock ML, et al. Changes in alveolar epithelial cell proportions during fetal and postnatal development in sheep. Am J Physiol Lung Cell Mol Physiol. 2003;285:L664–L679. doi: 10.1152/ajplung.00306.2002. [DOI] [PubMed] [Google Scholar]
- 10.Gallup JM, Kawashima K, et al. New quick method for isolating RNA from laser captured cells stained by immunofluorescent immunohistochemistry; RNA suitable for direct use in fluorogenic TaqMan one-step real-time RT-PCR. Biol Proced Online. 2005;7:70–92. doi: 10.1251/bpo107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garofalo RP, Haeberle H. Epithelial regulation of innate immunity to respiratory syncytial virus. Am J Respir Cell Mol Biol. 2000;23:581–585. doi: 10.1165/ajrcmb.23.5.f204. [DOI] [PubMed] [Google Scholar]
- 12.Garofalo RP, Hintz KH, Hill V, et al. A comparison of epidemiologic and immunologic features of bronchiolitis caused by influenza virus and respiratory syncytial virus. J Med Virol. 2005;75:282–289. doi: 10.1002/jmv.20268. [DOI] [PubMed] [Google Scholar]
- 13.Grubor B, Gallup JM, Meyerholz DK, et al. Enhanced surfactant protein and defensin mRNA levels and reduced viral replication during paramyxoviral pneumonia in neonatal lambs. Clin Diagn Lab Immunol. 2004;11:599–607. doi: 10.1128/CDLI.11.3.599-607.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harju K, Glumoff V, Hallman M. Ontogeny of Toll-like receptors Tlr2 and Tlr4 in mice. Pediatr Res. 2001;49:81–83. doi: 10.1203/00006450-200101000-00018. [DOI] [PubMed] [Google Scholar]
- 15.Haynes LM, Moore DD, Kurt-Jones EA, et al. Involvement of toll-like receptor 4 in innate immunity to respiratory syncytial virus. J Virol. 2001;75:10730–10737. doi: 10.1128/JVI.75.22.10730-10737.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He Y, Crouch EC, Rust K, et al. Proximal promoter of the surfactant protein D gene: regulatory roles of AP-1, forkhead box, and GT box binding proteins. J Biol Chem. 2000;275:31051–31060. doi: 10.1074/jbc.M003499200. [DOI] [PubMed] [Google Scholar]
- 17.Ikegami M, Jobe AH. Injury responses to different surfactants in ventilated premature lamb lungs. Pediatr Res. 2002;51:689–695. doi: 10.1203/00006450-200206000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Kotelkin A, Prikhod’ko EA, Cohen JI, et al. Respiratory syncytial virus infection sensitizes cells to apoptosis mediated by tumor necrosis factor-related apoptosis-inducing ligand. J Virol. 2003;77:9156–9172. doi: 10.1128/JVI.77.17.9156-9172.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kramer BW, Jobe AH, Ikegami M. Monocyte function in preterm, term, and adult sheep. Pediatr Res. 2003;54:52–57. doi: 10.1203/01.PDR.0000066621.11877.33. [DOI] [PubMed] [Google Scholar]
- 20.Krishnan S, Craven M, Welliver RC, et al. Differences in participation of innate and adaptive immunity to respiratory syncytial virus in adults and neonates. J Infect Dis. 2003;188:433–439. doi: 10.1086/376530. [DOI] [PubMed] [Google Scholar]
- 21.Lehmkuhl HD, Cutlip RC. Experimentally induced respiratory syncytial viral infection in lambs. Am J Vet Res. 1979;40:512–44. [PubMed] [Google Scholar]
- 22.Lehrer RI. Primate defensins. Nat Rev Microbiol. 2004;2:727–738. doi: 10.1038/nrmicro976. [DOI] [PubMed] [Google Scholar]
- 23.LeVine AM, Elliott J, Whitsett JA, et al. Surfactant protein-d enhances phagocytosis and pulmonary clearance of respiratory syncytial virus. Am J Respir Cell Mol Biol. 2004;31:193–199. doi: 10.1165/rcmb.2003-0107OC. [DOI] [PubMed] [Google Scholar]
- 24.Levy O, Martin S, Eichenwald E, et al. Impaired innate immunity in the newborn: newborn neutrophils are deficient in bactericidal/permeability-increasing protein. Pediatrics. 1999;104:1327–1333. doi: 10.1542/peds.104.6.1327. [DOI] [PubMed] [Google Scholar]
- 25.Maher CF, Hussell T, Blair E, et al. Recombinant respiratory syncytial virus lacking secreted glycoprotein G is attenuated, non-pathogenic but induces protective immunity. Microbes Infect. 2004;6:1049–1055. doi: 10.1016/j.micinf.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Meyerholz DK, Ackermann MR. Antimicrobial peptides and surfactant proteins in ruminant respiratory tract disease. Vet Immunol Immunopathol. 2005;108:91–96. doi: 10.1016/j.vetimm.2005.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyerholz DK, Gallup JM, Grubor BM, et al. Developmental expression and distribution of sheep beta-defensin-2. Dev Comp Immunol. 2004;28:171–178. doi: 10.1016/s0145-305x(03)00105-8. [DOI] [PubMed] [Google Scholar]
- 28.Meyerholz DK, Grubor B, Fach SJ, et al. Reduced clearance of respiratory syncytial virus in a preterm lamb model. Microbes Infect. 2004;6:1312–1319. doi: 10.1016/j.micinf.2004.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olszewska-Pazdrak B, Casola A, Saito T, et al. Cell-specific expression of RANTES, MCP-1, and MIP-1alpha by lower airway epithelial cells and eosinophils infected with respiratory syncytial virus. J Virol. 1998;72:4756–4764. doi: 10.1128/jvi.72.6.4756-4764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rose CE, Jr, Sung SS, Fu SM. Significant involvement of CCL2 (MCP-1) in inflammatory disorders of the lung. Microcirculation. 2003;10:273–288. doi: 10.1038/sj.mn.7800193. [DOI] [PubMed] [Google Scholar]
- 32.Viuff B, Tjornehoj K, Larsen LE, et al. Replication and clearance of respiratory syncytial virus: apoptosis is an important pathway of virus clearance after experimental infection with bovine respiratory syncytial virus. Am J Pathol. 2002;161:2195–2207. doi: 10.1016/S0002-9440(10)64496-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolach B, Dolfin T, Regev R, et al. The development of the complement system after 28 weeks gestation. Acta Paediatr. 1997;86:523–527. doi: 10.1111/j.1651-2227.1997.tb08924.x. [DOI] [PubMed] [Google Scholar]