Abstract
Recent research suggests bi-directional interactions between the experience of pain and the process of sleep; pain interferes with the ability to obtain sleep, and disrupted sleep contributes to enhanced pain perception. Our group recently reported, in a controlled experimental study, that sleep fragmentation among healthy adults resulted in subsequent decrements in endogenous pain inhibition. The present report follows up that observation by extending this line of research to a sample of patients experiencing persistent pain. Patients with chronic temporomandibular joint disorder (TMD) pain were studied using polysomnography and psychophysical evaluation of pain responses. We assessed whether individual differences in sleep continuity and/or architecture were related to diffuse noxious inhibitory controls (DNIC), a measure of central nervous system pain inhibition. Among 53 TMD patients, higher sleep efficiency and longer total sleep time were positively associated with better functioning of DNIC (r=.42 − .44, p< .01; p’s< .05 for the multivariate analyses). These results suggest the possibility that disrupted sleep may serve as a risk factor for inadequate pain-inhibitory processing and hint that aggressive efforts to treat sleep disturbance early in the course of a pain condition might be beneficial in reducing the severity or impact of clinical pain.
Keywords: pain sensitivity, sleep architecture, temporomandibular joint disorder, diffuse noxious inhibitory controls, idiopathic pain, central sensitivity
Introduction
Recent reviews have highlighted bi-directional interactions between sleep and pain (Lautenbacher et al., 2006;Smith and Haythornthwaite, 2004;Argoff, 2007). Many studies have suggested that experimental sleep disruption results in enhanced pain perception (Kundermann et al., 2004;Haack and Mullington, 2005), that poor sleep is correlated with elevated pain severity in chronic pain patients (Smith and Haythornthwaite, 2004), and that in the general population, individual differences in sleep impact on subsequent pain (Gupta et al., 2006;Edwards et al., 2008). An emerging consensus suggests that multiple mechanisms underlie this reciprocal association, with hypotheses ranging from shared neurobiological substrates (Smith et al., 2005) to inflammatory processes (Haack et al., 2007).
We and others have hypothesized (Lautenbacher et al., 2006) that sleep disruption produces maladaptive effects on central pain-modulatory systems, and we recently reported that fragmenting sleep among healthy adults results in next-day decrements in pain inhibition (Smith et al., 2007). Specifically, a loss of diffuse noxious inhibitory controls (DNIC), a measure of central nervous system (CNS) pain inhibition, was observed. DNIC, or counter-irritation, refers to one noxious stimulus inhibiting the pain produced by a second noxious stimulus; DNIC depends on opioid-mediated supraspinal mechanisms (Willer et al., 1990;Wilder-Smith et al., 2004), and is a sensitive measure of deficits in pain modulation in fibromyalgia (Edwards, 2005;Diatchenko et al., 2006). We previously reported that DNIC is inversely correlated with daily pain in non-clinical samples (Edwards et al., 2003b), and our recent study suggested that increases in clinical pain accompanied reductions in DNIC after sleep fragmentation (Smith et al., 2007). Moreover, others have confirmed that changes in DNIC parallel changes in clinical pain (Kosek and Ordeberg, 2000), and that DNIC prospectively predicts long-term post-surgical pain (Yarnitsky et al., 2008).
Collectively, this literature suggests that DNIC is a sensitive and clinically relevant measure of endogenous pain inhibition, and that processes which interfere with DNIC are likely to amplify clinical pain. Our previous study suggests that disturbed sleep may play such a role (Smith et al., 2007), but those findings in healthy subjects are potentially limited in their generalizability. It is not presently known whether naturally-occurring impairments in sleep in pain patients are also associated with decrements in DNIC. In this report, we analyze data from patients with chronic temporomandibular joint disorder (TMD) pain in order to evaluate whether poor sleep is related to less-functional pain inhibition, operationalized as DNIC. In particular, we assessed whether variables reflecting sleep continuity, such as sleep efficiency, were related to DNIC in multivariate analysis. TMD was selected as a diagnostic group of interest for three reasons: first, it is one of a cluster of “idiopathic” (Diatchenko et al., 2006) or “central sensitivity syndromes” (Yunus, 2008) in which deficits in pain-inhibitory processes such as DNIC are especially clinically relevant. Second, sleep in TMD is a relatively under-studied area, warranting increased research attention. Third, TMD patients show broad individual differences in many pain- and sleep-related parameters, making them a good group in which to study these associations (Auvenshine, 2007).
Methods
Participants
We recruited 53 temporomandibular joint disorder (TMD) patients from a university- based tertiary care facial pain clinic and from local media advertisements. Major eligibility criteria included: meeting research diagnostic criteria for primary myofascial TMD, reporting typical pain severity ≥ 2 out of 10, and having symptom duration ≥ 6 months. We excluded patients reporting primary pain conditions or serious medical disorders other than TMD, patients with active alcohol or drug abuse problems, or patients using narcotic/opioid analgesic medications, antidepressants, anticonvulsants, or muscle relaxants.
Procedures
Subjects underwent informed consent and completed panoramic X-Rays. An experienced dentist (EG) performed a dental exam according to research diagnostic procedures for TMD (Dworkin and LeResche, 1992) to establish diagnosis. Those who were eligible and enrolled in the study underwent 2 nights of polysomnography, and 4 sessions of laboratory pain testing.
Questionnaires
Subjects completed formal psychodiagnostic interviews (DSM-IV Structured Clinical Interview; e.g., (Kivioja et al., 2004)) conducted by a licensed clinical psychologist (MTS), and completed a battery of widely-used questionnaires, including the Beck Depression Inventory (Beck et al., 1961), the Spielberger State –Trait Anxiety Inventory (Spielberger et al., 1983), and the Brief Pain Inventory (Tan et al., 2004). Subjects then completed two weeks of electronic diary monitoring of sleep and pain. Standard sleep continuity items were completed each morning (Haythornthwaite et al., 1991).
Polysomnography (PSG)
After screening, subjects completed two consecutive overnight sleep studies in a sound-attenuated private room on an inpatient clinical research unit at Johns Hopkins. Signals were recorded using EMBLA N7000 polysomnographs and Somnolgica software at a sampling rate of 500 HZ. We provided subjects with an 8-hour sleep period during their preferred sleep phase. The Night 1 montage included placements for electroencephalography (EEG), electrooculography (EOG), and electromyography (EMG) according to Rechtschaffen & Kales (R&K))(Rechtschaffen and Kales, 1968), with the addition of bilateral masseter EMGs and auditory recordings as described by Lavigne (Lavigne et al., 1996). These consisted of digital audio recordings to detect tooth-grinding sounds, part of the research diagnostic criteria for bruxism. We acquired the following electrophysiologic signals: 4EEGs (C4-A1, C3-A2, O1-A2, O2-A1); right and left EOGs linked to a single mastoid; bilateral masseter EMGs for scoring sleep bruxism, submental EMGs, bilateral anterior tibialis EMGs; and an electrocardiogram (ECG) using a single modified ECG lead II, with torso placement. Respiratory function was measured using an oronasal thermister, a nasal air pressure transducer, pulse oximetry, and abdominal and thoracic strain gages. These assessments permitted a full, standardized assessment of sleep continuity, sleep architecture (e.g., the percentage of time spent in each stage of sleep), respiratory function during sleep, and sleep bruxism. On the second recording night, we used an abbreviated montage, removing all respiratory sensors and tibialis EMGs. Sleep continuity and architecture was scored according to R&K criteria (Rechtschaffen and Kales, 1968) by a registered polysomnographic technician, then reviewed and confirmed by a board-certified sleep specialist. The scoring technician was blind to subject characteristics, including diagnosis. Clinical sleep indices were scored as defined in the International Classification of Sleep disorders-II Manual (American Academy of Sleep Medicine, 2005).
Laboratory Pain Testing Procedures
Subjects completed a standardized quantitative sensory testing protocol that we have used in prior studies (Smith et al., 2007). Testing was conducted each afternoon between 15:00 and 17:00, and each morning following the sleep study, approximately 30 minutes after waking. We focus this report on our assessment of DNIC, which we showed to be sensitive to sleep deprivation in a prior study among healthy adults (Smith et al., 2007). All subjects refrained from using any analgesics or centrally acting agents within 24 hours prior to pain testing. Urine toxicology tests confirmed that individuals were not using sedatives, opioids, or common recreational drugs.
Diffuse Noxious Inhibitory Controls (DNIC)
DNIC is a non-invasive test of endogenous pain-inhibitory systems using a heterotopic noxious conditioning stimulation paradigm. Briefly, we assessed baseline pressure pain threshold (PPTh) according to standard procedures using a Somedic pressure algometer (Smith et al., 2007). PPTh ratings were obtained on the right brachioradialis and right trapezius in a random order. Immediately following this baseline assessment, participants underwent a series of 4 cold pressor tasks, similar to previous DNIC studies (Edwards et al., 2003a;Talbot et al., 1989). During each cold pressor task, participants immersed their contralateral hand (left) up to the wrist, in a circulating cold water bath maintained at 4°C. Twenty seconds after commencing hand immersion, PPTh was re-assessed on either the right brachioradialis or right trapezius (i.e., the same site as baseline assessment). After the PPTh assessment, participants removed their hands from the water. During each morning or afternoon testing session, a total of 4 DNIC tasks were performed, with two trials at each anatomical site. Two-min intervals were maintained between each cold pressor task. DNIC was measured as the percent change in PPTh during cold pressor, relative to baseline PPTh [i.e., (mean PPTh during cold pressor/mean PPTh prior to cold pressor)*100]. Generally, an increase in PPTh during cold pressor (i.e., percentage scores above 100) reflects normal functioning of pain-inhibitory processes. For example, an index of 130, a typical score for healthy subjects, reflects a 30% increase in PPTh during the cold pressor task. Inspection of the data revealed that all subjects reported the cold pressor task as painful at the time of DNIC testing.
Ideally, we would have also used an additional condition, with non-painful water, to control for non-specific effects. This would have substantially lengthened the testing session, though, and there is general agreement that DNIC effects are due to the activation of endogenous pain-inhibitory pathways, and not to factors such as altered attention (Lautenbacher et al., 2007). However, the present methodology does not allow us to state definitively the mechanism by which the DNIC effect (i.e., the elevation of PPTh by concurrent cold pressor stimulation) occurred in this study.
Data Analysis
For the initial analysis, data were averaged across assessment points in order to maximize reliability and stability. DNIC scores were averaged across the four pain testing sessions, and PSG variables were averaged across the 2 nights. PSG variables assessed in this study reflected standard measures of sleep continuity and architecture: sleep latency, total sleep time, wake after sleep onset (WASO) time, sleep efficiency, and the percentage of time spent in each sleep stage. In addition, measures of sleep-disordered breathing (i.e., the apnea-hypopnea index; AHI), periodic limb movements (PLM index), and sleep bruxism were included. Pearson correlations were computed to assess simple bivariate relationships between sleep and DNIC. Those sleep variables and demographic factors that were significantly or near-significantly correlated (p< .10) with DNIC were included as predictive factors in a mixed model analysis. This two-step approach (i.e., first evaluating simple bivariate associations, and then performing multivariate analysis) has some redundancy, but is commonly employed in studies of pain, and has the advantage of allowing examination of both simple univariate findings as well as “unique” (i.e. a particular predictor variable may be correlated with the dependent variable of interest even after statistically adjusting for other potential predictors, thus reducing potential confounders) multivariate relationships. Analyses were performed using SAS 9.1.
Results
Consistent with the demographics of TMD, the sample was 78% female, with a mean age of 34 years old. On average, participants had been living with TMD symptoms for quite some time, as the mean TMD duration was 9.0 years ± 8.1. In general, patients in the current sample reported mild levels of pain and distress. Pain severity scores on the Brief Pain Inventory averaged 3.2 ± 1.5 (on a 0–10 scale). A total of 15 of the 53 patients (28.3%) reported a current mood or anxiety disorder during the structured interview. In addition, the mean BDI score for the sample was 7.5 ± 7.0, and the mean STAI Trait Anxiety score was 36.3 ± 10.5.
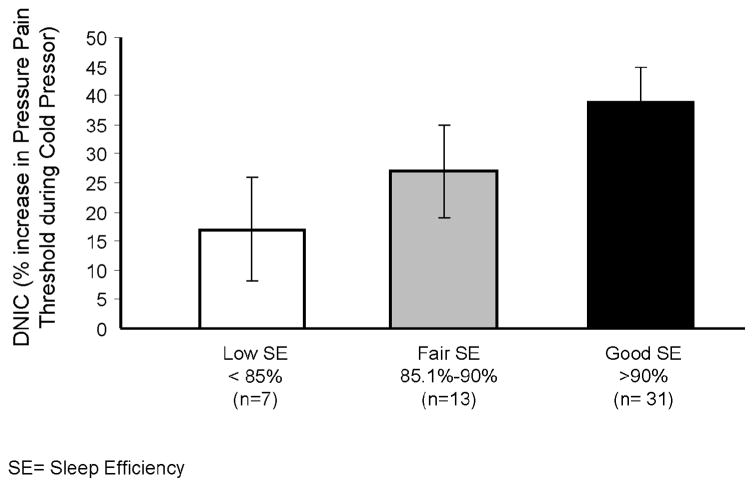
As described in the Methods section above, data were analyzed in two ways. First, simple bivariate correlations between DNIC and sleep variables were observed for total sleep time (r= .44, p= .001) and sleep efficiency (r= .42, p= .002); WASO tended to be inversely correlated with DNIC, but the association did not reach significance (r= −.29, p<.10). These findings suggest that better sleep continuity is associated with improved pain inhibition (i.e., a higher DNIC score). These data are presented graphically (using categories of sleep efficiency) in Fig 1. In terms of sleep architecture, a trend was observed for a positive association between DNIC and the percent of time spent in Stage 2 sleep (r= .25, p= .08). DNIC was not correlated with either the percentage of time spent in Stage 3/4 sleep (r= .06) or the percentage of time spent in REM sleep (r= .01). Several other sleep variables showed near-significant relationships: e.g., correlations for the PLM index (r= −.25, p= .09) and the Sleep Bruxism Index [events/hour sleep (r= .25, p= .08)] approached significance. We also evaluated other demographic and pain-related correlates of DNIC; no significant associations were observed for sex, duration of TMD, clinical pain severity, BDI, or STAI scores (all p’s > .10). DNIC tended to be inversely related to an individual’s body mass index (BMI) (r= −.26, p= .07). DNIC was also inversely associated with age (r= −.36, p= .01), as has been reported previously (Lariviere et al., 2007).
Figure 1.
Diffuse Noxious Inhibitory Controls (DNIC) as a function of PSG Sleep Efficiency. Data are presented as Mean ± SD.
Next, multivariate linear mixed-effects model were used to examine the relationship of predictors and DNIC assessed over repeated measurements. Multiple sleep variables (i.e., those that showed significant or near-significant univariate correlations with DNIC) were included as predictors in the model, including the PLM and sleep bruxism indices, stage 2 %, and sleep efficiency. Due to multicollinearity considerations, sleep efficiency and TST could not be included together in a model (i.e., r= .99 for these 2 variables), so 2 separate models were computed. Day and time were included as potential predictors of DNIC in order to evaluate whether DNIC changed over time or differed as a function of the time of day at which it was assessed. These effects were non-significant (see Table 2), suggesting relative stability of the DNIC response across study days. Although age was a significant correlate of DNIC in univariate analyses, it was non-significant in the multivariate analysis, perhaps reflecting the confounding effects of sleep continuity (e.g., older age is associated with reduced sleep efficiency, which accounts for the relationship between age and DNIC). Collectively, only sleep efficiency (and total sleep time) was predictive of DNIC scores, with better sleep efficiency being associated with improved DNIC (i.e., positive scores, reflecting greater pain inhibition). See Table 2.
Table 2.
Results of linear mixed-effects model assessing predictors of DNIC
| Variable | Standardized β | SEM | Unstandardized β | t-value | p-value |
|---|---|---|---|---|---|
| Age | −.06 | .12 | −.001 | −0.5 | .64 |
| Body Mass Index | .−.15 | .10 | −.007 | −1.5 | .15 |
| Time | −.07 | .09 | −.03 | −0.7 | .48 |
| Day | −.09 | .09 | −.03 | −1.0 | .32 |
| Sleep Variables | |||||
| PLM Index | −.12 | .11 | −.004 | −1.0 | .25 |
| Stage 2 % | .05 | .11 | .001 | 0.4 | .68 |
| Bruxism Index | .06 | .09 | .003 | 0.6 | .54 |
| PSG Sleep Efficiency* | .23 | .11 | .60 | 2.0 | .04 |
| PSG Total Sleep Time* | .24 | .11 | .001 | 2.1 | .04 |
Note: The estimates for Sleep Efficiency and Total Sleep Time were derived from separate models; the two variables are highly correlated and could not be included together in a model.
Discussion
Recently, increased attention has been focused on the inter-relationships between pain and sleep. Reviews have suggested that sleep may play an etiologic role in certain pain conditions such as fibromyalgia (Staud and Spaeth, 2008), that sleep disruption and pain are bi-directionally connected (Smith and Haythornthwaite, 2004), and that manipulations of sleep alter sensitivity to noxious stimuli (Lautenbacher et al., 2006). Classic studies from the 1970’s demonstrated that patients with chronic, widespread, musculoskeletal pain showed characteristic abnormalities of non-REM sleep, and that selective disruption of slow wave sleep could re-produce similar abnormalities as well as the associated pain symptoms (Moldofsky et al., 1975;Moldofsky and Scarisbrick, 1976). In addition, recent work in several laboratories has demonstrated that application of calibrated noxious stimuli during sleep produces arousals and disruptions of sleep continuity (Drewes et al., 1997;Lavigne et al., 2000), highlighting the bi-directional nature of the associations between sleep and pain.
Several recent findings have tied naturally-occurring disturbances of sleep to augmented pain perception and pain report in the general population (Gupta et al., 2006;Edwards et al., 2008), and in a longitudinal study of fibromyalgia patients (Bigatti et al., 2008) which reported that over the course of one year, those fibromyalgia patients whose sleep was most disrupted (based on self-report) showed the greatest increases in pain. Similar micro-longitudinal findings had been reported earlier, in which sleep quality on a given night was a predictor of pain report the following day (Affleck et al., 1996). Of note, an earlier study had also highlighted links between disturbed sleep and low pressure pain thresholds in fibromyalgia patients (Agargun et al., 1999).
To date, though, little research has examined the role of sleep in shaping TMD pain. Moreover, no research in clinical samples had evaluated associations between objective PSG-derived indices of sleep and measures of pain processing derived from laboratory-based quantitative sensory testing methods. In the present study, we find that measures of sleep efficiency and total sleep time are positively associated with better functioning of DNIC, a measure of endogenous pain-inhibition. Recent studies have suggested links between dysfunction in DNIC systems and dysfunction in endogenous opioid systems (Ram et al., 2008), and less-effective DNIC may serve as a risk factor for the development or maintenance of persistent pain complaints (Yarnitsky et al., 2008;Pielsticker et al., 2005). Our previous study suggested that fragmentation of sleep over the course of several nights could reduce or abolish DNIC in healthy volunteers, and also resulted in elevations in clinical pain (Smith et al., 2007). To our knowledge, the present study is the first to demonstrate, in a sample of patients with chronic pain, that better sleep continuity is related to better-functioning DNIC.
The present report is limited by its relatively small sample size, its focus on a single chronic pain condition (e.g., the present findings in patients with TMD, a quintessential “idiopathic” pain disorder, may or may not hold for other conditions such as neuropathic pain, inflammatory pain, etc), and its essentially cross-sectional design. Moreover, we were not able to measure and analyze numerous additional variables that might influence pain- and sleep-related processes, such as menstrual cycle phase (for women), personality factors, etc. Collectively, however, despite its limitations, the results are consistent with numerous other recent studies documenting associations between individual differences in sleep and the experience of pain. If replicated and extended to other samples, these findings would appear to support aggressive efforts to treat insomnia (particularly in patients demonstrating reduced sleep efficiency or total sleep time) early in the course of a painful condition. At present, caution is warranted in drawing such conclusions, since it has not yet been shown that improvements in disturbed sleep are accompanied by beneficial alterations in DNIC or other pain-modulatory systems. Additional studies, especially controlled trials with long-term follow-up, are needed to determine whether sleep-improving interventions are of prophylactic benefit in the context of chronic pain.
Table 1.
PSG-assessed sleep variables.
| Variable | Sample Mean | SD |
|---|---|---|
| Sleep Latency (min) | 10.4 | 8.7 |
| Wake after Sleep Onset (min) | 34.3 | 28.2 |
| Sleep Efficiency | .89 | .09 |
| Total Sleep Time (min) | 428.2 | 42.9 |
| Stage 1 Percentage | 8.3% | 6.9 |
| Stage 2 Percentage | 54.7% | 8.2 |
| Stage 3/4 Percentage | 15.9% | 12.0 |
| REM Percentage | 22.2% | 9.8 |
| Periodic Leg Movements/Hour | 1.8 | 7.3 |
| Apnea/Hypopnea Rate/Hour | 5.1 | 8.4 |
| Sleep Bruxism Episodes/Hour | 2.5 | 2.3 |
PSG= Polysomnography; REM= Rapid Eye Movement
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Affleck G, Urrows S, Tennen H, Higgins P, Abeles M. Sequential daily relations of sleep, pain intensity, and attention to pain among women with fibromyalgia. Pain. 1996;68:363–368. doi: 10.1016/s0304-3959(96)03226-5. [DOI] [PubMed] [Google Scholar]
- 2.Agargun MY, Tekeoglu I, Gunes A, Adak B, Kara H, Ercan M. Sleep quality and pain threshold in patients with fibromyalgia. Compr Psychiatry. 1999;40:226–228. doi: 10.1016/s0010-440x(99)90008-1. [DOI] [PubMed] [Google Scholar]
- 3.American Academy of Sleep Medicine. The International Classification of Sleep Disorders: Diagnostic and coding manual. Westchester, IL: 2005. [Google Scholar]
- 4.Argoff CE. The coexistence of neuropathic pain, sleep, and psychiatric disorders: a novel treatment approach. Clin J Pain. 2007;23:15–22. doi: 10.1097/01.ajp.0000210945.27052.b3. [DOI] [PubMed] [Google Scholar]
- 5.Auvenshine RC. Temporomandibular disorders: associated features. Dent Clin North Am. 2007;51:105–27. vi. doi: 10.1016/j.cden.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 7.Bigatti SM, Hernandez AM, Cronan TA, Rand KL. Sleep disturbances in fibromyalgia syndrome: relationship to pain and depression. Arthritis Rheum. 2008;59:961–967. doi: 10.1002/art.23828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diatchenko L, Nackley AG, Slade GD, Fillingim RB, Maixner W. Idiopathic pain disorders--pathways of vulnerability. Pain. 2006;123:226–230. doi: 10.1016/j.pain.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Drewes AM, Nielsen KD, Arendt-Nielsen L, Birket-Smith L, Hansen LM. The effect of cutaneous and deep pain on the electroencephalogram during sleep--an experimental study. Sleep. 1997;20:632–640. doi: 10.1093/sleep/20.8.632. [DOI] [PubMed] [Google Scholar]
- 10.Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord. 1992;6:301–355. [PubMed] [Google Scholar]
- 11.Edwards RR. Individual differences in endogenous pain modulation as a risk factor for chronic pain. Neurology. 2005;65:437–443. doi: 10.1212/01.wnl.0000171862.17301.84. [DOI] [PubMed] [Google Scholar]
- 12.Edwards RR, Almeida DM, Klick B, Haythornthwaite JA, Smith MT. Duration of sleep contributes to next-day pain report in the general population. Pain. 2008 doi: 10.1016/j.pain.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards RR, Fillingim RB, Ness TJ. Age-related differences in endogenous pain modulation: a comparison of diffuse noxious inhibitory controls in healthy older and younger adults. Pain. 2003a;101:155–165. doi: 10.1016/s0304-3959(02)00324-x. [DOI] [PubMed] [Google Scholar]
- 14.Edwards RR, Ness TJ, Weigent DA, Fillingim RB. Individual differences in diffuse noxious inhibitory controls (DNIC): An association with clinical variables. Pain. 2003b;106(3):427–437. doi: 10.1016/j.pain.2003.09.005. Ref Type: Journal (Full) [DOI] [PubMed] [Google Scholar]
- 15.Gupta A, Silman AJ, Ray D, Morriss R, Dickens C, Macfarlane GJ, Chiu YH, Nicholl B, McBeth J. Rheumatology. Oxford: 2006. The role of psychosocial factors in predicting the onset of chronic widespread pain: results from a prospective population-based study. [DOI] [PubMed] [Google Scholar]
- 16.Haack M, Mullington JM. Sustained sleep restriction reduces emotional and physical well-being. Pain. 2005;119:56–64. doi: 10.1016/j.pain.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30:1145–1152. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haythornthwaite JA, Hegel MT, Kerns RD. Development of a sleep diary for chronic pain patients. J Pain Symptom Manage. 1991;6:65–72. doi: 10.1016/0885-3924(91)90520-e. [DOI] [PubMed] [Google Scholar]
- 19.Kivioja J, Sjalin M, Lindgren U. Psychiatric morbidity in patients with chronic whiplash-associated disorder. Spine. 2004;29:1235–1239. doi: 10.1097/00007632-200406010-00013. [DOI] [PubMed] [Google Scholar]
- 20.Kosek E, Ordeberg G. Lack of pressure pain modulation by heterotopic noxious conditioning stimulation in patients with painful osteoarthritis before, but not following, surgical pain relief. Pain. 2000;88:69–78. doi: 10.1016/S0304-3959(00)00310-9. [DOI] [PubMed] [Google Scholar]
- 21.Kundermann B, Spernal J, Huber MT, Krieg JC, Lautenbacher S. Sleep deprivation affects thermal pain thresholds but not somatosensory thresholds in healthy volunteers. Psychosom Med. 2004;66:932–937. doi: 10.1097/01.psy.0000145912.24553.c0. [DOI] [PubMed] [Google Scholar]
- 22.Lariviere M, Goffaux P, Marchand S, Julien N. Changes in pain perception and descending inhibitory controls start at middle age in healthy adults. Clin J Pain. 2007;23:506–510. doi: 10.1097/AJP.0b013e31806a23e8. [DOI] [PubMed] [Google Scholar]
- 23.Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10:357–369. doi: 10.1016/j.smrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Lautenbacher S, Prager M, Rollman GB. Pain additivity, diffuse noxious inhibitory controls, and attention: a functional measurement analysis. Somatosens Mot Res. 2007;24:189–201. doi: 10.1080/08990220701637638. [DOI] [PubMed] [Google Scholar]
- 25.Lavigne G, Zucconi M, Castronovo C, Manzini C, Marchettini P, Smirne S. Sleep arousal response to experimental thermal stimulation during sleep in human subjects free of pain and sleep problems. Pain. 2000;84:283–290. doi: 10.1016/s0304-3959(99)00213-4. [DOI] [PubMed] [Google Scholar]
- 26.Lavigne GJ, Rompre PH, Montplaisir JY. Sleep bruxism: validity of clinical research diagnostic criteria in a controlled polysomnographic study. J Dent Res. 1996;75:546–552. doi: 10.1177/00220345960750010601. [DOI] [PubMed] [Google Scholar]
- 27.Moldofsky H, Scarisbrick P. Induction of neurasthenic musculoskeletal pain syndrome by selective sleep stage deprivation. Psychosom Med. 1976;38:35–44. doi: 10.1097/00006842-197601000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Moldofsky H, Scarisbrick P, England R, Smythe H. Musculoskeletal symptoms and non-REM sleep disturbance in patients with “fibrositis syndrome” and healthy subjects. Psychosomatic Medicine. 1975;37:341–351. doi: 10.1097/00006842-197507000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Pielsticker A, Haag G, Zaudig M, Lautenbacher S. Impairment of pain inhibition in chronic tension-type headache. Pain. 2005;118:215–223. doi: 10.1016/j.pain.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 30.Ram KC, Eisenberg E, Haddad M, Pud D. Oral opioid use alters DNIC but not cold pain perception in patients with chronic pain - New perspective of opioid-induced hyperalgesia. Pain. 2008 doi: 10.1016/j.pain.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 31.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Washington, D.C: 1968. [DOI] [PubMed] [Google Scholar]
- 32.Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30:494–505. doi: 10.1093/sleep/30.4.494. [DOI] [PubMed] [Google Scholar]
- 33.Smith MT, Edwards RR, Stonerock GL, McCann UD. Individual variation in rapid eye movement sleep is associated with pain perception in healthy women: preliminary data. Sleep. 2005;28:809–812. doi: 10.1093/sleep/28.7.809. [DOI] [PubMed] [Google Scholar]
- 34.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8:119–132. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 35.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (Palo Alto) 1983. [Google Scholar]
- 36.Staud R, Spaeth M. Psychophysical and neurochemical abnormalities of pain processing in fibromyalgia. CNS Spectr. 2008;13:12–17. doi: 10.1017/s109285290002678x. [DOI] [PubMed] [Google Scholar]
- 37.Talbot JD, Duncan GH, Bushnell MC. Effects of diffuse noxious inhibitory controls (DNICs) on the sensory-discriminative dimension of pain perception. Pain. 1989;36:231–238. doi: 10.1016/0304-3959(89)90028-6. [DOI] [PubMed] [Google Scholar]
- 38.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004;5:133–137. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Wilder-Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut. 2004;53:1595–1601. doi: 10.1136/gut.2003.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willer JC, Le Bars D, De Broucker T. Diffuse noxious inhibitory controls in man: involvement of an opioidergic link. European Journal of Pharmacology. 1990;182:347–355. doi: 10.1016/0014-2999(90)90293-f. [DOI] [PubMed] [Google Scholar]
- 41.Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben Nun A, Sprecher E, Best LA, Granot M. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain. 2008;138:22–28. doi: 10.1016/j.pain.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 42.Yunus MB. Central sensitivity syndromes: a new paradigm and group nosology for fibromyalgia and overlapping conditions, and the related issue of disease versus illness. Semin Arthritis Rheum. 2008;37:339–352. doi: 10.1016/j.semarthrit.2007.09.003. [DOI] [PubMed] [Google Scholar]