Abstract
Plant roots exhibit remarkable developmental plasticity in response to local soil conditions. It is shown here that mild salt stress stimulates a stress-induced morphogenic response (SIMR) in Arabidopsis thaliana roots characteristic of several other abiotic stresses: the proliferation of lateral roots (LRs) with a concomitant reduction in LR and primary root length. The LR proliferation component of the salt SIMR is dramatically enhanced by the transfer of seedlings from a low to a high NO3− medium, thereby compensating for the decreased LR length and maintaining overall LR surface area. Increased LR proliferation is specific to salt stress (osmotic stress alone has no stimulatory effect) and is due to the progression of more LR primordia from the pre-emergence to the emergence stage, in salt-stressed plants. In salt-stressed seedlings, greater numbers of LR primordia exhibit expression of a reporter gene driven by the auxin-sensitive DR5 promoter than in unstressed seedlings. Moreover, in the auxin transporter mutant aux1-7, the LR proliferation component of the salt SIMR is completely abrogated. The results suggest that salt stress promotes auxin accumulation in developing primordia thereby preventing their developmental arrest at the pre-emergence stage. Examination of ABA and ethylene mutants revealed that ABA synthesis and a factor involved in the ethylene signalling network also regulate the LR proliferation component of the salt SIMR.
Keywords: ABA, Arabidopsis thaliana, auxin, lateral root, salt stress, SIMR
Introduction
A major feature of plant development is that it is continuously modified by interactions with the environment thereby enabling plants to cope with the constraints of their sessile lifestyle. A quintessential example is the developmental plasticity of the three-dimensional deployment of the root (the root architecture) in response to the soil environment. This plasticity is critical for a plant's ability to forage the soil for nutrients and water and contributes to a plant's competitive fitness (Grime et al., 1986; Lynch, 1995). Root architecture is determined by the pattern of root branching (lateral root formation) and by the rate and direction of growth of individual roots (Malamy, 2005). The formation of lateral roots is a reiterative process. Lateral roots (LRs) form along the length of the primary root and these first order LRs give rise to second order LRs which themselves can form third order LRs etc. In Arabidopsis thaliana, LRs initiate from a subset of root pericycle cells adjacent to the two xylem-poles (Dubrovsky et al., 2000, 2001; Casimiro et al., 2001). These ‘founder’ cells undergo successive cell divisions to generate a dome-shaped structure of cells, the LR primordium (LRP). After establishment of a new meristem layer, the LRP expands and emerges through the epidermis of the parent root (Bhalerao et al., 2002).
Several studies have shown how differences in the composition of the growth medium can alter root architecture (reviewed in Malamy, 2005). A good example is the proliferation of LRs into resource-rich growth media zones such as those enriched in NO3− or phosphate (Drew et al., 1973; Drew and Saker, 1975; Zhang and Forde, 1998; Zhang et al., 1999; Malamy and Ryan, 2001; Linkohr et al., 2002). On the other hand, less is understood regarding how plant root architecture is modified in response to stressful conditions, even though this response may be vital for the plant's ability to compete for soil resources. A recent survey of plant responses to heavy metal stress, phosphorus deficiency, UV or mechanical stress indicated a common morphogenic response characterized by the inhibition of primary root or shoot elongation and enhanced production of LRs or axillary branching (Potters et al., 2007, 2009). The authors suggested the existence of a co-ordinated, generic ‘stress-induced morphogenic response’ (SIMR), which is stimulated by mild chronic stress and comprises (i) inhibition of elongation; (ii) localized stimulation of cell division; (iii) alterations in cell differentiation status. The SIMR is thus typified by growth redistribution rather than by the cessation of growth.
The response of roots to drought or salinity stress is less clear. Several reports have described the reduction in root elongation caused by the application of osmotic or salinity stress in cotton, maize, and bean (Kurth et al., 1986; Itoh et al., 1987; Sharp et al., 1988; Eshel and Waisel, 1996). In cotton and in seedlings of three boreal conifers, a reduction in LR number is also observed (Eshel and Waisel, 1996; Croser et al., 2001). In most studies using Arabidopsis, osmotic or salt stress severely represses the formation of LRs (Burssens et al., 2000; van der Wheele et al., 2000; Kim et al., 2004; West et al., 2004; Deak and Malamy, 2005; Xiong et al., 2006). Osmotic stress appears to inhibit LR formation after LR initiation and affects the formation of autonomous LRs from LRPs (Deak and Malamy, 2005). However, during their studies of AtNAC2, a transcription factor involved in salt responses and LR development, He et al. (2005) noticed that in both wild type and the nac2-1 mutant, salt stress led to increased LR number, although this finding was not explored further.
In light of the conflicting reports regarding the effect of salt stress on Arabidopsis root architecture, it was decided to investigate this question in more detail. In the present study, it is reported that mild salt stress causes a drastic reduction in primary root elongation and LR elongation but an increase in LR number, constituting a typical SIMR. Enriching the medium with NO3−, dramatically enhances the effects of salt stress on LR number such that the overall LR surface area for absorption is maintained. The SIMR is specifically caused by salt stress but not by osmotic stress alone. Stimulation of LR number by salt stress is due to the progression of more LRPs from the pre-emergence to the emergence stage of LR formation. Evidence is also presented suggesting that the LR proliferation component of the SIMR is mediated by auxin accumulation in developing LRPs and by the stress hormone ABA.
Materials and methods
Plant material and growth conditions
All wild-type and mutant Arabidopsis thaliana lines were in the Columbia background. Seeds were surface-sterilized in NaClO (3% w/v) for 5 min, rinsed five times with sterile water, and sown on plates containing basal nutrient agar (Wilson et al., 1990) including 1 mM KNO3. Seeds were stratified at 4 °C for 4 d and then placed vertically in a growth room at 22 °C, with a 16/8 h light/dark photoperiod. When primary root length was approximately 2 cm (4–5 d), seedlings were transferred to the top segment of a vertical glass plate divided into three segments by glass dividers with each segment containing nutrient media supplemented with 1 mM KNO3 plus/minus 50 mM NaCl or 100 mM mannitol (100 mM mannitol has an approximate equal osmolarity to 50 mM NaCl). Enough medium was added so that roots were able to grow from one segment to another and the top edge of the agar in the top segment was cut away before the transfer of seedlings to ensure that only roots, not shoots, were in contact with the medium (MacGregor et al., 2008). For low to high NO3− treatments, seeds were initially sown on basal nutrient medium containing 10 μM KNO3 instead of 1 mM KNO3.
Analysis of root growth
For visualization, roots were stained with Brilliant Cresyl Blue as described by Gruntman and Novoplansky (2004). Roots were photographed with a C-5050 ZOOM digital camera (Olympus, Center Valley, PA). For LRP analysis, seedlings were harvested 8 d after transfer to segmented plates, stained with 2% (w/v) acetocarmine (Sigma-Aldrich, St Louis, MO) for 30 min at 78 °C and then cleared and mounted according to Malamy and Benfey (1997). For histochemical staining of GUS activity, seedlings were collected in staining buffer (0.1 M TRIS pH 7.5, 2.9 mg ml−1 NaCl, 0.66 mg ml−1 K3Fe(CN)6, and 20% methanol) and stained for 5 d as described by Malamy and Benfey (1997). Roots sections were photographed with a digital camera DXM1200F (Nikon Instruments, Badhoevedorp, Netherlands) mounted on a Nikon Eclipse TE2000-U microscope. For experiments carried out for 21 d, data were statistically analysed using the Fisher's protected LSD test. However, for experiments carried out for 8 d or 12 d, and where the total number of LRs produced was much smaller, the more stringent Tukey's post-hoc test was performed.
Results
Mild salt stress inhibits primary and lateral root elongation and stimulates LR proliferation
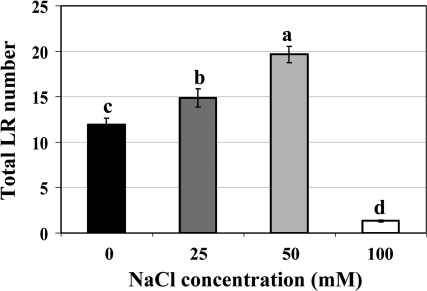
To examine how salt stress affects Arabidopsis LR proliferation, the dose response of Arabidopsis seedlings to increasing salt concentration was tested. Seedlings were germinated and grown on vertical nutrient agar plates until the root was ∼2 cm long (4–5 d) and then transferred to fresh plates with increasing levels of NaCl. At the time of transfer no LRs were visible. Figure 1 shows that increasing NaCl levels up to 50 mM stimulated the production of LRs, where seedlings exhibited virtually twice the number of first order LRs (emerged LRs) after 21 d than the control (0 mM NaCl) and also produced a few second order LRs (data not shown). However, the transfer of seedlings to 100 mM salt stress caused almost complete inhibition of LR development. These results suggest an acclimation response of LR development to mild levels of salt stress.
Fig. 1.
Effect of NaCl concentration on Arabidopsis lateral root number. Seedlings grown on 1 mM KNO3 medium for 4–5 d were transferred to the same medium plus the indicated concentrations of NaCl for 21 d. Data are mean emerged LRs ±SE (n=30). Bars with different letters indicate significant difference at P ≤0.05 (Fisher's protected LSD test). The data are representative of similar results in three independent experiments.
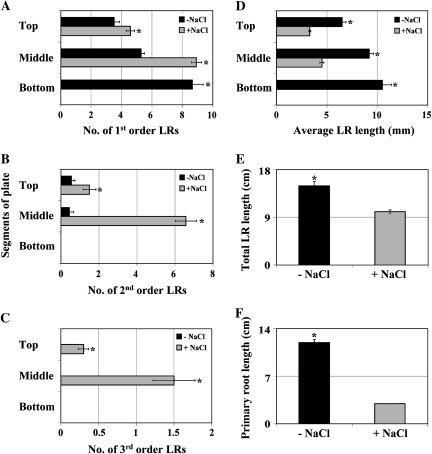
To investigate the stimulatory effect of mild salt stress on LR development further, 4–5 d-old seedlings were transferred to vertical segmented nutrient agar plates with or without 50 mM NaCl (the NaCl concentration that had displayed the greatest stimulatory effect) in all the segments. It was observed that first order LR number increased significantly in the top and middle segments of the plate compared with unstressed plants (Fig. 2A). Salt stress caused an even greater stimulation of second order LR proliferation where stressed seedlings produced 8-fold more LRs than unstressed seedlings (Fig. 2B). Furthermore, only stressed plants developed third order LRs (Fig. 2C). By contrast with its stimulatory effect on LR number, salt stress led to a reduction in average LR length (Fig. 2D) with stressed LRs exhibiting approximately half the length of unstressed LRs. Moreover, although salt stress led to an increase in LR number, an evaluation of total LR length (a function of both total LR number and average LR length) suggested that the salt-mediated increase in LR number could not fully compensate for the reduction in the average LR length (Fig. 2E). Primary root elongation was severely reduced in stressed seedlings, which displayed a 4-fold reduction in primary root length compared with unstressed seedlings (Fig. 2F). Indeed, only unstressed primary roots reached the bottom segment of the plate, hence the absence of any first order stressed LRs in the bottom segment (Fig. 2A). Overall, our results suggest that mild salt stress stimulates an SIMR phenotype in contrast to severe salt stress where LR proliferation is inhibited.
Fig. 2.
Salt stress causes an SIMR phenotype. (A–F) Seedlings grown on 1 mM KNO3 medium for 4–5 d were transferred to the same medium ±50 mM NaCl for 21 d. Data are mean ±SE (n=20). * Significant difference (P ≤0.05) between unstressed and stressed roots (Fisher's protected LSD test). The data are representative of similar results in three independent experiments. Top, Middle, Bottom: the different segments of the segmented agar plate.
Stimulation of LR proliferation by salt stress is enhanced upon transfer from low to high NO3−
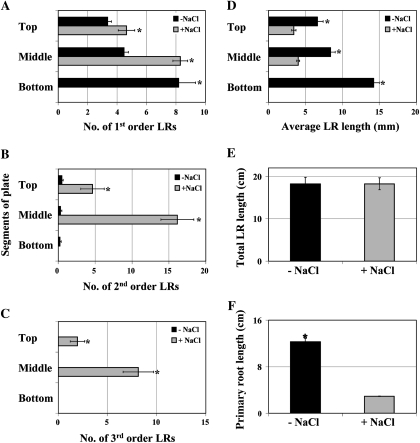
The stimulatory effect of nitrate-enriched media on LR proliferation has been well documented (reviewed in Zhang et al., 2007). In Arabidopsis, the transfer from NO3−-poor to NO3−-rich medium predominantly stimulates LR elongation (Zhang et al., 1999; Linkohr et al., 2002). It was therefore tested whether transfer from low NO3− to NO3−-rich medium would have a stimulatory effect on the LR component of the salt SIMR, by transferring seedlings grown on 10 μM KNO3 to plates containing 1 mM KNO3 with and without NaCl uniformly present across all plate segments. The enrichment of the NO3− supply led to a dramatic enhancement of the salt-induced increase in LR number. While first order LRs were induced to a similar extent as with salt alone (compare Figs. 2A and 3A) the mean number of second and third order LRs (16 and 8, respectively) exhibited a striking enhancement compared to salt stress alone (6.5 and 1.5, respectively) (compare Figs. 2B and 3B; 2C and 3C). On the other hand, when combined with salt treatment, the enrichment of the NO3− supply had no stimulatory effect on LR elongation (Fig. 3D); mean LR length was reduced to a similar degree as with salt stress alone (compare Figs. 2D and 3D). Strikingly, evaluation of total LR length showed that NO3− enhancement of the salt stress-induced increase in LR number fully compensated for the decreased average LR length (Fig. 3E). Primary root elongation was again severely inhibited in salt-stressed roots (Fig. 3F).
Fig. 3.
The LR proliferation component of the salt SIMR is enhanced by transfer from low to high NO3−. (A–F) Seedlings grown on 10 μM KNO3 medium for 4–5 d were transferred to 1 mM KNO3 medium ±50 mM NaCl for 21 d. Data are mean ±SE (n=20). * Significant difference (P ≤0.05) between unstressed and stressed roots (Fisher's protected LSD test). The data are representative of similar results in three independent experiments. Top, Middle, Bottom: the different segments of the segmented agar plate.
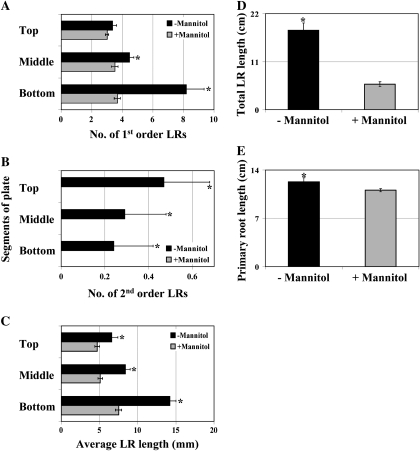
Salt stress comprises two stress components, namely osmotic stress and ionic stress. To determine whether the salt SIMR is specific to salt stress and not to osmotic stress alone, the effect of the latter on root architecture was tested. When seedlings were transferred from 10 μM KNO3 to 1 mM KNO3 supplemented with 100 mM mannitol, primary root elongation was only minimally affected by osmotic stress (compare Figs. 3F and 4E). Furthermore, instead of a stimulation of LR proliferation, a reduction in first order LR number was observed in stressed roots compared with unstressed roots (Fig. 4A). Moreover, second order LRs only appeared in unstressed roots (Fig. 4B) while no third order LRs were observed in either unstressed or stressed roots. The average LR length was reduced by osmotic stress in a similar manner to salt stress (Fig. 4C) and this reduction in average LR length combined with the decreased LR number led to a large fall in total LR length (Fig. 4D). Similar results were obtained when seedlings were transferred to mannitol but without the stimulatory effect of NO3−-enrichment (transfer of seedlings from 1 mM KNO3 to 1 mM KNO3 with or without mannitol [data not shown]). These findings show that osmotic stress alone or in combination with NO3− enrichment is not sufficient to stimulate a characteristic SIMR.
Fig. 4.
Osmotic stress alone inhibits LR elongation but does not stimulate LR proliferation. (A–E) Seedlings grown on 10 μM KNO3 medium for 4–5 d were transferred to 1 mM KNO3 medium ±100 mM mannitol for 21 d. Data are mean ±SE (n=20). * Significant difference (P ≤0.05) between unstressed and stressed roots (Fisher's protected LSD test). The data are representative of similar results in three independent experiments. Top, Middle, Bottom: the different segments of the segmented agar plate.
Greater numbers of LRPs develop into LRs during the NO3−-enhanced salt SIMR
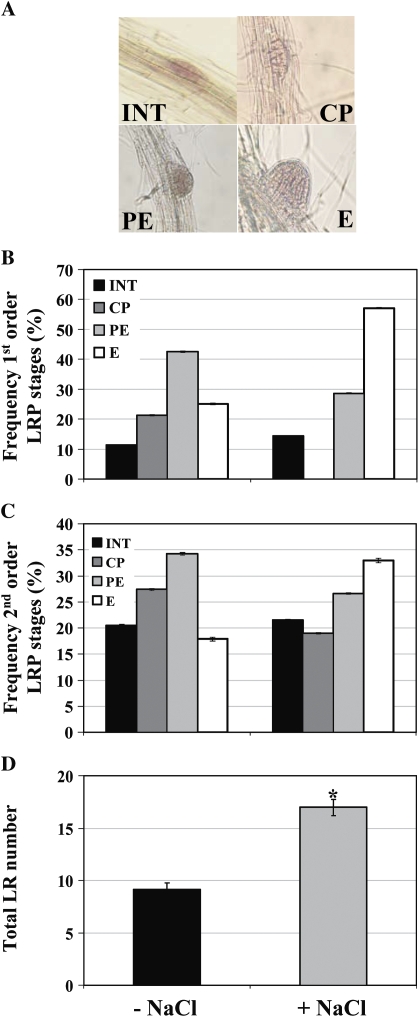
It was next investigated whether development of LR primordia (LRPs) is affected during the NO3−-enhanced salt SIMR. Seedlings were again grown on 10 μM KNO3 and transferred to plates containing 1 mM KNO3 with and without NaCl uniformly present across all plate segments. Visualization of LRPs was performed by staining with acetocarmine (Enstone et al., 2001) and the development of each primordium assessed by combining the detailed stages of LRP development assigned by Malamy and Benfey (1997) into four broad phases: (INT) initiation, (CP) cell proliferation, (PE) pre-emergence, and (E) emergence (Fig. 5A). Almost 60% of first order LRPs reached the E stage in stressed seedlings whereas the greatest proportion (42%) of LRPs in unstressed plants was still at the PE stage of development (Fig. 5B). Although similar numbers of INT stage LRPs in unstressed and stressed seedlings were observed, no CP stage LRPs in stressed seedlings were detected compared to 21% CP stage LRPs from unstressed seedlings. Similar results were observed with second order LRPs although, as expected, a greater percentage of LRPs from both unstressed and stressed seedlings were in the earlier stages of development than first order LRPs (Fig. 5C). Nevertheless, the highest proportion of second order LRPs in stressed plants was at the E stage of development whereas the greatest percentage of LRPs in unstressed plants was at the PE stage. These results suggest that while the proportion of LRPs being initiated in both unstressed and stressed plants is similar, the overall increase in LR number in stressed roots (Fig. 5D) is either due to the arrest of a proportion of LRPs in unstressed plants at the pre-emergence stage of development or, alternatively, LRPs in stressed roots develop at a faster rate.
Fig. 5.
LRP development during the NO3−-enhanced salt SIMR. Seedlings grown on 10 μM KNO3 medium for 4–5 d were transferred to 1 mM KNO3 medium ±50 mM NaCl for 8 d. (A) Stages of LRP development visualized by acetocarmine staining. INT, Initiation; CP, cell proliferation; PE, pre-emergence; E, emergence. (B, C) Frequency of first and second order LRP developmental stages, respectively. Stages of LRP development are indicated in the graph legend. (D) Total LR number. Data are mean ±SE (n=30). Tukey's post-hoc test showed a significant difference (P ≤0.05) in LRP developmental stages between unstressed and stressed roots. (D) * Significant difference (P ≤0.05) between unstressed and stressed roots (Fisher's protected LSD test). The data are representative of similar results in three independent experiments.
NO3−-enhanced, salt stress stimulation of LR proliferation is mediated by auxin
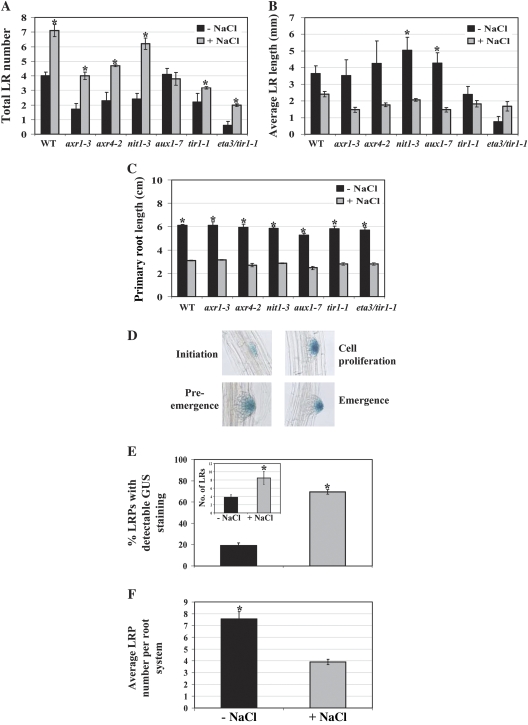
One of the major generic characteristics of root SIMR phenotypes is the increased proliferation of LRs (Potters et al., 2007). Auxin is a key regulator of LR development. It promotes LRP initiation by the local activation of root pericycle cells and also plays a role in LR emergence (Reed et al., 1998; Casimiro et al., 2001; Bhalerao et al., 2002; Marchant et al., 2002; De Smet et al., 2007; Okushima et al., 2007; Dubrovsky et al., 2008). It was therefore investigated whether the LR proliferation component of the NO3−-enhanced salt SIMR is mediated by auxin, by testing if salt stress can still induce LR proliferation in (i) an auxin synthesis mutant, nit1-3 (Normanly et al., 1997) [NIT1 is one of three nitrilases that catalyse a biosynthetic step in one of several IAA biosynthesis pathways (Woodward and Bartels, 2005)]; (ii) auxin transport mutants, axr4-2, aux1-7 (Picket et al., 1990; Dharmasiri et al., 2006); (iii) auxin signalling mutants, axr1-3, tir1-1, eta3/tir1-1 (the eta3 background enhances the tir-1-1 phenotype) (Lincoln et al., 1990; Ruegger et al., 1998; Gray et al., 2003; Dharmasiri et al., 2005; Kepinski et al., 2005). All auxin mutants exhibited decreases in either, or both, unstressed and stressed LR number compared to wild-type (Fig. 6A). However, while the stimulation of LR proliferation under salt stress was observed in the axr1-3, axr4-2, nit1-3, tir1-1, and eta3/tir1-1 mutants, it was completely blocked in the aux1-7 mutant, suggesting that auxin transport, at least via AUX1, is essential for the LR proliferation component of the salt SIMR.
Fig. 6.
Salt stress affects auxin distribution in LRPs. (A–C) Total LR number, average LR length, and primary root length, respectively, of WT and auxin mutants transferred from 10 μM KNO3 to 1 mM KNO3 medium ±50 mM NaCl for 8 d. Data are mean ±SE (n=20). * Significant difference (P ≤0.01) between unstressed and stressed roots (Tukey's post-hoc test). (D) LRP development visualized by DR5:GUS staining. Growth conditions were as described (Benkova et al., 2003). (E) Frequency of stained LRP. (F) LRP number per root system. Inset in (E), Total LR number. For (E), (F) and inset, data are mean ±SE (n=30). * Significant difference (P ≤0.05) between unstressed and stressed roots (Fisher's protected LSD test). The data are representative of similar results in three independent experiments.
A clear trend showing a stress-induced decrease in average LR length could be observed in wild-type, axr1-3, axr4-2, nit1-3, and aux1-7 roots, although this decrease was not statistically significant except for the nit1-3 and aux1-7 mutants (Fig. 6B). No significant salt-mediated reduction in average LR length could be seen in the tir1-1 mutant, but this was due to a large decrease in unstressed LR elongation with little effect on stressed LR length. This phenotype was enhanced in the eta3/tir1-1 mutant. All mutants exhibited the wild-type inhibition of primary root elongation by salt stress (Fig. 6C). Overall, the results suggest that auxin does not play a role in the root elongation component of the salt SIMR but is important for LR elongation under unstressed conditions.
Our results indicate that at least auxin transport is essential for the salt-mediated stimulation of LR proliferation. AUX1 is an influx carrier that imports auxin into developing LRPs to regulate both LRP initiation and emergence (Marchant et al., 1999, 2002; Swarup et al., 2001; Yang et al., 2006). To test whether the LR proliferation component of the NO3−-enhanced salt SIMR is associated with increased auxin accumulation, expression of the GUS reporter gene, driven by the auxin-sensitive DR5 promoter, was examined in developing LRPs. DR5 is an established indirect marker for auxin accumulation and is expressed from the earliest stages of LRP development (Ulmasov et al., 1997; Benkova et al., 2003; Dubrovsky et al., 2008). It was possible to detect LRP developmental stages INT to E by histochemical staining for GUS activity (Fig. 6D). Remarkably, only 20% of LRPs in unstressed seedlings exhibited GUS staining while 70% of LRPs in stressed seedlings were stained (Fig. 6E). Moreover, twice the number of LRPs per root system was observed in unstressed seedlings compared to stressed seedlings, whereas twice as many LRs had developed in stressed seedlings (Fig. 6F and inset in E). Taken together, these results suggest that the development of a greater number of LRP's is arrested in the absence of salt stress; the corollary being that salt stress stimulates the development of a larger proportion of LRPs and that this stimulation is associated with increased auxin accumulation in the LRPs. This finding correlates well with our observation that, while a similar proportion of LRPs in unstressed and stressed seedlings are initiated, a greater percentage of LRPs in roots of stressed seedlings reach developmental stage E than in unstressed seedlings where a proportion of LRPs appear to be arrested at the CP or PE stages (Fig. 5B, C).
ABA and an ethylene signalling factor regulate NO3−-enhanced, salt stress stimulation of LR proliferation
In contrast to auxin, ABA exerts an inhibitory effect on LR development at the post-emergence stage (De Smet et al., 2003, 2006). Evidence suggests that ABA action on root development may require, at least in part, a functioning ethylene signalling network and that ABA inhibition of root growth is attenuated in ethylene signalling mutants such as ein2 and etr1 (Beaudoin et al., 2000; Ghassemian et al., 2000; Cheng et al., 2002). It was therefore investigated whether ABA or ethylene signalling play a role in the NO3−-enhanced salt SIMR by testing whether salt stress leads to a different phenotype from the wild type in the following ABA and ethylene mutants: (i) ABA synthesis mutants, aba2-1, aba3-1 (Leon-Kloosterziel et al., 1996; Schwartz et al., 1997); (ii) the ABA signalling mutant, abi4-1 (Finkelstein, 1994; Soderman et al., 2000); (iii) ethylene signalling mutants, ein2-1, etr1-3 (Guzman and Ecker, 1990; Chang et al., 1993).
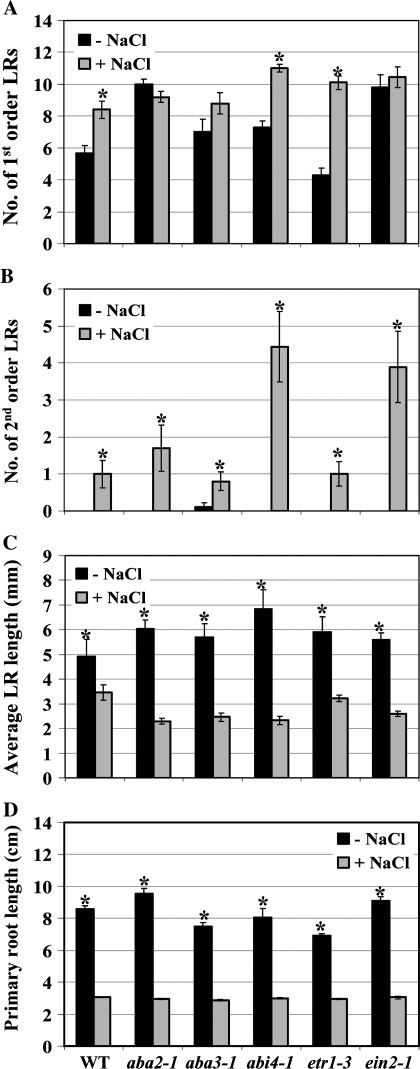
The aba2-1 mutant produced a greater number of first order LRs than the wild type in both unstressed and stressed seedlings (Fig. 7A) consistent with the inhibitory role of ABA in LR development. However, NO3−-enhanced salt stress stimulation of first order LR proliferation was completely abrogated in this mutant. The slightly weaker aba3-1 mutant (Leon-Kloosterziel et al., 1996) also displayed no significant difference in first order LR number between unstressed and stressed seedlings. The ein2-1 mutant exhibited a similar phenotype to the aba2-1 mutant with greater numbers of first order LRs in unstressed and stressed seedlings but no stimulation of first order LR proliferation in stressed seedlings. By contrast, salt stress stimulated first order LR proliferation in the abi4-1 and etr1-3 mutants. These results suggest that ABA synthesis is required for the first order LR proliferation component of the salt SIMR, that ABI4 is not involved in the signalling network and that at least one factor involved in the ethylene signal network is required.
Fig. 7.
ABA and an ethylene signalling factor regulate the LR proliferation component of the NO3−-enhanced salt SIMR. (A–D) Seedlings of wild-type, ABA mutants, and ethylene mutants grown on 10 μM KNO3 medium for 4–5 d were transferred to 1 mM KNO3 medium ±50 mM NaCl for 12 d. Data are mean ±SE (n=20). * Significant difference (P ≤0.01) between unstressed and stressed roots (Tukey's post-hoc test). The data are representative of similar results in three independent experiments.
Figure 7B shows that the response of second order LRs was different from that of first order LRs. Second order LRs were almost only observed in stressed roots in both wild-type and mutant seedlings. Both the aba mutants and the etr1-3 mutant exhibited a similar number of LRs to the wild type, whereas the abi4-1 and ein2-1 mutants displayed greater LR proliferation. These findings suggest that second order LRs are regulated in a different manner to first order LRs.
All the ABA and ethylene signalling mutants exhibited wild-type phenotypes of salt-mediated inhibition of LR and primary root length (Fig. 7C, D) suggesting that the root elongation component of the salt SIMR does not involve ABA or ethylene signalling.
Discussion
Several distinct abiotic stresses (heavy metals, UV, phosphorus-deficiency, mechanical stress) lead to a stress-induced morphogenic response (SIMR) which, in roots, consists of the inhibition of root elongation and an increase in LR number (Potters et al., 2007). However, due to conflicting studies in recent years regarding the effect of salt stress on Arabidopsis root architecture, it is unclear whether salinity causes a root SIMR. Our data show that mild salt stress leads to a characteristic SIMR: a reduction in Arabidopsis LR and primary root elongation with a concomitant stimulation of LR number and LR branching (Figs. 1, 2). On the other hand, a high level of salt stress leads to a reduction in LR number (Fig. 1) again typical of an SIMR, which is an active process and is therefore inhibited under severe stress (Potters et al., 2007). A closer inspection of the experimental systems used in different salinity studies might account for the contrasting reports of the effect of salt stress on LR development. For instance, one study that showed a salt-mediated reduction in Arabidopsis LR number used 3.5 times the concentration of NaCl used in our system (Burssens et al., 2000). Arabidopsis is a salt-sensitive plant and the reduction in LR number at such high salinity levels is likely to represent salt-induced damage rather than bona-fide acclimation.
The Malamy laboratory has shown the importance of experimental design on the interpretation of LR development data from Arabidopsis grown in culture. This group had shown in a previous paper that osmotic stress leads to a repression of LR formation in culture-grown Arabidopsis (Deak and Malamy, 2005). However, in a recent paper, they demonstrated that uptake of sucrose by aerial tissues in contact with the growth medium stimulated LR development (MacGregor et al., 2008). Moreover, the apparent repression of LR formation by osmotic stress was actually due to an osmotic stress-mediated decrease in the permeability of aerial tissues thereby reducing the uptake of sucrose and causing inhibition of LR development. This finding may also explain the results of a report showing a reduction in Arabidopsis LR number using mild salt stress but where aerial tissues were in contact with sucrose-containing growth medium (Kim et al., 2004). By contrast, our experiments were designed so that the top section of the agar was cut away and only the roots were in contact with the growth media. Our data thus provide strong evidence that the stimulation of LR proliferation under mild salt stress is an acclimation response.
It is not possible at this stage to distinguish whether the ionic or osmotic components of salt stress, or a combination of both, are responsible for the LR proliferation component of the salt SIMR. While it could be shown that osmotic stress (mannitol treatment) was not sufficient to promote an increase in LR number (Fig. 4), further experiments using a salt such as LiCl that imposes ionic toxicity at lower concentrations than NaCl would aid in determining the relative importance of the two components of salt stress (Tester and Davenport, 2003). On the other hand, the osmotic component of salt stress was probably responsible for the inhibition of LR and primary root elongation. This idea is supported by the observation that both salt stress and osmotic stress lead to shorter LRs and primary roots and by the data showing the salt stress stimulation of LR number implying that the inhibition of root elongation was not due to ion toxicity.
Our results show that the LR proliferation component of the salt SIMR is massively enhanced by the transfer of seedlings from low to high NO3− (Fig. 3). Indeed, the increased LR number fully compensates for the salt stress-mediated reduction in LR length, thereby maintaining the overall LR surface area for absorbing nutrients and water. The stimulatory effect of transfer from low to high NO3− upon Arabidopsis LR development has been well documented (Zhang and Forde, 1998; Zhang et al., 1999, 2000, 2007; Linkohr et al., 2002). However, the predominant effect is the stimulation of LR elongation. An increase in LR elongation was also noted upon transfer to high NO3− in the absence of salt, compared to low NO3−, with no effect on LR number (data not shown). Moreover, our data demonstrate that transfer from low to high NO3− without salt does not lead to LR proliferation. This evidence supports the idea that the signal stimulating an increase in LR number in our experiments is salt stress. Transfer from low to high NO3− only enhances the salt stress signal. The mechanism by which NO3− enhances the LR proliferation component of the salt SIMR is unclear. It is possible that the increase in NO3− supply indirectly promotes LR proliferation by affecting cotyledon and leaf size. However, in transfer experiments where seedlings experienced greater NO3− supply throughout the experiment leading to bigger seedlings at the time of transfer (e.g. Figs 1, 2: NO3− supply was kept constant at 1 mM KNO3 before and after transfer), salt stress stimulation of LR proliferation was less than when smaller seedlings initially grown on low (10 μM) NO3− were transferred to high NO3−. This suggests that the NO3−-mediated enhancement is due to the plant's ability to sense changes in the external NO3− supply upon transfer from low to high NO3−. Such a NO3− sensing system has been demonstrated in Arabidopsis (Zhang and Forde, 1998; Remans et al., 2006). In this system, the NO3− ion itself, acts as a signalling molecule and evidence suggests that the nitrate transporter NRT1.1 may act as a key NO3− sensor regulating expression of ANR1, a MADS box transcription factor thought to transduce the NO3− signal internally. However, this NO3− sensing system is associated with NO3− stimulation of LR elongation. Whether it is also part of the sensing system regulating NO3− enhancement of the salt stress-mediated increase in LR number requires further investigation.
It was possible to correlate the NO3−-enhanced salt SIMR with the development of more LRPs into LRs than was observed in unstressed roots (Fig. 5). Although speculative, the fact that unstressed roots possess a large proportion of LRPs whose development appears to be arrested suggests that an optimal number of LRs are produced under favourable conditions but that new developing roots are ‘primed’ with an excess of LRPs as a contingency against sub-optimal soil conditions. Seventy per cent of LRPs in stressed seedlings showed DR5:GUS staining compared with 20% of LRPs in unstressed seedlings (Fig. 6E). It has previously been shown that expression from the auxin-responsive DR5 promoter is undetectable in LRPs that fail to develop into LRs (Benkova et al., 2003; De Smet et al., 2003). Thus, our results suggest that salt stress prevents the developmental arrest of a large proportion of LRPs that would not normally progress into mature LRs in unstressed conditions rather than increasing the rate of LR development. In addition, it has recently been demonstrated that the DR5 promoter is the earliest marker for lateral root founder cells in the pericycle and that activation of the promoter absolutely correlates with subsequent LRP formation (Dubrovsky et al., 2008). Furthermore, the authors provide evidence suggesting that DR5 promoter activity does not reflect a higher sensitivity of auxin signalling but rather increased cellular auxin levels. Thus, our data showing reduced DR5:GUS expression in unstressed roots suggest that arrest of LRPs observed in unstressed plants is due to reduced auxin accumulation and, conversely, that the LR proliferation component of the salt SIMR is mediated by the salt stress promotion of auxin accumulation in developing LRPs. This contention is supported by our results showing that AUX1 is essential for the LR proliferation component of the salt SIMR. Initiation and development of LRs appears to be dependent on auxin transport via both influx and efflux carriers (Marchant et al., 2002; Friml et al., 2002; Benkova et al., 2003). During LR development, AUX1 is thought to be involved in facilitating LR outgrowth by translocating auxin from newly formed leaf primordia and by the uptake of auxin into developing LRPs (Marchant et al., 2002). At later stages of LR development, an auxin gradient is gradually established with the maximum auxin level at the tip of the primordium (Benkova et al., 2003). Our results thus fit in well with the hypothesis arising from the tight link between auxin and root or shoot branching, that alterations in auxin distribution and metabolism are likely to be a general feature of the SIMR phenotype in response to diverse stresses. Hence, the phosphorus-deficiency SIMR in Arabidopsis is linked to changes in auxin sensitivity (Lopez-Bucio et al., 2002; Perez-Torres et al., 2008), the cadmium SIMR in Arabidopsis and the aluminium SIMR are correlated with changes in auxin distribution (Kollmeier et al., 2000; Doncheva et al., 2005) and the UV-B SIMR is associated with altered auxin metabolism (Jansen et al., 2001).
It is unclear at present how diverse stresses affect auxin homeostasis, but one possibility is that reactive oxygen species, which are produced by many abiotic and biotic stresses, act as signalling intermediates leading to changes in auxin homeostasis and thereby stimulating the SIMR (Potters et al., 2009). Treatments that cause oxidative stress lead to an SIMR phenotype and enhance auxin-dependent growth cycle reactivation (Pasternak et al., 2005). Altering the cellular levels of antioxidants can interfere with auxin responses and also affect the cell cycle (Potters et al., 2002; Pignocchi et al., 2003). Cellular redox states can also impinge on auxin-mediated changes in cell elongation (reviewed in Potters et al., 2009).
Another attractive candidate as a signalling intermediate between diverse stresses and the SIMR phenotype is the universal stress hormone, abscisic acid. ABA is synthesized in response to many abiotic stresses and activates the expression of numerous stress-responsive genes (Zeevaart and Creelman, 1988; Seki et al., 2002). Moreover, ABA has a well-documented role in lateral root formation (De Smet et al., 2006). In general, ABA and auxin act antagonistically; whereas auxin acts to promote LR proliferation, ABA has been shown to inhibit LR development by suppression of the post-emergence activation of the LR meristem (De Smet et al., 2003). In our experiments, this inhibitory role of ABA can be observed in the aba2-1 mutant, which exhibits increased first order LR numbers in both unstressed and stressed roots (Fig. 7). However, the abolition of the first order LR proliferation component of the salt SIMR in the aba2-1 and aba3-1 mutants suggests that ABA can also act as a positive signal in LR development. A positive role for ABA in LR development is supported by the demonstration that stimulation of LR elongation upon transfer of seedlings from low to high NO3− levels, is mediated by ABA (Signora et al., 2001). It is thus also conceivable that the enhancement of the LR proliferation component of the salt SIMR by NO3− could be regulated via ABA.
Our data indicate that ABA does not exert its effect on the first order LR proliferation component of the salt SIMR via the ABA signalling factor, ABI4. However, the stimulation of first order LR proliferation was abrogated in the ein2-1 mutant whose LR phenotype was similar to the aba2-1 mutant. This suggests that the stimulatory effect of ABA on the salt SIMR may involve factors of the ethylene signalling pathway. On the other hand, ethylene may regulate the LR proliferation component of the salt SIMR independently of ABA. For instance, two recent reports suggest that ethylene regulates Arabidopsis root branching via interactions with auxin (Ivanchenko et al., 2008; Negi et al., 2008).
Intriguingly, the response of second order LRs in the wild type, and in ABA and ethylene mutants differed from that of first order LRs. In fact, the higher order LRs showed the greatest NO3−-enhanced, salt stress stimulation of LR number throughout this study suggesting either increased uptake of salt or/and NO3−, or increased sensitivity to the salt-mediated signal, the NO3− signal or a combination of the two. Little is understood regarding both the intrinsic and extrinsic regulation of higher order LRs and whether, or how, this regulation differs from that of first order LRs. Our results demonstrate the importance of separating the analysis of LRs of different order to begin to dissect the mechanisms that control root branching.
What might be the physiological and ecological significance of the salt SIMR and its enhancement by NO3−-rich growth media? It has been suggested that SIMRs in general are a mechanism for stress evasion and represent a re-direction of growth away from localized sources of stress (e.g. heavy metals) or a means to reduce stress exposure (e.g. UV-B penetration) (Potters et al., 2007). This hypothesis could conceivably apply to the salt SIMR as well. On the other hand, the reduction in both LR length and primary root length under salt stress would reduce root surface area for absorption. This would occur at a time when a reduction in soil water potential due to increased salt concentration would make it more difficult to maintain water flow into the plant. However, a rise in LR number (the LR proliferation component of the SIMR) would partly compensate for the reduction in LR length by providing greater root surface area for absorption of water and nutrients albeit at a reduced absorption rate. The significance of enhancement of the LR proliferation component of the salt SIMR by NO3− may be more complex. It has been shown that an extensive root system is only required for relatively immobile nutrients such as phosphate ions whereas mobile ions such as nitrate can be efficiently taken up by a more restricted root architecture (Robinson, 1996; Fitter et al., 2002). Root proliferation for mobile ions becomes important only when plants are in competition for those ions (Hodge et al., 1999; Robinson et al., 1999). However, Fitter et al. (2002) showed that under extreme conditions of high N and high P, a larger root system might be important for exploiting NO3−. Salt stress might represent an extreme condition, where, in addition to the inhibition of LR elongation, the increased concentration of Na+ ions could interfere with NO3− for root uptake sites (Sagi et al., 1997). Under these circumstances, integration of the salt SIMR and NO3− signalling pathways leading to a massive increase in LR proliferation would facilitate exploitation of mobile nutrients, thereby allowing the plant to compete better for resources under sub-optimal soil conditions. Furthermore, seedlings (upon which the present work is based), are highly vulnerable to such conditions, which can have severe effects upon seedling growth and establishment. Indeed, the seedling stage has been considered as a bottleneck in a species’ life history (Leck et al., 2008). Seedling responses such as an SIMR could aid in seedling survival and establishment, which ultimately underlies the sustainability of plant communities.
Acknowledgments
We would like to express our appreciation to the Arabidopsis Biological Resource Center (Ohio State University) for supplying the axr1-3, axr4-2, aux1-7, nit1-3, tir1-1, abi4-1, aba3-1, ein2-1, and etr1-3 mutants. We are also grateful to Professors Gadi Galili, William M Gray, and Thomas Guilfoyle for their kind gift of the aba2-1, era3/tir1-1, and DR5:GUS lines, respectively. We are indebted to Ruti Shaked and Rami Mosly for excellent technical assistance. Finally, thanks to the Sonnenfeldt-Goldman Career Development Chair for Desert Research for funding this research.
References
- Beaudoin N, Serizet C, Gosti F, Giraudat J. Interactions between abscisic acid and ethylene signalling cascades. The Plant Cell. 2000;12:1103–1015. doi: 10.1105/tpc.12.7.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, Friml J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- Bhalerao RP, Eklof J, Ljung K, Marchant A, Bennett M, Sandberg G. Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. The Plant Journal. 2002;29:325–332. doi: 10.1046/j.0960-7412.2001.01217.x. [DOI] [PubMed] [Google Scholar]
- Burssens S, Himanen K, van de Cotte B, Beeckman T, van Montagu M, Inzé D, Verbruggen N. Expression of cell cycle regulatory genes and morphological alterations in response to salt stress in Arabidopsis thaliana. Planta. 2000;211:632–640. doi: 10.1007/s004250000334. [DOI] [PubMed] [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, et al. Auxin transport promotes Arabidopsis lateral root initiation. The Plant Cell. 2001;13:843–852. doi: 10.1105/tpc.13.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM. Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science. 1993;262:539–544. doi: 10.1126/science.8211181. [DOI] [PubMed] [Google Scholar]
- Cheng WH, Endo A, Zhou L, et al. A unique short-chain dehydrogenase/reductase in Arabidopsis glucose signalling and abscisic acid biosynthesis and functions. The Plant Cell. 2002;14:2723–2743. doi: 10.1105/tpc.006494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croser C, Renault S, Franklin J, Zwiazek J. The effect of salinity on the emergence and seedling growth of Picea mariana, Picea glauca, and Pinus banksiana. Environmental Pollution. 2001;115:9–16. doi: 10.1016/s0269-7491(01)00097-5. [DOI] [PubMed] [Google Scholar]
- De Smet I, Signora L, Beeckman T, Inzé D, Foyer CH, Zhang H. An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis. The Plant Journal. 2003;33:543–555. doi: 10.1046/j.1365-313x.2003.01652.x. [DOI] [PubMed] [Google Scholar]
- De Smet I, Zhang H, Inzé D, Beeckman T. A novel role for abscisic acid emerges from underground. Trends in Plant Science. 2006;11:434–439. doi: 10.1016/j.tplants.2006.07.003. [DOI] [PubMed] [Google Scholar]
- De Smet I, Tetsumura Y, De Rybel B, et al. Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development. 2007;134:681–690. doi: 10.1242/dev.02753. [DOI] [PubMed] [Google Scholar]
- Deak KI, Malamy J. Osmotic regulation of root system architecture. The Plant Journal. 2005;43:17–28. doi: 10.1111/j.1365-313X.2005.02425.x. [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005;435:441–445. doi: 10.1038/nature03543. [DOI] [PubMed] [Google Scholar]
- Dharmasiri S, Swarup R, Mockaitis K, et al. AXR4 is required for localization of the auxin influx facilitator AUX1. Science. 2006;312:1218–1220. doi: 10.1126/science.1122847. [DOI] [PubMed] [Google Scholar]
- Doncheva S, Amenos M, Poschenrieder C, Barcelo J. Root cell patterning: a primary target for aluminium toxicity in maize. Journal of Experimental Botany. 2005;56:1213–1220. doi: 10.1093/jxb/eri115. [DOI] [PubMed] [Google Scholar]
- Drew MC, Saker LR, Ashley TW. Nutrient supply and the growth of the seminal root system in barley. I. The effect of nitrate concentration on the growth of axes and laterals. Journal of Experimental Botany. 1973;24:1189–1202. [Google Scholar]
- Drew MC, Saker LR. Nutrient supply and the growth of the seminal root system of barley. II. Localized, compensatory increases in lateral root growth and rates of nitrate uptake when nitrate supply is restricted to only part of the root system. Journal of Experimental Botany. 1975;26:79–90. [Google Scholar]
- Dubrovsky JG, Doerner P, Colon-Carmona A, Rost TL. Pericycle cell proliferation and lateral root initiation in Arabidopsis. Plant Physiology. 2000;124:1648–1654. doi: 10.1104/pp.124.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky JG, Rost TL, Colon-Carmona A, Doerner P. Early primordium morphogenesis during lateral root initiation in Arabidopsis thaliana. Planta. 2001;214:30–36. doi: 10.1007/s004250100598. [DOI] [PubMed] [Google Scholar]
- Dubrovsky JG, Sauer M, Napsucialy-Mendivil S, Ivanchenko MG, Friml J, Shishkova S, Celenza J, Benkova E. Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proceedings of the National Academy of Sciences, USA. 2008;105:8790–8794. doi: 10.1073/pnas.0712307105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enstone DE, Peterson CA, Hallgren SW. Anatomy of seedling tap roots of loblolly pine (Pinus taeda L.) Trees. 2001;15:98–111. [Google Scholar]
- Eshel A, Waisel Y. Multiform and multifunction of various constituents of one root system. In: Waisel Y, Eshel A, Kafkafi U, editors. Plant roots: the hidden half. New York, NY: Marcel Dekker Inc; 1996. pp. 175–192. [Google Scholar]
- Finkelstein RR. Mutations at two new Arabidopsis ABA response loci are similar to the abi3 mutations. The Plant Journal. 1994;5:765–771. [Google Scholar]
- Fitter A, Williamson L, Linkohr B, Leyser O. Root system architecture determines fitness in an Arabidopsis mutant in competition for immobile phosphate ions but not for nitrate ions. Proceedings of the Royal Society London B. 2002;269:2017–2022. doi: 10.1098/rspb.2002.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Benkova E, Blilou I, et al. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell. 2002;108:661–673. doi: 10.1016/s0092-8674(02)00656-6. [DOI] [PubMed] [Google Scholar]
- Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, McCourt P. Regulation of abscisic acid signalling by the ethylene response pathway in Arabidopsis. The Plant Cell. 2000;12:1117–1126. doi: 10.1105/tpc.12.7.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, Muskett PR, Chuang HW, Parker JE. Arabidopsis SGT1b is required for SCF (TIR1)-mediated auxin response. The Plant Cell. 2003;15:1310–1319. doi: 10.1105/tpc.010884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grime JP, Crick JC, Rincon JE. The ecological significance of plasticity. In: Jennings DH, Trewavas AJ, editors. Plasticity in plants. Cambridge: Company of Biologists, Cambridge University Press; 1986. pp. 5–29. [Google Scholar]
- Gruntman M, Novoplansky A. Physiologically mediated self/non-self discrimination in roots. Proceedings of the National Academy of Sciences, USA. 2004;101:3863–3867. doi: 10.1073/pnas.0306604101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman P, Ecker JR. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. The Plant Cell. 1990;2:513–523. doi: 10.1105/tpc.2.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XJ, Mu RL, Cao WH, Zhang ZG, Zhang JS, Chen SY. AtNAC2, a transcription factor downstream of ethylene and auxin signalling pathways, is involved in salt stress response and lateral root development. The Plant Journal. 2005;44:903–916. doi: 10.1111/j.1365-313X.2005.02575.x. [DOI] [PubMed] [Google Scholar]
- Hodge A, Robinson D, Griffiths BS, Fitter AH. Why plants bother: root proliferation results in increased nitrogen capture from an organic patch when two grasses compete. Plant, Cell and Environment. 1999;22:811–820. [Google Scholar]
- Itoh K, Nakamura Y, Kawata H, Yamada T, Ohta E, Sakata M. Effect of osmotic stress on turgor pressure in mung bean root cells. Plant and Cell Physiology. 1987;28:987–994. [Google Scholar]
- Ivanchenko MG, Muday GK, Dubrovsky JG. Ethylene–auxin interactions regulate lateral root initiation and emergence in Arabidopsis thaliana. The Plant Journal. 2008;55:335–347. doi: 10.1111/j.1365-313X.2008.03528.x. [DOI] [PubMed] [Google Scholar]
- Jansen MAK, van den Noort RE, Tan MYA, Prinsen E, Lagrimini LM, Thorneley RNF. Phenol-oxidizing peroxidases contribute to the protection of plants from ultraviolet radiation stress. Plant Physiology. 2001;126:1012–1023. doi: 10.1104/pp.126.3.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepinski S, Leyser O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature. 2005;435:446–451. doi: 10.1038/nature03542. [DOI] [PubMed] [Google Scholar]
- Kim S, Kang JY, Cho DI, Park JH, Kim SY. ABF2, an ABRE-binding bZIP factor, is an essential component of glucose signalling and its overexpression affects multiple stress tolerance. The Plant Journal. 2004;40:75–87. doi: 10.1111/j.1365-313X.2004.02192.x. [DOI] [PubMed] [Google Scholar]
- Kollmeier M, Felle HH, Horst WJ. Genotypical differences in aluminium resistance of maize are expressed in the distal part of the transition zone. Is reduced basipetal auxin flow involved in inhibition of root elongation by aluminium? Plant Physiology. 2000;122:945–956. doi: 10.1104/pp.122.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth E, Cramer GR, Lauchli A, Epstein E. Effects of NaCl and CaCl2 on cell enlargement and cell production in cotton roots. Plant Physiology. 1986;82:1102–1106. doi: 10.1104/pp.82.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leck MA, Simpson RL, Parker VT. Why seedlings? In: Leck MA, Parker VT, Simpson RL, editors. Seedling ecology and evolution. Cambridge University Press; 2008. pp. 3–11. [Google Scholar]
- Leon-Kloosterziel KM, Gil MA, Ruijs GJ, Jacobsen SE, Olszewski NE, Schwartz SH, Zeevaart JAD, Koornneef M. Isolation and characterization of abscisic acid-deficient Arabidopsis mutants at two new loci. The Plant Journal. 1996;10:655–661. doi: 10.1046/j.1365-313x.1996.10040655.x. [DOI] [PubMed] [Google Scholar]
- Lincoln C, Britton J, Estelle M. Growth and development of the axr1 mutants of Arabidopsis. The Plant Cell. 1990;2:1071–1080. doi: 10.1105/tpc.2.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkohr BI, Williamson LC, Fitter AH, Leyser HMO. Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis. The Plant Journal. 2002;29:751–760. doi: 10.1046/j.1365-313x.2002.01251.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Bucio J, Hernandez-Abreu E, Sanchez-Calderon L, Nieto-Jacobo MF, Simpson J, Herrera-Estrella L. Phosphate availability alters architecture and causes changes in hormone sensitivity in the Arabidopsis root system. Plant Physiology. 2002;129:244–256. doi: 10.1104/pp.010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J. Root architecture and plant productivity. Plant Physiology. 1995;109:7–13. doi: 10.1104/pp.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor DR, Deak KI, Ingram PA, Malamy JE. Root system architecture in Arabidopsis grown in culture is regulated by sucrose uptake in the aerial tissues. The Plant Cell. 2008;20:2643–2660. doi: 10.1105/tpc.107.055475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy J. Intrinsic and environmental response pathways that regulate root system architecture. Plant, Cell and Environment. 2005;28:67–77. doi: 10.1111/j.1365-3040.2005.01306.x. [DOI] [PubMed] [Google Scholar]
- Malamy JE, Benfey P. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development. 1997;124:33–44. doi: 10.1242/dev.124.1.33. [DOI] [PubMed] [Google Scholar]
- Malamy JE, Ryan KS. Environmental regulation of lateral root initiation in Arabidopsis. Plant Physiology. 2001;127:899–909. [PMC free article] [PubMed] [Google Scholar]
- Marchant A, Kargul J, May ST, Muller P, Delbarre A, Perrot-Rechenmann C, Bennett M. AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO Journal. 1999;18:2066–2073. doi: 10.1093/emboj/18.8.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant A, Bhalerao R, Casimiro I, Eklof J, Casero PJ, Bennett M, Sandberg G. AUX1 promotes lateral formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. The Plant Cell. 2002;14:589–597. doi: 10.1105/tpc.010354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi S, Ivanchenko MG, Muday GK. Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana. The Plant Journal. 2008;55:175–187. doi: 10.1111/j.1365-313X.2008.03495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normanly J, Grisafi P, Fink GR, Bartel B. Arabidopsis mutants resistant to the auxin effects of indole-3-acetonitrile are defective in the nitrilase encoded by the NIT1 gene. The Plant Cell. 1997;9:1781–1790. doi: 10.1105/tpc.9.10.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okushima Y, Fukaki H, Onoda M, Theologis A, Tasakaa M. ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. The Plant Cell. 2007;19:118–130. doi: 10.1105/tpc.106.047761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak T, Potters G, Caubergs R, Jansen MAK. Complementary interactions between oxidative stress and auxins control plant growth responses at plant, organ, and cellular level. Journal of Experimental Botany. 2005;56:1991–2001. doi: 10.1093/jxb/eri196. [DOI] [PubMed] [Google Scholar]
- Perez-Torres CA, Lopez-Bucio L, Cruz-Ramırez A, Ibarra-Laclette E, Dharmasiri S, Estelle M, Herrera-Estrella L. Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. The Plant Cell. 2008;20:3258–3272. doi: 10.1105/tpc.108.058719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett FB, Wilson AK, Estelle M. The aux1 mutation of Arabidopsis confers both auxin and ethylene resistance. Planta. 1990;194:439–442. doi: 10.1104/pp.94.3.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignocchi C, Foyer CH. Apoplastic ascorbate metabolism and its role in the regulation of cell signalling. Current Opinion in Plant Biology. 2003;6:379–389. doi: 10.1016/s1369-5266(03)00069-4. [DOI] [PubMed] [Google Scholar]
- Potters G, De Gara L, Asard H, Horemans N. Ascorbate and glutathione: guardians of the cell cycle, partners in crime? Plant Physiology and Biochemistry. 2002;40:537–548. [Google Scholar]
- Potters G, Pasternak TP, Guisez Y, Jansen MAK. Stress-induced morphogenic responses: growing out of trouble? Trends in Plant Science. 2007;12:98–105. doi: 10.1016/j.tplants.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Potters G, Pasternak TP, Guisez Y, Jansen MAK. Different stresses, similar morphogenic responses: integrating a plethora of pathways. Plant, Cell and Environment. 2009;32:158–169. doi: 10.1111/j.1365-3040.2008.01908.x. [DOI] [PubMed] [Google Scholar]
- Reed RC, Brady SR, Muday GK. Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiology. 1998;118:1369–1378. doi: 10.1104/pp.118.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remans T, Nacry P, Pervent M, Filleur S, Diatloff E, Mounier E, Tillard P, Forde BG, Gojon A. The Arabidopsis NRT1.1 transporter participates in the signalling pathway triggering root colonization of nitrate-rich patches. Proceedings of the National Academy of Sciences, USA. 2006;103:19206–19211. doi: 10.1073/pnas.0605275103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Gray WM, Hobbie L, Turner J, Estelle M. The TIR1 protein of Arabidopsis functions in auxin response and is related to human SKP2 and yeast Grr1p. Genes and Development. 1998;12:198–207. doi: 10.1101/gad.12.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D. Resource capture by localised root proliferation: why do plants bother? Annals of Botany. 1996;77:179–186. [Google Scholar]
- Robinson D, Hodge A, Griffiths BS, Fitter AH. Plant root proliferation in nitrogen-rich patches confers competitive advantage. Proceedings of the Royal Society London B. 1999;265:431–435. [Google Scholar]
- Sagi M, Dovrat A, Kipnis T, Lips SH. Ionic balance and the production of biomass and organic nitrogen as affected by salinity and N source in annual ryegrass (Lolium multiflorum Lam) Journal of Plant Nutrition. 1997;20:1291–1316. [Google Scholar]
- Schwartz SH, Léon-Kloosterziel KM, Koornneef M, Zeevaart JAD. Biochemical characterization of the aba2 and aba3 mutants in Arabidopsis thaliana. Plant Physiology. 1997;114:161–166. doi: 10.1104/pp.114.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki M, Ishida J, Narusaka M, et al. Monitoring the expression pattern of around 7000 Arabidopsis genes under ABA treatments using a full length cDNA microarray. Functional and Integrative Genomics. 2002;2:282–291. doi: 10.1007/s10142-002-0070-6. [DOI] [PubMed] [Google Scholar]
- Sharp RE, Silk WK, Hsiao TC. Growth of the maize primary root at low water potentials. Plant Physiology. 1988;87:50–57. doi: 10.1104/pp.87.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signora L, De Smet I, Foyer CH, Zhang H. ABA plays a central role in mediating the regulatory effects of nitrate on root branching in Arabidopsis. The Plant Journal. 2001;28:655–662. doi: 10.1046/j.1365-313x.2001.01185.x. [DOI] [PubMed] [Google Scholar]
- Soderman EM, Brocard IM, Lynch TJ, Finkelstein RR. Regulation and function of the Arabidopsis ABA-insensitive4 gene in seed and abscisic acid response signalling networks. Plant Physiology. 2000;124:1752–1765. doi: 10.1104/pp.124.4.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R, Friml J, Marchant A, Ljung K, Sandberg G, Palme K, Bennett M. Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes and Development. 2001;15:2648–2653. doi: 10.1101/gad.210501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tester M, Davenport R. Na+ tolerance and Na+ transport in higher plants. Annals of Botany. 2003;91:503–527. doi: 10.1093/aob/mcg058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. The Plant Cell. 1997;9:1963–1971. doi: 10.1105/tpc.9.11.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wheele CM, Spollen WG, Sharp RE, Baskin TI. Growth of Arabidopsis thaliana seedlings under water deficit studied by control of water potential in nutrient agar media. Journal of Experimental Botany. 2000;51:1555–1562. doi: 10.1093/jexbot/51.350.1555. [DOI] [PubMed] [Google Scholar]
- West G, Inzé D, Beemster GT. Cell cycle modulation in the response of the primary root of Arabidopsis to salt stress. Plant Physiology. 2004;135:1050–1058. doi: 10.1104/pp.104.040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AK, Pickett FB, Turner JC, Estelle M. A dominant mutation in Arabidopsis confers resistance to auxin, ethylene and abscisic acid. Molecular and General Genetics. 1990;222:377–383. doi: 10.1007/BF00633843. [DOI] [PubMed] [Google Scholar]
- Woodward AW, Bartels B. Auxin: regulation, action, and interaction. Annals of Botany. 2005;95:707–735. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L, Wang RG, Mao G, Koczan JM. Identification of drought tolerance determinants by genetic analysis of root response to drought stress and abscisic acid. Plant Physiology. 2006;142:1065–1074. doi: 10.1104/pp.106.084632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Hammes UZ, Taylor CG, Schachtman DP, Nielsen E. High-affinity auxin transport by the AUX1 influx carrier protein. Current Biology. 2006;16:1–5. doi: 10.1016/j.cub.2006.04.029. [DOI] [PubMed] [Google Scholar]
- Zeevaart JAD, Creelman RA. Metabolism and physiology of abscisic acid. Annual Review of Plant Physiology and Plant Molecular Biology. 1988;39:439–473. [Google Scholar]
- Zhang H, Forde BG. An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science. 1998;279:407–409. doi: 10.1126/science.279.5349.407. [DOI] [PubMed] [Google Scholar]
- Zhang HM, Jennings A, Barlow PW, Forde BG. Dual pathways for regulation of root branching by nitrate. Proceedings of the National Academy of Sciences, USA. 1999;96:6529–6534. doi: 10.1073/pnas.96.11.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Jennings AJ, Forde BG. Regulation of Arabidopsis root development by nitrate availability. Journal of Experimental Botany. 2000;51:51–59. [PubMed] [Google Scholar]
- Zhang H, Rong H, Pilbeam D. Signalling mechanisms underlying the morphological responses of the root system to nitrogen in Arabidopsis thaliana. Journal of Experimental Botany. 2007;58:2329–2338. doi: 10.1093/jxb/erm114. [DOI] [PubMed] [Google Scholar]