Abstract
Nodule CO2 fixation via PEPC provides malate for bacteroids and oxaloacetate for N assimilation. The process is therefore of central importance for efficient nitrogen fixation. Nodule CO2 fixation is known to depend on external CO2 concentration. The hypothesis of the present paper was that nitrogen fixation in alfalfa plants is enhanced when the nodules are exposed to elevated CO2 concentrations. Therefore nodulated plants of alfalfa were grown in a hydroponic system that allowed separate aeration of the root/nodule compartment that avoided any gas leakage to the shoots. The root/nodule compartments were aerated either with a 2500 μl l−1 (+CO2) or zero μl l−1 (–CO2) CO2-containing N2/O2 gas flow (80/20, v/v). Nodule CO2 fixation, nitrogen fixation, and growth were strongly increased in the +CO2 treatment in a 3-week experimental period. More intensive CO2 and nitrogen fixation coincided with higher per plant amounts of amino acids and organic acids in the nodules. Moreover, the concentration of asparagine was increased in both the nodules and the xylem sap. Plants in the +CO2 treatment tended to develop nodules with higher %N concentration and individual activity. In a parallel experiment on plants with inefficient nodules (fix–) the +CO2 treatment remained without effect. Our data support the thesis that nodule CO2 fixation is pivotal for efficient nitrogen fixation. It is concluded that strategies which enhance nodule CO2 fixation will improve nitrogen fixation and nodule formation. Moreover, sufficient CO2 application to roots and nodules is necessary for growth and efficient nitrogen fixation in hydroponic and aeroponic growth systems.
Keywords: Alfalfa, amino acid, 13CO2, H2 evolution, Medicago sativa, N2 fixation, nitrogen fixation, nodule CO2 fixation, PEPC, xylem sap
Introduction
Numerous studies have shown that legumes react to increased CO2 concentrations around the shoots with an orchestrated increase in root and nodule growth (Phillips et al., 1976; Murphy, 1986; Aranjuelo et al., 2009). With relatively few contradictory reports, nodule specific activity remains unchanged (Cabrerizo et al., 2001) and the higher N demand of the more intensely growing shoots at high CO2 concentrations is met by the improved nitrogen fixation capacity of more and bigger nodules. Improved assimilate supply to nodules has no short-term effect on nodule specific activity (Vance and Heichel, 1991) and, in turn, an erratic assimilate supply to the nodules is buffered through nodule carbon pools like starch, α-polyhydroxybutyrate, and glycogen (Wang et al., 2007). A decline in nodule activity at night is apparently more a reaction to lower temperatures than to a lower assimilate supply (Schweitzer and Harper, 1980). Rather than a lack of available sugar, nodule specific activity appears to be limited by the ability of the nodule to cleave sucrose through sucrose synthase (Gordon et al., 1999; Baier et al., 2007) and to form organic acids (Vance, 1998; Wang et al., 2007). In particular, malate formation is important, since malate is the principal source of energy for the bacteroids (Driscoll and Finan, 1993), and at the same time functions as a carbon skeleton for N assimilation after reconversion to oxaloacetate (Rosendahl et al., 1990). Moreover, malate might be involved in a putative osmoregulatory function of the nodule oxygen diffusion barrier that controls microaerobic conditions in the nodule infected zone (Minchin, 1997). The microaerobic conditions inside the nodule are part of the reason that nodule carbon metabolism is shunted towards organic acid, namely malate, formation. Phosphoenolpyruvate (PEP) rather than being decarboxylated, is transformed into oxaloacetate and malate by the combined activity of carbonic anhydrase (CA) (Atkins et al., 2001), phosphoenolpyruvate carboxylase (PEPC) (Vance et al., 1994), and malate dehydrogenase (MDH) (Schulze et al., 2002). For PEPC and MDH, nodule-enhanced forms have been described (Suganuma et al., 1997; Miller et al., 1998) and CA shows nodule-specific expression in various legumes (de la Pena et al., 1997; Atkins et al., 2001). In fact, overexpression of nodule-enhanced MDH (neMDH) in alfalfa nodules not only increased nitrogen fixation per plant but also the specific activity of individual nodules (Denton et al., 2002). The importance of the biochemical pathway towards malate in nodules is highlighted by the fact that PEPC and MDH activity occur alongside nitrogenase expression and activity in emerging nodules (Vance et al., 1983; Egli et al., 1989). PEPC is found to be 10–15-fold greater in nodules than in roots and can comprise up to 2% of the soluble protein fraction of nodules (Vance and Stade, 1984; Vance et al., 1994). The post-translational regulation of PEPC activity occurs through reversible phosphorylation (Schuller and Werner, 1993). Studies with labelled CO2 reveal that nodules do indeed have considerable CO2 fixation rates (Warembourg and Roumet, 1989) and the down-regulation of PEPC activity in nodules through an antisense strategy impairs nitrogen fixation (Schulze et al., 1998). Although leaf PEPC has a low Km for CO2 concentration, in situ saturation of the enzyme capacity might strongly depend on the ongoing drainage of its products (Willmer et al., 1990; Kromer et al., 1996). PEPC is tightly regulated in part by the nodule malate concentration (Zhang et al., 1995). Christeller et al. (1977) have shown that nodule CO2 fixation in lupin is a function of external CO2 concentration. The apparent saturation is reached between 20–40 ml l−1 CO2 in the air around the nodules. However, these measurements were made on excised nodules, in which the use of malate might progressively decline due to less N2 fixation and N assimilation. The CO2 concentration in the soil gaseous phase is high, and depends strongly on microbial activity. Concentrations of up to 5000 μl l−1 have been reported (Buyanovsky and Wagner, 1983). In experimental systems with sand culture but, in particular, in aeroponic and hydroponic systems, CO2 concentrations around roots and nodules are often very low since the systems need to be intensely aerated to secure the available oxygen for the nodules and roots. This aeration is usually made with ambient air (around 360 μl l−1 CO2) and, in particular, the roots of young plants do not add any significant additional CO2 from respiration. There are some scattered reports that nodule activity is increased through long-term high CO2 concentrations around the roots and nodules (Mulder and Van Veen, 1960; Grobbelaar et al., 1971; Yamakawa et al., 1997, 2004). Such experiments, however, necessitate the strict separation of the shoots and a root/nodule compartment to avoid the CO2 feeding of leaves and thus a mixture of shoot and root effects. The hypothesis of the present paper was that long-term high CO2 concentration around the roots and nodules (2500 μl l−1 versus zero μl l−1) would improve the nitrogen fixation of young alfalfa plants due to increased CO2 fixation, resulting in a better provision of organic acids for driving N2 fixation and supporting N assimilation in nodules. Particular emphasis was put on the avoidance of any side-effect through any accidental additional CO2 feeding of the shoots.
Materials and methods
Experimental design
The experimental approach was to study the effect of different CO2 concentrations around alfalfa (Medicago sativa L.) roots and nodules on growth and nitrogen fixation over a longer period of time (3 weeks) avoiding side-effects through accidental additional CO2 feeding of the shoots. This was achieved by establishing a hydroponic cultivation system which allows the CO2 concentration in the root/nodule compartments to be continuously controlled while keeping the ambient CO2 concentration around shoots. In the –CO2 treatment, the root/nodule compartment was aerated with a CO2-free N2/O2 (80/20; v/v) gas flow while in the +CO2 treatment, the gas flow was enriched with 2500 μl l−1 CO2. These treatments were kept for a 3-week experimental period. The effect of the treatments were studied on ‘Saranac’ (normal N2-fixing plants) and ‘Insaranac’ (fix– plants, fed with mineral N) to compare the effects of the CO2 concentration around the roots and nodules. The H2 evolution of ‘Saranac’ plants was measured as a parameter of nodule N2 fixation activity during the experimental period. In addition, plant development in terms of the number of leaves and branches was recorded. At harvest, the plants were separated into shoot, root, and nodules for the determination of dry matter and N%. Amino acid (AA) export out of the nodules into the shoot was determined by analysing the xylem sap at harvest time.
In a second experiment, ‘Saranac’ plants were grown exactly as in the first experiment in a –CO2 and a +CO2 treatment to determine the CO2 fixation and N2 fixation capacity of the nodules at the end of the 3-week experimental period by measuring short-term nodule 13CO2 in relation to N2 fixation measured as H2 evolution. In addition, the nodules were analysed for AA and organic acids (OA) concentration.
Plant growth
Cuttings of alfalfa plants cvs ‘Saranac’ and ‘Insaranac’ were made from approximately 4-week-old plants grown in nutrient solution. The cultivation of plants from cuttings enabled the selection of plants with very homogeneous development for further growth in nutrient solution culture. ‘Insaranac’ forms inactive nodules with regard to N2 fixation (fix–) (Viands et al., 1979; Barnes et al., 1990) whereas ‘Saranac’ plants have active nodules. Cuttings were treated with hormone rooting mix, planted in containers with fine quartz sand, and maintained in a controlled environment chamber with a 16/8 h day/night cycle at temperatures of approximately 25/18 °C and a relative humidity of about 70%. The light intensity was 360 μmol m−2 s−1. The quartz sand was kept at about 70% of its maximum water-holding capacity (21% of its dry weight) by the addition of an N-free nutrient solution of the following composition: macronutrients (mM): K2SO4 0.7, MgSO4 0.5, CaCl2 0.8, KH2PO4 0.015; and micronutrients (μM): H3BO3 4.0, Na2MoO4 0.1, ZnSO4, 1.0, MnCl2 2.0, Co(NO3)2 0.2, CuCl2 1.0, and FeNaEDTA 10. The pH was buffered with 0.25 mM MES and adjusted to 6.5 by applying KOH. In addition, at 7 d and 14 d after planting, each tray, containing approximately 50 cuttings, received 5 μmol P as KH2PO4 and 1 mmol N as urea. After rooting, cuttings were inoculated with Sinorhizobium meliloti strain 102F51. Nodules appeared 6–7 d after inoculation. Three weeks after inoculation 12 cuttings of even size were selected and carefully transferred to glass tubes (h, 600 mm; inner diameter, 20 mm) with nutrient solution. The tubes were closed with a rubber stopper at the lower side. Plants were inserted through a hole in the rubber stopper on the upper side of the tube and held in place with sponge. The hydroponic cultivation of alfalfa plants in the glass tubes is described in Schulze and Drevon (2005). The glass tubes were filled with the nutrient solution described above except for phosphorus and nitrogen. Legumes show susceptibility to P-toxicity in hydroponic culture when supplied with excessive amounts of P (Bell et al., 1990; Tang et al., 2001) and this agrees with our own observations on Medicago truncatula (Gaertn.) and Medicago sativa (L.). Based on pre-experiments the P-supply in our system was optimized in order to achieve intensive but not P-limited growth. Each plant received 3 μmol or 7.5 μmol P as KH2PO4 d−1 during the first or second week after transplanting, respectively. Subsequently, the P application was increased to 15 μmol P plant−1 d−1. This P supply resulted in a P concentration in the nutrient solution of 12, 30, or 60 μM, respectively. Since ‘Insaranac’ forms ineffective (fix–) nodules, the plants received 2.5 mg N d−1 as KNO3, while the ‘Saranac’ plants did not receive any mineral nitrogen. The solution was changed daily and aerated with ambient air at a flow rate of about 1.2 vols min−1 until the introduction of treatments.
Application of different CO2 concentrations to the root/nodule compartment
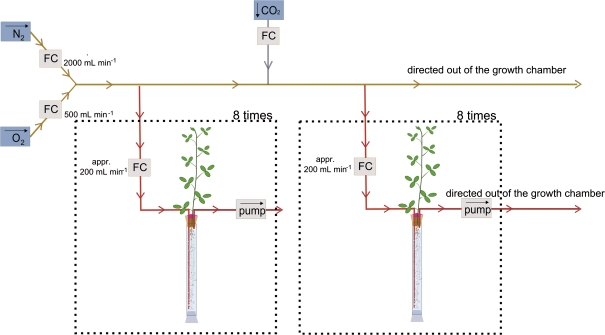
Plants were kept in the glass tubes for 4 d and aerated with ambient air to allow them to adapt. At day 5 the root/nodule compartment was sealed for the measurement of H2 and CO2 evolution and for the long-term application of air with different CO2 concentrations. For that purpose the hole in the upper rubber stopper was sealed with plasticine material with a high beeswax content. The beeswax gave the material a soft and pliable consistency that ensured a tight fit to the rubber stopper and the plant stem. The material is non plant-toxic. Before sealing, stiff inflow and outflow tubing for the sealed root/nodule compartment was inserted through the hole in the upper rubber stopper and also sealed with the same material. The inflow tubing reached to the lower end of the glass cylinder while the outflow was put above any nodules on the lower side of the upper rubber stopper. The inflow and outflow of the glass tubes were subsequently connected to a gas flow (N2/O2, 80/20, v/v) with either zero (–CO2) or 2500 μl l−1 (+CO2) CO2 concentration (Fig. 1). The respective gas flows were sucked through the sealed root nodule compartment at a flow rate of 200 ml min−1 and then directed outside the growth chamber. Root/nodule respiration resulted in a concentration of approximately 30–100 μl l−1 CO2 in the outflowing gas stream in the –CO2 treatment. Any possible leakage in the system would result in a dilution of the CO2 concentration of the applied air in the +CO2 treatment, while a leakage in the –CO2 treatment would result in a CO2 concentration beyond that caused by root/nodule respiration. The CO2 content of the outflowing gas streams was under constant surveillance. No CO2-enriched gas was able to reach the shoots. Repeated measurements of the CO2 concentration around the shoots showed ambient CO2 concentrations. The two CO2 levels in the root/nodule compartment were maintained for 3 weeks.
Fig. 1.
Experimental set-up for the long-term application of an N2/O2 mixture (80/20; v/v) with different CO2 concentrations to the root/nodule compartments. The parts enclosed in dotted lines are repeated eight times each. The gas input comes from pressurized gas bottles. The N2 and O2 gas was free of any CO2 or H2 contamination. FC, flow controller.
H2 evolution measurements
The measurement of H2 evolution is an indirect parameter for the determination of N2 fixation activity of legume nodules (Hunt and Layzell, 1993). The Sinorhizobium meliloti strain 102F51 used here has no uptake hydrogenase (hup–) (Blumenthal et al., 1997). For the H2 evolution measurement, the sealed root/nodule compartment was connected to an open-flow gas exchange measurement system that allowed the application of a mixture of N2/O2 (80/20, v/v) to the root/nodule compartment. For the measurements, the level of the nutrient solution was lowered to about 1/3 of the glass cylinder, leaving the lower virtually nodule-free part of the root system in solution. A gas flow of 200 ml min−1 (about 1.2 vols min−1) was applied to the root compartment. A subsample (100 ml min−1) of the outflowing gas was taken, dried (ice trap and MgClO4) and passed through an H2 analyser (S121 Hydrogen analyser, Qubit Systems, Canada). When a stable H2 outflow from the root/nodule compartment was reached, this value was taken as the apparent nitrogenase activity (ANA). Subsequently, the composition of the air in the inflowing airstream was changed to Ar/O2 (80/20, v/v). Argon is inert to nitrogenase and thus the whole electron flow is diverted to H+. Consequently, H2 evolution under argon represents the total enzyme activity (total nitrogenase activity, TNA). The peak value taken 3–5 min after switching to Ar/O2 was regarded as the TNA value. The electron allocation coefficient (EAC) of nitrogenase activity was calculated as 1–(ANA/TNA). The amount of fixed nitrogen per time and per plant or unit nodule was calculated on the basis of the ANA and TNA measurements (Schulze et al., 2006). ANA, TNA, and the EAC were measured before the introduction of the treatments, 2 d after treatment introduction, and at the end (after 3 weeks) of the experimental period.
Xylem harvest
Xylem sap was harvested and analysed for amino acid composition at the end of the experimental period. For xylem sap collection, the shoot was cut directly at the stem base. To avoid any contamination, the cut surface of the root part was rinsed for about 15 s with 1 M CaCL2 solution, resulting in the closure of the phloem and the removal of the cell bleeding sap. The root was subsequently placed in a pressure chamber (Model 600 Pressure Chamber Instrument, PMS Instrument Co, Corvallis, Oregon, USA), and subjected to 300 MPa pressure. The xylem sap was collected for a period of 10 min. During the whole procedure the xylem sap was kept on ice and then immediately frozen (–20 °C).
13CO2 application
In a second experiment ‘Saranac’ plants were grown under the exact same conditions as described for the first experiment. The objective of the second experiment was to measure 13CO2 fixation capacity of roots and nodules at the end of the experimental period. Moreover, nodule AA and OA concentration was also determined at this time. After 3 weeks of ±CO2 treatment, the nodules from both treatments were exposed to a 13CO2 concentration of 2500 μl l−1 13CO2 (98 atom% enriched, Cambridge Isotope Laboratories, Andover MA, USA) for 15 min. Thus 13CO2 uptake indicates the physiological capability of the nodules to fix CO2 and the uptake is not influenced by different CO2 concentrations around the nodules during labelling. The application was made to alternate plants from the +CO2 and the –CO2 treatments. The airstream was set up in the same way as to the CO2-feeding system, i.e. the 13CO2-enriched gas flow was sucked through the root/nodule compartment to avoid accidental feeding of the shoots. At the end of the labelling period the root/nodule compartment was thoroughly and quickly flushed with ambient air and the plants were immediately taken out of the tubes and submerged in liquid nitrogen. The plants were subsequently divided into shoots, roots, and nodules and vacuum-dried. A subsample of nodules was taken for AA and OA determination before drying. Reference plants were harvested in a growth chamber separated from the 13CO2 application.
Nodule amino acid and organic acid concentration
Nodules were taken from intact plant roots with attached nodules and directly frozen in liquid nitrogen. The nodules collected were stored at –20 °C until analysis. For analysis of free AA and OA, nodules were homogenized with liquid N2 using a mortar and pestle. Subsequently, 0.5 mg of the material was extracted with 3 ml of 50% ethanol (v/v) in a 40 °C water bath for 20 min. The solution was centrifuged for 30 min at 8000 g and 4 °C. The supernatant was immediately used for HPLC analyses after filtration (0.45 μm). AA were detected with a fluorescence detector after precolumn derivatization by orthophthaldialdehyde (Chen et al., 1979). OAs were separated through HPLC and were detected by a photodiode array detector. For analytical details see Keutgen and Pawelzik (2008).
Dry matter, N, C, and 13C concentration
The plants in experiments one and two were divided in shoots, roots, and nodules. The fractions were dried to a constant weight at 60 °C (experiment one) or through vacuum application (experiment two). Dried material from experiments one and two was ground to a fine powder in a pebble mill. The powdered material was subsequently analysed using a C/N analyser (NA 2500, CE-Instruments, Milano, Italy) and a mass spectrometer (Finnigan MAT, model 252, Bremen, Germany). The 13CO2 uptake was determined by multiplying the C content of a fraction with the 13C excess of this fraction over the 13C% of an unlabelled reference group.
Statistical analyses
Experimental data were analysed with the Sigmastat 2.03 statistical program (SPSS Inc., 1992–1997). All data sets were tested for a normal distribution. In the case of homogeneous sample variances, mean separation procedures were carried out using the t test.
Results
Growth, nodulation, and %N
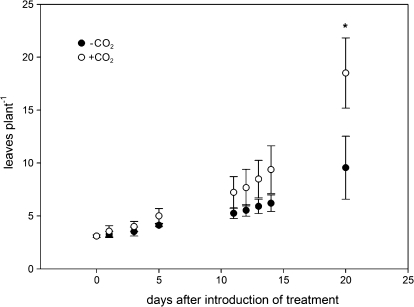
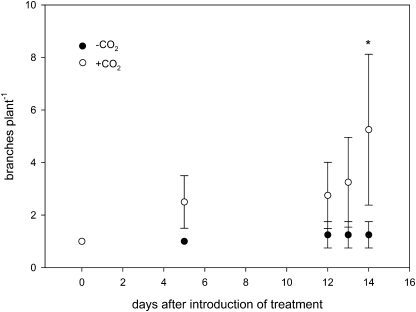
Growth of nodulated plants with effective nodules was significantly increased in the +CO2 treatment (Table 1; Fig. 2). Nodules of ‘Saranac’ plants were bigger and appeared pink while nodules of ‘Insaranac’ plants were white. Total dry matter formation in ‘Saranac’ was increased by 250% through the application of CO2 to the root/ nodule compartment, while this treatment was without any effect on ‘Insaranac’ plants, neither on total dry matter formation nor on any particular plant organ. In relative terms, the increase in shoot and roots of ‘Saranac’ plants was about equal but greater when compared with nodules. Plants with CO2 application tended to form fewer and bigger nodules. There was a large variability in nodule number, nodule per plant dry matter, and individual nodule dry matter. The mean value for the nodule individual dry matter was about 5-fold higher in the +CO2 treatment, statistically significantly different at P ≤0.1. CO2 application had no effect on the fix–-nodules of ‘Insaranac’ plants. ‘Saranac’ plants in the +CO2 treatment achieved the growth advantage by progressively more leaf and branch formation during the experimental period (Figs 3, 4). Between 14–20 d after the introduction of the CO2 treatments both parameters became significantly different. At the end of the experimental period %N concentration showed no significant difference in shoots or roots between the treatments either in ‘Saranac’ or ‘Insaranac’ plants (Table 2). However, nodules of +CO2 ‘Saranac’ plants had about 180% N concentration when compared with nodules of the –CO2 plants. Nodule %N was not affected by CO2 application in ‘Insaranac’ plants.
Table 1.
Dry matter, nodule number, and nodule individual weight of +CO2 and –CO2 alfalfa plants
| Parameter | Treatments |
|||
| Saranac |
Insaranac |
|||
| +CO2 | –CO2 | +CO2 | –CO2 | |
| Shoot dry matter (mg plant−1) | 373±160 | 143*±84 | 243±40 | 253±43 |
| Root dry matter (mg plant−1) | 153±56 | 61*±20 | 109±29 | 114±6 |
| Nodule dry matter (mg plant−1) | 24.3±9.8 | 12.1±7.9 | 3.7±1.7 | 4.6±2.3 |
| Total dry matter (mg plant−1) | 550±220 | 216*±114 | 355 ±70 | 370 ±48 |
| Nodule number | 24±19 | 40±11 | 38±15 | 56±40 |
| Nodule individual dry weight (mg nodule−1) | 1.60±1.06 | 0.31±0.22 | 0.11±0.07 | 0.12±0.12 |
Plants were grown for 3 weeks with different levels of CO2 concentration in the root/nodule compartment. ‘Insaranac’ forms fix–-nodules. Nitrogen nutrition of ‘Saranac’ depended on nitrogen fixation while ‘Insaranac’ plants received 2.5 mg N d−1 as KNO3. Data are means of four replicates ±sd. An asterisk indicates a statistically significant difference from the +CO2 treatment of ‘Saranac’ or ‘Insaranac’ (t test, P ≤0.05).
Fig. 2.
Nodulated alfalfa plants ‘Saranac’ grown for 3 weeks with either −CO2 (left) or +CO2 (right) application to a separated root/nodule compartment. Nodules of plants from both treatments are shown below the plants.
Fig. 3.
Development of leaf number per plant (‘Saranac’) during the course of a 3-week experimental period with the application of an N2/O2 mixture (80/20; v/v) with either zero (–CO2) or 2500 μl l−1 CO2 (+CO2) to the root/nodule compartment. Data are means of four replicates. Bars represent the standard deviation. An asterisk indicates a statistically significant difference from the +CO2 treatment (t test, P ≤0.05).
Fig. 4.
Development of branch number per plant (‘Saranac’) during the course of a 3-week experimental period with the application of an N2/O2 mixture (80/20; v/v) with either zero (–CO2) or 2500 μl l−1 CO2 (+CO2) to the root/nodule compartment. Data are means of four replicates. Bars represent standard deviation. An asterisk indicates a statistically significant difference from the +CO2 treatment (t test, P ≤0.05).
Table 2.
%N concentration in shoots, roots and nodules of +CO2 and −CO2 alfalfa plants
| Parameter | Treatments |
|||
| Saranac |
Insaranac |
|||
| +CO2 | –CO2 | +CO2 | –CO2 | |
| Shoot N concentration (% N) | 2.3±0.4 | 2.0±0.6 | 2.3±0.2 | 2.4±0.2 |
| Root N concentration (% N) | 2.8±0.2 | 2.7±0.3 | 2.8±0.2 | 3.1±0.2 |
| Nodule N concentration (% N) | 9.2±0.9 | 5.1*±0.6 | 4.8±0.9 | 5.2±1.2 |
Plants were grown for 3 weeks with different levels of CO2 concentration in the root/nodule compartment. ‘Insaranac’ forms fix–-nodules. Nitrogen nutrition of ‘Saranac’ depended on nitrogen fixation while ‘Insaranac’ plants received 2.5 mg N d−1 as KNO3. Data are means of four replicates ±SD. An asterisk indicates a statistically significant difference from the +CO2 treatment of ‘Saranac’ or ‘Insaranac’ (t test, P ≤0.05).
Nitrogen fixation
N2 fixation was determined on the basis of nodule H2 evolution measurements which enabled the parallel monitoring of the N2 fixation activity during the experimental period. The N2 fixation activity did not differ between treated plants before the introduction of the different CO2 application (Table 3). ‘Insaranac’ plants showed no measureable H2 evolution during the course of the experiment. Two days after the introduction of the CO2 treatments, a significant differentiation in N2 fixation of the ‘Saranac’ plants occurred (Table 3). Nitrogen fixation in the +CO2 plants was about 225% of that in the –CO2 plants. The differentiation in N2 fixation did not show significant further change from 2 d until the end of the experimental period.
Table 3.
N2 fixation of alfalfa plants (‘Saranac’) before and during application of different levels of CO2 concentration to the root/nodule compartment
| Parameter | Treatments |
|
| +CO2 | –CO2 | |
| Total N2 fixation activity before introduction of treatments (mg N plant−1 d−1) | 192±42 | 171±31 |
| Total N2 fixation activity 2 d after introduction of treatments (mg N plant−1 d−1) | 283±134 | 126* 50 |
| Total N2 fixation activity 21 d after introduction of treatments (mg N plant−1 d−1) | 965±279 | 415*±241 |
| EAC 21 d after introduction of treatments | 0.59±0.01 | 0.61±0.11 |
| Specific N2 fixation 21 d after introduction of treatments (mg N g−1nodule dry matter d−1) | 43±15 | 39±14 |
| N2 fixation activity of an individual nodule 21 d after introduction of treatments (μg N nodule−1 d−1) | 61±40 | 11*±7 |
Plants were grown for 3 weeks with different levels of CO2 concentration in the root/nodule compartment. N2-fixation was calculated from nodule H2 evolution according to Schulze et al. (2006). Data are means of four replicates ±sd. An asterisk indicates a statistically significant difference from the +CO2 treatment (t test, P ≤0.05).
Amino acids in nodule and xylem sap
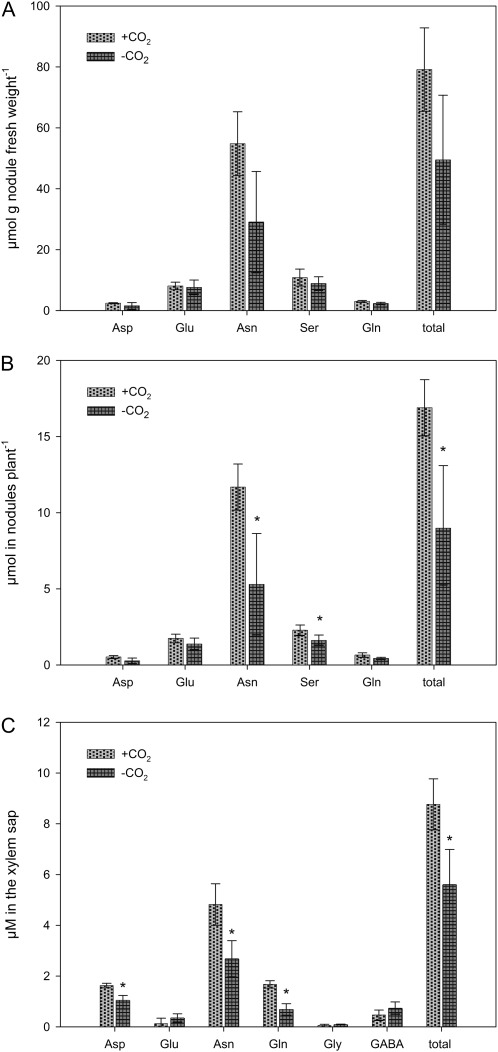
The higher nitrogen fixation activity of plants in the +CO2 treatment is supported by a tendency towards a higher concentration of asparagine (Asn) in the nodules. Figure 5A shows the proportion of asparagine among the five most abundant amino acids measured. The amount of amino acids in nodules per plant is significantly increased in the +CO2 treatment (Fig. 5B). These facts resulted in a higher total concentration of amino acids in the xylem sap (Fig. 5C). This higher total concentration was the result of particular increases in asparagine, glutamine, and aspartate.
Fig. 5.
Amino acids in nodules and in the xylem sap 3 weeks after beginning the application of an N2/O2 mixture (80/20; v/v) with either zero (–CO2) or 2500 μl l−1 CO2 (+CO2) to the root/nodule compartment. Data are the means of four replicates. Bars represent standard deviation. An asterisk indicates a statistically significant difference from the +CO2 treatment (t test, P ≤0.05). (A) Concentrations of the five most abundant amino acids in the nodules. (B) Total amount in nodules per plant of the five most abundant nodule amino acids. In addition to the amino acids shown, Ala, Gaba, Tyr, Arg, Try, Lys, Val, Thr, Leu, His, Ile, Gly, and Prol. were detected in concentrations below 0.5 μmol g−1 nodule fresh weight. (C) Concentrations of amino acids found in the xylem sap in concentrations above 0.1 μM.
Root/nodule CO2 fixation
Root and nodule CO2 fixation was determined based on 13CO2 application to the root/nodule compartment (Fig. 1). Apparent CO2 fixation per unit root or nodule was stronger in the +CO2 treatment by approximately 3- or 4-fold, respectively (Table 4). No 13C-label was detected in shoots after the 15 min labelling period.
Table 4.
CO2 fixation capacity of alfalfa (‘Saranac’) roots and nodules after 3 weeks of growth at different CO2 concentrations in the root/nodule compartment
| Parameter | Treatments |
|
| +CO2 | –CO2 | |
| Root CO2 fixation (μg C g−1 root dry matter h−1) | 42±22 | 14±12 |
| Nodule CO2 fixation (μg C g−1 nodule dry matter h−1) | 82±25 | 22*±14 |
| Nodule CO2 fixation per N2 reduced (mg C g−1 N) | 88±47 | 35±15 |
CO2 fixation was determined through a 15 min application of 13CO2 (2500 μl l−1 13CO2) to the root/nodule compartments and subsequent measurement of 13C in roots and nodules. N2-fixation was calculated from nodule H2 evolution according to Schulze et al. (2006). Data are means of three replicates ±sd. An asterisk indicates a statistically significant difference compared to the +CO2 treatment (t test, P ≤0.05).
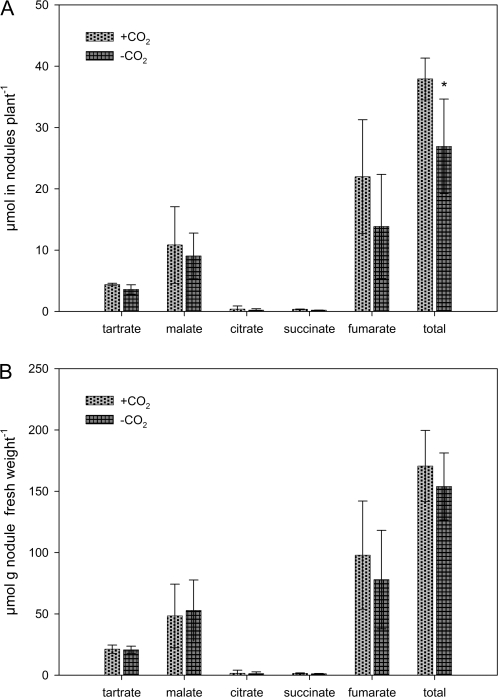
Nodule organic acid composition
Organic acid formation per plant was increased in the +CO2 plants (Fig. 6A) by approximately 30%. This was the result of more nodule fresh weight per plant while the concentration of organic acids in nodules did not increase (Fig. 6B). Among the organic acids detected, fumarate, malate, and tartrate were the most abundant, while succinate and citrate were only found in low concentrations.
Fig. 6.
Nodule organic acid compostition. Data are the means of four replicates. Bars represent standard deviation. An asterisk indicates a statistically significant difference from the +CO2 treatment (t test, P ≤0.05). (A) Total amount of organic acids in nodules per plant. (B) Concentration of detected organic acids in nodules.
Discussion
The results of our study highlight the importance of nodule CO2 fixation for nitrogen fixation and the growth of legumes. Nodule CO2 fixation is known to be tightly coupled to N2 fixation, as proven, for example, by the concomitant expression of PEPC in nodules and emerging nitrogenase activity (Vance et al., 1983) or through decreased N2 fixation as a result of decreased PEPC expression in response to transformation with an antisense PEPC construct (Nomura et al., 2006). The extent of nodule CO2 fixation of lupin roots depends on the external CO2 concentration with an apparent saturation at 20–40 ml l−1 CO2 in the soil atmosphere (Christeller et al., 1977). Increased nitrogen fixation and growth were found at CO2 concentrations of about 2500 μl l−1 versus zero μl l−1 around nodules and roots in alfalfa plants. Thus, although the CO2 concentration in the +CO2 treatment is close to that found in the soil atmosphere, it might still not have been saturating for nodule CO2 fixation. Effects of high CO2 concentrations around nodules are reported for soybean, pea, and common bean (Mulder and Van Veen, 1960; Grobbelaar et al., 1971; Yamakawa et al., 2004). These reports found not only a consistent effect on N2 fixation per plant but also on nodulation and nodule size. However, in most of these experiments a certain concomitant CO2 feeding of shoots and thus a mix of effects on nodule and shoot CO2 fixation cannot be completely ruled out. Our experimental set-up meant that any additional CO2 from the root/nodule compartment reaching the shoots was avoided. Sucking the CO2-enriched air through the root/nodule compartment rather than pressing it, would have resulted in a CO2 dilution in the airstream in the event of any possible leakage. Repeated measurements of the CO2 concentration in the outflowing air from the root/nodule compartments, in addition to measurements of the CO2 concentration around the shoots and around the whole experimental set-up proved the validity and viability of the system with respect to specific CO2 feeding of roots and nodules. Moreover, the observed effects were restricted to plants with efficient nodules, while plants nourished with nitrate displayed no effect on growth. In addition, a more or less equal increase in shoot and root growth through CO2 feeding of roots and nodules was observed, while CO2 feeding of the shoots tends preferentially to support root growth (Schulze and Merbach, 2008). A distinctly higher N2 fixation per plant in the +CO2 treatment is shown by the H2 evolution measurements and also by the significantly higher total amino acid content in the nodules and by the higher amino acid concentrations in the xylem sap. At the end of the experimental period better nitrogen fixation per plant in the +CO2 treatment was largely a result of bigger nodules with higher individual efficiency. Two days after the introduction of the treatment, nitrogen fixation per plant in the +CO2 treatment was strongly increased while it was more or less constant in the –CO2 treatment. This increment in H2 evolution probably results from an increase in specific nodule activity because the emergence of active nodules takes at least 6–7 d in alfalfa; thus it is unlikely that significantly more active nodules had been formed 2 d after the introduction of the treatments. It is conceivable that CO2 feeding accelerated the development of young nodules that were already established when the CO2 treatment commenced. However, this is not consistent with the fact that a tendency towards lower numbers of nodules was noted in the +CO2 treatment at the end of the experimental period. In our experiment, a noticeable initial increase in nodule specific activity was found, as well as the formation of increasingly larger nodules with higher per nodule activity. By contrast, shoot CO2 feeding in most reported experiments shows neither a short- nor a long term-effect on nodule specific activity (Vance and Heichel, 1991; Cabrerizo et al., 2001).
The observed increase in growth and nitrogen fixation was connected with the development of more new leaves and the development of additional branches. Improved C fixation may have improved C nutrition at the whole plant level, by at least partly supporting the C costs arising from nitrogen fixation and/or nodule growth. This might have resulted in the development of additional leaves and branches. Nodule growth and nitrogen fixation consumes considerable amounts of carbon (Schulze, 2004). A substantial contribution of nodule CO2 fixation to the overall root/nodule carbon balance is indicated by several long-term experiments at the whole plant level (Warembourg and Roumet, 1989; Schulze et al., 2006). Nitrogen fixation in the –CO2 treatment could obviously not fully support the growth potential of the alfalfa plants. Better legume growth with nitrate nutrition as opposed to exclusive nitrogen fixation has been reported repeatedly (Herrmann et al., 2001). Under natural soil conditions, a mixed supply of nitrogen from the nodules and soil solution is normal and apparently the optimal way to meet the plants’ nitrogen requirements (Lamb et al., 1995).
Increased nitrogen fixation in the +CO2 treatment was accompanied by a higher asparagine content in nodules per plant and an increased asparagine concentration in the xylem sap. However, nodule concentration in the detected organic acids or, in particular, in malate was not improved by the +CO2 treatment. For analysis, the nodules had been fixed in liquid nitrogen while adhering to the roots and subsequently had not been allowed to melt before extraction. Thus organic acid analysis and, in particular, that for malate, allows a one-off insight into a steady-state turnover in which the organic acids are intensely drained through uptake by the symbiosome and respiration and also through carbon skeleton provision for the increasingly available ammonium. Consequently, the equal concentrations in nodules with strongly different nitrogen fixation and therefore concurrent malate use indicate improved malate production brought about by nodule CO2 fixation. In fact, in experiments with pea plants, it was found that organic acid concentration was higher in senescent nodules compared with active ones and also in nodules left detached yet otherwise intact for a certain period of time (Ahmed, 2007). Both observations indicate that a decrease in nodule nitrogen fixation activity is connected with organic acid accumulation, which is suggested to have a negative regulatory impact on nitrogenase activity (Roux et al., 2008).
These measurements on root/nodule CO2 fixation show that it was higher in the +CO2 treatment, thus supporting the thesis of improved organic acid formation. In addition, the long-term +CO2 treatment also improved the root/nodule CO2 fixation capacity, since the measurements were made with equal 13CO2 concentrations in both treatments (2500 μl l−1). Thus sufficient CO2 around the nodules apparently contributes to the emergence of efficient nodules in terms of CO2 and N2 fixation activity. At the time of the 13CO2 fixation measurements, the shoots (and thus the shoot N demand in the +CO2 treatment) were already considerably greater when compared with the −CO2 treatment. This might have played a role in the measured higher CO2 fixation indicating a possible feedback effect of shoot N demand on root/nodule CO2 fixation.
In conclusion, our results support the thesis that short- and long-term CO2 concentration around the nodules is of importance for nitrogen fixation activity and for the formation of efficient nodules in alfalfa. This has implications for experimental procedures measuring nodule gas exchange, in particular, in hydroponic and aeroponic systems. Measurements using pure N2/O2 mixtures or ambient air might underestimate nitrogen fixation. Moreover, long-term hydroponic growth with aeration of the nutrient solution with ambient air might impair the formation of optimally efficient nodules, in particular in young plants when root/nodule respiration does not sufficiently increase the nodule internal and external CO2 concentrations. The biochemical pathway leading from nodule CO2 fixation to malate production and use can be influenced through breeding and techniques of plant genetic transformation. Both strategies might improve nitrogen fixation activity, in particular in the early stages of growth in alfalfa plants. Moreover, agronomic measures improving soil respiration and thus CO2 concentration in the soil atmosphere might contribute to more efficient legume growth.
Acknowledgments
We are indebted to Carroll P Vance, University of Minnesota, for providing seeds of ‘Saranac’ and ‘Insaranac’. We would like to express our gratitude for the outstanding technical assistance of Marlies Niebuhr, Susanne Koch, and Ute Ronsoehr from the Department of Crop Sciences, University of Goettingen, and Reinhard Langel from the Centre for Stable Isotope Research and Analysis, University of Goettingen. We would like to thank John Coates for correcting the language. The study was supported by the German Science Foundation (DFG, SCHU 1602/3-1). We wish to thank anonymous referees for helpful and constructive comments.
Glossary
Abbreviations
- ANA
apparent nitrogenase activity measured as H2 evolution in an N2/O2 mixture (80/20, v/v)
- TNA
total nitrogenase activity measured as H2 evolution in an Ar/O2 mixture (80/20, v/v)
- EAC
electron allocation coefficient (1–ANA/TNA)
- AA
amino acids
- OA
organic acids
References
- Ahmed MM. Nitrogen fixation of pea (Pisum sativum L.) and common bean (Phaseolus vulgaris L.) at various phosphorus supply levels. 2007 PhD thesis, Georg-August-University Goettingen, Goettingen. [Google Scholar]
- Aranjuelo I, Irigoyen JJ, Nogues S, Sanchez-Diaz M. Elevated CO2 and water-availability effect on gas exchange and nodule development in N2-fixing alfalfa plants. Environmental and Experimental Botany. 2009;65:18–26. [Google Scholar]
- Atkins C, Smith P, Mann A, Thumfort P. Localization of carbonic anhydrase in legume nodules. Plant, Cell and Environment. 2001;24:317–326. [Google Scholar]
- Barnes DK, Heichel GH, Vance CP, Peaden RN. Registration of ineffective agate and ineffective saranac non-N2-fixing alfalfa germplasms. Crop Science. 1990;30:752–753. [Google Scholar]
- Baier MC, Barsch A, Kuster H, Hohnjec N. Antisense repression of the Medicago truncatula nodule-enhanced sucrose synthase leads to a handicapped nitrogen fixation mirrored by specific alterations in the symbiotic transcriptome and metabolome. Plant Physiology. 2007;145:1600–1618. doi: 10.1104/pp.107.106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RW, Edwards DG, Asher CJ. Growth and nodulation of tropical food legumes in dilute solution culture. Plant and Soil. 1990;122:249–258. [Google Scholar]
- Blumenthal JM, Russelle MP, Vance CP. Nitrogenase activity is affected by reduced partial pressures of N2 and NO3−. Plant Physiology. 1997;114:1405–1412. doi: 10.1104/pp.114.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyanovsky GA, Wagner GH. Annual cycles of carbon dioxide level in soil air. Soil Science Society of America Journal. 1983;47:1139–1145. [Google Scholar]
- Cabrerizo PM, Gonzalez EM, Aparicio-Tejo PM, Arrese-Igor C. Continuous CO2 enrichment leads to increased nodule biomass, carbon availability to nodules and activity of carbon-metabolising enzymes but does not enhance specific nitrogen fixation in pea. Physiologia Plantarum. 2001;113:33–40. [Google Scholar]
- Chen RF, Scott C, Trepman E. Fluorescence properties of ortho-phthaldialdehyde derivatives of amino-acids. Biochimica et Biophysica Acta. 1979;576:440–455. doi: 10.1016/0005-2795(79)90419-7. [DOI] [PubMed] [Google Scholar]
- Christeller JT, Laing WA, Sutton WD. Carbon-dioxide fixation by lupin root-nodules. 1. Characterization, association with phosphoenolpyruvate carboxylase, and correlation with nitrogen-fixation during nodule development. Plant Physiology. 1977;60:47–50. doi: 10.1104/pp.60.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Pena TC, Frugier F, McKhann HI, Bauer P, Brown S, Kondorosi A, Crespi M. A carbonic anhydrase gene is induced in the nodule primordium and its cell-specific expression is controlled by the presence of Rhizobium during development. The Plant Journal. 1997;11:407–420. doi: 10.1046/j.1365-313x.1997.11030407.x. [DOI] [PubMed] [Google Scholar]
- Denton MD, Schulze J, Temple SJ, Vance CP, Allan DL, Michael RP, Samac DA. Overexpression of a nodule enhanced malate dehydrogenase increases nitrogen fixation in alfalfa. Plant Biology. 2002;2002:136–137. [Google Scholar]
- Driscoll BT, Finan TM. NADP-malic enzyme of Rhizobium meliloti. Abstracts of the General Meeting of the American Society for Microbiology. 1993;93:298. [Google Scholar]
- Egli MA, Griffith SM, Miller SS, Anderson MP, Vance CP. Nitrogen assimilating enzyme-activities and enzyme protein during development and senescence of effective and plant gene-controlled ineffective alfalfa nodules. Plant Physiology. 1989;91:898–904. doi: 10.1104/pp.91.3.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AJ, Minchin FR, James CL, Komina O. Sucrose synthase in legume nodules is essential for nitrogen fixation. Plant Physiology. 1999;120:867–878. doi: 10.1104/pp.120.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobbelaar N, Hough MC, Clarke B. Nodulation and nitrogen fixation of isolated roots of Phaseolus vulgaris L. 3. Effect of carbon dioxide and ethylene. Plant and Soil. 1971;35:215–278. [Google Scholar]
- Herrmann B, Jones SK, Fuhrer J, Feller U, Neftel A. N budget and NH3 exchange of a grass/clover crop at two levels of N application. Plant and Soil. 2001;235:243–252. [Google Scholar]
- Hunt S, Layzell DB. Gas exchange of legume nodules and the regulation of nitrogenase activity. Annual Review of Plant Physiology and Plant Molecular Biology. 1993;44:483–511. [Google Scholar]
- Keutgen AJ, Pawelzik E. Contribution of amino acids to strawberry fruit quality and their relevance as stress indicators under NaCl salinity. Food Chemistry. 2008;111:642–647. [Google Scholar]
- Kromer S, Gardestrom P, Samuelsson G. Regulation of the supply of oxaloacetate for mitochondrial metabolism via phosphoenolpyruvate carboxylase in barley leaf protoplasts. 2. Effects of metabolites on PEPC activity at different activation states of the protein. Biochimica et Biophysica Acta. 1996;1289:351–361. doi: 10.1016/0304-4165(95)00165-4. [DOI] [PubMed] [Google Scholar]
- Lamb JFS, Barnes DK, Russelle MP, Vance CP, Heichel GH, Henjum KI. Ineffectively and effectively nodulated alfalfas demonstrate biological nitrogen-fixation continues with high nitrogen fertilization. Crop Science. 1995;35:153–157. [Google Scholar]
- Miller SS, Driscoll BT, Gregerson RG, Gantt JS, Vance CP. Alfalfa malate dehydrogenase (MDH): molecular cloning and characterization of five different forms reveals a unique nodule-enhanced MDH. The Plant Journal. 1998;15:173–184. doi: 10.1046/j.1365-313x.1998.00192.x. [DOI] [PubMed] [Google Scholar]
- Minchin FR. Regulation of oxygen diffusion in legume nodules. Soil Biology and Biochemistry. 1997;29:881–888. [Google Scholar]
- Mulder EG, Van Veen WL. The influence of carbon dioxide on symbiotic nitrogen fixation. Plant and Soil. 1960;13:265–278. [Google Scholar]
- Murphy PM. Effect of light and atmospheric carbon dioxide concentration on nitrogen fixation by herbage legumes. Plant and Soil. 1986;95:399–409. [Google Scholar]
- Nomura M, Mai HT, Fujii M, Hata S, Izui K, Tajima S. Phosphoenolpyruvate carboxylase plays a crucial role in limiting nitrogen fixation in Lotus japonicus nodules. Plant and Cell Physiology. 2006;47:613–621. doi: 10.1093/pcp/pcj028. [DOI] [PubMed] [Google Scholar]
- Phillips DA, Newell KD, Hassell SA, Felling CE. Effect of CO2 enrichment on root nodule development and symbiotic N2 reduction in Pisum sativum L. American Journal of Botany. 1976;63:356–362. [Google Scholar]
- Rosendahl L, Vance CP, Pedersen WB. Products of dark CO2 fixation in pea root nodules support bacteroid metabolism. Plant Physiology. 1990;93:12–19. doi: 10.1104/pp.93.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux MRL, Khan S, Valentine AJ. Organic acid accumulation may inhibit N2 fixation in phosphorus-stressed lupin nodules. New Phytologist. 2008;177:956–962. doi: 10.1111/j.1469-8137.2007.02305.x. [DOI] [PubMed] [Google Scholar]
- Schuller KA, Werner D. Phosphorylation of soybean (Glycine max L.) nodule phosphoenolpyruvate carboxylase in vitro decreases sensitivity to inhibition by L-malate. Plant Physiology. 1993;101:1267–1273. doi: 10.1104/pp.101.4.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze J. How are nitrogen fixation rates regulated in legumes? Journal of Plant Nutrition and Soil Science. 2004;167:125–137. [Google Scholar]
- Schulze J, Drevon JJ. P-deficiency increases the O2 uptake per N2 reduced in alfalfa. Journal of Experimental Botany. 2005;56:1779–1784. doi: 10.1093/jxb/eri166. [DOI] [PubMed] [Google Scholar]
- Schulze J, Merbach WF. Nitrogen rhizodeposition of young wheat plants under elevated CO2 and drought stress. Biology and Fertility of Soils. 2008;44:417–423. [Google Scholar]
- Schulze J, Shi LF, Blumenthal J, Samac DA, Gantt JS, Vance CP. Inhibition of alfalfa root nodule phosphoenolpyruvate carboxylase through an antisense strategy impacts nitrogen fixation and plant growth. Phytochemistry. 1998;49:341–346. [Google Scholar]
- Schulze J, Temple G, Temple SJ, Beschow H, Vance CP. Nitrogen fixation by white lupin under phosphorus deficiency. Annals of Botany. 2006;98:731–740. doi: 10.1093/aob/mcl154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze J, Tesfaye M, Litjens R, Bucciarelli B, Trepp G, Miller S, Samac D, Allan D, Vance CP. Malate plays a central role in plant nutrition. Plant and Soil. 2002;247:133–139. [Google Scholar]
- Schweitzer LE, Harper JE. Effect of light, dark, and temperature on root nodule activity (acetylene reduction) of soybeans. Plant Physiology. 1980;65:51–56. doi: 10.1104/pp.65.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganuma N, Okada Y, Kanayama Y. Isolation of a cDNA for nodule-enhanced phosphoenolpyruvate carboxylase from pea and its expression in effective and plant-determined ineffective pea nodules. Journal of Experimental Botany. 1997;48:1165–1173. [Google Scholar]
- Tang C, Hinsinger P, Drevon JJ, Jaillard B. Phosphorus defciency impairs early nodule functioning and enhances proton release in roots of Medicago truncatula L. Annals of Botany. 2001;88:131–138. [Google Scholar]
- Vance C. Nodule carbon metabolism: organic acids for N2 fixation. In: Elmerich C, Kondorosi A, Newton WE, editors. Biological nitrogen fixation for the 21st century. Dordrecht: Kluwer Academic Publishers; 1998. pp. 443–448. [Google Scholar]
- Vance CP, Gregerson RG, Robinson DL, Miller SS, Gantt JS. Primary assimilation of nitrogen in alfalfa nodules - molecular-features of the enzymes involved. Plant Science. 1994;101:51–64. [Google Scholar]
- Vance CP, Heichel GH. Carbon in N2 fixation: limitation or exquisite adaptation. Annual Review of Plant Physiology and Plant Molecular Biology. 1991;42:373–392. [Google Scholar]
- Vance CP, Stade S. Alfalfa root nodules carbon-dioxide fixation. 2. Partial purification and characteriaztion of root nodule phosphoenol pyruvate carboxylase. Plant Physiology. 1984;75:261–264. doi: 10.1104/pp.75.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance CP, Stade S, Maxwell CA. Alfalfa root nodule carbon-dioxide fixation. 1. Association with nitrogen-fixation and incorporation into amino-acids. Plant Physiology. 1983;72:469–473. doi: 10.1104/pp.72.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viands DR, Vance CP, Heichel GH, Barnes DK. Ineffective nitrogen-fixation trait in alfalfa. Crop Science. 1979;19:905–908. [Google Scholar]
- Wang CX, Saldanha M, Sheng XY, Shelswell KJ, Walsh KT, Sobral BWS, Charles TC. Roles of poly-3-hydroxybutyrate (PHB) and glycogen in symbiosis of Sinorhizobium meliloti with Medicago sp. Microbiology. 2007;153:388–398. doi: 10.1099/mic.0.29214-0. [DOI] [PubMed] [Google Scholar]
- Warembourg FR, Roumet C. Why and how to estimate the cost of symbiotic N2 fixation: a progressive approach based on the use of 14C and 15N isotopes. Plant and Soil. 1989;115:167–177. [Google Scholar]
- Willmer CM, Petropoulou Y, Manetas Y. No light activation and high malate sensitivity of phosphoenolpyruvate carboxylase in guard-cell protoplasts of Commelina communis L. Journal of Experimental Botany. 1990;41:1103–1107. [Google Scholar]
- Yamakawa T, Ikeda T, Ishizuka J. Effects of CO2 concentration in rhizosphere on nodulation and N2 fixation of soybean and cowpea. Soil Science and Plant Nutrition. 2004;50:713–720. [Google Scholar]
- Yamakawa T, Tanaka S, Ishizuka J. Effect of CO2-free air treatment on nitrogen fixation of soybeans inoculated with Bradyrhizobium japonicum and Rhizobium fredii. Soil Science and Plant Nutrition. 1997;43:819–826. [Google Scholar]
- Zhang XQ, Li B, Chollet R. In vivo regulatory phosphorylation of soybean nodule phosphoenolpyruvate carboxylase. Plant Physiology. 1995;108:1561–1568. doi: 10.1104/pp.108.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]