Abstract
Loquat (Eriobotrya japonica Lindl.) is a subtropical fruit, with some cultivars such as ‘Luoyangqing’ (LYQ) susceptible to chilling injury (CI), while others such as ‘Baisha’ (BS) are resistant. Although loquats are non-climacteric, modulation of ethylene has an effect on ripening-related post-harvest CI. Therefore the role of ethylene signalling in the development of CI was investigated in fruit of both the LYQ and BS cultivars. Three ethylene receptor genes, one CTR1-like gene, and one EIN3-like gene were isolated and characterized in ripening fruit. All of these genes were expressed differentially within and between fruit of the two cultivars. Transcripts either declined over fruit development (EjERS1a in both cultivars and EjEIL1 in LYQ) or showed an increase in the middle stages of fruit development before declining (EjETR1, EjERS1b, and EjCTR1 in both cultivars and EjEIL1 in BS). The main cultivar differences were in levels rather than in patterns of expression during post-harvest storage. EjETR1, EjCTR1, and EjEIL1 genes showed increased expression in response to low temperature and this was particularly notable for EjETR1, and EjEIL1 during CI development in LYQ fruit. The genes were also differentially responsive to ethylene treatment, 1-methycyclopropene (1-MCP) and low temperature conditioning, confirming a role for ethylene in regulation of CI in loquat fruit.
Keywords: Chilling injury, ethylene signal transduction, loquat, low temperature conditioning, non-climacteric fruit, 1-MCP
Introduction
Ethylene signalling in ripening fruit is largely investigated in climacteric fruit such as tomato (Alexander and Grierson, 2002; Barry and Giovannoni, 2007), apple (Dal Cin et al., 2005; Tatsuki et al., 2007; Wang et al., 2007; Wiersma et al., 2007), and kiwifruit (Yin et al., 2008, 2009). However, much less is known about the pathway in non-climacteric fruit and such studies are limited to the ethylene receptor level. Katz et al. (2004) reported the transition from system II-like ethylene in young fruitlets to system I behaviour during the development of citrus fruit where CsETR1 might be involved in the production of system I ethylene and CsERS1 modulates the differential sensitivity to ethylene in fruitlets versus mature fruit. In strawberry, three ethylene receptors showed increased expression during fruit ripening and there was a concomitant increase in ethylene receptors such as FaETR2 (Trainotti et al., 2005). The production of small amounts of ethylene was proposed to be sufficient to trigger ripening-related physiological responses (Trainotti et al., 2005). There has been little further investigation of the ethylene signal transduction pathway, especially the downstream components, in non-climacteric fruit.
Another area where the role of ethylene and its signalling pathway dynamics are unclear is the response of fruit to low temperature. The effects of ethylene on the development of chilling injury (CI) symptoms are different depending on the fruit species. Exogenous ethylene accelerated CI symptoms in avocado (Pesis et al., 2002) and plum (Candan et al., 2008), while it alleviated the disorders caused by CI in nectarine during 0 °C storage (Zhou et al., 2001). Similarly, the effects of 1-methylcyclopropene (1-MCP), an ethylene action inhibitor, on the development of CI vary with fruit. 1-MCP treatment can reduce low temperature disorders in persimmon (Salvador et al., 2004), mandarin (Salvador et al., 2006), and plum (Candan et al., 2008) while it accelerates the appearance of symptoms in banana (Jiang et al., 2004), peach (Girardi et al., 2005), and nectarine (Dong et al., 2001). These results suggest that ethylene is involved in the incidence and development of fruit CI, although the reasons for the inconsistencies stated above, and the mechanisms, largely remain unknown.
Results on ethylene signal transduction elements in response to low temperature in fruit have been reported in the past few years. PcETR1a in winter pear (Pyrus communis L.) (El-Sharkawy et al., 2003), PpCTR1 and PpEIN2 genes in stony hard peach (Begheldo et al., 2008), and AdERS1a, AdETR2, AdETR3, AdCTR1, and four AdEIL genes in kiwifruit (Yin et al., 2009), all showed increased expression patterns in response to low temperature. But these patterns in winter pear and stony hard peach were most probably associated with fruit ripening. In kiwifruit, ethylene receptor genes AdERS1b and AdETR1, in contrast to AdERS1a, AdETR2, and AdETR3, were suppressed by low temperature (Yin et al., 2009).
Loquat (Eriobotrya japonica Lindl.) is a non-climacteric fruit belonging to the Rosaceae family. It can be divided into two categories of cultivar, white-fleshed and red- or orange-fleshed (Zhou et al., 2007). At harvest, the two types of fruit vary in properties such as fruit size and shape, and sugar, acid, and carotenoid contents (Zhou et al., 2007). It has been shown that the red-fleshed ‘Luoyangqing’ (LYQ) fruit developed lignification not only as a ripening characteristic at 20 °C, but also as a CI symptom during 0 °C storage (Cai et al., 2006a, b, c, d). Under both post-harvest conditions, increase in fruit firmness in relation to lignin accumulation was significantly correlated and post-harvest treatments such as low temperature conditioning (LTC) and 1-MCP can alleviate such lignification while ethylene accelerates it (Cai et al., 2006a, c, d). However, no significant lignification was observed in ripening white-fleshed ‘Baisha’ (BS) fruit nor as a CI symptom of such fruit; and the fruit softened continuously at 20 °C or maintained firmness at 0 °C (Yang et al., 2008). By studying the enzymes and expression of genes associated with lignification in both LYQ and BS fruit, the enzyme activities of cinnamyl alcohol dehydrogenase (CAD) and peroxidase (POD) and the transcript expression levels of EjCAD1 and EjPOD were most associated temporally with lignification of LYQ flesh and their expression levels were all low in BS flesh (Shan et al., 2008). These partially explained the different texture changes and ripening patterns for LYQ and BS fruit at 20 °C.
As a non-climacteric fruit, the ethylene release from loquats is at a low level during post-harvest ripening. However, the significant effects shown by ethylene or 1-MCP treatment on ripening and CI development of LYQ fruit suggested an effective involvement of the ethylene signal transduction pathway in fruit of this cultivar. LYQ and BS fruit differed significantly in their post-harvest behaviours, and whether this indicates that ethylene acts differentially on fruit of these two cultivars is yet to be determined. Since no evident CI symptoms have been found in BS fruit, a comparison of fruit from these two cultivars might serve as a means to characterize ethylene signal transduction involvement in the CI of loquat fruit.
In this study, three ethylene receptor genes, one CTR1-like gene, and one EIN3-like gene were cloned from loquat flesh tissue. The transcript abundance of the five genes was estimated in different tissues and at several fruit development stages as well as during post-harvest ripening in LYQ and BS cultivars. Treatments of LTC and 1-MCP were used to alleviate the CI in LYQ during 0 °C storage and the responses of these genes to such treatments were studied. The possible role played by ethylene signal transduction elements in CI development of loquat fruit is discussed.
Materials and methods
Plant materials and treatments
Fruit of two loquat (Eriobotrya japonica Lindl.) cultivars, ‘Luoyangqing’ (LYQ) and ‘Baisha’ (BS, also described in Zhou et al., 2007, as ‘Luqiaobaisha’), were picked at commercial maturity, as described by Zhou et al. (2007) from an orchard in Luqiao, Zhejiang, China, and transported to the laboratory on the day of harvest. For loquat fruit, the maturity was assessed by colour (Morton, 1987). In our experiment, harvest was based on colour, with the fruit picked showing no green on the skin. To ensure consistent maturity, fruit with a colour index of 4–5 for BS and 10–11 for LYQ were selected. The colour was measured with MiniScan XE plus (Hunter Associates Laboratory Inc.) and the colour index was calculated according to Jimenez-Cuesta et al. (1981).
Fruit were screened and selected for uniform size and maturity and the absence of disease and mechanical damage. Two lots of each cultivar were placed at 20 °C and 0 °C, respectively, and stored for 8 d.
In two separate sets of experiments, LTC and 1-MCP treatments plus 0 °C storage, were designed to alleviate CI of LYQ loquat. Both treatments were carried out according to Cai et al. (2006a, d). LTC treatment: fruit were stored 6 d at 5 °C prior to 0 °C storage, and control fruit were stored at 0 °C directly. 1-MCP treatment plus 0 °C storage: fruit were treated with 5 μl l−1 1-MCP for 12 h at 20 °C in air-tight containers, and then transferred to 0 °C storage, and control fruit were stored 20 °C with no treatment in air-tight containers, and then transferred to 0 °C.
In order to study the response of the ethylene signal transduction components to ethylene, a separate experiment was done where fruit were treated with and without 100 μl l−1 ethylene gas in air-tight containers for 12 h at 20 °C, and then all were transferred to 20 °C storage. The controls were as above.
During all the experiments, three replicates of five fruit were sampled at each sampling time, and the fruit flesh (excluding skin) was cut into small pieces and frozen in liquid nitrogen immediately, then stored at –80 °C for further use.
For analysis of different plant tissues and fruit at different developmental stages, young leaves, roots, stems, petals at anthesis, and young fruit of the two cultivars were collected. Fruit at different developmental stages were taken every 4 weeks, from 4 weeks after anthesis (WAA) until the time of commercial harvest (16 WAA). All tissue samples were immediately frozen in liquid nitrogen and stored at –80 °C for further use.
Fruit firmness
Nine individual fruit were used each time to determine fruit firmness, using a TA-XT plus Texture Analyser (Stable Micro Systems, UK) with a 5 mm diameter probe, a penetration depth of 4 mm, and a penetration rate of 1 mm s−1. Measurements were made on two sides of each fruit after removal of a small piece of peel, and the data were expressed in newtons (N).
Lignin determination and histochemical tests
Lignin extraction and determination was performed according to the method of Shan et al. (2008). Frozen tissue powder was homogenized in 5 ml washing buffer (100 mM K2HPO4/KH2PO4, pH 7.8, 0.5% Triton X-100, 0.5% PVP), slowly stirred for 30 min at room temperature, and centrifuged. The pellet was resuspended and washed twice in washing buffer as above. Then the pellet was washed four times in 100% methanol and dried at 80 °C for 12 h. Ten milligrams of dried residue was placed into a 10 ml screw-cap tube, and 1 ml of 2 M HCl and 0.1 ml of thioglycolic acid were added. The sample was heated at 100 °C for 8 h, cooled on ice and centrifuged at 15 000 g for 20 min at 4 °C. The pellet was washed with distilled water, and resuspended in 2 ml 1 M NaOH. After agitating gently at room temperature for 18 h, the solution was centrifuged at 15 000 g for 20 min, and 0.5 ml of the supernatant was transferred to a test tube. One hundred microlitres of concentrated HCl was added to the test tube and the lignin thioglycolic acid was allowed to precipitate at 4 °C for 4 h. After centrifugation at 15 000 g for 20 min, the pellet was dissolved in 1 ml of 1 M NaOH. The absorbance was measured against a NaOH blank at 280 nm. Data were expressed on a fresh weight basis, and all measurements were done in triplicate.
Histochemical tests were carried out as described in Shan et al. (2008). Each fruit was cut into two halves vertically, sections near the calyx end were taken and one quarter of the cross-sections of 2 mm thickness was used for staining. Two quarters were taken from each fruit, and a total of three fruit were analysed using phloroglucinol/hydrochloric acid staining and a Leica MZ95 stereomicroscope.
Gene cloning and amino acid sequence analysis
Total RNA was extracted from different loquat tissues following our previously published protocol (Shan et al., 2008). The degenerate primers isolating ETRs, CTRs, and EILs were described as follows: ETRUP, 5′-GAGACGGG[A/C/T]AG[G/A]CATGT[A/C/G/T]AG[G/A]ATG-3′, ETRDP, 5′-CATGGG[A/C]GTTCTCATTTCATG[G/A]TTCAT-3′; CTRUP, 5′-ATGGAGCAAGA[C/T]TT[C/T]CATGCTGAGCG-3′, CTRDP, 5′-ATCTCG[A/C]T[G/T]AACTTC[A/C/G/T]GGTGCCATCC-3′; EILUP, 5′-T[G/T]GAGA[G/A]GAGGATGTGGAG[A/G]GAC-3′, EILDP, 5′-ATAAT[A/G]GCAAGCCA[A/T/G]GT[A/T]GCAC-3′, designed according to known gene sequences of other plants from the National Center for Biotechnology Information (NCBI) database. The 3′-untranslated regions (UTR) of candidate sequences were amplified using the SMART™ RACE cDNA amplification Kit (Clontech). PCR products were cloned into pMD18-T vector (Takara). The positive clones were analysed by PCR before being sequenced by Invitrogen (Shanghai, China). Finally, one ETR1 gene, two ERS1-type genes, one CTR1-like gene, and one EIN3-like gene were obtained from 3′-RACE. Comparisons of nucleotide and deduced amino acid sequences were carried out by the basic local alignment search tool (BLAST) program online (http://www.ncbi.nlm.nih.gov/BLAST). Protein alignments were analysed with ClustalX (version 1.81) and phylogenetic trees were constructed by MEGA (version 4.0) with default parameters (Tamura et al., 2007).
Real-time PCR analysis
Four micrograms of total RNA was pretreated with DNase I (Fermentas) to remove contaminating genomic DNA. The concentration of total RNA was measured using a spectrophotometer. First-strand cDNA was synthesized by Revert Aid™ First Strand cDNA Synthesis kit (Fermentas) using 2.0 μg of treated total RNA. The cDNA was then diluted 10-fold with DEPC treated water, and 2 μl of the diluted cDNA was used as a template for real-time PCR analysis. PCR reactions were performed in a total volume of 20 μl, including 1 μl of each primer (10 μM), and 10 μl of 2× iQ SYBR Green Supermix (Bio-Rad) on an iCycler iQ real-time PCR instrument (Bio-Rad). The real-time PCR program was initiated with a preliminary step of 5 min at 94 °C, followed by 45 cycles of 94 °C for 10 s, and 60 °C for 30 s. No-template controls for each primer pair were included in each run.
The oligonucleotide primers for real-time PCR analysis were designed on the basis of the 3′-UTR of individual genes, using Primer3 on line (http://frodo.wi.mit.edu). The length of all real-time PCR products ranged from 150 bp to 200 bp. The gene specificity of these primers was tested by the method described by Zhang et al. (2006). The primers for real-time PCR are shown in Table 1. The EjACT gene was used as an internal control to normalize small differences in template amounts with the forward primer 5′-GGATTTGCTGGTGATGATGC-3′ and the reverse primer 5′-CCGTGCTCAATGGGATACTT-3′ (Shan et al., 2008). Expression levels produced by real-time PCR were expressed as a ratio relative to the fruit harvest time point, which was set to 1.
Table 1.
Oligonucleotide primers for real-time PCR analysis
| Gene | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) |
| EjETR1 | ACCGGAACATTACTCCAGCA | GCTCTTCACAGGCAAGTTCC |
| EjERS1a | GAATGCCAATGGTCAGAGTC | CGGCATCGTTCAGTTTTACA |
| EjERS1b | GAATGCCAGTGGACACACAA | TTCGGACGAGGATTTAACGC |
| EjCTR1 | AATTGATGTGTGGCGAGGAT | ACAATGGCTCTGCAACCAG |
| EjEIL1 | GGGGTGTGTGTGTGTGTTGT | AGTACGGAACAACGGATTGG |
Statistical analysis
A completely randomized design was performed in all experiments. Figures were drawn by Origin 7.0 (Microcal Software Inc., Northampton, MA, USA), and LSDs (α=0.05) were calculated for mean separations using the Data Processing System (DPS, version 3.01, Zhejiang University, Hangzhou, China).
Results
Gene isolation and sequence analysis
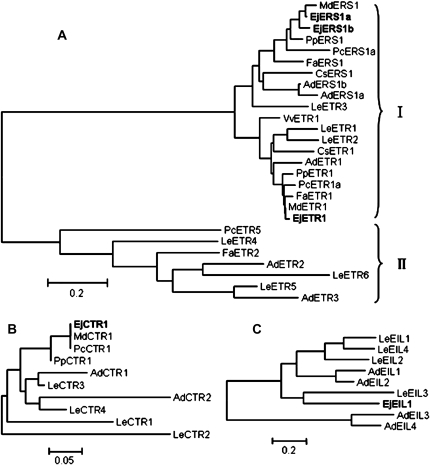
Twenty individual recombinant plasmids were sequenced and three ethylene receptor gene sequences were obtained. For cloning of a CTR1-like gene, cDNA clones from five different recombinant plasmids gave the same sequence. Fifteen different recombinant plasmids were sequenced and provided the same sequence as an EIN3-like gene. These five genes were designated as EjETR1 (FJ624867), EjERS1a (FJ624871), EjERS1b (FJ624870), EjCTR1 (FJ624869), and EjEIL1 (FJ624868), respectively, based on sequence alignment. Phylogenetic analysis of deduced amino acid sequences suggested that the three ethylene receptors from loquat were all clustered into the ETR1 subfamily (Fig. 1A). EjERS1a had 89% identity with EjERS1b in alignable regions of the deduced amino acid sequences. Loquat is a rosaceous plant, and the ethylene receptors had higher homologies to those of rosaceous plants than to those of other fruit species. EjETR1 and apple MdETR1a (AAC31123) had 99% amino acid identity, while EjERS1a and EjERS1b showed high sequence identities with MdERS1 (BAE97296) at 96% and 88%, respectively (Fig. 1A). EjCTR1 was close to MdCTR1 (AAV85951) with 99% identity (Fig. 1B), and EjEIL1 was 60% identical to LeEIL3 (AAK58859) (Fig. 1C).
Fig. 1.
Phylogenetic analysis of ETRs (A), CTRs (B), and EILs (C) from loquat and other fruit. The deduced amino acid sequences were analysed using ClustalX (version 1.81), and the phylogenetic trees were conducted with MEGA (version 4.0) software using a Neighbor–Joining test and default parameters. Loquat sequences are indicated in bold type. I represents the ETR1 subfamily, and II represents the ETR2 subfamily. The accession numbers of amino acid sequences used to build trees were as follows: LeETR1 (AAA85479), LeETR2 (AAC02214), LeETR3 (AAC49124), LeETR4 (AAU34076), LeETR5 (AAD31397), LeETR6 (AAL86614), LeCTR1 (AAL87456), LeCTR2 (CAA06334), LeCTR3 (AAR89820), LeCTR4 (AAR89822), LeEIL1 (AAK58857), LeEIL2 (AAK58858), LeEIL3 (AAK58859), LeEIL4 (BAC99307), MdETR1 (AAC31123), MdERS1 (BAE97296), MdCTR1(AAV85951), PpETR1 (AAM73756), PpERS1 (AAL30116), PpCTR1(AAY21209), PcETR1a (AAL66191), PcETR5 (AAL66193), PcERS1a (AAL66197), PcCTR1 (AAL66190), FaERS1 (CAC48385), FaETR1 (CAC48384), FaETR2 (CAC48386), VvETR1 (CAN73257), CsETR1 (CAB76929), CsERS1 (AAC99435), AdETR1 (ABY28264), AdETR2 (ABY28265), AdETR3 (ABY28266), AdERS1a (ABY28262), AdERS1b (ABY28263), AdCTR1 (ABY28267), AdCTR2 (ABY28268), AdEIL1 (ABY28269), AdEIL2 (ACJ70675), AdEIL3 (ACJ70676), AdEIL4 (ACJ70677).
Tissue specificity and expression patterns during fruit development
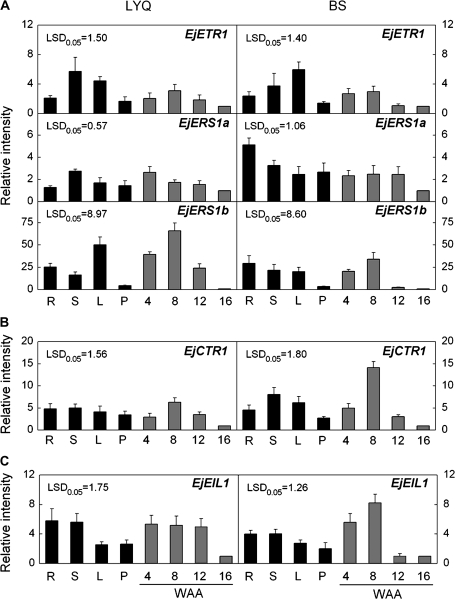
Real-time PCR was used to test the tissue specificity and expression profiles of the five genes during fruit development in both LYQ and BS cultivars. Results showed that none of the five genes were fruit specific and their expression patterns were slightly different in root, stem, leaf, and petal. Transcript levels of EjETR1 were relatively high in stem and leaf, while EjERS1b showed low expression level in petals and mature fruit of both cultivars (Fig. 2A). Expression of EjERS1a varied with different tissues and cultivars. In LYQ, high expression of EjERS1a was found in the stem, while its transcript level appeared relatively high in the root of BS loquat (Fig. 2A). Compared with the mature fruit, EjCTR1 showed relatively higher transcript levels in the root, stem, leaf, and petal of both cultivars (Fig. 2B). EjEIL1 displayed similar expression patterns in different tissues of both cultivars (Fig. 2C).
Fig. 2.
Expression of different ethylene signal transduction elements in various tissues (black columns) and during loquat fruit development stages (grey columns). The letters and numbers represent: R, root; S, stem; L, leaf; P, petal; 4, four weeks after anthesis (WAA); 8, eight WAA; 12, twelve WAA; 16, sixteen WAA (the harvest time point). Real-time PCR was used to analyse the expression patterns of EjETRs (A), EjCTR1 (B), and EjEIL1 (C). Each value represents the mean ±standard error of three replicates. Expression levels were expressed as a ratio relative to the harvest time point (16 WAA), which was set at 1.
During fruit development, expression patterns of three ethylene receptor genes could be divided into two types: (i) transcript levels of EjETR1 and EjERS1b in both cultivars peaked at 8 WAA followed by a decline; (ii) transcript levels of EjERS1a in two cultivars remained relatively high for 12 WAA, followed by a decrease in the expression levels at harvest (Fig. 2A). In both cultivars, EjCTR1 expression patterns were similar to those of EjETR1 and EjERS1b (Fig. 2B). Transcript levels of EjEIL1 during fruit development dropped sharply 4 weeks earlier in BS than in LYQ fruit (Fig. 2C).
Fruit firmness and lignin content
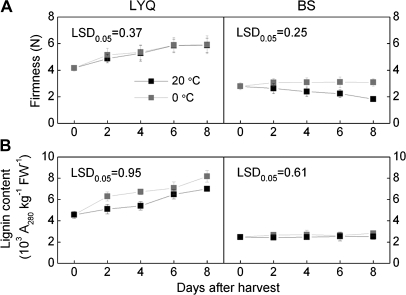
During post-harvest ripening, LYQ and BS fruit underwent different firmness changes (Fig. 3A). At 20 °C, fruit firmness increased steadily by 41% from 4.2 N to 5.9 N in LYQ fruit after 8 d storage, whereas it decreased by 53% from 2.8 N to 1.8 N in BS fruit. During the same storage period at 0 °C, there also was a 43% increase in LYQ fruit firmness (eventually 5.9 N), while firmness remained constant in BS fruit (from 2.8 N to 3.0 N). Such changes were consistent with changes in lignin contents in both cultivars where 53% and 79% increases were observed in LYQ fruit stored at 20 °C and 0 °C, respectively, while no significant changes were observed in BS fruit under both storage conditions (Fig. 3B). Furthermore, histochemical analysis confirmed that LYQ flesh contained more lignin-staining cells (from 168 to 437) compared with BS fruit (from 20 to 25) during 8 d storage at 0 °C.
Fig. 3.
Changes in firmness (A) and lignin content (B) of LYQ and BS loquat fruit during storage at 20 °C or 0 °C. Each value represents the mean ±standard error of nine (firmness) or three (lignin content) replicates.
Gene expression patterns during post-harvest ripening
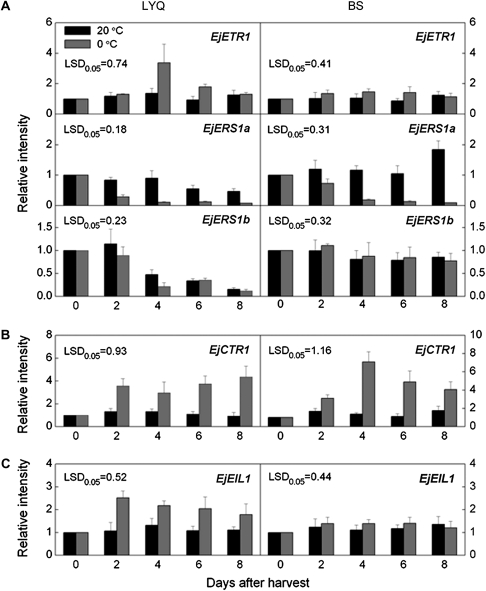
The expression profiles of the five genes were studied further in the two cultivars during storage at two temperatures. Divergent expression patterns of the three receptor genes were observed in the two cultivars at 20 °C and 0 °C (Fig. 4A). Only EjETR1 was strongly induced by low temperature and peaked at 4 d in LYQ fruit, but no significant difference was observed in the transcript accumulation of EjETR1 in BS fruit at either storage temperature. EjERS1a was down-regulated with duration of low temperature storage in both cultivars, but it exhibited different expression patterns at 20 °C storage. In LYQ fruit, the expression levels of EjERS1a decreased slightly while they maintained constant levels and then increased at day 8 in BS fruit held at 20 °C. EjERS1b expression in BS fruit was fairly constant throughout storage at both temperatures, whereas in LYQ fruit its expression declined sharply after 2 d at both temperatures.
Fig. 4.
Expression of different ethylene signal transduction elements in LYQ and BS fruit stored at 20 °C (black columns) and 0 °C (grey columns). Real-time PCR was used to analyse EjETRs (A), EjCTR1 (B), and EjEIL1 (C) expression patterns. Each value represents the mean ±standard error of three replicates. Expression levels were expressed as a ratio relative to the harvest time point (0 d), which was set at 1.
EjCTR1 had similar expression patterns in both cultivars under both storage conditions (Fig. 4B). During 20 °C storage, transcript levels of EjCTR1 in LYQ and BS fruit displayed no significant changes, but expression was strongly induced by low temperature, and the highest transcript abundances were approximately 4-fold and 7-fold greater than those at harvest in LYQ and BS fruit, respectively. EjEIL1 expression was very similar and constant in LYQ and BS fruit at 20 °C. By contrast, its expression was induced after 2 d storage at 0 °C in LYQ but not in BS fruit (Fig. 4C).
EjETR1 and EjEIL1 were the only genes uniquely up-regulated in association with CI (which was only observed in LYQ fruit), when the two cultivars were compared (Fig. 4). Therefore, these two genes were chosen to study further expression in LTC or 1-MCP treated LYQ fruit in order to confirm a close association with the development of CI in this cultivar.
Expression patterns of EjETR1 and EjEIL1 with LTC, 1-MCP, and ethylene treatments
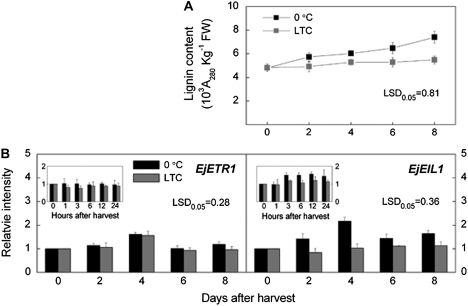
No significant changes in lignin content were found in LTC-treated fruit during the 8 d storage period, while lignin content increased by 53% in the control fruit held at 0 °C (Fig. 5A). LTC treatment strongly inhibited the expression of EjEIL1 while it had little effect on the expression of EjETR1 (Fig. 5B). A more detailed time-course of gene expression showed that the LTC treatment significantly reduced the low temperature-induced up-regulation of EjEIL1 observed in controls 3 h after storage at 0 °C (Fig. 5B).
Fig. 5.
Changes in lignin content (A) and expression patterns of EjETR1 and EjEIL1 (B) in LYQ fruit stored at LTC (stored at 5 °C for 6 d, then transferred to 0 °C, grey columns) and 0 °C (control fruit, black columns). Each value represents the mean ±standard error of three replicates. Expression levels were expressed as a ratio relative to the harvest time point (0 d), which was set at 1.
1-MCP treatment significantly inhibited lignification in chilling-injured LYQ fruit during the 8 d storage period at 0 °C. The lignin content increased 75% after 8 d in fruit held at 0 °C, while it only increased by 42% in the 1-MCP treated fruit (Fig. 6A). 1-MCP treatment inhibited the expression of EjETR1 while it had little effect on the expression of EjEIL1 (Fig. 6B).
Fig. 6.
Effect of 1-MCP treatment plus 0 °C (5 μl l−1 1-MCP treated for 12 h at 20 °C, then transferred to 0 °C, grey columns) on the changes in lignin content (A) and expression patterns (B) of EjETR1 and EjEIL1 of LYQ fruit stored at 0 °C. Each value represents the mean ±standard error of three replicates. Expression levels were expressed as a ratio relative to the harvest time point (0 d), which was set at 1.
The effects of ethylene treatment on the expression of EjETR1 and EjEIL1 were in accordance with the results of 1-MCP treatment; EjETR1 expression was induced by a 12 h ethylene treatment (100 μl l−1) while the expression of EjEIL1 was not affected by such a treatment (Fig. 7).
Fig. 7.
Effect of exogenous ethylene treatment (100 μl l−1 ethylene treated for 12 h at 20 °C, grey columns) on the expression of EjETR1 and EjEIL1 in LYQ loquat fruit at 20 °C. Each value represents the mean ±standard error of three replicates. Expression levels were expressed as ratio relative to the harvest time point (–12 h), which was set at 1.
Discussion
Five genes from three levels of the ethylene signalling pathway were isolated from ripening loquat fruit. The three receptor genes were all classified into the ETR1 subfamily with relatively high sequence homologies to those from other rosaceous species such as pear (El-Sharkawy et al., 2003), strawberry (Trainotti et al., 2005), apple and peach (Dal Cin et al., 2006; Tatsuki et al., 2007). Similarly, CTR1-like genes from rosaceous fruit were clustered into one group (Fig. 1). To date, EjEIL1 is the first EIN3-like gene that has been cloned from rosaceous fruits, and showed 51–60% identity at the deduced amino acid level with LeEILs from tomato fruit.
None of the five genes were fruit specific, and most genes were expressed at all stages of fruit development, and similarly in both cultivars, except for EjEIL1 where expression levels dropped sharply 4 weeks earlier in BS than in LYQ fruit. Transcripts of EjETR1, EjERS1b, and EjCTR1 peaked at about 8 WAA before decreasing during the late stage of development, while EjERS1a maintained relatively high expression until fruit were harvested. LYQ fruit growth was rapid after 8 WAA, mean flesh weight being 2.20 g at 8 WAA and 9.32 g at 12 WAA. BS fruit showed a similar profile during this period, increasing from 4.05 g to 13.76 g. Generally, about 50% of loquat fruit flesh weight at harvest maturity (16 WAA) is reached at about 12 WAA. The present study showed that genes involved in ethylene signalling had relatively high expression levels from 8 WAA to 12 WAA, suggesting that these genes may be associated with rapid fruit growth. Similar results have been found for AdETR2, AdERS1b, and AdCTRs in developing kiwifruit (Yin et al., 2008). To date, there is little information on transcript levels of EIN3-like genes during non-climacteric fruit development, except that two CmEILs maintained constant expression levels in developing PI161375 melon fruit (non-climacteric phenotype) (Périn et al., 2002). The high expression levels of loquat EjEIL1 prior to harvest is similar to those found with MaEIL3 and MaEIL4 during ‘Grande Naine’ banana (ethylene-sensitive cultivar) fruit development (Mbéguié-A-Mbéguié et al., 2008), while it differed from the pattern of EIN3-like genes in developing kiwifruit (Yin et al., 2008). A positive regulatory role of EIN3-like genes throughout tomato plant development has been suggested from the reduced expression of each LeEIL (Tieman et al., 2001).
There were no notable changes in expression of the three receptor genes during post-harvest ripening, except for some reduction in EjERS1a and EjERS1b expression levels during the first 2 d or 4 d after harvest. Similar results were found in mature citrus fruit (another non-climacteric fruit) where CsETR1 and CsERS1 were constantly expressed during post-harvest ripening (Katz et al., 2004). However, the results from loquat differ significantly from those of other rosaceous fruits; a marked increase in receptor gene expression has been found in climacteric peach (PpERS1; Rasori et al., 2002), pear (PcETR1a, PcERS1a, and PcETR5; El-Sharkawy et al., 2003), apple (MdETR1; Dal Cin et al., 2006), and non-climacteric strawberry (FaERS1; Trainotti et al., 2005) fruit. In addition, the receptors isolated from non-climacteric fruit showed different responses to exogenous ethylene. The EjETR1, EjERS1a, and EjERS1b genes in loquat and FaERS1 in strawberry can be induced by ethylene (Trainotti et al., 2005; Wang et al, unpublished data in loquat), but CsETR1 and CsERS1 in citrus showed little response to ethylene and 1-MCP treatments (Katz et al., 2004). The general expression patterns found in loquat, however, are perhaps what might be expected from a non-climacteric fruit that can still show small stimulatory responses to external ethylene and has ripening retarded by 1-MCP (Cai et al., 2006c). The constant expression level of EjCTR1 during post-harvest storage was similar to that of rosaceous peach PpCTR1 (Dal Cin et al., 2006) while it differed from the up-regulated expression of tomato LeCTR1 (Leclercq et al., 2002; Adams-Phillips et al., 2004) and kiwifruit AdCTR1 (Yin et al., 2008). EjEIL1 showed no response during post-harvest loquat fruit storage in either cultivar. Similar results have been found with AdEIL1 and MaEIL1/3/4 in ripening kiwifruit (Yin et al., 2008) and ‘Grande Naine’ banana (Mbéguié-A-Mbéguié et al., 2008), respectively. However, transcripts of four LeEILs accumulated during fruit ripening (Yokotan et al., 2003), and MaEIL2 was induced by exogenous acetylene treatment during the ripening process (Mbéguié-A-Mbéguié et al., 2008). Tieman et al. (2001) pointed out that LeEIL played a role in determining ethylene sensitivity during fruit ripening.
The low temperature responses of these genes are of some greater interest, particularly where there were cultivar differences. EjETR1, EjCTR1, and EjEIL1 transcripts were all increased by low temperature, but mostly in LYQ fruit. EjERS1a transcripts were decreased in fruit of both cultivars. Such low temperature sensitivity has also been observed in other species, for example, up-regulation of PcETR1a in winter pear, PpCTR1 in stony hard peach, AdETR2, AdETR3, AdCTR1, and four AdEIL genes in kiwifruit, and down-regulation of AdERS1b in kiwifruit have been found during low temperature fruit storage (El-Sharkawy et al., 2003; Begheldo et al., 2008; Yin et al., 2009). Since ethylene has been implicated in the development of CI in loquat (Cai et al., 2006a), these gene responses may be linked to differences in low temperature response between the two cultivars. EjCTR1 showed a similar up-regulated expression pattern in response to low temperatures in both LYQ and BS fruit, suggesting that the differences in chilling response are not associated with this gene or at this level in the signalling pathway. However, the up-regulation of EjETR1 at 4 d and of EjEIL1 from 2 d onwards was unique to LYQ fruit, suggesting the possible involvement of these genes in the chilling response.
The LTC treatment showed a further difference in low temperature response between EjETR1 and EjEIL1. The results of the treatment further indicated a relationship between up-regulation of the latter gene by low temperature and LYQ loquat CI since LTC reduced CI and also reduced EjEIL1 expression levels. By contrast, a strong inhibition of EjETR1 gene expression in 1-MCP treated fruit was not mimicked with the EjEIL1 levels. Regulation of CI development by ethylene may differ in LTC and 1-MCP treatments, i.e. LTC may alleviate CI in LYQ through regulation of EjEIL1 while 1-MCP may alleviate CI through the regulation of EjETR1 at the transcriptional level. The accumulation of lignin was strongly inhibited by LTC and 1-MCP treatments, while the effect was more pronounced with LTC treatment, suggesting that EjEIL1 might be a stronger regulation site for preventing CI in LYQ fruit.
To date, the involvement of ethylene signal transduction in cold stress has mainly reported at the ERF level. For example, CBF/DREB is an ERF subfamily, and has been suggested to have a regulatory role in response to low temperature and other stresses (Nakano et al., 2006; Pino et al., 2008). However, there is little information in terms of a relationship between EIL genes and low temperature. Induced expression of kiwifruit AdEILs suggests a strong response to low temperature (Yin et al., 2009). In the present study, EjEIL1 of LYQ (the low temperature-sensitive loquat cultivar) tended to accumulate during cold storage, and thus could be associated with low temperature or other stress responses. Chilling-induced ethylene has been found in other fruit such as winter pears and stony hard peaches (El-Sharkawy et al., 2003; Begheldo et al., 2008), but was not detected in chilling-injured LYQ fruit. Therefore, how ethylene might regulate the chilling response in LYQ fruit and whether ethylene signal transduction elements are involved in cross-talk with the low temperature stress response remains unclear. Lignin content tends to increase in response to abiotic stresses such as low temperatures (Zheng et al., 2000; Maldonado et al., 2002; Luo et al., 2008). Loquat EjCAD1, showed similar expression patterns as those of EjEIL1 during storage at the low temperature, EjCAD1 being one of the critical lignification genes (Shan et al., 2008). The possible role of EjEIL1 in mediating the low temperature response, such as lignin accumulation in post-harvest loquat, needs further study.
Recent studies in Arabidopsis showed constitutive expression of EIN3/EILs genes, emphasizing the regulation of EIN3/EILs at the protein level. During Arabidopsis development, turnover rates of EIN3/EIL1 could be regulated by EBF1 and EBF2, and possible models have been proposed for the mechanism for ethylene-mediated stabilization of EIN3/EILs (Guo and Ecker, 2003; Binder et al., 2007). However, the present data and previous studies have shown that changes in transcript level of EIN3/EILs also played an important role in plant development and responses to stresses. In tobacco, TEIL (60% identical to Arabidopsis EIN3 at the amino acid level) mRNA abundance was enhanced upon wounding, and expression of PR1 and PR5 was induced in TEIL-overexpressed lines and inhibited in TEIL-suppressed lines (Hibi et al., 2007). Expression of carnation DcEIL3 (54% identical to Arabidopsis EIN3 at the amino acid level) was also influenced by wounding (Iordachescu and Verlinden, 2005). In addition, EIL expression was affected following low temperature treatment in kiwifruit (Yin et al., 2009) and loquat fruit. Achard et al. (2006) reported that EIN3 had a role in salt tolerance by enhancing the DELLA function. Recently, it has also been reported that EILs can bind to a sequence containing the 8 bp TEIL-binding site (tebs), which is found in promoters of many defence-related genes (Kosugi and Ohashi, 2000; Hibi et al., 2007). These results indicated that EIL genes were involved in response to wounding or other stresses such as low temperature.
In conclusion, five components of the ethylene signal transduction pathway were identified from ripening loquat fruit and their possible role in CI was investigated in this study. The low temperature responses of specific genes such as EjETR1 and EjEIL1 suggest that these may be involved in CI development.
Acknowledgments
We wish to thank Dr Andrew Allan (The New Zealand Institute for Plant and Food Research) for critical reading of the manuscript. This research was supported by the National Nature Science Foundation in China (No. 30871759), National Project of Scientific and Technical Supporting Programs Funded by Ministry of Science & Technology of China (2006BAD22B01, 2006BAD22B05), The University Doctoral Foundation of China (No. 200803350091) and the 111 project (No. B06014). It is part of a collaborative programme between Zhejiang University, China, and The New Zealand Institute for Plant and Food Research.
References
- Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Mortiz T, Van Der Straeten D, Peng J, Harberd NP. Integration of plant responses to environmentally activated phytohormonal signals. Science. 2006;311:91–94. doi: 10.1126/science.1118642. [DOI] [PubMed] [Google Scholar]
- Adams-Phillips L, Barry C, Kannan P, Leclercq J, Bouzayen M, Giovannoni J. Evidence that CTR1-mediated ethylene signal transduction in tomato is encoded by a multigene family whose members display distinct regulatory features. Plant Molecular Biology. 2004;54:387–404. doi: 10.1023/B:PLAN.0000036371.30528.26. [DOI] [PubMed] [Google Scholar]
- Alexander L, Grierson D. Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. Journal of Experimental Botany. 2002;53:2039–2055. doi: 10.1093/jxb/erf072. [DOI] [PubMed] [Google Scholar]
- Barry CS, Giovannoni JJ. Ethylene and fruit ripening. Journal of Plant Growth Regulation. 2007;26:143–159. [Google Scholar]
- Begheldo M, Manganaris GA, Bonghi C, Tonutti P. Different post-harvest conditions modulate ripening and ethylene biosynthetic and signal transduction pathways in Stony Hard peaches. Post-harvest Biology and Technology. 2008;48:84–91. [Google Scholar]
- Binder BM, Walker JM, Gagne JM, Emborg TJ, Hemmann G, Bleecker AB, Vierstrab RD. The Arabidopsis EIN3 binding F-Box proteins EBF1 and EBF2 have distinct but overlapping roles in ethylene signaling. The Plant Cell. 2007;19:509–523. doi: 10.1105/tpc.106.048140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C, Chen KS, Xu WP, Zhang WS, Li X, Ferguson IB. Effect of 1-MCP on post-harvest quality of loquat fruit. Post-harvest Biology and Technology. 2006a;40:155–162. [Google Scholar]
- Cai C, Li X, Chen KS. Acetylsalicylic acid alleviates chilling injury of post-harvest loquat (Eriobotrya japonica Lindl.) fruit. European Food Research and Technology. 2006b;223:533–539. [Google Scholar]
- Cai C, Xu CJ, Li X, Ferguson IB, Chen KS. Accumulation of lignin in relation to change in activities of lignification enzymes in loquat fruit flesh after harvest. Post-harvest Biology and Technology. 2006c;40:163–169. [Google Scholar]
- Cai C, Xu CJ, Shan LL, Li X, Zhou CH, Ferguson IB, Chen KS. Low temperature conditioning reduces post-harvest chilling injury in loquat fruit. Post-harvest Biology and Technology. 2006d;41:252–259. [Google Scholar]
- Candan AP, Graell J, Larrigaudière C. Roles of climacteric ethylene in the development of chilling injury in plums. Post-harvest Biology and Technology. 2008;47:107–112. [Google Scholar]
- Dal Cin V, Danesin M, Boschetti A, Dorigoni A, Ramina A. Ethylene biosynthesis and perception in apple fruitlet abscission (Malus domestica L. Bork) Journal of Experimental Botany. 2005;56:2995–3005. doi: 10.1093/jxb/eri296. [DOI] [PubMed] [Google Scholar]
- Dal Cin V, Rizzini FM, Botton A, Tonutti P. The ethylene biosynthetic and signal transduction pathways are differently affected by 1-MCP in apple and peach fruit. Post-harvest Biology and Technology. 2006;42:125–133. [Google Scholar]
- Dong L, Zhou HW, Sonego L, Lers A, Lurie S. Ethylene involvement in the cold storage disorder of ‘Flavortop’ nectarine. Post-harvest Biology and Technology. 2001;23:105–115. [Google Scholar]
- El-Sharkawy I, Jones B, Li ZG, Lelièvre JM, Pech JC, Latché A. Isolation and characterization of four ethylene perception elements and their expression during ripening in pears (Pyrus communis L.) with/without cold requirement. Journal of Experimental Botany. 2003;54:1615–1625. doi: 10.1093/jxb/erg158. [DOI] [PubMed] [Google Scholar]
- Girardi CL, Corrent AR, Lucchetta L, Zanuzo MR, da Costa TS, Brackmann A, Twymand RM. Effect of ethylene, intermittent warming and controlled atmosphere on post-harvest quality and the occurrence of woolliness in peach (Prunus persica cv. Chiripá) during cold storage. Post-harvest Biology and Technology. 2005;38:25–33. [Google Scholar]
- Guo HW, Ecker JR. Plant responses to ethylene gas are mediated by SCFEBF1/EBF2-dependent proteolysis of EIN3 transcription factor. Cell. 2003;115:667–677. doi: 10.1016/s0092-8674(03)00969-3. [DOI] [PubMed] [Google Scholar]
- Hibi T, Kosugi S, Iwai T, Kawata M, Seo S, Mitsuhara I, Ohashi Y. Involvement of EIN3 homologues in basic PR gene expression and flower development in tobacco plants. Journal of Experimental Botany. 2007;58:3671–3678. doi: 10.1093/jxb/erm216. [DOI] [PubMed] [Google Scholar]
- Iordachescu M, Verlinden S. Transcriptional regulation of three EIN3-like genes of carnation (Dianthus caryophyllus L. cv. Improved White Sim) during flower development and upon wounding, pollination, and ethylene exposure. Journal of Experimental Botany. 2005;56:2011–2018. doi: 10.1093/jxb/eri199. [DOI] [PubMed] [Google Scholar]
- Jiang YM, Joyce DC, Jiang WB, Lu WJ. Effects of chilling temperatures on ethylene binding by banana fruit. Plant Growth Regulation. 2004;43:109–115. [Google Scholar]
- Jimenez-Cuesta M, Cuquerella J, Martinez-Javega JM. Determination of color index for citrus fruit degreening. Proceedings of the International Society of Citriculture. 1981;2:750–753. [Google Scholar]
- Katz E, Lagunes PM, Riov J, Weiss D, Goldschmidt EE. Molecular and physiological evidence suggests the existence of system II-like pathway of ethylene production in non-climacteric Citrus fruit. Planta. 2004;219:243–252. doi: 10.1007/s00425-004-1228-3. [DOI] [PubMed] [Google Scholar]
- Kosugi S, Ohashi Y. Cloning andDNA-binding properties of a tobacco Ethylene-Insensitive3 (EIN3) homolog. Nucleic Acids Research. 2000;28:960–967. doi: 10.1093/nar/28.4.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq J, Adams-Phillips LC, Zegzouti H, Jones B, Latché A, Giovannoni JJ, Pech JC, Bouzayen M. LeCTR1, a tomato CTR1-like gene, demonstrates ethylene signalling ability in Arabidopsis and novel expression pattern in tomato. Plant Physiology. 2002;130:1132–1142. doi: 10.1104/pp.009415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo ZS, Xu XL, Yan BF. Use of 1-methylcyclopropene for alleviating chilling injury and lignification of bamboo shoot (Phyllostachys praecox f. prevernalis) during cold storage. Journal of the Science of Food and Agriculture. 2008;88:151–157. [Google Scholar]
- Maldonado R, Molina-Garcia AD, Sanchez-Ballesta MT, Escribano MI, Merodio C. High CO2 atmosphere modulating the phenolic response associated with cell adhesion and hardening of Annona cherimola fruit stored at chilling temperature. Journal of Agricultural and Food Chemistry. 2002;50:7564–7569. doi: 10.1021/jf020629g. [DOI] [PubMed] [Google Scholar]
- Mbéguié-A-Mbéguié D, Hubertb O, Fils-Lycaonc B, Chilletd M, Baurens FC. EIN3-like gene expression during fruit ripening of Cavendish banana (Musa acuminata cv. Grande naine) Physiologia Plantarum. 2008;133:435–448. doi: 10.1111/j.1399-3054.2008.01083.x. [DOI] [PubMed] [Google Scholar]
- Morton JF. Fruits of warm climates. Miami, FL: Published by Julia F Morton; 1987. Loquat; pp. 103–108. [Google Scholar]
- Nakano T, Suzuki K, Fujimura T, Shinshi H. Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiology. 2006;140:411–432. doi: 10.1104/pp.105.073783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Périn C, Gomez-Jimenez MC, Hagen L, Dogimont C, Pech JC, Latché A, Pitrat M, Lelièvre JM. Molecular and genetic characterization of a non-climacteric phenotype in melon reveals two loci conferring altered ethylene response in fruit. Plant Physiology. 2002;129:209–300. doi: 10.1104/pp.010613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesis E, Ackerman M, en-Arie R, Feygenberg O, Feng XQ, Apelbaum A, Goren R, Prusky D. Ethylene involvement in chilling injury symptoms of avocado during cold storage. Post-harvest Biology and Technology. 2002;24:171–181. [Google Scholar]
- Pino MT, Skinner JS, Jeknić Z, Hayes PM, Soeldner AH, Thomashow MF, Chen THH. Ectopic AtCBF1 over-expression enhances freezing tolerance and induces cold acclimation-associated physiological modifications in potato. Plant, Cell and Environment. 2008;31:393–406. doi: 10.1111/j.1365-3040.2008.01776.x. [DOI] [PubMed] [Google Scholar]
- Rasori A, Ruperti B, Bonghi C, Tonutti P, Ramina A. Characterization of two putative ethylene receptor genes expressed during peach fruit development and abscission. Journal of Experimental Botany. 2002;53:2333–2339. doi: 10.1093/jxb/erf097. [DOI] [PubMed] [Google Scholar]
- Salvador A, Arnal L, Monterde A, Cuquerella J. Reduction of chilling injury symptoms in persimmon fruit cv. ‘Rojo Brillante’ by 1-MCP. Post-harvest Biology and Technology. 2004;33:285–291. [Google Scholar]
- Salvador A, Carvalho CP, Monterde A, Martínez-Jávega JM. 1-MCP effect on chilling injury development in ‘Nova’ and ‘Ortanique’ mandarins. Food Science and Technology International. 2006;12:165–170. [Google Scholar]
- Shan LL, Li X, Wang P, Cai C, Zhang B, Sun CD, Zhang WS, Xu CJ, Ferguson IB, Chen KS. Characterization of cDNAs associated with lignification and their expression profiles in loquat fruit with different lignin accumulation. Planta. 2008;227:1243–1254. doi: 10.1007/s00425-008-0696-2. [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biology and Evolution. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Tatsuki M, Endob A, Ohkawa H. Influence of time from harvest to 1-MCP treatment on apple fruit quality and expression of genes for ethylene biosynthesis enzymes and ethylene receptors. Post-harvest Biology and Technology. 2007;43:28–35. [Google Scholar]
- Tieman DM, Ciardi JA, Taylor MG, Klee HJ. Members of the tomato LeEIL (EIN3-like) gene family are functionally redundant and regulate ethylene responses throughout plant development. The Plant Journal. 2001;26:47–58. doi: 10.1046/j.1365-313x.2001.01006.x. [DOI] [PubMed] [Google Scholar]
- Trainotti L, Pavanello A, Casadoro G. Different ethylene receptors show an increased expression during the ripening of strawberries: does such an increment imply a role for ethylene in the ripening of these non-climacteric fruits? Jounal of Experimental Botany. 2005;56:2037–2046. doi: 10.1093/jxb/eri202. [DOI] [PubMed] [Google Scholar]
- Wang A, Tan D, Takahashi A, Li TZ, Harada T. MdERFs, two ethylene-response factors involved in apple fruit ripening. Journal of Experimental Botany. 2007;58:3743–3748. doi: 10.1093/jxb/erm224. [DOI] [PubMed] [Google Scholar]
- Wiersma PA, Zhang H, Lua C, Quail A, Toivonen PMA. Survey of the expression of genes for ethylene synthesis and perception during maturation and ripening of ‘Sunrise’ and ‘Golden Delicious’ apple fruit. Post-harvest Biology and Technology. 2007;44:204–211. [Google Scholar]
- Yang SL, Sun CD, Wang P, Shan LL, Cai C, Zhang B, Zhang WS, Li X, Ferguson IB, Chen KS. Expression of expansin genes in post-harvest lignification and softening of ‘Luoyangqing’ and ‘Baisha’ loquat fruit under different storage conditions. Post-harvest Biology and Technology. 2008;49:46–53. [Google Scholar]
- Yin XR, Chen KS, Allan AC, Wu RM, Zhang B, Lallu N, Ferguson IB. Ethylene-induced modulation of genes associated with the ethylene signalling pathway in ripening kiwifruit. Journal of Experimental Botany. 2008;59:2097–2108. doi: 10.1093/jxb/ern067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin XR, Allan AC, Zhang B, Wu RM, Burdon J, Wang P, Ferguson IB, Chen KS. Ethylene-related genes show a differential response to low temperature during ‘Hayward’ kiwifruit ripening. Post-harvest Biology and Technology. 2009;52:9–15. [Google Scholar]
- Yokotani N, Tamura S, Nakano R, Inaba A, Kubo Y. Characterization of a novel tomato EIN3-like gene (LeEIL4) Journal of Experimental Botany. 2003;54:2775–2776. doi: 10.1093/jxb/erg308. [DOI] [PubMed] [Google Scholar]
- Zhang B, Chen KS, Bowen J, Allan A, Espley R, Karunairetnam S, Ferguson IB. Differential expression within the LOX gene family in ripening kiwifruit. Journal of Experimental Botany. 2006;57:3825–3836. doi: 10.1093/jxb/erl151. [DOI] [PubMed] [Google Scholar]
- Zheng YH, Li SY, Xi YF. Changes of cell wall substances in relation to flesh woodiness in cold-stored loquat fruits. Acta Phytophysiol Sinica. 2000;26:306–310. (in Chinese) [Google Scholar]
- Zhou CH, Xu CJ, Sun CD, Li X, Chen KS. Carotenoids in white- and red-fleshed loquat fruits. Journal of Agricultural and Food Chemistry. 2007;55:7822–7830. doi: 10.1021/jf071273h. [DOI] [PubMed] [Google Scholar]
- Zhou HW, Dong L, Ben-Arie R, Lurie S. The role of ethylene in the prevention of chilling injury in nectarines. Journal of Plant Physiology. 2001;158:55–61. [Google Scholar]