Abstract
Both cross-sectional studies of chronic pain and sleep deprivation experiments suggest a bi-directional relationship between sleep and pain. Few longitudinal studies, however, have assessed whether acute-insomnia following traumatic injury predicts the development of persistent pain. We sought to evaluate: 1) whether in-hospital insomnia independently predicts long-term pain after burn injury and 2) whether in-hospital pain predicts future insomnia symptoms. We analyzed data on 333 subjects hospitalized for major burn injury (72.7% male; mean age = 41.1 years ±14.5) who were participating in the multi-site, Burn Model System project. Subjects completed measures of health, function (SF-36), and psychological distress (Brief Symptom Inventory) while in-hospital, at 6, 12, and 24 months after discharge. Participants were categorized as either having or not having sleep onset insomnia at discharge. Linear mixed effects analyses revealed that persons reporting insomnia at discharge (40.5%) had significantly decreased improvement in pain and increased pain severity during long-term follow up (p<.001). More severe pain during the week preceding hospital discharge, time from injury, lack of college education and older age also contributed independent effects on chronic pain (p<.05). In a reciprocal model (N = 299), more severe pain during the week preceding discharge predicted increased rates of long-term sleep onset insomnia. In-hospital insomnia and preburn mental health symptoms were also highly significant predictors of insomnia. This study provides support for a long-term, prospective and reciprocal interaction between insomnia and pain. Future work should ascertain whether treatment of insomnia and pain during acute injury can prevent or minimize chronic pain.
Keywords: Pain, insomnia, sleep, burn injury, neuropathic pain, depression, anxiety, Burn Model Systems
Introduction
Sleep deprivation causes hyperalgesia in both animals and humans (26) and may impair pain inhibitory mechanisms implicated in the pathophysiology of chronic pain (46). Several sleep diary studies in individuals with intractable pain have found evidence for a bi-directional relationship between pain and sleep measured proximally. Increased daytime pain is linked with poor nighttime sleep and poor sleep is, in turn, associated with augmented next day pain (1;40;41;48). Few studies, however, have examined whether sleep disturbance predicts the development of chronic pain.
Nicassio and Wallston (1992) reported that sleep disturbance failed to predict pain 6 months later in rheumatoid arthritis (RA), but baseline pain predicted sleep disturbance (37). Another RA study, however, found a reciprocal relationship between prolonged nighttime awakening and pain severity measured at 6 months (13). Mikkelsson et al. (1999) have reported that sleep disturbance and depression severity increase the 1-year risk for children with neck pain to develop widespread pain (34). Similarly, Gupta et al. (2006) found that subjective sleep problems increased risk for developing widespread pain within 15 months (16).
These data suggest that sleep disturbance and psychosocial distress may both contribute to the exacerbation and spreading of pain over time. It remains unclear whether insomnia symptoms, however, predict incident cases of chronic pain, because the available data are derived largely from populations with established chronic pain conditions, and/or the analyses did not control for preexisting pain. One acute injury model that might be useful in this regard is serious burn injury. Because serious burns involve substantial nerve damage, tissue loss, and scarring, patients are at high risk for chronic pain. Prevalence rates range between 35% – 52% (8;10;32) and persistent neuropathic symptoms are experienced by as many as 80% of patients (31).
In addition to pain, both insomnia and psychological distress have also been identified as significant burn injury sequealae (9;15;49;51). During hospitalization, multiple proximal factors interfere with sleep, including: hypermetabolism, circadian dysregulation, pain (40;43), and environmental factors (19). Rates of sleep disturbance range between 50–73% (3;27) and several studies have documented that sleep complaints persist at high levels several months to 11 years post discharge (23;27;30).
In this secondary data analysis of the Burn Model System Study, we sought to evaluate: 1) whether insomnia symptoms during the week prior to discharge independently predict chronic pain at follow-up, after controlling for discharge pain severity, burn injury severity, psychological distress, premorbid pain and health status and 2) conversely, the extent to which pain during the week prior to discharge predicts long-term insomnia symptoms, after controlling for insomnia at time of discharge, psychological distress and other relevant covariates.
Methods
Study Population
Participants were recruited from consecutive admissions to 1 of the 3 regional burn centers contributing data on adults to the Burn Model System database, i.e., Johns Hopkins University, University of Texas, and University of Colorado. All subjects included in this analysis met American Burn Association criteria for major burns, and provided informed consent for the longitudinal Burn Model Systems study, sponsored by the National Institute on Disability and Rehabilitation Research (22). The data reported here were gathered between 05/01/1994 and 12/31/2001 as part of this larger study. It should be noted, however, that the Brief Symptom Inventory (BSI), used to identify sleep onset insomnia complaints, was dropped from the Burn Model System study methods to reduce participant burden on 06/01/2000. The protocol was approved by the institutional review boards for each of the universities involved. For the purposes of our primary aim, which was to evaluate whether insomnia status during the week prior to discharge predicted pain on long-term follow-up, after controlling for burn injury severity, pre-injury pain, discharge pain and psychological distress, we included only individuals who had completed, at a minimum, the SF-36 Health Survey’s Bodily Pain subscale, the sleep item (#25) from the BSI at discharge, and at least 1 follow-up SF-36 Bodily Pain Index (SF-36 BP) measure. We focused our analyses on adults ≥ 18 years of age. The initial sample size meeting this minimal inclusion criteria was N = 333. For the secondary aim, evaluating whether discharge pain predicted long-term insomnia, we included individuals who had completed at least 1 follow up BSI administration. Because the BSI was discontinued on 06/01/2000, the sample size for this analysis reduced to N=299.
Table 1 describes sample characteristics, clinical burn parameters, and retrospectively measured premorbid physical and psychological function for the 333 subjects whose data were used in the primary analysis. These characteristics are similar for the 299 subjects whose data were used for the secondary analysis reporting whether discharge pain predicts long-term insomnia. Injury circumstances were categorized as work-related (24%), non-work, non-intentional (46%), or, suspected abuse, assault, self-inflicted or arson (29%). Injury agents included flame (57%), scalding (13%), or grease (8.7%). Amputations were performed in 6.0% of cases. These characteristics are similar to data reported in the National Burn Registry (NBR) for the period 1995 – 2005 with records on more than 126,000 individuals admitted to hospitals in 30 states and the District of Columbia with burn injury (35).
Table 1.
Sample Characteristics
| (n = 333) | |
|---|---|
| Demographics | |
| Age [mean ± sd (median)] | 40.9 ± 14.0 (40.3) |
| Male (n & %) | 232 (66.9%) |
| Ethnicity (n & %) | |
| White | 237 (72.0%) |
| African American | 67 (20.4%) |
| Hispanic | 19 (5.8%) |
| Other | 6 (1.8%) |
| Marital Status (n & %) | |
| Currently Married | 156 (47.3%) |
| Single (Never Married, Divorced,Widowed) | 175 (52.7%) |
| Employment Status (n & %) | |
| Employed | 256 (74.1%) |
| Not working | 76 (25.9%) |
| Burn Injury Severity [mean ± sd (median)] | |
| ICU days | 7.8 ± 15.0 (0) |
| O.R. trips | 1.7 ± 1.6 (1) |
| Ventilation days | 2.9 ± 8.0 (0) |
| TBSA% Burned | 18.8 ± 16.4 (14) |
| TBSA% Grafted | 9.2 ± 10.9 (5.0) |
| Burn Locations (n & %) | |
| Hand | 209 (63.9%) |
| Face, Head or Neck | 156 (48.3%) |
| Trunk | 178 (55.1%) |
| Arm | 217 (66.2%) |
| Leg | 180 (55.4%) |
| Feet | 87 (27.0%) |
| Pre-burn SF-36 mean ± sd (median)] | |
| Physical Functioning | 89 ± 25.1 (100) |
| Role-Physical | 84.2 ± 27.3 (100) |
| Bodily Pain | 83.7 ± 24.4 (100) |
| General Health | 78.0 ± 19.4 (82) |
| Vitality | 69.7 ± 21.0 (75) |
| Social Functioning | 85.9 ± 23.5 (100) |
| Role-Emotional | 85.7 ± 25.4 (100) |
| Mental Health | 79.0 ± 19.7 (85) |
Procedures
Following a description of study procedures, subjects signed informed consent agreeing to participate in the 2-year study. All self-report measures were administered by trained personnel, using standard procedures as previously described in detail (22). Baseline measures were obtained prior to discharge. The follow-up protocol involved scheduling an in-person interview @ 6 months, 1 year, and 2 year follow-up time points. If contact was not made or data gathering not yet completed, this was followed by a first mailing, then a second mailing to non-responders, and finally, a search for new address, phone number or listed contacts. The allowable period of time to complete the assessment at a given follow-up was defined as starting 1 month prior to the scheduled date, and ending 2 months after the scheduled date. Thus, redundancy was built into the design to maximize data collection, since attrition is common in this population due to problems such as, frequent changes of address from home destruction, loss of job, avoidance of reminders of the injury, and fear of collection agency contact, etc.
Measures
For the purpose of this report, we analyzed data from the following measures:
Demographic, Injury and Treatment Variables
In addition to demographics, standard clinical burn injury and treatment parameters reported in Table 1 were obtained by chart review, e.g., inhalation injury requiring endotracheal intubation; percent total body surface area burned (TBSA); percent TBSA grafted; location and presence of deep partial thickness or full-thickness burn injuries, burn-related amputations, etc.
Sleep Onset Insomnia
Brief Symptom Inventory (BSI)
The BSI is a widely used, well-validated 53-item version of the Symptom Checklist 90-Revised (11). To assess the presence of sleep onset insomnia symptoms at each time point, we evaluated the response to the BSI sleep item (#25), “How much were you distressed in the past 7 days by trouble falling asleep.” Responses, “moderately,”“quite a bit,” or “extremely,” were classified as positive for sleep onset insomnia symptoms. Responses, “not all” and “a little bit,” were classified as negative for insomnia.
Pain Severity and Health
Health Survey Short Form - 36 (SF-36)
The SF-36 is a 36-item quality of life measure that includes summary scales reflecting patient-reported physical and psychological function and 8 primary index scales. The acute form of the SF-36 was administered within a day or so prior to discharge from acute hospitalization, with reference to health and function during the 7 day period prior to discharge. The SF-36 is one of the most widely-used health status measures in the world; it possesses excellent psychometric properties across a wide variety of patient and non-patient samples (5;33;52). Scale scores range from “0,” indicating extreme dysfunction or symptom severity to “100,” indicating optimal function. The following 3 scales were used as a described below. The Bodily Pain (BP) scale is a two-item scale that taps pain severity and interference. The Mental Health Scale is a 5-item scale measuring depressed mood and anxiety. The General Health Scale is a 5-item self-evaluation of overall health status. Additionally, during their hospital stay, patients completed the SF-36 in a retrospective manner, with reference to their pre-injury functioning during the 1 month prior to injury. Patients’ estimates of pre-injury General Health (GH) and Bodily Pain (BP) on the SF-36 were included in the models as control variables. The SF-36 pain scale has been used in several burn studies (28;36) and shown to differentiate burn patients with contractures from those without contractures (28). A particular advantage of the SF-36 is that it allows comparison with population norms, including patients with burn injury (36). The SF-36 Bodily Pain Index has also been used in other prospective studies of pain following acute trauma, such as spinal cord injury, e.g., (20) and it has been found to be sensitive/responsive to treatment in chronic sciatic-related pain (14). At least one psychometric study has used the SF-36 bodily pain score as a "gold standard" for the assessment of persistent pain, against which other, newer instruments were compared (45).
Analyses
Sample Attrition and Incomplete Data
Of the initial 333 subjects who were age 18 or older, who had BSI data at discharge, and at least one follow up SF-36 Bodily Pain observation, we found the following attrition rates due to drop out or intermittent non-response: 6 month, 23% (n = 255); 1 year, 8.3% (n = 234); 2 year, 18.8% (n =190). All available follow up data were utilized, such that if a subject missed the 3 month follow up, but completed the 6 month follow up, this subject was retained in the multilevel models. These attrition rates are typical for this population. For the analyses predicting SF-36 bodily pain: 106 (31.8%) subjects had 2 observations, 109 (32.8%) had 3 observations and 118 (35.4%) had all 4 observations. Conversely, for the analyses predicting BSI insomnia, 122 (40.8%) had 2 observations, 111 (37.1%) had 3 observations, and 66 (22.1%) had 4 observations. Because BSI administration was discontinued after 6/01/00, analyses evaluating whether discharge pain predicts long-term insomnia are based on 299 subjects with some of these missing observations due to the fact that the BSI was discontinued while subject were in the long-term follow up. To determine whether attrition may have biased the findings, we conducted logistic regression analyses, which revealed that neither BSI insomnia status at anytime point, nor SF-36 pain scores at any time point, predicted missing data (p values ranged from .21 to .41). Therefore, we are confident that our findings with respect to pain and sleep are not skewed by study attrition. Not surprisingly, however, there were significant, differential attrition rates by ethnicity, age, education, sex and burn severity (p<0.05). Thus, we included these factors as covariate predictors in the primary regression analyses as described below.
Statistical Procedures
To assess the contribution of self-reported insomnia at discharge to changes in pain over time, we used repeated-measures analytical techniques. Both conditional full likelihood-based linear mixed-effects models (LME) (25) and marginal quasi-score Generalized Estimating Equations (GEE) (53) were used to model the correlation structure of the repeated measures within each patient. Both methods allow for estimation of population effects while accounting for within-subject correlations. Results from LME were very similar to those given by GEE. We therefore chose to report the results from LME for normally distributed response variables (SF-36 BP), because this method is valid under less constrictive missing data assumptions. We report GEE for dichotomous dependent variables as is commonly practiced (12). Different structures for the subject-specific error covariance were examined, including compound symmetry, variance components, Huynh-Feldt and Toeplitz. The latter was chosen because it maximized the model likelihood.
We conducted analyses to evaluate the distributions of the BSI insomnia item and the SF-36 bodily pain indices for use both as independent and dependent variables in the regression models. Because the BSI sleep-item displayed a positively skewed distribution (discharge BSI frequencies: response 0 = 112 (33.6%); response 1 = 91(27.3%); response 2 = 55(16.5%); response 3 = 48 (14.4%); response 4 = 27(8.1%), we dichotomized this item. Responses, 2-“moderately,” 3-“quite a bit,” or 4-“extremely,” were classified as positive for sleep onset insomnia symptoms. Responses, 0-“not all” and 1-“a little bit,” were classified as negative for insomnia. We selected this cut point, based on both the wording of the item and the sample distribution; achieving a median split and a dichotomization reflecting those reporting a moderate to severe degree of sleep onset insomnia symptoms and those reporting only mild to minimal symptoms. We then ran separate regression models with the 5- item BSI and the dichotomous BSI variable, both as an independent variable predicting pain and as the dependent variable, predicted by pain. Although the 5-item BSI scores are ordinal data, we treated these raw BSI scores as a continuous dependent variable in the LME models as supported by the central limit theorem and delta method (2;39). The analyses using the dichotomized BSI dependent variable (GEE) yielded a similar pattern of significant predictors compared to the model using raw, ordinal BSI scores. We, therefore, conducted a Kolmogorov-Smirnov test to examine model residuals for normality in the BSI raw score model and a Hosmer-Lemenshow Goodness-of-Fit test for the model with the dichotomized BSI as the dependent variable. The LME model using raw BSI values as the dependent variable, was found to have a non-normal residual distribution (D=0.18, p=.01), whereas the GEE model treating the BSI dichotomously did not have a problematic goodness of fit as determined by the Hosmer-Lemenshow Goodness-of-Fit test (ChiSq = 5.3,8 df, p>.1). Since non-normal residual distributions may lead to questionable model inferences (18) and to simplify presentation of the data, we report here the findings using the dichotomized BSI item.
For binary response variables (insomnia) we used GEE, because GEE provides marginalized parameter estimates, whereas Generalized Linear Mixed Models yields parameter estimates that are conditional on subject specific random effects. An exchangeable correlation structure was used for GEE. All analyses were performed using SAS Version 9.1 using the GENMOD and MIXED procedures.
To model the effects of discharge sleep onset insomnia symptom severity on long-term pain, we adopted two analytic approaches: 1) utilized the change in the SF-36 BP score from baseline to six months, one year and two years as the primary outcome (SF-36 BP@ follow up time points –SF36 BP @ discharge) and 2) the other used the actual Sf-36 BP at each time point as the primary dependent variable. While both approaches are similar, the former permits a more direct evaluation of whether discharge insomnia impacts the trajectory of pain over time, i.e., whether insomnia predicts decreased improvement in pain severity after serious burn injury. For both the change score model and the model predicting pain severity scores, we selected key discharge and pre-burn covariates, which have been shown in previous studies to predict pain or burn injury outcome. Additionally, since pain was expected to reduce as a function of time, we include time from discharge as a predictor. Covariate predictors included in the models were: % total body surface area burned, % total body surface area skin grafted, number of days in intensive care unit, time since discharge (measured at 6 months, 1 year and 2 years), SF-36 Mental Health and Bodily Pain scores at the time of discharge, SF-36 General Health, Bodily Pain, and Mental Health Indices before burn injury (measured retrospectively), sex, age in years at the time of burn injury, and ethnicity (white vs. non-white). Of the 333 subjects, all had completed the BSI insomnia item at discharge, but 57 had at least one missing discharge or pre-burn covariate. Therefore, we used Markov Chain Monte Carlo (MCMC) Multiple Imputation on those persons so that they could be included in the analysis using the SAS MI procedure. Estimates, standard errors, and degrees of freedom for MI were calculated according to Little and Rubin (2002) (29). On average, each subject had two follow-up observations with complete data.
Results
Table 2 presents summary statistics for the linear mixed effect model predicting change in pain from baseline with: 1) discharge sleep onset insomnia status, 2) discharge Bodily Pain Index, 3) % TBSA burned, 4) %TBSA grafted, 5) days in ICU, 6) time since discharge, 7) discharge Mental Health Index, 8) preburn Mental Health Index, 9) preburn General Health Index, 10) preburn Bodily Pain Index, 11) sex, 12) age, 13) education and 14) race included in the model. The overall model was found to be significant (ChiSq = 135, 2 df, p < 0.0001). Time since discharge and discharge pain were the two strongest predictors of change in pain, i.e. pain severity improved with the progression of time and greater improvement was reported by individuals with less severe discharge pain (p<.001). Interestingly, having at least some college education was also associated with greater improvement in pain severity relative to individuals reporting no college education (p = .001). Age also predicted change in pain, such that older age was associated with decrease improvement in pain severity over time (p =.04). As hypothesized, discharge sleep onset insomnia was associated with decreased improvement in pain at subsequent observations (p=.006). More severe mental health symptoms at discharge strongly trended toward predicting reduced change in pain (p=.07). When the preburn Mental Health Index was removed from the model, which modestly overlapped with discharge mental health (r =.45, p = .0001), discharge mental health was a significant predictor of change in pain (p=.03). Discharge mental health also predicted pain in preliminary models where BSI item #25 raw scores were used instead of the dichotomized scores (p=.05). None of the other predictors explained a significant amount of variance in pain severity. We also tested for interactions between self-reported insomnia at discharge and time from burn and discharge SF-36 Bodily Pain Index. These interactions were non-significant (p>.1) and were thus dropped from the models.
Table 2.
Model Coefficients for Discharge Insomnia Predicting Overall Change in Pain Over Two Year Follow-up Period
| Variable | Unstandardized Beta |
Standardized Beta |
Std. Error | p-value |
|---|---|---|---|---|
| Discharge Sleep Onset Insomnia* | −8.30 | −0.13 | 3.00 | 0.006 |
| Discharge Bodily Pain Index (SF-36) | −0.81 | −0.60 | 0.06 | <0.001 |
| % Total Body Surface Area Burned | −0.13 | −0.06 | 0.11 | 0.260 |
| % Total Body Surface Area Grafted | −0.24 | −0.10 | 0.20 | 0.340 |
| Days in Intensive Care Unit | −0.04 | −0.01 | 0.13 | 0.750 |
| Time Since Discharge (Years.5, 1 & 2) | 4.30 | 0.08 | 1.20 | <0.001 |
| Discharge Mental Health Index (SF-36) | 0.14 | 0.09 | 0.07 | 0.070 |
| Preburn Mental Health Index (SF-36) | 0.10 | 0.05 | 0.08 | 0.240 |
| Preburn General Health Index (SF-36) | 0.01 | 0.00 | 0.09 | 0.930 |
| Preburn Bodily Pain Index (SF-36) | 0.08 | 0.06 | 0.07 | 0.210 |
| Sex | −1.20 | −0.02 | 2.90 | 0.680 |
| Age (years) | −0.20 | −0.09 | 0.10 | 0.040 |
| College Educated | 10.00 | 0.14 | 3.00 | 0.001 |
| White (vs. non-White) | −2.40 | −0.03 | 3.00 | 0.430 |
Note: lower scores on the SF-36 indicate more sever symptoms or decreased function in that domain
Brief Symptom Inventory Item# 25 (insomnia = endorsed moderate to extreme distress with trouble falling asleep in the past 7 days)
Null model likelihood ratio test: ChiSq = 135, 2 df, p < 0.0001
The linear mixed effect model predicting raw pain scores over time yielded very similar findings (ChiSq = 136, 2 df, p < 0.0001), i.e. discharge sleep onset insomnia, more severe discharge pain severity, and older age predicted more severe pain over follow up periods, where as, time from discharge and having some college education was prospectively associated with less pain severity (p<.05 for all).
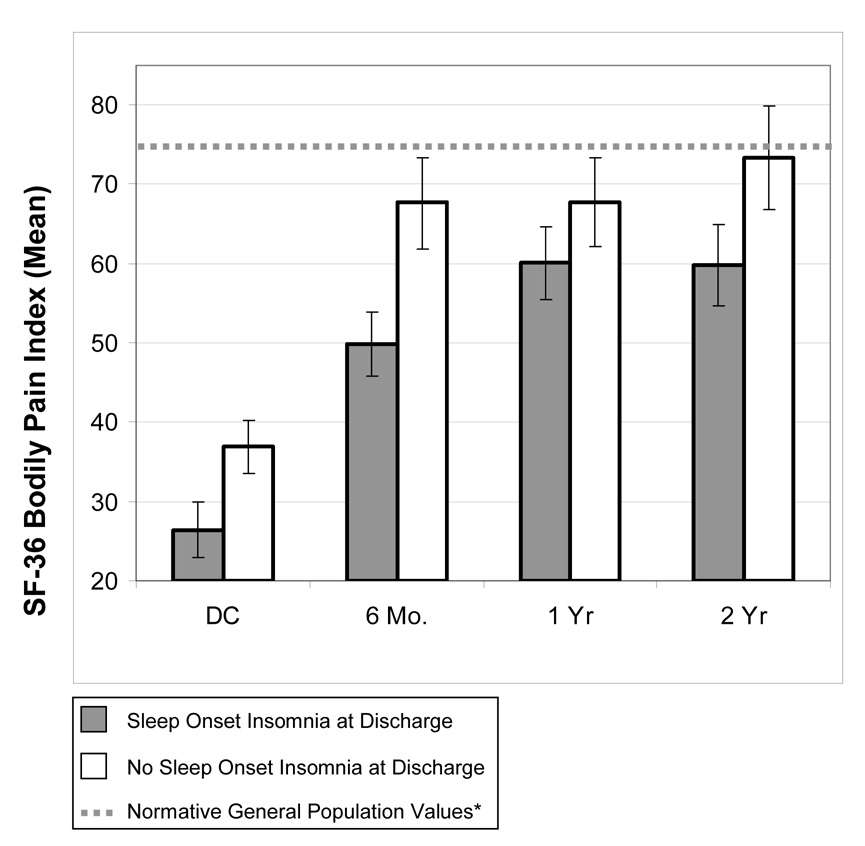
Figure 1 depicts the unmarginalized mean SF-36 Bodily Pain scores with 95% confidence intervals by discharge insomnia classification at each time point. This figure illustrates that individuals reporting baseline sleep onset insomnia reported significantly increased pain severity during the 2 year monitoring period. Furthermore, at two years, the group reporting no baseline insomnia achieved SF-36 bodily pain scores within the normative range, whereas the insomnia group continued to demonstrate elevations on the SF-36.
Figure 1. Long-term Clinical Pain Severity by Discharge Sleep Onset Insomnia Status (SF-36 Mean Bodily Pain Scores with 95% confidence Intervals).
Note: DC = Discharge; SF-36 Score Range = 0 to 100; lower score = more severe bodily pain (N: DC= 333; 6 Mo = 255 ; 1 Yr= 234; 2 Yr = 190). *Ware et al., (1992)
Please note that when groups are matched on pain level at discharge by creating a subsample (n=294), the revised figure yields a nearly identical long-term pain severity profile as depicted in Figure 1, i.e., the insomnia group shows differentially increased pain levels on long-term follow-up.
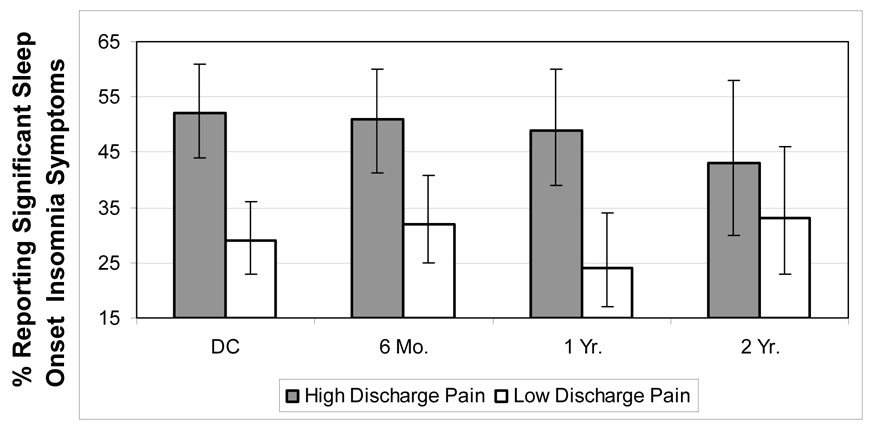
To test the possibility that discharge pain severity might also confer increased prospective risk for sleep onset insomnia, we reversed this model by letting insomnia status at follow up be the dependent variable predicted by discharge pain severity. Table 3 summarizes the results of the generalized estimating equation predicting insomnia. A test of the overall model demonstrated significance (ChiSq = 8.07, 1 df, p = 0.005). As shown, the only significant independent predictors of prospective sleep onset insomnia symptoms were discharge Bodily Pain (OR: 0.88 per 10 unit change, 95% CI: 0.80, 0.97, p=.006), preburn Mental Health (OR: 0.81 per 10 unit change, 95% CI: 0.71, 0.92, p=.002), and discharge sleep onset insomnia (OR: 1.99, 95% CI: 1.27, 3.16, p<.003). We calculated odd ratios to facilitate evaluation of clinical significance by natural exponentiation of the unstandardized beta weights (44) reported in Table 2. Interpretation of the odds ratios indicates that even after controlling for the other predictors, for every 10 point increase in SF-36 Bodily Pain Score (increase = less severe pain), the odds of having sleep onset insomnia at follow-up decreased by 12%. Similarly, for every 10 point increase in the preburn Sf-36 Mental Health Index Score (increase = better mental health), the long-term odds of reporting sleep onset insomnia decreased by 19%. Reporting discharge sleep onset insomnia increased the odds of having sleep onset insomnia at any follow time point, two-fold. To further illustrate the effects of discharge pain on prospective insomnia symptoms, we dichotomized the sample by conducting a median split on discharge pain severity to form a high (more severe pain) discharge pain group and a low (less severe pain) discharge pain group. Figure 2 depicts the unadjusted rates (%) of insomnia with 95% confidence intervals at each time point by discharge pain status. As shown, patients categorized as having high pain at discharge, on average, demonstrated a greater percentage of insomnia symptoms at every time point, except at two years (ChiSq =17.8, df=1, p<0.0001 at discharge; ChiSq =8.4, df=1, p = 0.004 at 6 months; ChiSq = 13.1, df = 1, p = 0.003 at 12 months, p>.05 at 2 years). This figure also illustrates that both groups, on average, demonstrated relatively high rates of sleep onset insomnia symptoms, ranging between 29% and 52%. Of the individuals who were classified as high in discharge pain (n=167), rates of continuing to meet this high pain level categorization (SF-36 BP ≤ 30) were as follows: 6 months = 21.8% (n=24 out of 110 cases); 12 months = 18.6% (19 out of 102 cases); 24 months = 12.4% 10 out 81 cases).
Table 3.
Model Coefficients for Pain Predicting Insomnia over Two Year Follow-up Period*
| Variable | Unstandardized Beta |
Standardized Beta |
Unstandardized Odds Ratio (95% CI) |
p-value |
|---|---|---|---|---|
| Discharge Insomnia | 0.69 | 0.34 | 1.99 (1.27, 3.16) |
0.003 |
| Discharge SF-36 Bodily Pain IndexΩ | −0.13 | −0.30 | 0.88 (0.80, 0.97) |
0.006 |
| Discharge SF-36 Mental Health IndexΩ | −0.06 | −0.12 | 0.95 (0.84,1.06) |
0.33 |
| Preburn SF-36 Mental Health IndexΩ | −0.21 | −0.42 | 0.81 (0.71, 0.92) |
0.002 |
| Preburn SF-36 General Health IndexΩ | 0.03 | 0.05 | 1.03 (0.90, 1.16) |
0.63 |
| Preburn SF-36 Bodily Pain IndexΩ | 0.04 | 0.09 | 1.04 (0.94, 1.15) |
0.48 |
| Sex | 0.30 | 0.13 | 1.35 (0.87, 2.10) |
0.19 |
| Time Since Discharge | −0.14 | −0.09 | 0.87 (0.63, 1.21) |
0.42 |
| Percent Body Surface Area Burned | 0.01 | 0.10 | 1.01 (0.99, 1.02) |
0.51 |
| Percent Body Surface Area Grafted | −0.00 | −0.09 | 0.99 (0.96, 1.02) |
0.58 |
| Days in I.C.U | 0.01 | 0.23 | 1.02 (0.99,1.04) |
0.16 |
| Age (years) | 0.01 | 0.09 | 1.01 (0.99,1.02) |
0.42 |
| College Educated | −0.34 | −0.15 | 0.72 (0.45, 1.15) |
0.15 |
| White (vs. non-White) | 0.22 | 0.10 | 1.25 (0.79,2.0) | 0.36 |
Note: lower scores on the SF-36 indicate more severe symptoms or decreased function in that domain
Brief Symptom Inventory Item# 25 (insomnia = endorsed moderate to extreme distress with trouble falling asleep in the past 7 days)
Null model likelihood ratio test: ChiSq = 8.07, 1 df, p = 0.005
Values are expressed in units of 10 to facilitate interpretation of odds ratios. Thus, for SF-36 bodily pain, the O.R. = .88, indicates that every 10 point increase in the bodily pain index (increase = less pain) decreased the risk of insomnia by 12%
Figure 2. Rates of Prospective Sleep Onset Insomnia Symptoms by Discharge Pain Severity (% with 95% Confidence intervals).
Note: High Discharge Pain = SF-36 Bodily Pain Index ≤ 30; Low Discharge Pain = SF-36 BPI >30. Insomnia = reported “quite a bit” or “extreme” trouble falling asleep in the past 7 days; N: DC = 333; 6 Mo. = 245; 1 Yr = 192; 2 Yr. = 122
Discussion
In this prospective, multi-site study of major burn injury survivors, we evaluated whether discharge sleep onset insomnia symptoms predicted long-term pain over two year follow-up and conversely whether pain severity at discharge predicted subsequent insomnia symptoms. We found that sleep onset insomnia predicted both decreased improvement and degree of long-term pain, even after controlling for: premorbid pain, self-reported health status, standard burn severity markers (% total body surface area burned, % total body surface area grafted, days spent in ICU), demographic factors, discharge mental health, and preburn mental health., Increased discharge pain severity, lack of college education, and older age also independently predicted pain on long-term follow-up. Discharge mental health symptoms strongly trended toward predicting pain. Furthermore, in support of a reciprocal relationship between sleep and pain, discharge pain severity and preburn mental health were significant predictors of long-term sleep onset insomnia, even after controlling for presence of insomnia at discharge, which was also a robust predictor of long-term insomnia. These data are among the first to directly support that insomnia may be an important, independent risk factor for developing chronic pain following traumatic injury. Because premorbid insomnia was not measured, however, it is unclear to what extent pre-existing insomnia, insomnia associated with the injury, or both confers the greatest risk.
Prior work has found that insomnia symptoms appear to be associated with an aggravation and encroachment of musculosketal pain from a regional to a widespread condition (16;34), but few, if any, studies have evaluated whether sleep disturbance predicts rate of improvement in pain after acute injury in a population unselected for a pre-existing pain disorder. We are aware of only one other study, which reported that sleep disturbance 3 months after lower limb trauma predicted pain severity 7 years later (6).
Our finding that acute pain severity and a trend toward mental health symptoms predicting chronic pain is consistent with prior work, which has identified anxiety, depressive symptoms, and acute pain severity as predictors of chronic pain following surgical procedures/traumatic injury (7;21;38). These studies, however, did not measure sleep, which could potentially confound the psychological distress risk factor findings due to overlap between psychological distress and sleep disturbance. The present study, however, suggests that sleep disturbance is an important additional predictor of long-term pain, independent from these more well established risk factors. Our findings are also consistent with prior work demonstrating that older age confers increased risk for developing chronic pain, e.g., (17). Moreover, these analyses replicate a relatively under-identified protective factor for the development of chronic pain following traumatic injury, i.e. having at least some college education. Two other studies have also reported a similar finding in patients recovering from surgical procedures for lower extremity threatening injuries (4;6). Future work will be necessary explain how education may be protective.
Using recovery from burn injury as a model to shed light on the question of whether sleep disturbance predicts the development of chronic pain has advantages and disadvantages. One advantage is that rates of persistent pain following burn injury survivors are relatively high, which enhances statistical power. Furthermore, while some participants may have suffered chronic pain and or insomnia, prior to burn injury, it is doubtful, based on the relatively young age of the sample (mean = 42 years old), that the majority of individuals had a preexisting pain or sleep disorder. We would argue that this model, like other acute injury models, more clearly supports sleep disturbance as a true independent risk factor for the development of chronic pain, because it is not largely confounded a by prior history of chronic pain. Further strengthening confidence in the insomnia finding is that we controlled for premorbid pain, general health status and mental health status. It should be acknowledged, however, that retrospective ratings of pre-burn status are likely to provide a somewhat biased estimate of premorbid status. Furthermore, while we were able to control for premorbid pain, health and mental health status, premorbid insomnia symptoms were not measured and so it remains possible that premorbid insomnia is also an important risk factor for developing chronic pain after acute injury. In support of this possibility, Raymond and colleagues have reported that retrospective ratings of pre-burn sleep problems predicted more intense pain during burn care procedures, more pain during the night, and more pain upon awakenings in hospitalized burn patients (41).
While the burn injury model has some unique advantages, a major disadvantage is the possibility that these findings might not generalize well to other chronic pain conditions. Burn injury survivors, as a group, might possess unique characteristics that predispose to both traumatic injury and pain.
Our findings are, however, consistent with several time series investigations, including two in hospitalized burn patients, which have found a reciprocal relationship between daytime pain and sleep, measured proximally, i.e. poor sleep is linked with increased next day pain and daytime pain is associated with disrupted sleep that night (1;13;40;41;48). The present study also extends prior work reporting that pain is associated with risk for long-term insomnia (37;42). Our finding that the degree of premorbid mental health symptoms, in addition to acute pain and insomnia is a robust predictor of long-term insomnia highlights the complexity of interacting psychological and neurobiologic factors involved in the pain-sleep relationship. Our group and others have argued that this reciprocal relationship, between pain and sleep, which can be observed in both, long and short time frames, in pain patients and in healthy subjects (50), may reflect a neurobiologic overlap between brainstem descending pain modulatory systems and ascending arousal systems, regulating consciousness (24;47). This reciprocal relationship suggests the possibility of a scarring effect such that intense pain or unmitigated insomnia may have downstream neurophysiologic consequences for both pain processing and sleep systems.
Whether or not these down stream effects can be reversed or prevented by intervening on either system remains a largely unexplored question. Our findings suggest that future work aimed at identifying and clarifying factors associated with the transition from acute to chronic pain should include an assessment of sleep. A number of qualifications and limitations should be highlighted when interpreting these findings. Despite the advantages of the prospective design, the findings should not be considered causal. These data, do however, support a reciprocal interrelationship between sleep and pain. It remains possible that the pain and sleep problems observed in burn patients are both the consequence of a common underlying pathophysiology.
As mentioned previously, this study like, other prospective studies of serious burn injury, involved a relatively high attrition rate. We found, however, that attrition was not associated with pain or insomnia symptoms measured at any time point. This suggests that differential dropout does not appear to have influenced the findings. Another limitation is the BSI single-item, self-report assessment of sleep onset insomnia. While there are no widely agreed upon single item measures developed for use in epidemiologic or large scale prospective studies, the BSI only measures symptoms over the past seven days and conflates both trouble falling asleep and associated distress into one item. This might limit both reliability and validity. Similarly, although widely used, the SF-36 Bodily Pain Index is only comprised of two items and therefore does not provide a comprehensive measure of pain severity. The possibility of residual confounding can not be entirely eliminated. These data will support future work that employs both objective and more comprehensive assessments of sleep and pain to identify which aspects of sleep are most predictive of chronic pain development. To further reduce the possibility of residual confounding, future work might utilize more comprehensive measures and match groups on degree of discharge pain, rather than relying on statistical control. Finally, while this study endeavored to control for several factors that have not been previously taken into account, e.g., premorbid pain, health and mental health status, several potential confounds were not evaluated. Discharge medications, for example, were not assessed. It remains possible that patients exhibiting high discharge insomnia, pain, and psychological symptoms received differential after care. Although this is unknown, we would expect that these patients might receive more aggressive medical and psychological intervention. This, however, might be expected to improve rather than hinder recovery. It should also be noted that in cases where covariates were missing, we imputed data, which may weaken confidence in the models’ performance to optimally control for the effects of these parameters. In conclusion, these data are particularly encouraging; because they highlight several risk factors for chronic pain and insomnia beyond acute injury severity, i.e. sleep onset insomnia, pain severity, anxiety and depressive symptoms, which are all highly modifiable. Future work should focus on whether aggressively treating sleep disturbance following traumatic injury prevents or mitigates the development of intractable pain.
Acknowledgements
This study was supported by the National Institute on Disability and Rehabilitation Research, U.S. Department of Education (H133A020101) and the U.S. National Institutes of Health (1K23 NS47168; Smith). None of the authors have any conflicts of interest to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Affleck G, Urrows S, Tennen H, Higgins P, Abeles M. Sequential daily relations of sleep, pain intensity, and attention to pain among women with fibromyalgia. Pain. 1996;68:363–368. doi: 10.1016/s0304-3959(96)03226-5. [DOI] [PubMed] [Google Scholar]
- 2.Agresti A. Categorical Data Analysis. 2 ed. Hoboken, New Jersey: Wiley; 2002. [Google Scholar]
- 3.Boeve SA, Aaron LA, Martin-Herz SP, Peterson A, Cain V, Heimbach DM, et al. Sleep disturbance after burn injury. J Burn Care Rehabil. 2002;23(1):32–38. doi: 10.1097/00004630-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Bosse MJ, MacKenzie EJ, Kellam JF, Burgess AR, Webb LX, Swiontkowski MF, et al. An analysis of outcomes of reconstruction or amputation after leg-threatening injuries. N Engl J Med. 2002;347(24):1924–1931. doi: 10.1056/NEJMoa012604. [DOI] [PubMed] [Google Scholar]
- 5.Bronfort G, Bouter LM. Responsiveness of general health status in chronic low back pain: a comparison of the COOP charts and the SF-36. Pain. 1999;83(2):201–209. doi: 10.1016/s0304-3959(99)00103-7. [DOI] [PubMed] [Google Scholar]
- 6.Castillo RC, MacKenzie EJ, Wegener ST, Bosse MJ. Prevalence of chronic pain seven years following limb threatening lower extremity trauma. Pain. 2006;124(3):321–329. doi: 10.1016/j.pain.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 7.Caumo W, Schmidt AP, Schneider CN, Bergmann J, Iwamoto CW, Adamatti LC, et al. Preoperative predictors of moderate to intense acute postoperative pain in patients undergoing abdominal surgery. Acta Anaesthesiol Scand. 2002;46(10):1265–1271. doi: 10.1034/j.1399-6576.2002.461015.x. [DOI] [PubMed] [Google Scholar]
- 8.Choiniere M, Melzack R, Papillon J. Pain and paresthesia in patients with healed burns: an exploratory study. J Pain Symptom Manage. 1991;6(7):437–444. doi: 10.1016/0885-3924(91)90043-4. [DOI] [PubMed] [Google Scholar]
- 9.Choiniere M, Racine M, Raymond-Shaw I. Epidemiology of Pain and Sleep Disturbances and their Reciprocal Interrelationships. In: Lavigne G, Choiniere M, Soja PJ, editors. Sleep and Pain. Seattle: IASP PRESS; 2007. pp. 267–285. [Google Scholar]
- 10.Dauber A, Osgood PF, Breslau AJ, Vernon HL, Carr DB. Chronic persistent pain after severe burns: a survey of 358 burn survivors. Pain Med. 2002;3(1):6–17. doi: 10.1046/j.1526-4637.2002.02004.x. [DOI] [PubMed] [Google Scholar]
- 11.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13(3):595–605. [PubMed] [Google Scholar]
- 12.Diggle PJ, Heagerty P, Liang KY, Zeger SL. Analysis of Longitudinal Data. Second ed. Oxford University Press; 2002. [Google Scholar]
- 13.Drewes AM, Nielsen KD, Hansen B, Taagholt SJ, Bjerregard K, Svendsen L. A longitudinal study of clinical symptoms and sleep parameters in rheumatoid arthritis. Rheumatology (Oxford) 2000;39(11):1287–1289. doi: 10.1093/rheumatology/39.11.1287. [DOI] [PubMed] [Google Scholar]
- 14.Edwards RR, Klick B, Buenaver L, Max MB, Haythornthwaite JA, Keller RB, et al. Symptoms of distress as prospective predictors of pain-related sciatica treatment outcomes. Pain. 2007;130(1–2):47–55. doi: 10.1016/j.pain.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Gottschlich MM, Jenkins ME, Mayes T, Khoury J, Kramer M, Warden GD, et al. The 1994 Clinical Research Award. A prospective clinical study of the polysomnographic stages of sleep after burn injury. J Burn Care Rehabil. 1994;15(6):486–492. [PubMed] [Google Scholar]
- 16.Gupta A, Silman AJ, Ray D, Morriss R, Dickens C, Macfarlane GJ, et al. The role of psychosocial factors in predicting the onset of chronic widespread pain: results from a prospective population-based study. Rheumatology (Oxford) 2006 doi: 10.1093/rheumatology/kel363. [DOI] [PubMed] [Google Scholar]
- 17.Gureje O, Simon GE, Von Korff M. A cross-national study of the course of persistent pain in primary care. Pain. 2001;92(1–2):195–200. doi: 10.1016/s0304-3959(00)00483-8. [DOI] [PubMed] [Google Scholar]
- 18.Harrell FE. Regression Modeling Strategies. New york: Springer; 2001. [Google Scholar]
- 19.Helton MC, Gordon SH, Nunnery SL. The correlation between sleep deprivation and the intensive care unit syndrome. Heart Lung. 1980;9(3):464–468. [PubMed] [Google Scholar]
- 20.Jensen MP, Hoffman AJ, Cardenas DD. Chronic pain in individuals with spinal cord injury: a survey and longitudinal study. Spinal Cord. 2005;43(12):704–712. doi: 10.1038/sj.sc.3101777. [DOI] [PubMed] [Google Scholar]
- 21.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 22.Klein MB, Lezotte DL, Fauerbach JA, Herndon DN, Kowalske KJ, Carrougher GJ, et al. The national institute on disability and rehabilitation research burn model system database: a tool for the multicenter study of the outcome of burn injury. J Burn Care Res. 2007;28(1):84–96. doi: 10.1097/BCR.0b013E31802C888E. [DOI] [PubMed] [Google Scholar]
- 23.Kravitz M, McCoy BJ, Tompkins DM, Daly W, Mulligan J, McCauley RL, et al. Sleep disorders in children after burn injury. J Burn Care Rehabil. 1993;14(1):83–90. doi: 10.1097/00004630-199301000-00018. [DOI] [PubMed] [Google Scholar]
- 24.Kshatri AM, Baghdoyan HA, Lydic R. Cholinomimetics, but not morphine, increase antinociceptive behavior from pontine reticular regions regulating rapid-eye-movement sleep. Sleep. 1998;21(7):677–685. doi: 10.1093/sleep/21.7.677. [DOI] [PubMed] [Google Scholar]
- 25.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–974. [PubMed] [Google Scholar]
- 26.Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10(5):357–369. doi: 10.1016/j.smrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Lawrence JW, Fauerbach J, Eudell E, Ware L, Munster A. The 1998 Clinical Research Award. Sleep disturbance after burn injury: a frequent yet understudied complication. J Burn Care Rehabil. 1998;19(6):480–486. doi: 10.1097/00004630-199811000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Leblebici B, Adam M, Bagis S, Tarim AM, Noyan T, Akman MN, et al. Quality of life after burn injury: the impact of joint contracture. J Burn Care Res. 2006;27(6):864–868. doi: 10.1097/01.BCR.0000245652.26648.36. [DOI] [PubMed] [Google Scholar]
- 29.Little RJA, Rubin DB. Statistical analysis with missing data. John Wiley & Sons; 1992. [Google Scholar]
- 30.Low JF, Dyster-Aas J, Willebrand M, Kildal M, Gerdin B, Ekselius L. Chronic nightmares after severe burns: risk factors and implications for treatment. J Burn Care Rehabil. 2003;24(4):260–267. doi: 10.1097/01.BCR.0000075847.26303.37. [DOI] [PubMed] [Google Scholar]
- 31.Malenfant A, Forget R, Amsel R, Papillon J, Frigon JY, Choiniere M. Tactile, thermal and pain sensibility in burned patients with and without chronic pain and paresthesia problems. Pain. 1998;77(3):241–251. doi: 10.1016/S0304-3959(98)00096-7. [DOI] [PubMed] [Google Scholar]
- 32.Malenfant A, Forget R, Papillon J, Amsel R, Frigon JY, Choiniere M. Prevalence and characteristics of chronic sensory problems in burn patients. Pain. 1996;67(2–3):493–500. doi: 10.1016/0304-3959(96)03154-5. [DOI] [PubMed] [Google Scholar]
- 33.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Mikkelsson M, Sourander A, Salminen JJ, Kautiainen H, Piha J. Widespread pain and neck pain in schoolchildren. A prospective one-year follow-up study. Acta Paediatr. 1999;88(10):1119–1124. doi: 10.1080/08035259950168199. [DOI] [PubMed] [Google Scholar]
- 35.Miller SF, Bessey PQ, Schurr MJ, Browning SM, Jeng JC, Caruso DM, et al. National Burn Repository 2005: a ten-year review. J Burn Care Res. 2006;27(4):411–436. doi: 10.1097/01.BCR.0000226260.17523.22. [DOI] [PubMed] [Google Scholar]
- 36.Moi AL, Wentzel-Larsen T, Salemark L, Wahl AK, Hanestad BR. Impaired generic health status but perception of good quality of life in survivors of burn injury. J Trauma. 2006;61(4):961–968. doi: 10.1097/01.ta.0000195988.57939.9a. [DOI] [PubMed] [Google Scholar]
- 37.Nicassio PM, Wallston KA. Longitudinal relationships among pain, sleep problems, and depression in rheumatoid arthritis. J Abnorm Psychol. 1992;101(3):514–520. doi: 10.1037//0021-843x.101.3.514. [DOI] [PubMed] [Google Scholar]
- 38.Poleshuck EL, Katz J, Andrus CH, Hogan LA, Jung BF, Kulick DI, et al. Risk factors for chronic pain following breast cancer surgery: a prospective study. J Pain. 2006;7(9):626–634. doi: 10.1016/j.jpain.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao CR. Linear Statistical inference and its Applications. 2 ed. New York: Wiley; 1973. [Google Scholar]
- 40.Raymond I, Ancoli-Israel S, Choiniere M. Sleep disturbances, pain and analgesia in adults hospitalized for burn injuries. Sleep Med. 2004;5(6):551–559. doi: 10.1016/j.sleep.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 41.Raymond I, Nielsen TA, Lavigne G, Manzini C, Choiniere M, et al. Quality of sleep and its daily relationship to pain intensity in hospitalized adult burn patients. Pain. 2001;92(3):381–388. doi: 10.1016/S0304-3959(01)00282-2. [DOI] [PubMed] [Google Scholar]
- 42.Riley JL, III, Benson MB, Gremillion HA, Myers CD, Robinson ME, Smith CL, Jr, et al. Sleep disturbance in orofacial pain patients: pain-related or emotional distress? Cranio. 2001;19(2):106–113. doi: 10.1080/08869634.2001.11746159. [DOI] [PubMed] [Google Scholar]
- 43.Rose M, Sanford A, Thomas C, Opp MR. Factors altering the sleep of burned children. Sleep. 2001;24(1):45–51. [PubMed] [Google Scholar]
- 44.Rosner B. Fundamentals of Biostatistics. 5 ed. Singapore: Duxbury Thomson Learning; 2000. [Google Scholar]
- 45.Salaffi F, Stancati A, Grassi W. Reliability and validity of the Italian version of the Chronic Pain Grade questionnaire in patients with musculoskeletal disorders. Clin Rheumatol. 2006;25(5):619–631. doi: 10.1007/s10067-005-0140-y. [DOI] [PubMed] [Google Scholar]
- 46.Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30(4):494–505. doi: 10.1093/sleep/30.4.494. [DOI] [PubMed] [Google Scholar]
- 47.Smith MT, Edwards RR, Stonerock GL, McCann UD. Individual variation in rapid eye movement sleep is associated with pain perception in healthy women: Preliminary data. Sleep. 2005;28(7):809–812. doi: 10.1093/sleep/28.7.809. [DOI] [PubMed] [Google Scholar]
- 48.Stone AA, Broderick JE, Porter LS, Kaell AT. The experience of rheumatoid arthritis pain and fatigue: examining momentary reports and correlates over one week. Arthritis Care Res. 1997;10(3):185–193. doi: 10.1002/art.1790100306. [DOI] [PubMed] [Google Scholar]
- 49.Thombs BD, Bresnick MG, Magyar-Russell G. Depression in survivors of burn injury: a systematic review. Gen Hosp Psychiatry. 2006;28(6):494–502. doi: 10.1016/j.genhosppsych.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 50.Totterdell P, Reynolds S, Parkinson B, Briner RB. Associations of sleep with everyday mood, minor symptoms and social interaction experience. Sleep. 1994;17(5):466–475. doi: 10.1093/sleep/17.5.466. [DOI] [PubMed] [Google Scholar]
- 51.Van Loey NE, Maas CJ, Faber AW, Taal LA. Predictors of chronic posttraumatic stress symptoms following burn injury: results of a longitudinal study. J Trauma Stress. 2003;16(4):361–369. doi: 10.1023/A:1024465902416. [DOI] [PubMed] [Google Scholar]
- 52.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36) Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 53.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]