Abstract
In plant cells, antioxidants keep reactive oxygen species at low concentrations, avoiding oxidative damage while allowing them to play crucial functions in signal transduction. However, little is known about the role of antioxidants during fruit maturation, especially in legumes. Snap pea (Pisum sativum) plants, which have edible fruits, were grown under nodulating and non-nodulating conditions. Fruits were classified in three maturity stages and antioxidants were determined in the seeds and seedless pods. Maturation or prolonged storage of fruits at 25 °C led to a decline in antioxidant activities and metabolites and in γ-glutamylcysteine synthetase protein. Notable exceptions were superoxide dismutase activity and glutathione peroxidase protein, which increased in one or both of these processes. During maturation, cytosolic peroxiredoxin decreased in seeds but increased in pods, and ascorbate oxidase activity was largely reduced in seeds. In stored fruits, ascorbate oxidase activity was nearly abolished in seeds but doubled in pods. It is concluded that symbiotic nitrogen fixation is as effective as nitrogen fertilization in maintaining the antioxidant capacity of pea fruits and that, contrary to climacteric fruits, a general decrease in antioxidants during maturation does not involve oxidative stress. Results underscore the importance of the antioxidant system in reproductive organs and point to ascorbate–glutathione metabolism and cytosolic peroxiredoxin as key players in pea fruit development.
Keywords: Antioxidants, ascorbate, fruit maturation, fruit storage, glutathione, nitrogen fixation, pea, peroxiredoxins
Introduction
Leguminous plants such as pea (Pisum sativum), bean (Phaseolus vulgaris), or alfalfa (Medicago sativa) are crops of major economical value as a protein source for human and animal consumption (Graham and Vance, 2003). They are also essential for sustainable agricultural systems because of their ability to establish nitrogen-fixing symbioses with soil bacteria, thus providing a biological alternative to chemical fertilization. The site of nitrogen fixation by the rhizobia–legume symbiosis is the root nodule, where bacterial nitrogenase produces ammonia which is assimilated in the host cells and exported to the shoot in the form of amides or ureides. Thus, rhizobia provide the plant with fixed nitrogen, whereas the plant supplies the nodule with carbon substrates, mainly sucrose, derived from photosynthesis.
These two processes, nitrogen fixation and photosynthesis, along with respiration and peroxisomal metabolism, involve electron transfer reactions that give rise to reactive oxygen species (ROS). In general, ROS are potentially toxic and their uncontrolled production can result in oxidative damage of cellular components (Halliwell and Gutteridge, 2007). However, some ROS such as the superoxide radical and hydrogen peroxide (H2O2), at low concentrations, fulfil important roles in stress perception, photosynthesis regulation, pathogen recognition, programmed cell death, and plant development (Mittler et al., 2004). Antioxidants modulate the steady-state concentrations of ROS, avoiding their potential cytotoxicity while allowing them to function as signal molecules (Mittler et al., 2004). Antioxidant enzymes include the superoxide dismutases (SODs), catalases, peroxiredoxins (PRXs), glutathione peroxidases (GPXs), and the four enzymes of the ascorbate–glutathione pathway (Dietz, 2003; Matamoros et al., 2003; Mittler et al., 2004; Navrot et al., 2006). In this pathway, ascorbate peroxidase (APX) catalyses the reduction of H2O2 to water by ascorbate producing monodehydroascorbate and dehydroascorbate. Ascorbate is regenerated by monodehydroascorbate reductase (MR) and dehydroascorbate reductase (DR) using NADH and reduced glutathione, respectively. The oxidized form of glutathione generated by DR activity is reduced by glutathione reductase (GR) using NADPH (Dalton et al., 1986; Jiménez et al., 2002a).
Two key metabolites of this pathway, ascorbate and glutathione, are major water-soluble antioxidants and redox buffers in plant cells but also have crucial functions in stress responses and organ development (Arrigoni and De Tullio, 2002; Noctor et al., 2002). Ascorbate can be oxidized to monodehydroascorbate by ascorbate oxidase (AO) in the apoplast. This enzyme can therefore modulate the redox state of the apoplastic ascorbate pool, which is important for controlling cell elongation and triggering signal transduction cascades in response to external stimuli (Kato and Esaka, 2000; Pignocchi et al., 2006). Glutathione participates in the induction of defence genes, sulphur transport and storage, and heavy metal detoxification, and its concentration needs to be tightly controlled at several levels. Major mechanisms are the transcriptional, translational, and post-translational regulation of the enzyme γ-glutamylcysteine synthetase (γECS), which catalyses the first step of glutathione biosynthesis in all organisms (May et al., 1998; Xiang and Oliver, 1998; Noctor et al., 2002; Hicks et al., 2007).
The antioxidants of legume leaves and nodules have been examined in considerable detail (see, for example, Matamoros et al., 2003; Palma et al., 2006), but similar information on legume fruits is lacking. In fact, most extensive studies on the role of ROS and antioxidants in fruit development and maturation (ripening) have been conducted on climacteric fruits such as pear (Pyrus communis; Brennan and Frenkel, 1977), saskatoon fruit (Amelanchier alnifolia; Rogiers et al., 1998), and tomato (Lycopersicon esculentum; Jiménez et al., 2002a). Ripening of these fruits is characterized by a burst of ethylene production and respiratory activity. The involvement of ROS and antioxidants during fruit ripening has been also investigated in the chloroplasts (Bouvier et al., 1998) and mitochondria (Jiménez et al., 2002b) of pepper (Capsicum annuum) fruit, which exhibits non-climacteric physiology. In this case, ripening involves an intense chloroplast-to-chromoplast (green-to-red fruit) transition (Bouvier et al., 1998). Many non-climacteric fruits like those of pea (Pisum sativum) do not exhibit those major changes during maturation, and hence the conclusion drawn from the above studies that there is an oxidative process needs to be assessed.
The study of antioxidants in fruits is important for several reasons. First, antioxidants may protect fruit tissues from potentially toxic ROS and thereby contribute to the stress tolerance of crops (Mittler et al., 2004; Van Breusegem et al., 2008). Second, fruits may have nutritional value for animal and/or human consumption. Third, in many cases fruits have a relatively short shelf-life following harvest, during which they undergo changes in texture, colour, and flavour, which may be accompanied by a decline in antioxidants (Davey and Keulemans, 2004; Malacrida et al., 2006; Stevens et al., 2008). However, the role of antioxidants in the development, maturation, and post-harvest storage of legume fruits is poorly defined. The implication of antioxidants and oxidative stress in these processes has been investigated here using a commercial variety of pea with edible seeds and pods (Basterrechea and Hicks, 1991). In addition, the effect of sustainable agricultural practices (nitrogen fixation versus combined nitrogen) on the antioxidant composition, nutritional value, and post-harvest shelf life of pea fruits has been assessed.
Materials and methods
Plant growth and metabolic parameters
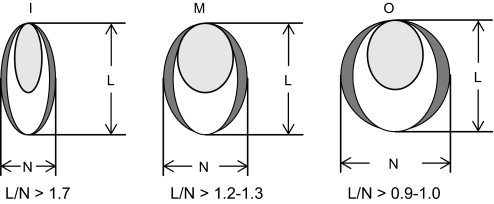
Sugar snap pea (Pisum sativum L. cv. Sugar Lace) seed was provided by Bonduelle (Milagro, Navarra, Spain). Nodulated plants (inoculated with Rhizobium leguminosarum bv. viciae strain NLV8) were grown in pots containing a 1:1 (v:v) mixture of Perlite and Vermiculite under controlled environment conditions (photon flux density of 350 μmol m−2 s−1, day length of 16 h, day/night regime of 25/20 °C temperature, and 70/85% relative humidity). Non-nodulated plants were grown under the same conditions, except that the nutrient solution (Gogorcena et al., 1997) was supplemented with 2.5 mM NH4NO3. After 45–60 d, fruits were harvested and classified into three maturity stages based on the L/N ratio (Fig. 1). In the immature stage (I) the seeds do not fill the hull, in the mature stage (M) the seeds fill the hull without causing deformation, and in the overmature stage (O) the seeds deform the hull (Basterrechea and Hicks, 1991).
Fig. 1.
Developmental stages of pea fruits. These were harvested from nodulated plants being 45–60-d-old and were classified as immature (I), mature (M), or overmature (O) according to their L/N ratios. The scheme shows transverse sections of pods.
To characterize these maturity stages further, fruits were separated into seed and seedless pods, and the fresh weights and water and protein contents were determined. Total protein was quantified by the Bradford microassay (Bio-Rad) using bovine serum albumin as the standard. The content of H2O2 in whole fruits was measured, just before the beginning of the photoperiod, as an indicator of metabolic activity. Extraction of H2O2 from pea fruits was performed immediately after harvest with trichloroacetic acid and activated charcoal. Extracts were adjusted to pH 8.4 with ammonia, and H2O2 was quantified following its reaction with 4-aminoantipyrine and phenol to form a stable red product in the presence of peroxidase (Zhou et al., 2006). Blanks containing catalase were run for each sample, as well as for the calibration with H2O2 standards which were added to the extraction medium in parallel to the samples.
For biochemical and molecular analyses, samples of seeds and seedless pods of representative fruits of the three maturity stages were flash-frozen in liquid nitrogen immediately after harvest. To investigate the effects of storage, fruits were kept at 25 °C for 4 d prior to freezing in liquid nitrogen. All plant material was stored at –80 °C.
Antioxidant enzymes
For enzyme extraction, 100 mg of seeds or seedless pods were ground in liquid nitrogen; this was left to boil dry and the powder was homogenized in 500 μl of the following optimized media.
SOD
50 mM potassium phosphate buffer (pH 7.8), 0.1 mM EDTA, 1% (w/v) soluble polyvinylpyrrolidone (PVP-10), and 0.1% (v/v) Triton X-100.
APX and catalase
50 mM potassium phosphate buffer (pH 7.0) and 0.5% PVP-10 (for the APX assay, this buffer was supplemented with 5 mM ascorbate to avoid the inactivation of the plastidic isoform).
MR, DR, and GR
50 mM potassium phosphate buffer (pH 7.8), 1% PVP-10, 0.2 mM EDTA, and 10 mM β-mercaptoethanol.
AO: 10 mM sodium phosphate buffer (pH 6.5).
The extracts were centrifuged (13 000 g for 10 min at 4 °C) and the enzyme activities were assayed in the supernatants. All activities were assayed at 25 °C within linear range.
Total SOD activity was assayed by a method based on the inhibition of cytochrome c reduction by superoxide at 550 nm and catalase activity by following the decomposition of H2O2 at 240 nm (Rubio et al., 2002). One SOD unit (U) was defined as the amount of SOD required to inhibit the reduction of ferric cytochrome c by 50% (McCord and Fridovich, 1969). APX and DR activities were determined by monitoring ascorbate oxidation at 290 nm (Asada, 1984) and ascorbate formation at 265 nm (Nakano and Asada, 1981), respectively. MR and GR activities were assayed by following the oxidation of NADH (Dalton et al., 1993) and NADPH (Dalton et al., 1986) at 340 nm, respectively. To assay AO activity, extracts were centrifuged (13 000 g for 15 min) and the pellets were resuspended by vigorous shaking for 10 min in 10 mM sodium phosphate buffer (pH 6.5) containing 1 M NaCl. The extracts were centrifuged again and AO activity was determined in the supernatant by following ascorbate oxidation at 265 nm (Pignocchi et al., 2003).
Antioxidant metabolites
Ascorbate was extracted from 100 mg of plant tissue with 500 μl of HClO4. The extract was centrifuged (13 000 g for 10 min at 4 °C) and the supernatant neutralized with 1 M K2CO3. For determination of total ascorbate (reduced ascorbate+dehydroascorbate), samples were incubated for 15 min at room temperature in the dark with 0.4 mM dithioerythritol; then 100 μl of the mixture was added to 900 μl of 100 mM HEPES (pH 5.6) and the decrease of ascorbate concentration was monitored at 265 nm after the addition of 0.05 units of AO (Sigma-Aldrich). For the determination of reduced ascorbate, the same procedure was used omitting the incubation with dithioerythritol, and dehydroascorbate was calculated as the difference between the concentrations of total and reduced ascorbate.
Glutathione was quantified after derivatization with monobromobimane using an HPLC with fluorescence detection (Matamoros et al., 1999). The concentrations of reduced and oxidized forms of glutathione were determined spectrophotometrically using an enzymatic cycling assay (Griffith, 1980). The redox states of ascorbate and glutathione were calculated as 100× [ascorbate/(ascorbate+dehydroascorbate)] and 100×[reduced glutathione/(reduced+oxidized glutathione)], respectively.
Markers of oxidative stress
The oxidative damage of lipids was estimated as the content of malondialdehyde after reaction with thiobarbituric acid (TBA) by HPLC (Iturbe-Ormaetxe et al., 1998). Briefly, lipid peroxides were extracted with 5% (w/v) metaphosphoric acid and 0.04% (w/v) butylated hydroxytoluene. After centrifugation, the chromogen was formed by mixing 100 μl of supernatant, 10 μl of 2% butylated hydroxytoluene, 50 μl of 1% (w/v) TBA, and 50 μl of 25% (v/v) HCl, and by incubating the reaction mixtures at 95 °C for 30 min. The chromogen was extracted with 1-butanol and the organic phase was evaporated under N2 and kept at –80 °C until analysis. The samples were resuspended in 60 μl of HPLC solvent and the (TBA)2-malondialdehyde adduct was resolved on an Ultrasphere C18 column (5 μm, 25 cm×4.6 mm; Beckman) and was eluted with 5 mM potassium phosphate buffer (pH 7.0) containing 15% acetonitrile and 0.6% tetrahydrofuran. The flow rate was 1 ml min−1 and detection was at 532 nm. Calibration curves were made using 1,1,3,3-tetraethoxypropane. The oxidative damage of proteins was estimated as the content of total carbonyl groups. Proteins were separated on 12.5% sodium dodecyl sulphate (SDS) gels, and carbonyls were quantified by derivatization with 2,4-dinitrophenylhydrazine using the OxyBlot Protein Oxidation Detection kit following the manufacturer's instructions (Chemicon, Temecula, CA, USA).
Immunoblot analyses
Proteins were extracted, separated on 15% SDS gels, and transferred onto polyvinylidene fluoride membranes as described by Loscos et al. (2008). Equal loading of lanes and transfer quality were verified by Ponceau staining of membranes. Immunoblot analyses were performed using rabbit polyclonal antibodies against γECS of common bean (Phaseolus vulgaris; MR Clemente, unpublished results), GPX3.2 of poplar (Populus trichocarpa; Navrot et al., 2006), or 2-CysPrx (Laxa et al., 2007), PrxQ (Lamkemeyer et al., 2006), PrxIIC (Horling et al., 2003), PrxIIF (Finkemeier et al., 2005), and plastidial DR (DRp) of Arabidopsis (Arabidopsis thaliana). For immunoblots of cytosolic DR (DRc), a guinea pig polyclonal antibody generated against Arabidopsis DRc (Eltayeb et al., 2006) was used. The antibody against PrxIIC also recognizes two other PRX isoforms of Arabidopsis, PrxIIB and PrxIID, that are localized to the cytosol (Horling et al., 2003). Consequently, cytosolic PrxII isoforms will collectively be designated as PrxIIc in this work. Primary antibodies were used at dilutions of 1:1000 (γECS and GPX), 1:500 (2-CysPrx), 1:3000 (PrxQ, PrxIIc, PrxIIF, and DRp), or 1:2500 (DRc). Secondary antibodies were goat anti-guinea pig (DRc) or anti-rabbit (other enzymes) IgG horseradish peroxidase conjugated antibodies and were used at dilutions of 1:5000 or 1:20000, respectively. Incubations with the primary and secondary antibodies were performed in TTBS [20 mM TRIS-HCl (pH 7.8), 0.5 M NaCl, 0.05% (v/v) Tween-20] supplemented with 5% (w/v) skimmed milk to reduce background signal. Immunoreactive proteins were visualized using the SuperSignal West Pico (Pierce) chemiluminescent reagent for peroxidase detection.
Gene expression analyses
Total RNA was isolated from seeds using the RNAqueous kit (Ambion, Cambridgeshire, UK) and processed as described by Loscos et al. (2008). Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis was carried out with the iCycler iQ System using the iQ SYBR-Green Supermix (Bio-Rad) and gene-specific primers for γECS (5′-CCCTCTTGAAACCCTGCATC-3′; 5′-CTCCCACTTTGGCTGGAAAC-3′) and actin (5′-GTGTCTGGATTGGAGGATCAATC-3′, 5′-GGCCACGCTCATCATATTCA-3′). The PCR programme was as previously reported (Loscos et al., 2008) and the amplification efficiency of the primers, calculated by serial dilutions of seed cDNA, was >90%. The γECS mRNA levels were normalized with actin as the reference gene and were expressed using the method of Livak and Shmittgen (2001). All the reactions were set up in duplicate (two technical replicates) using three RNA preparations (biological replicates) from different fruits.
Results and discussion
Antioxidant protection is similar in pea fruits from nodulated and non-nodulated plants
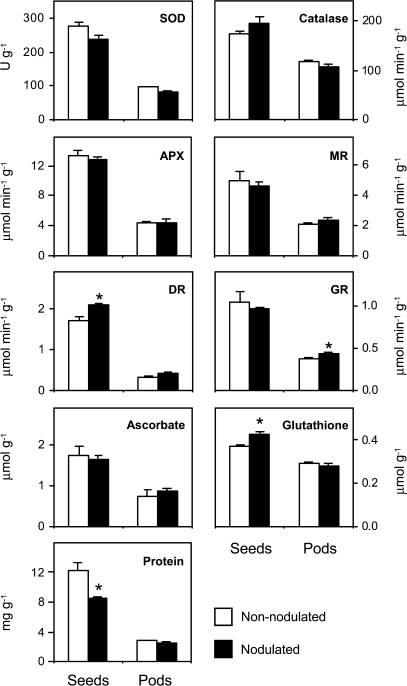
The first objective of this study was to evaluate the effect of symbiotic nitrogen fixation on the antioxidant composition of pea fruits. To this end, the antioxidant enzymatic activities and metabolite contents of fruits were determined at the mature stage from nodulated plants and from plants fed combined nitrogen (Fig. 2). For all measurements, fruits were separated into seeds and seedless pods immediately after harvest. In general, the antioxidant content, when expressed on a fresh weight basis, was greater in the seeds, but there were also significant amounts in the pods. For example, DR activity was 5-fold greater in the seeds than in the pods, whereas catalase activity and glutathione content were only c. 1.7-fold greater in the seeds (Fig. 2). However, in an average mature fruit, the pod accounts for c. 70% of the fresh weight (Table 1). These data should be taken into consideration when calculating the absolute values of antioxidants present in the seeds and pod of an individual fruit. Also, it can be concluded that the fruits from plants that were dependent on nitrogen fixation had similar antioxidant levels to those that were supplied with ammonium nitrate. In fact, the DR activity and glutathione content of the seeds were c. 22% and 17% higher, respectively, in the nodulated plants. In seedless pods, the antioxidant content was roughly similar in plants grown on any of the two nitrogen nutrition sources, with a slight increase of GR activity (14%) in nodulated plants (Fig. 2). It is also worth noting that nodulation led to an increase in the specific activities of the antioxidant enzymes in the seeds (data not shown). Consequently, the loss of 30% of the total protein content in the mature seeds of nodulated plants (Fig. 2) did not compromise their overall antioxidant capacity. Recently, Palma et al. (2006) compared the oxidative metabolism of leaves of nodulated and non-nodulated pea, and concluded that nodulation accelerates the decline in the antioxidant capacity of leaves during senescence. Also, Vanacker et al. (2006) observed higher lipid and protein oxidation levels in senescing leaves of nodulated plants compared with nitrate-fed plants, and suggested that systemic signals produced by the breakdown of the symbiosis were the responsible factors. Instead it was found here that the pea fruits of nodulated plants retained a similar antioxidant capacity to those of non-nodulated plants. This discrepancy between the responses of the antioxidant capacity of leaves and fruits during senescence and maturation, respectively, may be attributed to metabolic features inherent in these two plant organs and underscores the importance of ROS protection in reproductive tissues.
Fig. 2.
Antioxidants in seeds and seedless pods of non-nodulated (nitrogen-fed) and nodulated (nitrogen-fixing) pea plants. All parameters are expressed per gram of fresh weight. The contents of ascorbate and glutathione are the sum of the corresponding reduced and oxidized forms. Values are means ±SE of 4–6 replicates from at least three series of plants grown independently. For each tissue, means marked with an asterisk are significantly different at P <0.05 based on Student's t test. Abbreviations: SOD, superoxide dismutase; APX, ascorbate peroxidase; MR, monodehydroascorbate reductase; DR, dehydroascorbate reductase; GR, glutathione reductase.
Table 1.
Some growth and metabolic parameters of seeds and seedless pods of pea fruits
| Parameter | Unitsa | Developmental stage |
||
| Immature | Mature | Overmature | ||
| Fresh weight (seeds) | g FW per seed | 0.04±0.01 a | 0.24±0.01 b | 0.56±0.02 c |
| Fresh weight (pods) | g FW per pod | 1.81±0.20 a | 2.81±0.19 b | 2.66±0.24 b |
| Water content (seeds) | % | 82.48±0.49 a | 82.60±0.13 a | 75.74±0.77 b |
| Water content (pods) | % | 87.69±0.50 a | 84.91±0.79 b | 85.56±0.64 b |
| Protein (seeds) | mg g−1 FW | 11.00±0.53 a | 8.59±0.13 b | 9.28±0.73 b |
| Protein (pods) | mg g−1 FW | 3.24±0.13 a | 2.51±0.23 b | 2.07±0.08 b |
| H2O2 (whole fruits) | μmol g−1 FW | 1.30±0.34 a | 1.21±0.25 a | 1.10±0.43 a |
FW, fresh weight.
Peroxiredoxins are differentially expressed in pea tissues
Thiol peroxidases, which include the closely related GPX and PRX families, catalyse the reduction of peroxides and play important roles in antioxidant defence and stress signalling. Several GPX and PRX isoforms can be distinguished based on their catalytic mechanism, substrate specificity, and subcellular localization (Dietz, 2003; Navrot et al., 2006). The information on the distribution of thiol peroxidases in legumes is scarce and the relative abundance of these proteins has therefore been examined in seeds and seedless pods of mature pea fruits using immunoblots. This was a necessary prerequisite to study the effects of fruit development and storage on these proteins. For comparison, leaves, roots, and nodules harvested at the same time as the fruits were included in the screening of thiol peroxidases. It was found that GPXs are expressed in the five plant organs examined (data not shown), but the presence of specific isoforms could not be ascertained because the antibody against poplar GPX3.2 may recognize several of them (Navrot et al., 2006). Immunolocalization studies showed that, in legumes, this antibody detects at least a GPX isoform in leaf chloroplasts and in root and nodule plastids (Ramos et al., 2009), and hence it was concluded that pea fruits contain GPX in the plastids of seeds and seedless pods.
By contrast, the high specificities of the four antibodies available against Arabidopsis PRXs allowed the localization of the proteins to be investigated in different organs of pea plants (Fig. 3). Our results indicate that the PrxQ and 2-CysPrx isoforms are abundantly expressed in leaves, in agreement with their proposed role in the protection of photosynthesis against ROS (Dietz, 2003). Notably, the PrxQ protein was not detected in roots, nodules, seeds, or seedless pods, whereas the 2-CysPrx protein was detectable in pods, albeit at low levels. The PrxIIc proteins immunologically related to Arabidopsis PrxIIC were clearly present in seeds and pods, but were barely detectable in roots, nodules, or leaves (Fig. 3). It should be noted, however, that these organs were harvested from the same plants, and at the same time, as fruits and hence PrxIIc might well be detectable in younger tissues. Contrary to the pattern observed for the other PRXs, it was found that the mitochondrial isoform PrxIIF was expressed in all five pea organs examined (Fig. 3). Groten et al. (2006) have reported that the PrxIIF isoform of pea nodules is not regulated by ascorbate and its level does not change appreciably with senescence. Together with our results, it is concluded that PrxIIF, along with manganese–SOD and the ascorbate–glutathione pathway enzymes (Jiménez et al., 2002b), represents a general defence mechanism of fruit mitochondria against ROS generated during respiration.
Fig. 3.
Relative abundance of peroxiredoxin proteins in leaves, roots, nodules, seeds, and seedless pods of pea plants. Gels were loaded with 15 μg of protein per lane. The apparent molecular mass of the proteins (kDa) is indicated on the right. Blots are representative of three to five gels loaded with extracts from different plants.
Pea fruit maturation involves a general decrease in antioxidant capacity but not oxidative stress
Maturation (ripening) has been described as an oxidative process in climacteric fruits (Brennan and Frenkel, 1977; Rogiers et al., 1998). Conceivably then, the antioxidant system may be involved in the control of fruit maturation. To gain an insight into the mechanisms underlying the maturation of pea fruits, antioxidant activities and metabolites were quantified in seeds and seedless pods at the three maturity stages described in Fig. 1. Fruit maturation caused a decrease in the water and protein contents of seeds and pods but had no significant effect on the H2O2 content of whole fruits (Table 1).
Although all determinations of antioxidants were performed both in nodulated and non-nodulated plants, the results were very similar and only those with nodulated plants are presented here for simplicity. Antioxidant enzyme activities and metabolites decreased during the maturation of pea seeds, except APX activity, which was not affected, and SOD and DR activities, which increased by 30–50% from the immature to the overmature stages (Fig. 4A). Thus, catalase and MR activities and ascorbate and glutathione contents were c. 30–50% lower in the mature and/or overmature seeds than in immature seeds, whereas GR activity declined by c. 10–25% (Fig. 4A). The availability of antibodies against the DRc and DRp of Arabidopsis enabled us to investigate whether the high DR activity observed in overmature seeds was due to an increase in the protein level. Surprisingly, during seed development, the content of DRp protein did not change and that of DRc protein was even moderately reduced (data not shown). This suggests that DR activity is post-translationally regulated during fruit development or that the fruit tissue contains additional proteins with DR activity. For example, thioredoxins, glutaredoxins, disulphide isomerases, and glutathione peroxidases display DR activity in plants and animals (De Tullio et al., 2002). The high DR activity in overmature seeds could be explained as a response to the low levels of ascorbate at this developmental stage. If dehydroascorbate is not rapidly reduced back to ascorbate, it may be catabolized to oxalate and tartrate; therefore, an improved efficiency of dehydroascorbate recycling could influence the ascorbate pool size and delay its degradation.
Fig. 4.
Changes in antioxidant levels during the maturation of pea fruits. These were classified as immature (I), mature (M), or overmature (O) as described in Fig. 1. (A) Enzyme activities and metabolite contents of seeds and seedless pods. Abbreviations and other details are as described in Fig. 2. Values are means ±SE of 4–6 replicates from at least three series of plants grown independently. For each tissue, means denoted by the same letter do not significantly differ at P<0.05 based on Duncan's multiple range test. (B) Immunoblot analysis of cytosolic peroxiredoxin II (PrxIIc) in seeds and pods. Gels were loaded with 15 μg of protein per lane, and blots are representative of three to five gels loaded with extracts from different plants. (C) Expression (mRNA level and protein) of γ-glutamylcysteine synthetase (γECS) in seeds. The γECS mRNA levels were normalized with actin as the internal reference gene and were expressed relative to those of immature seeds, which were arbitrarily given a value of 1. Values represent means ±SE of 3–4 RNA extractions from seeds of at least two series of plants grown independently. For immunoblots, gels were loaded with 50 μg of protein per lane. The blot shown in (C) is representative of three independent blots. For (B) and (C), the apparent molecular mass (kDa) of the proteins is indicated on the right.
The changes in the antioxidants of pods were also studied during fruit development (Fig. 4A). Except for SOD, MR, and DR activities, which remained constant or slightly decreased in pods, the antioxidant activities and metabolites followed similar trends in seeds and pods. However, the content of PrxIIc protein showed a contrasting pattern during fruit development (Fig. 4B). The protein decreased in seeds but consistently accumulated in pods from overmature fruits (Fig. 4B), which also contained less ascorbate than the immature or mature pods (Fig. 4A). Because oxidative damage was not detected in seeds or pods at any maturity stage, PrxIIc may be differently regulated in pea fruits and in Arabidopsis leaves, and this regulation could involve roles during seed and pod growth. This hypothesis is consistent with the proposal of Bréhélin et al. (2003) that the expression of three cytosolic PrxII isoforms have developmental functions in Arabidopsis. The results suggest that ascorbate or oxidative stress, described as regulatory cues in leaves (Horling et al., 2003) and nodules (Groten et al., 2006), do not regulate expression of PrxIIc in pea fruits.
Despite our finding that the maturation of pea fruits is accompanied by a decrease in antioxidant defences, progressive oxidative damage was not detected using lipid and protein oxidation as markers (data not shown). The lack of accumulation of oxidatively damaged products is consistent with the observation that H2O2 levels remained rather constant during maturation (Table 1). In addition, although the ascorbate and glutathione contents declined during fruit development, their redox states remained largely unchanged. Thus, >90% of the ascorbate and glutathione pool were in a reduced form in immature, mature, and overmature fruits. However, it should be kept in mind that the glutathione redox potential is sensitive to concentration since two molecules of reduced glutathione are oxidized to one molecule of glutathione disulphide; thus, with decreasing glutathione concentration the redox midpoint potential increases to more positive values even if the ratio of reduced to oxidized glutathione remains unchanged (Schafer and Buettner, 2001). Because the drop in concentration was less than 2-fold, this effect is <20 mV. Despite this, and contrary to the situation described for climacteric fruits, a decrease in the antioxidant capacity does not trigger oxidative stress in developing fruits of nodulated pea plants. Also, it is surmised that the low levels of protein and lipid oxidation during the reproductive phase may be part of a strategy to limit the transfer of oxidatively damaged components to the offspring.
To investigate the mechanism behind the decrease in glutathione in seeds and seedless pods during pea fruit maturation further, the expression of γECS was analysed at the mRNA and protein levels (Fig. 4C). It was found that the γECS mRNA level remained fairly constant during seed maturation, whereas the corresponding protein level sharply decreased at the overmature stage, indicating that the γECS activity of pea fruits is regulated, at least in part, at the translational level. These results, together with our previous work with bean nodules (Loscos et al., 2008), are consistent with the proposal that a translational repression of γECS is part of a multilevel regulation of glutathione homeostasis in plant cells (Xiang and Oliver, 1998; Noctor et al., 2002). Our results also suggest that the significant decline of glutathione in overmature seeds was caused by a decrease in the content of γECS protein and that additional factors, such as glutathione degradation, utilization or transport, may be involved in the decrease in glutathione in mature seeds.
Prolonged storage of pea fruits decreases antioxidant capacity but does not cause oxidative damage
Post-harvest storage of fruits usually results in physiological disorders that affect both commercial and nutritional qualities. On the other hand, the antioxidant capacity has been positively correlated with better storage properties (Davey and Keulemans, 2004; Stevens et al., 2008). Thus, it was decided to assess the involvement of ROS and oxidative damage in the deterioration of fruits from nodulated pea plants during storage at room temperature. For this purpose, the antioxidant content of mature fruits that had been flash-frozen in liquid nitrogen immediately after harvest was compared with that of fruits that had been kept at 25 °C for 4 d. In addition, oxidative damage levels were determined in both sets of fruits. These results showed that the storage of pea fruits at room temperature caused a general decrease in their antioxidant content. In seeds from stored fruits, APX, MR, and DR activities were 16%, 25%, and 47% lower, respectively, than in seeds from frozen fruits (Fig. 5A). Similarly, the ascorbate content of seeds decreased by 78% with storage. By contrast, glutathione did not vary and SOD activity was enhanced by 37% (Fig. 5A). In pods, prolonged storage of fruits decreased the ascorbate and glutathione contents by 35–55% and MR and GR activities by 10–35%, whereas it doubled the SOD activity and had no effect on the activities of catalase or the other enzymes of the ascorbate–glutathione pathway (Fig. 5A).
Fig. 5.
Changes in antioxidants during the storage of pea fruits. Control fruits were frozen in liquid nitrogen immediately after harvest, whereas stored fruits were kept at 25 °C for 4 d. (A) Enzyme activities and metabolite contents in seeds and seedless pods. Abbreviations and other details are as described in Fig. 2. Values are means ±SE of 4–6 replicates from at least three series of plants grown independently. For each tissue, means marked with an asterisk are significantly different at P <0.05 based on Student's t test. (B) Immunoblot analysis of cytosolic dehydroascorbate reductase (DRc), cytosolic peroxiredoxin II (PrxIIc), and glutathione peroxidase (GPX) in seeds. Gels were loaded with 50 μg (DRc, GPX) or 15 μg (PrxIIc) of protein per lane. (C) Expression (mRNA level and protein) of γ-glutamylcysteine synthetase (γECS) in seeds. The γECS mRNA levels were normalized with actin as the internal reference gene and were expressed relative to those of control seeds, which were arbitrarily given a value of 1. Values are means ±SE of 3–4 RNA extractions from seeds of at least two series of plants grown independently, and asterisks denote up-regulation of the gene (R >2). For immunoblots, gels were loaded with 50 μg of protein per lane. For (B) and (C), blots are representative of four gels loaded with seed extracts from different plants, and the apparent molecular mass (kDa) of the proteins is indicated on the right.
Further studies on the effects of pea fruit storage on selected antioxidant enzymes were performed using immunoblots (Fig. 5B). These revealed that the decrease of DR activity in the seeds during storage was caused, at least in part, by a consistent decrease in DRc protein. Similarly, fruit storage decreased the content of PrxIIc protein in the seeds but had the opposite effect on GPX protein (Fig. 5B). The levels of γECS protein were also determined in the seeds during fruit storage, along with those of the corresponding transcript (Fig. 5C). Most notably, the mRNA and protein levels showed contrasting patterns to the point that the γECS protein was hardly detectable in extracts of stored fruits, further confirming a translational regulation of the enzyme. As concluded above from the results of fruit maturation, the lack of correlation among γECS mRNA, γECS protein, and glutathione in stored fruits reflects a high degree of complexity in the regulation of glutathione homeostasis in plant cells.
To determine whether the decreases in ascorbate, glutathione, and some enzyme activities of the ascorbate–glutathione pathway caused oxidative stress in the stored fruits, lipid peroxidation and protein oxidation were measured as markers. Instead of the accumulation of oxidative products, a decrease of 68% was found in malondialdehyde (from 25.6 to 8.2 nmol g−1 fresh weight) and of 61% in protein carbonyl groups (as estimated by densitometric analysis of Western blots), and the pools of ascorbate and glutathione remained >90% in the reduced form. The more likely explanation for the absence of measurable oxidative damage or altered redox poise in cells is a general slow-down of metabolism during storage. These fruits would exhibit lower ROS production, yet they maintain sufficient antioxidant protection to cope with them. In fact, there were marked increases in SOD activity and GPX protein content in the stored fruits (Fig. 5B), which are enzymes involved in the detoxification of superoxide radicals and lipid peroxides, respectively.
Previous results about the effects of storage on the antioxidants of fruits are difficult to interpret because of their considerable variability. This may be due to differences in the plant species, type of fruits, experimental designs, or plant growth conditions (for example, see Jiménez et al., 2003; Malacrida et al., 2006). Interestingly, Davey and Keulemans (2004) reported that different apple fruit (Malus) cultivars differed substantially in their ability to maintain ascorbate levels during storage, and that the capacity to maintain the ascorbate and glutathione pool was related to better storage properties.
Apoplastic AO activity is important for pea fruit development
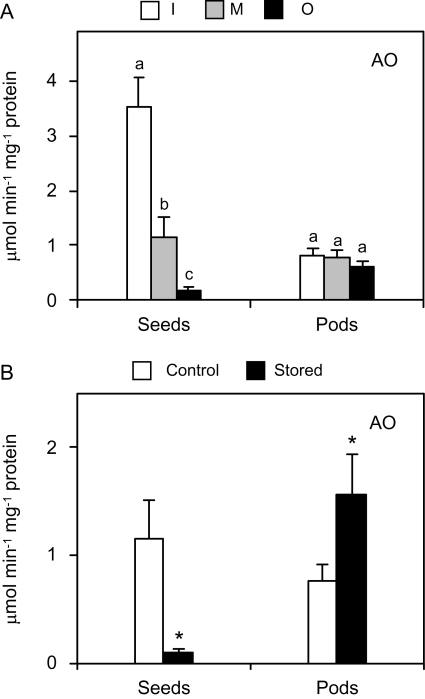
Ascorbate is not only a potent antioxidant but is also involved in the control of cell elongation as a substrate of AO (Pignocchi et al., 2003). Because seed maturation, especially from the immature to the mature stage, entails a rapid growth (Fig. 1) and AO expression is high in expanding tissues (Kato and Esaka, 2000), this enzyme activity was determined during maturation (Fig. 6A) and after prolonged storage (Fig. 6B) of the fruits. Indeed, the AO activity of mature and overmature seeds was 70% and 95% lower, respectively, than that found in immature seeds; however, this activity remained constant in pods (Fig. 6A). This distinct response of AO activity in seeds and pods suggests differences in the growth pattern of both organs during maturation.
Fig. 6.
Ascorbate oxidase (AO) activity in seeds and seedless pods of pea fruits. (A) Changes in AO activity during maturation. Fruits were classified as immature (I), mature (M), or overmature (O) as described in Fig. 1. Values are means ±SE of 4–6 samples from at least two series of plants grown independently. For each tissue, means denoted by the same letter do not significantly differ at P <0.05 based on Duncan's multiple range test. (B) AO activity in seeds and pods from control and stored fruits. Values are means ±SE of 4–6 samples from at least two series of plants grown independently, and asterisks indicate significant differences from control seeds based on Student's t test (P <0.05).
Our results are consistent with the implication of AO in cell growth and, specifically, in legume fruit development. Previous studies on the changes of AO activity in fruits showed that AO activity was high in rapidly growing young fruits of pumpkin (Cucurbita maxima; Esaka et al., 1992) and zucchini (Cucurbita pepo; Lin and Varner, 1991). Likewise, AO expression was enhanced in young and mature fruits of melon (Cucumis melo; Sanmartin et al., 2007). The major decreases in AO activity that were found in pea fruits during seed maturation or storage are consistent with an important role of the enzyme in cell growth. The observation that AO activity is very high in immature seeds, which consist of actively growing tissues (Table 1; see also Figs 1 and 2 in Dam et al., 2009), supports this conclusion. The mechanism by which AO activity modulates cell growth may involve changes in the apoplastic pool of oxidized ascorbate (Pignocchi et al., 2006). Despite the high AO activity in immature seeds, however, we failed to detect a corresponding increase in the dehydroascorbate levels (data not shown). This may be explained considering that the apoplastic pool of ascorbate constitutes only 5–10% of the total ascorbate cell content (Pignocchi et al., 2006), and thus determination of the ascorbate redox state in whole seeds may mask detection of any increase in the oxidative state of the apoplast.
Prolonged storage of pea fruits at room temperature nearly abolished AO activity in seeds but doubled the activity in pods (Fig. 6B). The down-regulation of AO activity during storage may decrease ascorbate oxidation in the cell wall and probably contributes to improved fruit tolerance to stressful conditions imposed by detachment from the plant. Other studies have shown a relationship between AO expression and stressful conditions. Thus, AO gene expression was repressed in response to wounding (Diallinas et al., 1997), and tobacco (Nicotiana tabacum) and Arabidopsis plants with suppressed expression of the apoplastic AO gene were more tolerant to salt stress (Yamamoto et al., 2005).
In summary, in this work the impact of sustainable agricultural practices on the antioxidant content of pea fruits was assessed. This may have implications for both the nutritional value and post-harvest shelf life of the fruits. Maturation and storage of pea fruits probably entail a ‘downwards’ modulation of ROS and antioxidant levels, which would explain the absence of oxidative damage. These results underscore the importance of ascorbate, glutathione, PrxIIc, and AO as part of the antioxidant network in these two processes
Acknowledgments
We thank Antolín Imas (Bonduelle) for providing snap pea seed, Amin E Eltayeb and Kiyoshi Tanaka for providing the DRc antibody, Nicolas Rouhier for providing the GPX antibody, and Carmen Pérez-Rontomé for excellent technical assistance. This work was funded by Ministerio de Ciencia e Innovación (grant AGL2008-01298) and Gobierno de Aragón (grant PIP137/2005 and group A53).
Glossary
Abbreviations
- AO
ascorbate oxidase
- APX
ascorbate peroxidase
- DRc
cytosolic dehydroascorbate reductase
- DRp
plastidic dehydroascorbate reductase
- γECS
γ-glutamylcysteine synthetase
- GPX
glutathione peroxidase
- GR
glutathione reductase
- MR
monodehydroascorbate reductase
- PRXs
peroxiredoxins
- qRT-PCR
quantitative reverse transcription-polymerase chain reaction
- ROS
reactive oxygen species
- SDS
sodium dodecyl sulphate
- SOD
superoxide dismutase
References
- Arrigoni O, De Tullio MC. Ascorbic acid, much more than just an antioxidant. Biochimica et Biophysica Acta. 2002;1569:1–9. doi: 10.1016/s0304-4165(01)00235-5. [DOI] [PubMed] [Google Scholar]
- Asada K. Chloroplasts: formation of active oxygen and its scavenging. Methods in Enzymology. 1984;105:422–429. [Google Scholar]
- Basterrechea M, Hicks JR. Effect of maturity on carbohydrate changes in sugar snap pea pods during storage. Scientia Horticulturae. 1991;48:1–8. [Google Scholar]
- Bouvier F, Backhaus RA, Camara B. Induction and control of chromoplast-specific carotenoid genes by oxidative stress. Journal of Biological Chemistry. 1998;273:30651–30659. doi: 10.1074/jbc.273.46.30651. [DOI] [PubMed] [Google Scholar]
- Bréhélin C, Meyer EH, de Souris J-P, Bonnard G, Meyer Y. Resemblance and dissemblance of Arabidopsis type II peroxiredoxins: similar sequences for divergent gene expression, protein localization, and activity. Plant Physiology. 2003;132:2045–2057. doi: 10.1104/pp.103.022533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan T, Frenkel C. Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiology. 1977;59:411–416. doi: 10.1104/pp.59.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton DA, Baird LM, Langeberg L, Taugher CY, Anyan WR, Vance CP, Sarath G. Subcellular localization of oxygen defense enzymes in soybean (Glycine max [L.]Merr.) root nodules. Plant Physiology. 1993;102:481–489. doi: 10.1104/pp.102.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton DA, Russell SA, Hanus FJ, Pascoe GA, Evans HJ. Enzymatic reactions of ascorbate and glutathione that prevent peroxide damage in soybean root nodules. Proceedings of the National Academy of Sciences, USA. 1986;83:3811–3815. doi: 10.1073/pnas.83.11.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam S, Laursen BS, Ørnfelt JH, et al. The proteome of seed development in the model legume Lotus japonicus. Plant Physiology. 2009;149:1325–1340. doi: 10.1104/pp.108.133405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey MW, Keulemans J. Determining the potential to breed for enhanced antioxidant status in Malus: mean inter- and intravarietal fruit vitamin C and glutathione contents at harvest and their evolution during storage. Journal of Agricultural and Food Chemistry. 2004;52:8031–8038. doi: 10.1021/jf048531k. [DOI] [PubMed] [Google Scholar]
- De Tullio MC, Paciolla C, Arrigoni O. Identification and analysis of proteins sharing dehydroascorbate reductase activity. Biologia Plantarum. 2002;45:145–147. [Google Scholar]
- Diallinas G, Pateraki I, Sanmartin M, Scossa A, Stilianou E, Panopoulos NJ, Kanellis AK. Melon ascorbate oxidase: cloning of a multigene family, induction during fruit development and repression by wounding. Plant Molecular Biology. 1997;34:759–770. doi: 10.1023/a:1005851527227. [DOI] [PubMed] [Google Scholar]
- Dietz KJ. Plant peroxiredoxins. Annual Review of Plant Biology. 2003;54:93–107. doi: 10.1146/annurev.arplant.54.031902.134934. [DOI] [PubMed] [Google Scholar]
- Eltayeb AE, Kawano N, Badawi GH, Kaminaka H, Sanekata T, Morishima I, Shibahara T, Inanaga S, Tanaka K. Enhanced tolerance to ozone and drought stresses in transgenic tobacco overexpressing dehydroascorbate reductase in cytosol. Physiologia Plantarum. 2006;127:57–65. [Google Scholar]
- Esaka M, Fujisawa K, Goto M, Kisu Y. Regulation of ascorbate oxidase expression in pumpkin by auxin and copper. Plant Physiology. 1992;100:231–237. doi: 10.1104/pp.100.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkemeier I, Goodman M, Lamkemeyer P, Kandlbinder A, Sweetlove LJ, Dietz KJ. The mitochondrial type II peroxiredoxin F is essential for redox homeostasis and root growth of Arabidopsis thaliana under stress. Journal of Biological Chemistry. 2005;280:12168–12180. doi: 10.1074/jbc.M413189200. [DOI] [PubMed] [Google Scholar]
- Gogorcena Y, Gordon AJ, Escuredo PR, Minchin FR, Witty JF, Moran JF, Becana M. N2 fixation, carbon metabolism, and oxidative damage in nodules of dark-stressed common bean plants. Plant Physiology. 1997;113:1193–1201. doi: 10.1104/pp.113.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham PH, Vance CP. Legumes: importance and constraints to greater use. Plant Physiology. 2003;131:872–877. doi: 10.1104/pp.017004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith OW. Determination of glutathione and glutathione disulphide using glutathione reductase and 2-vinylpyridine. Analytical Biochemistry. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Groten K, Dutilleul C, van Heerden PD, Vanacker H, Bernard S, Finkemeier I, Dietz KJ, Foyer CH. Redox regulation of peroxiredoxin and proteinases by ascorbate and thiols during pea root nodule senescence. FEBS Letters. 2006;580:1269–1276. doi: 10.1016/j.febslet.2006.01.043. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 4th edn. Oxford, UK: Oxford University Press; 2007. [Google Scholar]
- Hicks LM, Cahoon RE, Bonner ER, Rivard RS, Sheffield J, Jez JM. Thiol-based regulation of redox-active glutamate-cysteine ligase from Arabidopsis thaliana. The Plant Cell. 2007;19:2653–2661. doi: 10.1105/tpc.107.052597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horling F, Lamkemeyer P, König J, Finkemeier I, Kandlbinder A, Baier M, Dietz KJ. Divergent light-, ascorbate-, and oxidative stress-dependent regulation of expression of the peroxiredoxin gene family in Arabidopsis. Plant Physiology. 2003;131:317–325. doi: 10.1104/pp.010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturbe-Ormaetxe I, Escuredo PR, Arrese-Igor C, Becana M. Oxidative damage in pea plants exposed to water deficit or paraquat. Plant Physiology. 1998;116:173–181. [Google Scholar]
- Jiménez A, Creissen G, Kular B, Firmin J, Robinson S, Verhoeyen M, Mullineaux P. Changes in oxidative processes and components of the antioxidant system during tomato fruit ripening. Planta. 2002a;214:751–758. doi: 10.1007/s004250100667. [DOI] [PubMed] [Google Scholar]
- Jiménez A, Gómez JM, Navarro E, Sevilla F. Changes in the antioxidative systems in mitochondria during ripening of pepper fruits. Plant Physiology and Biochemistry. 2002b;40:515–520. [Google Scholar]
- Jiménez A, Romojaro F, Gómez JM, Llanos MR, Sevilla F. Antioxidant systems and their relationship with the response of pepper fruits to storage at 20 °C. Journal of Agricultural and Food Chemistry. 2003;51:6293–6299. doi: 10.1021/jf030052i. [DOI] [PubMed] [Google Scholar]
- Kato N, Esaka M. Expansion of transgenic tobacco protoplasts expressing pumpkin ascorbate oxidase is more rapid than that of wild-type protoplasts. Planta. 2000;210:1018–1022. doi: 10.1007/s004250050712. [DOI] [PubMed] [Google Scholar]
- Lamkemeyer P, Laxa M, Collin V, et al. Peroxiredoxin Q of Arabidopsis thaliana is attached to the thylakoids and functions in context of photosynthesis. The Plant Journal. 2006;45:968–981. doi: 10.1111/j.1365-313X.2006.02665.x. [DOI] [PubMed] [Google Scholar]
- Laxa M, König J, Dietz KJ, Kandlbinder A. Role of the cysteine residues in Arabidopsis thaliana cyclophilin CYP20-3 in peptidyl-prolyl cis-trans isomerase and redox-related functions. Biochemical Journal. 2007;401:287–297. doi: 10.1042/BJ20061092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Varner JE. Expression of ascorbic acid oxidase in zucchini squash (Cucurbita pepo L.) Plant Physiology. 1991;96:159–165. doi: 10.1104/pp.96.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Loscos J, Matamoros MA, Becana M. Ascorbate and homoglutathione metabolism in common bean nodules under stress conditions and during natural senescence. Plant Physiology. 2008;146:1282–1292. doi: 10.1104/pp.107.114066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malacrida C, Valle E, Boggio S. Postharvest chilling induces oxidative stress response in the dwarf tomato cultivar Micro-Tom. Physiologia Plantarum. 2006;127:10–18. [Google Scholar]
- Matamoros MA, Dalton DA, Ramos J, Clemente MR, Rubio MC, Becana M. Biochemistry and molecular biology of antioxidants in the rhizobia–legume symbiosis. Plant Physiology. 2003;133:499–509. doi: 10.1104/pp.103.025619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matamoros MA, Moran JF, Iturbe-Ormaetxe I, Rubio MC, Becana M. Glutathione and homoglutathione synthesis in legume root nodules. Plant Physiology. 1999;121:879–888. doi: 10.1104/pp.121.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May MJ, Vernoux T, Sánchez-Fernández R, Van Montagu M, Inzé D. Evidence for posttranscriptional activation of γ-glutamylcysteine synthetase during plant stress responses. Proceedings of the National Academy of Sciences, USA. 1998;95:12049–12054. doi: 10.1073/pnas.95.20.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) Journal of Biological Chemistry. 1969;244:6049–6055. [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends in Plant Science. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase is spinach chloroplasts. Plant and Cell Physiology. 1981;22:867–880. [Google Scholar]
- Navrot N, Collin V, Gualberto J, Gelhaye E, Hirasawa M, Rey P, Knaff DB, Issakidis E, Jacquot JP, Rouhier N. Plant glutathione peroxidases are functional peroxiredoxins distributed in several subcellular compartments and regulated during biotic and abiotic stresses. Plant Physiology. 2006;142:1364–1379. doi: 10.1104/pp.106.089458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Gomez L, Vanacker H, Foyer CH. Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signalling. Journal of Experimental Botany. 2002;53:1283–1304. doi: 10.1093/jexbot/53.372.1283. [DOI] [PubMed] [Google Scholar]
- Palma JM, Jiménez A, Sandalio LM, Corpas FJ, Lundqvist M, Gómez M, Sevilla F, del Río A. Antioxidative enzymes from chloroplasts, mitochondria, and peroxisomes during leaf senescence of nodulated pea plants. Journal of Experimental Botany. 2006;57:1747–1758. doi: 10.1093/jxb/erj191. [DOI] [PubMed] [Google Scholar]
- Pignocchi C, Fletcher JM, Wilkinson JE, Barnes JD, Foyer CH. The function of ascorbate oxidase in tobacco. Plant Physiology. 2003;132:1631–1641. doi: 10.1104/pp.103.022798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignocchi C, Kiddle G, Hernández I, Foster SJ, Asensi A, Taybi T, Barnes J, Foyer CH. Ascorbate oxidase-dependent changes in the redox state of the apoplast modulate gene transcript accumulation leading to modified hormone signalling and orchestration of defense processes in tobacco. Plant Physiology. 2006;141:423–435. doi: 10.1104/pp.106.078469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos J, Matamoros M, Naya L, James EK, Rouhier N, Sato S, Tabata S, Becana M. The glutathione peroxidase gene family of Lotus japonicus. Characterization of genomic clones, expression analyses and immunolocalization in legumes. New Phytologist. 2009;181:103–114. doi: 10.1111/j.1469-8137.2008.02629.x. [DOI] [PubMed] [Google Scholar]
- Rogiers SY, Kumar GNM, Knowles NR. Maturation and ripening of fruit of Amelanchier alnifolia Nutt. are accompanied by increasing oxidative stress. Annals of Botany. 1998;81:203–211. [Google Scholar]
- Rubio MC, González EM, Minchin FR, Webb KJ, Arrese-Igor C, Ramos J, Becana M. Effects of water stress on antioxidant enzymes of leaves and nodules of transgenic alfalfa overexpressing superoxide dismutases. Physiologia Plantarum. 2002;115:531–540. doi: 10.1034/j.1399-3054.2002.1150407.x. [DOI] [PubMed] [Google Scholar]
- Sanmartin M, Pateraki I, Chatzopoulou F, Kanellis AK. Differential expression of the ascorbate oxidase multigene family during fruit development and in response to stress. Planta. 2007;225:873–885. doi: 10.1007/s00425-006-0399-5. [DOI] [PubMed] [Google Scholar]
- Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulphide/glutathione couple. Free Radicals in Biology and Medicine. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- Stevens R, Page D, Gouble B, Garchery C, Zamir D, Causse M. Tomato fruit ascorbic acid content is linked with monodehydroascorbate reductase activity and tolerance to chilling stress. Plant, Cell and Environment. 2008;31:1086–1096. doi: 10.1111/j.1365-3040.2008.01824.x. [DOI] [PubMed] [Google Scholar]
- Vanacker H, Sandalio LM, Jiménez A, et al. Roles for redox regulation in leaf senescence of pea plants grown on different sources of nitrogen nutrition. Journal of Experimental Botany. 2006;57:1735–1745. doi: 10.1093/jxb/erl012. [DOI] [PubMed] [Google Scholar]
- Van Breusegem F, Bailey-Serres J, Mittler R. Unraveling the tapestry of networks involving reactive oxygen species in plants. Plant Physiology. 2008;147:978–984. doi: 10.1104/pp.108.122325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang C, Oliver DJ. Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis. The Plant Cell. 1998;10:1539–1550. doi: 10.1105/tpc.10.9.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A, Bhuiyan MNH, Waditee R, Tanaka Y, Esaka M, Oba K, Jagendorf AT, Takabe T. Suppressed expression of the apoplastic ascorbate oxidase gene increases salt tolerance in tobacco and Arabidopsis plants. Journal of Experimental Botany. 2005;56:1785–1796. doi: 10.1093/jxb/eri167. [DOI] [PubMed] [Google Scholar]
- Zhou B, Wang J, Guo Z, Tan H, Zhu X. A simple colorimetric method for determination of hydrogen peroxide in plant tissues. Plant Growth Regulation. 2006;49:113–118. [Google Scholar]