Abstract
The role of S in legume growth, N uptake, and N2 fixation was investigated using white clover (Trifolium repens L.) as a model species. We examined whether the effect of sulphate addition on N fixation resulted from a stimulation of host plant growth, a specific effect of S on nodulation, or a specific effect of S on nodule metabolism. Clones of white clover, inoculated with Rhizobium leguminosarum, were grown for 140 d in a hydroponic system with three levels of sulphate concentration (0 mM, 0.095 mM, and 0.380 mM). Nodule morphological and biochemical traits, such as root length, nodule biomass and volume, nodule protein contents (nitrogenase and leghaemoglobin obtained by an immunological approach), and root amino acid concentrations, were used to analyse the effect of sulphate availability on N2 fixation. The application of sulphate increased whole plant dry mass, root length, and nodule biomass, expressed on a root-length basis. N uptake proved less sensitive than N2 fixation to the effects of S-deficiency, and decreased as a consequence of the lower root length observed in S-deficient plants. N2 fixation was drastically reduced in S-deficient plants as a consequence of a low nodule development, but also due to low nitrogenase and leghaemoglobin production. This effect is likely to be due to down-regulation by a N-feedback mechanism, as, under severe S-deficiency, the high concentration of whole plant N and the accumulation of N-rich amino acids (such as asparagine) indicated that the assimilation of N exceeded the amount required for plant growth.
Keywords: Amino acids, legumes, N2-fixation, nitrogenase, nodules, sulphur (S) deficiency
Introduction
Legumes are environmentally, agriculturally, and economically important in many cropping systems because of their ability to reduce atmospheric N2 symbiotically. Because the use of nitrogen (N) fertilizer contributes substantially to environmental pollution, this biological alternative has received increasing attention in agricultural practices in the last few years. Legumes are particularly sensitive to a wide range of environmental limitations. Studies on mineral requirements of the Legume–Rhizobia symbiosis, phosphorus (P), and to a lesser extent potassium (K), have received considerable attention because N, P, and K are frequently the most limiting nutrients for plant growth in numerous ecosystems (Høgh-Jensen, 2003; Olivera et al., 2004). It is now well documented that legume growth and nitrogen (N) fixation are highly responsive to P and K supplies.
The effect of sulphur (S) supply on N2 fixation has received less attention, as sulphate availability in soil has not been considered in the past as a limiting factor for plant growth. However, crop deficiencies of S have been reported with increasing frequency in the last decade, caused by decreasing anthropogenic S input and by the lack of input through S fertilization to compensate for exportation (Scherer, 2001). S is accumulated in plants in low concentrations compared to N, but is an essential element as a constituent of proteins, Cysteine-containing peptides such as glutathione, or numerous secondary metabolites. S deficiencies have been observed in Brassiceae, Poaceae, but also in Fabaceae. Fabaceae species require a large quantity of S, probably because of their high protein concentration (Aulakh et al., 1976) and because S is crucial for N2 fixation (Scherer and Lange, 1996; Krusell et al., 2005; Scherer et al., 2008).
S-deficiency causes a decrease in N acquisition by plants as a result of a decrease in nitrate uptake (Clarkson et al., 1989; Karmoker et al., 1991) or N2 fixation (Zhao et al., 1999; Scherer et al., 2008). The low N2 fixation observed in S-deficient plants can result in lower plant growth (Scherer and Lange, 1996) or in a specific effect of S on N2 fixation (Zhao et al., 1999; Pacyna et al., 2006; Scherer et al., 2008). Low N2 fixation is due to the effect of S on nodule growth (Schulze and Drevon, 2005; Habtemichial et al., 2007) or nodule metabolism (Pacyna et al., 2006; Scherer et al., 2008). In a previous study, a specific effect of S-deficiency on N2 fixation in white clover was observed (Varin et al., 2009a). However, the mechanisms accounting for this decreased activity have not been elucidated. It has recently been proposed that the low nitrogenase activity observed in S-deficient plants is due to a limitation of the energy supply to the nodule, as well as a decrease in leghaemoglobin and ferredoxin concentrations (Pacyna et al., 2006; Scherer et al., 2008). Even if it is known that (i) N2 fixation can be reduced as a consequence of low nitrogenase concentration in plants subjected to nutrient stresses (Bolaños et al., 2006) and (ii) S is a central component of this Fe–S cluster enzyme (Curatti et al., 2006), the effect of S supply on nitrogenase concentrations has not been considered in any of these studies.
The objective of this study was to reconcile conflicting reports on the role of S in symbiotic N fixation by investigating this effect in white clover (Trifolium repens L.), a major legume in Western Europe grasslands and a species highly sensitive to sulphate supply (Tallec et al., 2008; Varin et al., 2009a, b). The following questions were addressed. Does the stimulation of N2 fixation by S supply result from (i) a stimulation of host plant growth; (ii) a specific effect of S on nodulation; (iii) a specific effect of S on nodule metabolism? To answer these questions, white clover plants of the same genotype have been subjected to three sulphate availability levels, and nodule morphological and biochemical parameters have been analysed in relation to the modification of plant dry mass.
Materials and methods
Plant material, clone production, and growth conditions
Clones of Trifolium repens L. cv. Huia were obtained by vegetative multiplication of the stolon of one individual chosen as an average individual from a population. Each individual consisted of a 20 mm stolon section including a node. When the primary leaf appeared, each fragment was transferred to a hydroponic system supplying a continuously aerated nutrient solution in 1.0 l black plastic bottles to maintain darkness in the rooting environment.
Plants were grown in a greenhouse with light measured at the plant level equal to 400 μmol m−2 s−1 (sodium high pressure ‘phytoclaude 400 W’ and sun) providing a 16/8 h photoperiod with 25/16 °C day/night. The plants were inoculated with Rhizobium leguminosarum bv. trifolii T354 three times a week during the four weeks after planting. The nutrient solution was prepared with demineralized water, changed weekly, and contained 2 mM KNO3, 0.18 mM CaCO3, 0.4 mM KH2PO4, 0.15 mM K2HPO4, 3 mM CaCl2, 0.2 mM EDTA 2NaFe (3H2O), 14 μM H3BO3, 3 μM ZnCl2, 0.7 μM CuCl2, 0.7 μM (NH4)6Mo7O24, 0.1 μM CoCl2. S was added as MgSO4 and three treatments were chosen; ‘zero S’, ‘low S’ (0.095 mM ), and ‘high S’ (0.380 mM ). MgSO4 was partly or entirely replaced by equimolar amount of MgCl2 and KCl for the zero and low S treatments. The sulphate concentration of the zero S solution was measured by HPLC and found to be nil, showing that no other source of sulphate was available for plant growth. The nutrient solution contained 15NO3 0.5 atom% excess to allow the measurement of absorbed from the nutrient solution and N2 fixed from the atmosphere.
Plants were harvested 70 d and 140 d after transfer into the hydroponic solution, separated into shoots, roots, and nodules and then dried at 70 °C and weighed when mass was constant. The determination of the relative chlorophyll concentration using the non-destructive SPAD (Soil Plant Analysis Development) chlorophyll meter (Minolta, SPAD-502 model, Tokyo, Japan) was also performed on leaves. Aliquots of roots and nodules were collected in an ice bath and frozen for morphological and biochemical analysis (amino acids and proteins).
Root and nodule morphological analysis
Root and nodule morphological parameters were measured using an EPSON Expression 1000XL scanner (Regent instruments Inc, Quebec, Canada). Individual root systems and nodules were spread out on a clear tray in an isotonic solution of KCl 9 g l−1 and placed on a flat-bed scanner. Root length and nodule volume were measured using the WinRHIZO software.
Isotopic analysis
All organ dry matter was reduced to a fine powder for elemental and isotopic analyses. Total N, total S, and 15N were determined from 1 mg of plant material using an elemental analyser (EA3000, EuroVector, Milan, Italy) linked to an isotope ratio mass spectrometer (Isoprime, GV instrument, Manchester, UK). The use of 15NO3− allowed the measurement of the relative contributions of N derived from the nutrient solution (N absorbed) and N derived from the atmosphere (N fixed) (Høgh-Jensen and Schjoerring, 1994):
where QN is the amount of N per plant. A non-fixing plant, ryegrass, grown in the same conditions was used as a control to estimate atom% excess of nutrient solution (Høgh-Jensen and Schjoerring, 1994). The amount of N fixed was estimated as the difference between QN and N absorbed:
Extraction and quantification of proteins from nodules
Total proteins were extracted from nodules using the method described by Wang et al. (2003) and Desclos et al. (2009). Frozen nodule samples were ground to a fine powder with a semi-automatic crusher in the presence of liquid nitrogen and resuspended in 2 ml of cold acetone containing 10% TCA (w/v). After centrifugation at 16 000 g for 3 min at 4 °C, the supernatant was discarded and the pellet was resuspended with 1.75 ml ammonium acetate (0.1 M)/methanol (80%) buffer for the precipitation of proteins. The extract was centrifuged at 16 000 g (3 min, 4 °C). The pellet was washed with acetone (80%), and dried under vacuum (Speedvac concentrator 5301, Eppendorf, France) for 5 min at 50 °C. The dried pellet was resuspended with 0.8 ml of phenol (pH 7.9) and 0.8 ml of SDS buffer [30% saccharose (w/v), 2% SDS (w/v), 0.1 M TRIS-HCl (w/v), 0.5% β-mercaptoethanol (v/v), pH 8]. After centrifugation (16 000 g, 3 min, 4 °C), the phenolic phase was separated from the supernatant and precipitated with 2 ml ammonium acetate (0.1 M)/methanol (80%) at –20 °C for 10 min. After centrifugation (16 000 g, 10 min, 4 °C), the pellet was washed with methanol (100%) and acetone (80%).
For SDS-PAGE, the protein pellet was resuspended in Laemmli lysis buffer (Laemmli, 1970) and denaturated for 5 min at 100 °C with β-mercaptoethanol (5%, v/v). For two-dimensional gel electrophoresis (2-DE), the protein extract was resuspended in 100 μl of R2D2 buffer as described by Mechin et al. (2003) containing dithiothreitol (DTT, 20 mM), thiourea (2 M), urea (5 M), CHAPS (2%, w/v), N-decyl-N,N-dimethyl-3-ammonio-1-propane-sulphonate (2%, w/v), TCEP (5 mM), IPG buffer (2%, v/v; GE Healthcare, Saclay, France) and used for the determination of the protein concentration in the nodule extract by the method of Bradford (1976).
SDS-PAGE and two-dimensional electrophoresis (2-DE)
For SDS-PAGE, an equal amount of protein (4 μg for silver nitrate staining and 250 μg for immunoblotting) was loaded per lane and proteins were separated on 15% polyacrylamide gels at a constant current (250 V, 75 mA, 1 h). For 2-DE, the extract of total proteins was prepared in rehydration R2D2 buffer (300 μg in 330 μl) and was first separated according to charge in the electrofocusing PROTEAN IEF system (Bio-Rad), at 20 °C, using 18 cm gel strips forming an immobilized linear pH gradient from 4 to 7 (GE Healthcare). Conditions for isoelectrofocusing, the equilibration step, and second dimension separation (SDS-PAGE) were monitored as described by Desclos et al. (2009). Proteins were visualized using the silver staining procedure described by Blum et al. (1987). The Millipore BioImage computerized image analysis system (Millipore) was used to determine the molecular mass of nodule proteins by comparison with a set of protein molecular mass markers (Precision Protein Dual Color, Bio-Rad, Marne-la-Coquette, France).
Western blotting and immunodetection of nitrogenase and leghaemoglobin
The abundance of FeMoCo-nitrogenase and leghaemoglobin in nodules was determined after Western blotting of total proteins separated by SDS-PAGE or 2-DE. After SDS-PAGE or 2-DE, Western blots were carried out on a polyvinylidene fluoride (PVDF) membrane (Immobilon-PVDF, Millipore) by semi-dry electroblotting (Milli Blot-graphite electroblotter system, Millipore). Conditions for protein transfer and immunoblotting were carried out as previously described by Noquet et al. (2004). Briefly, after Western blotting, PVDF membranes were treated with polyclonal anti-protein primary antibodies: anti-FeMoCo-nitrogenase from IgG antibodies (Agrisera, SE911 Vanâdas, Sweden; dilution 1:750) or anti-leghaemoglobin 17 kDa from soybean IgG antibodies (Goulas et al., 2001; dilution 1:750). The antigen–antibody complex was visualized with alkaline phosphatase linked to rabbit anti-chicken immunoglobulin Y (IgY), dilution 1:1000 (Noquet et al., 2004). Independently performed gels and Western blots were scanned and analysed using the Millipore Bioimage computerized image analysis system to determine the molecular mass of proteins.
Protein identification by LC MS/MS
Protein spots of interest were manually excised from the gel, washed several times with water, and dried for a few minutes. Trypsin digestion was performed overnight with a dedicated automated system (MultiPROBE II, PerkinElmer). The gel fragments were subsequently incubated twice for 15 min in a H2O/CH3CN solution to allow extraction of peptides from the gel pieces. Peptide extracts were then dried and dissolved in starting buffer for chromatographic elution, consisting of 3% CH3CN and 0.1% HCOOH in water. Peptides were enriched and separated using lab-on-a-chip technology (Agilent, Massy, France) and fragmented using an on-line XCT mass spectrometer (Agilent). The fragmentation data were interpreted using the DataAnalysis program (version 3.4, Bruker Daltonic, Billerica, USA). For protein identification, tandem mass spectrometry peak lists were extracted and compared with the protein database using the MASCOT Daemon (version 2.1.3; Matrix Science, London, UK) search engine. The searches were performed with no fixed modification and with variable modifications for the oxidation of methionines, and with a maximum of one missed cleavage. Tandem mass spectrometry spectra were searched with a mass tolerance of 1.6 Da for precursor ions and 0.8 for MS/MS fragments. The LC MS/MS data were converted into DTA-format files which were further searched for proteins with MASCOT Daemon. Only peptides matching an individual ion score >51 were considered. Proteins with two or more unique peptides matching the protein sequence were automatically considered as a positive identification. Measured peptides were searched in the NCBInr–protein sequence databases, Viridiplantae (green plants) and Bacteria (Eubacteria).
Amino acid extraction and analysis
Frozen plant material was freeze-dried and amino acids were extracted from 10±5% mg of milled plant material in 400 μl of methanol containing 200 μM DL-3-aminobutyric acid as an internal standard and samples were agitated at 1500 rpm for 15 min. Subsequently, 200 μl of chloroform was added and samples were agitated again for 5 min. Finally, 400 μl of ultra-pure water was added and samples were then vigorously vortexed and centrifuged at 13 000 g for 5 min. The upper phase was transferred to a microtube and dried under vacuum over-night. Dry residues were resuspended in an appropriate volume of ultra-pure water and 10 μl of the resulting extract was sampled for amino acid derivatization according to the AccQ Tag Ultra Derivitization Kit protocol (Waters Corp., Milford, USA). Amino acids were analysed using an Acquity™ UPLC system (Waters Corp., Milford, USA) by injecting 1 μl of the derivatization mix onto an Acquity™ UPLC BEH C18 1.7 μm 2.1×100 mm column heated at 55 °C. Amino acids were eluted at a 0.7 ml min−1 flow with a mix of 10-fold diluted AccQ Tag Ultra Eluent (A) and acetonitrile (B) according to the following gradient: initial, 99.9% A; 0.54 min, 99.9% A; 6.50 min, 90.9% A, curve 7; 8.50 min, 78.8% A, curve 6; 8.90 min, 40.4% A, curve 6; 9.50 min, 40.4% A, curve 6; 9.60 min, 99.9% A, curve 6; 10.10 min, 99.9% A. Derivatized amino acids were detected at 260 nm using a photo-diode array detector.
Statistical analysis
Five replicates per treatment were used. Because some data did not fit the parametric test conditions, the choice was made to analyse the effect of S treatments by the Kruskall–Wallis non parametric test (Sokal and Rohlf, 2003), and then by the Signed-Ranks test.
Results
Growth parameters
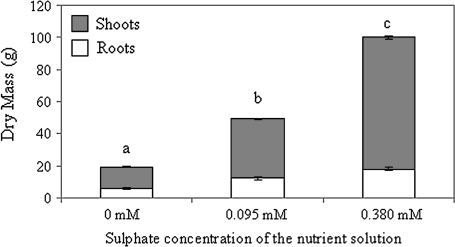
An increase in sulphate application markedly increased the total biomass of plants grown for 140 d (Fig. 1; H=12.50, P <0.01). Even if this change was due to the increase of both shoots (stolons plus leaves) and nodulated roots, shoot biomass increased more than root biomass with sulphate application, and the highest S concentration in the nutrient solution doubled the shoot/root ratio compared with non-treated plants. Chlorophyll concentration increased significantly (H=12.50, P <0.01) with S availability in the nutrient solution (data not shown).
Fig. 1.
Shoot and root dry mass of white clover plants grown for 140 d at three sulphate concentrations in the nutrient solution (0, 0.095, and 0.380 mM). Values are presented as means ±SE (n=5). Different letters on bars indicate significant differences of total dry mass between S treatments (P <0.01).
Sulphur and nitrogen acquisition
A higher N concentration was observed in nodules, and was independent of S treatments in this organ (Table 1). Sulphate application increased N concentration in roots but decreased N concentration in leaves and stolons. A higher S concentration was also observed in nodules (Table 1), and in this organ, S concentration was increased by sulphate application. S concentration was lower and independent of S treatments in all other organs. As a result of the stimulation of plant biomass by sulphate application, the amount of nitrate absorbed from the nutrient solution and the amount of N derived from nitrogen fixation were both significantly higher under the high S treatment (Table 2). However, S supply appeared to have no significant effect on nitrate uptake when the results are expressed per unit of root length (Table 2). By contrast, S supply appeared to have a specific effect on N2 fixation, as a higher amount of N fixed per metre of roots was observed for the high S treatment. Moreover, the percentage of N derived from fixation increased from 38±2% for plants grown in the absence of S to 59±2% of total N for the high S treatment, confirming that N2 fixation is more dependent on S nutrition than N uptake.
Table 1.
Concentration of total N and total S (mg g−1 dry mass) in leaves, stolons, roots, and nodules of white clover plants grown for 140 d at three sulphate concentrations in the nutrient solution
| (mM) | Total N concentration (mg g−1 DM) |
Total S concentration (mg g−1 DM) |
||||||
| Leaves | Stolons | Roots | Nodules | Leaves | Stolons | Roots | Nodules | |
| 0 | 20.45 b | 31.83 b | 17.24 a | 36.62 a | 0.83 a | 0.19 a | 0.36 a | 1.18 a |
| 0.095 | 14.84 a | 15.70 a | 17.42 a | 35.39 a | 0.37 a | 0.16 a | 0.28 a | 2.57 b |
| 0.380 | 14.23 a | 13.19 a | 23.14 b | 36.01 a | 0.40 a | 0.15 a | 0.34 a | 3.09 b |
| H | 9.98 | 10.50 | 9.56 | 0.77 | 5.06 | 4.50 | 2.63 | 11.58 |
| P | <0.01 | <0.01 | <0.01 | ns | ns | ns | ns | <0.01 |
Values are presented as means (n=5). Different letters indicate significant differences between S treatments (P <0.01). H and P: Kruskall–Wallis statistics (ns: non significant).
Table 2.
Amounts of N coming from nitrate absorption (N absorbed) or atmospheric N fixation (N fixed) of white clover plants grown for 140 d at three sulphate concentrations in the nutrient solution, expressed per plant or per metre of roots
| (mM) | N absorbed |
N fixed |
||
| (g plant−1) | (g m−1 roots) | (g plant−1) | (g m−1 roots) | |
| 0 | 0.23 a | 0.30 a | 0.14 a | 0.18 a |
| 0.095 | 0.35 b | 0.22 a | 0.29 b | 0.19 a |
| 0.380 | 0.51 c | 0.44 a | 0.74 c | 0.94 b |
| H | 12.50 | 5.66 | 10.50 | 9.50 |
| P | <0.01 | ns | <0.01 | <0.01 |
White clover plants were grown at three sulphate concentration of the nutrient solution (0, 0.095, and 0.380 mM) and 2 mM K15NO3 (0.5 atom% excess) to measure N absorbed from the nutrient solution (N absorbed) and N derived from the atmosphere (N fixed). Values are presented as means (n=5). Different letters indicate significant differences between S treatments (P <0.01). H and P: Kruskall–Wallis statistics (ns: non significant).
Nodulation parameters
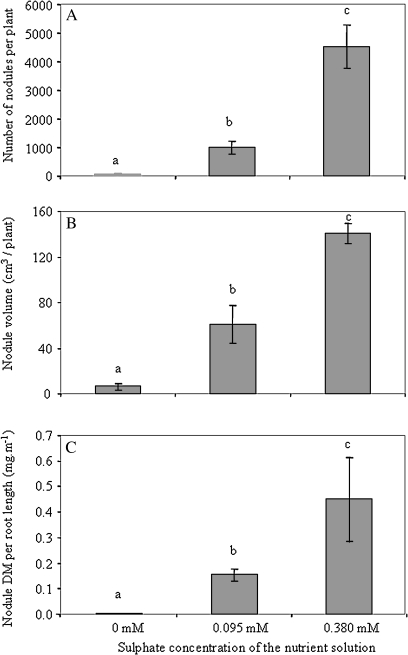
In order to clarify the ways that a deficiency in S diminishes atmospheric N2 fixation, nodule morphological and biochemical traits were further investigated. A very strong effect of S nutrition on nodulation was observed. As Fig. 2A shows, a very small number of nodules were observed when plants were grown in the absence of S, and this number increased dramatically with S treatment (H=12.50, P <0.01). S supply had a similar effect on nodule dry mass per plant (H=11.18, P <0.05) (data not shown), and nodule volume per plant (H=12.02, P <0.05) (Fig. 2B), with these two parameters being closely correlated (Pearson correlation: r=0.893, P <0.001). S appeared to have a specific effect on nodulation as nodule dry mass increased strongly when expressed per unit of root length (H=12.02, P <0.01; Fig. 2C). Plants grown in the absence of S exhibited a very low nodule mass per root length and a higher nodule mass per root length was observed for white clover plants grown under the high S treatment. The nodule morphological parameters (nodule DM/root length; total nodule volume per plant) were correlated significantly with the percentage of N derived from fixation (Pearson correlation: r=0.714, P <0.01 and r=0.873, P <0.05, respectively). In addition, the nodules of plants grown in the absence of S appeared pale in contrast with the pink nodules observed in the presence of S.
Fig. 2.
Nodulation of white clover plants grown for 140 d at three sulphate concentrations in the nutrient solution (0, 0.095, and 0.380 mM). (A) Number of nodules per plant, (B) total volume of nodules per plant, and (C) dry mass (DM) of nodules per unit of root length. Values are presented as means ±SE (n=5). Different letters on bars indicate significant differences between S treatments (P <0.05).
Nodule proteins
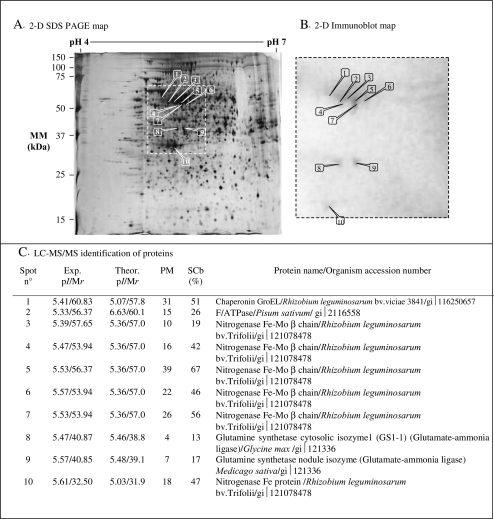
Nodule protein concentration increased with S supply (Table 3). Using 2-DE and immunological analysis, 10 spots were immunodetected using anti-FeMoCo nitrogenase antibodies (Fig. 3A, B). Identification by LC MS/MS demonstrated that five of these proteins are nitrogenase Fe-Mo βchain (Spots 3 to 7) and one (Spot 10) is nitrogenase Fe protein (Fig. 3C).
Table 3.
Protein concentrations (expressed as mg g−1 fresh mass) of nodules of white clover plants grown for 140 d at three sulphate concentrations in the nutrient solution
| (mM) | Total soluble proteins (mg g−1 FM) | Nitrogenase Fe Mo β chain (mg g−1 FM) | Nitrogenase Fe protein (mg g−1 FM) | Leghaemoglobin (mg g−1 FM) |
| 0 | 1.97 a | 0.16 a | 0.13 ab | 0.06 a |
| 0.095 | 3.18 ab | 0.49 b | 0.06 a | 0.16 ab |
| 0.380 | 5.51 b | 0.32 b | 0.22 b | 0.33 b |
| H | 8.61 | 10.35 | 9.74 | 10.35 |
| P | <0.05 | <0.01 | <0.01 | <0.01 |
White clover plants were grown for 140 d at three sulphate concentrations of the nutrient solution (0, 0.095, and 0.380 mM). Total soluble proteins were determined by the Bradford method (1976). Nitrogenase and leghaemoglobin were quantified from image analysis of SDS-PAGE gels shown in Fig. 4. Values are presented as means (n=5). Different letters indicate significant differences between S treatments (P <0.05). H and P: Kruskall–Wallis statistics (ns: non significant).
Fig. 3.
Proteomic maps of nodule proteins from white clover plants grown for 140 d at three sulphate concentrations in the nutrient solution (0, 0.095, and 0.380 mM). (A) Nodule proteins were separated by 2-D SDS-PAGE (12% acrylamide) and stained with silver nitrate. (B) Nodule proteins were revealed after immunoblot with anti-NifH. Immunodetected spots are numbered from the highest to the lowest molecular mass. (C) Identification of nodule proteins immunodetected by the anti-FeMoCo-nitrogenase antibodies. Experimental and theorical pI and molecular mass are indicated as Exp. and theor. pI/Mr. The assigned protein of best match is given with the organism in which it was identified and its GenBank protein accession number: PM: number of LC-MS/MS matched peptides, SCb (%): percentage of sequence coverage of the protein.
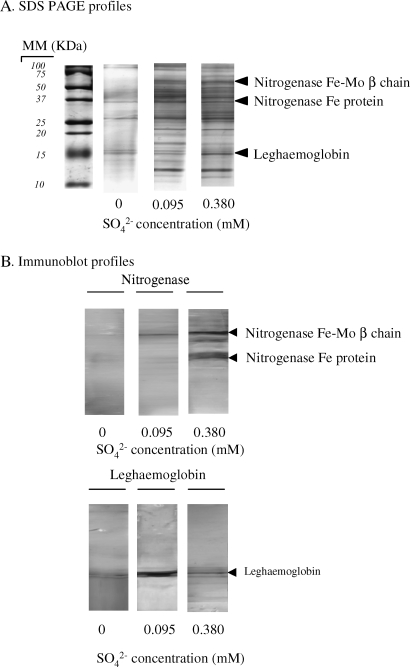
Both nitrogenase and leghaemoglobin were detectable in nodules of white clover grown in the absence and in the presence of sulphate in the nutrient solution (Fig. 4). Image analysis of SDS-PAGE electrophoresis of nodule soluble proteins (Fig. 4A) and a comparison with data from immunoblots (Fig. 4B) indicated that the amount of both nitrogenase Fe-Mo protein and leghaemoglobin were dependent on S supply (Table 3). Nodules of plants grown in the absence of S contained significantly less nitrogenase Fe-Mo protein and less leghaemoglobin than plants grown with the high S treatment. The lowest nitrogenase Fe protein content was observed for the 0.095 mM S treatment, the zero S treatment being intermediary between treatments and not significantly different from other treatments.
Fig. 4.
2-D electrophoresis profiles from white clover plants grown for 140 d at three sulphate concentrations in the nutrient solution (0, 0.095, and 0.380 mM). (A) SDS-PAGE profiles of nodule protein. (B) Immunodetection of the nitrogenase NifH component and leghaemoglobin following SDS-PAGE and Western blotting of nodule extracts with specific antibodies. MM: molecular markers (kDa).
Nodulated root amino acids
A strong accumulation of amino acids was observed in nodulated roots of white clover grown in the absence of S (Table 4), with the amino acid concentration in roots being 4-times higher in the zero S treatment than in the high S treatment. A similar effect of S availability on amino acids was observed in shoots (data not shown). Asparagine was the major amino acid found in white clover roots, and the proportion of asparagine increased from 71±7% of total free amino acids for the high S concentration nutrient solution, to 85±2% for the zero S roots. A strong accumulation of other N-rich amino acids, arginine and histidine, was also observed for zero S roots, while the concentration of other amino acids like glutamate and aspartate was independent of S availability. The concentration of cysteine was too low in all treatments to be measured accurately, and the other sulphur amino acid, methionine, constituted less than 0.1% of total amino acids in all treatments. As expected, this proportion of methionine increased significantly with S availability (H=9.38, P <0.01).
Table 4.
Free amino acid concentration (expressed as μmol g−1 dry mass) of nodulated roots of white clover plants grown for 140 d at three sulphate concentrations in the nutrient solution
| Amino acid | Amino acid concentration (μmol g−1 DM) |
H | P | ||
|
(mM) |
|||||
| 0 mM | 0.095 mM | 0.mM | |||
| Asparagine | 1055 c | 416 b | 221 a | 12.02 | <0.01 |
| Arginine | 103 b | 57 a | 30 a | 12.02 | <0.01 |
| Histidine | 10 b | 5 a | 3 a | 11.58 | <0.01 |
| Aspartate | 12 a | 11 a | 13 a | 5.12 | ns |
| Glutamate | 10 a | 8 a | 8 a | 6.61 | <0.05 |
| Glutamine | 2 a | 2 a | 2 a | 1.81 | ns |
| Others | 37 a | 27 a | 24 a | 6.50 | ns |
| Total AA | 1230 b | 525 a | 301 a | 12.02 | <0.01 |
Values are presented as means (n=5). Different letters indicate significant differences between S treatments (P <0.01). H and p: Kruskall-Wallis statistics (ns: non significant).
Discussion
Plant growth and N acquisition
Plants are able to use both pedospheric S (sulphate) and atmospheric S as sources of S to support growth. The observation that white clover can grow hydroponically without S added to the nutrient solution suggests that this legume is able to use atmospheric sulphur as a significant source of S because the amount of S contained in each plant at the beginning of the experiment (67 μg) was not sufficient to support plant growth for 140 d. Atmospheric S can replace soil sulphate as the sulphur source for other plant species, especially when the sulphur supply to the roots is low (Cowling et al., 1973; De Kok et al., 2007). The main sources of atmospheric S are H2S reduced in cysteine via O-acetylserine lyase and SO2 oxidized to sulphate via sulphite oxidase (Van Der Kooij et al., 1997; Hänsch and Mendel, 2005; Durenkamp et al., 2007). Our study suggests that clover can use these sources and a more detailed experiment is needed to quantify the ability of legume plants such as clover to use H2S or SO2 as S sources.
Work by Zhao et al. (1999) has shown that the effect of S-deficiency on pea growth was likely to be caused by the shortage of N, due to decreased N2 fixation. Accordingly, the effect of S-deficiency on clover growth was associated here with a strong reduction of N2 fixation. However, the higher N concentration in shoot tissues observed for the zero S treatment indicates that shoot growth was not limited by N assimilation. The low amount of N acquired from the nutrient solution and from the atmosphere shows that both pathways of N assimilation, soluble N uptake and N2 fixation, were reduced in S-deficient clover. A decrease in nitrate uptake in S-starved plants has previously been observed in barley (Clarkson et al., 1989; Karmoker et al., 1991) and spinach (Prosser et al., 2001). In clover, the reduction of nitrate uptake is mainly explained by the strong reduction in root length in the zero S and low S treatments rather than a specific effect of S availability on soluble N uptake, as shown by the similar amount of nitrate taken up per unit of root length that was observed in all treatments.
S-deficiency had a stronger effect on the process of atmospheric N2 fixation than on soluble N uptake as shown by the low amount of N fixed per metre of roots for the zero S and low S treatments. The high requirement of S for the process of N fixation, also evidenced by the high nodule S concentration, is in accordance with previous work (Pacyna et al., 2006; Zhao et al., 1999).
Nodule morphology and metabolism
Nodulation was evaluated here as the number, volume, and dry mass of nodules. The strong reduction of nodulation observed with the zero and low S treatments resulted mainly in a much lower dry mass of nodules per unit of root length, and not only in a lower root production as observed for other legumes (Gilbert and Robson, 1984; Scherer and Lange, 1996). This result confirms that traits associated with N2 fixation are more responsive to S availability than host plant growth. This specific effect of S on nodulation can be explained by the role of S in stimulating the formation of nodules in the early events of symbiosis establishment (Schwedock and Long, 1992; Crockard et al., 2002). The effect of S-deficiency appears similar to those of P deficiency in preventing nodulation in white clover (Almeida et al., 2000). This strong reduction of nodulation could result from an N feedback mechanism, as a strong accumulation of amino acids was observed in the zero and low S treatments. It has been shown that such an accumulation can reduce nodule growth (Parsons et al., 1993).
The close relationship observed between nodule volume per plant and the percentage of N derived from fixation suggests that the low N2 fixation observed can be primarily explained by the low nodulation. However, our biochemical and immunological approach shows that nodule metabolism was also markedly dependent on sulphate availability. A close relationship was found between S supply and protein content in the nodules, and both the proteins measured, nitrogenase and leghaemoglobin, were dependent on S supply. It has been proposed that low N2 fixation observed in S-deficient legumes is due to a low ATP supply and to an inhibition of nitrogenase by high levels of free O2 (Pacyna et al., 2006; Scherer et al., 2008). ATP was not measured in the present study, but the low N2 fixation could also be due to a high level of O2 in clover nodules. Indeed, it was observed that leghaemoglobin content in clover roots is highly dependent on sulphate availability, confirming a recent result by Scherer et al. (2008) working with pea and alfalfa. As the role of leghaemoglobin in legume nodules is to maintain a low free O2 concentration within the nodule (Gordon et al., 2001), the decrease in its content may have resulted in a higher O2 concentration.
S starvation generally leads to the accumulation of non-S-containing amino acids and O-acetylserine, the precursor for the synthesis of the first organic S compound, cysteine, and a decrease in numerous S compounds, primarily cysteine, methionine, and S-adenosyl methionine (SAM), (Prosser et al., 2001; Nikiforova et al., 2003; Saito, 2004; Hoefgen and Nikiforova, 2008). In clover leaves, a reduction in chlorophyll concentration was observed, as mentioned for other species grown with insufficient S supply (Lunde et al., 2008). This chlorosis could be explained by the decrease in the SAM that served as a methyl-group donor in different branches of plant metabolism and especially in chlorophyll synthesis (Hoefgen and Nikiforova, 2008). In nodules, this disruption of N metabolism was evidenced by the decrease in protein content and the strong accumulation of non-S-containing amino acids. The low level of the nitrogenase Fe–Mo protein is in line with reports showing that iron–sulphur proteins and haem biosynthesis can be repressed when sulphate availability decreases (Nikiforova et al., 2003; Touraine et al., 2004; Hausman et al., 2008). Both components of the nitrogenase complex, the Fe protein and the Fe–Mo protein, contain high proportions of S (Krusell et al., 2005; Curatti et al., 2006). However, the differentiated behaviour of both nitrogenase components with respect to S availability makes the relationship between S availability and nitrogenase difficult to interpret. It is noteworthy that nodulated clovers grown in the absence of S in the nutrient solution were able to form both components of the nitrogenase complex, and the strikingly high total S concentrations in nodules for all S treatments confirms that nodules are a strong sink for S in legumes. A full metabolic analysis could help to clarify the interactions between S and N metabolism in nodules.
Even though nodulation and Fe–Mo nitrogenase content were very low under S-deficiency, 38% of the total N assimilated by these plants originated from N2 fixation. Nodule dry weight was reduced by 99% in zero S clover compared with high S clover and the amount of N fixed per plant was only decreased by 81%. This indicates that S-deficiency inhibited nodulation to a greater extent than N2 fixation. Specific nitrogenase activity was not measured directly in the present study, and it is difficult to estimate this activity expressed on a quantity, volume or mass of nodules basis as neither the kinetics of fixation nor those of nodule development are known. Nevertheless, this comparison suggests that S-deficiency could increase the specific nitrogenase activity expressed g−1 of nodule. A similar increase in specific nitrogenase activity has been found under other conditions that hindered the development of nodules such as under P deficiency (Almeida et al., 2000) or high mineral N supply (Zanetti et al., 1998). In the case of P deficiency, it has been proposed that this high specific nitrogenase activity could be the result of an increase in O2 permeability, and further work is needed to investigate the effect of S-deficiency on O2 permeability in the nodules.
Concluding remarks
From our experimental data, it is proposed that the reduced symbiotic N2 fixation observed in S-deficient white clover is an adjustment to the lower demand for N due to a lower plant growth. The high N concentration observed in leaves and stolons of S-deficient clover, compared with other treatments, shows that shoot growth was not limited by N assimilation under S-deficiency and thus supports this hypothesis. The lowered N2 fixation may have resulted from an N-feedback mechanism down-regulating nodule development and nitrogenase and leghaemoglobin production, as indicated by the increased asparagine concentration observed. Such regulatory behaviour has already been observed for other nutrient stresses in white clover and other legumes (Hartwig, 1998; Almeida et al., 2000; Vadez et al., 2000; Høgh-Jensen et al., 2002).
Acknowledgments
We are grateful to Anne-Françoise Ameline, Marie-Paule Bataillé, Michelle Coustenoble, Josiane Pichon, Dominique Ballois, Patrick Beauclair, Hugues Renault, and Raphaël Ségura for technical assistance. We wish to thank Jessica Bryant and Laurence Cantrill for critical comments and advice on the manuscript.
References
- Almeida JPF, Hartwig UA, Frehner M, Nösberger J, Lüscher A. Evidence that P deficiency induces N feedback regulation of symbiotic N2 fixation in white clover (Trifolium repens L.) Journal of Experimental Botany. 2000;51:1289–1297. [PubMed] [Google Scholar]
- Aulakh MS, Dev G, Arora BR. Effect of sulphur fertilization on the nitrogen–sulphur relationships in alfalfa (Medicago sativa L. Pers.) Plant and Soil. 1976;45:75–80. [Google Scholar]
- Blum H, Beier H, Gross HJ. Improved silver staining of plant proteins, RNA and DNA in polyacrylamid gel. Electrophoresis. 1987;8:93–99. [Google Scholar]
- Bolaños L, Martin M, El Hamdaovi A, Rivilla R, Bonilla I. Nitrogenase inhibition in nodules from pea plants grown under salt stress occurs at the physiological level and can be alleviated by B and Ca. Plant and Soil. 2006;280:135–142. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities utilizing the principle of protein–dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Clarkson DT, Saker LR, Purves JV. Depression of nitrate and ammonium transport in barley plants with diminished sulphate status. Evidence of co-regulation of nitrogen and sulphate intake. Journal of Experimental Botany. 1989;40:953–963. [Google Scholar]
- Cowling DW, Jones LHP, Lockyer DR. Increased yield through correction of sulphur deficiency in ryegrass exposed to sulphur dioxide. Nature. 1973;243:479–480. [Google Scholar]
- Crockard MA, Bjourson AJ, Dazzo FB, Cooper JE. A white clover nodulin gene, dd23b, encoding a cysteine cluster protein, is expressed in roots during the very early stages of interaction with Rhizobium leguminosarum biovar trifolii and after treatment with chitolipooligosaccharide Nod factors. Journal of Plant Research. 2002;115:439–447. doi: 10.1007/s10265-002-0053-7. [DOI] [PubMed] [Google Scholar]
- Curatti L, Ludden PW, Rubio LM. Nif B-dependent in vitro synthesis of the iron–molybdenum cofactor of nitrogenase. Proceedings of the National Academy of Sciences, USA. 2006;104:5207–5301. doi: 10.1073/pnas.0601115103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kok LJ, Durenkamp M, Yang L, Stulen I. Atmospheric sulfur. In: Hawkesford MJ, De Kok LJ, editors. Sulfur in plants: an ecological perspective. Dordrecht: Springer-Verlag; 2007. pp. 91–106. [Google Scholar]
- Desclos M, Etienne P, Coquet L, Cosette P, Bonnefoy J, Segura R, Reze S, Ourry A, Avice J-C. A combined 15N tracing/proteomics study in Brassica napus reveals the chronology of proteomics events associated to N remobilization during leaf senescence induced by nitrate limitation or starvation. Proteomics. 2009;9:3580–3608. doi: 10.1002/pmic.200800984. [DOI] [PubMed] [Google Scholar]
- Durenkamp M, De Kok LJ, Kopriva S. Adenosine 5′-phosphosulfate reductase is regulated differently in Allium cepa L. and Brassica oleracea L. upon exposure to H2S. Journal of Experimental Botany. 2007;58:1571–1579. doi: 10.1093/jxb/erm031. [DOI] [PubMed] [Google Scholar]
- Gilbert MA, Robson AD. The effect of sulfur supply on the root characteristics of subterranean clover and annual ryegrass. Plant and Soil. 1984;77:377–380. [Google Scholar]
- Gordon AJ, Lea PJ, Rosenberg C, Trinchant JC. Nodule formation and function. In: Lea PJ, Morot-Gaudry JF, editors. Plant nitrogen. Berlin, Heidelberg, New York: Springer-Verlag; 2001. pp. 101–146. [Google Scholar]
- Goulas E, Le Dily F, Teissedre L, Corbel G, Robin C, Ourry A. Vegetative storage proteins in white clover (Trifolium repens L.): quantitative and qualitative features. Annals of Botany. 2001;88:789–795. [Google Scholar]
- Habtemichial KH, Singh BR, Aune JB. Wheat response to N2 fixed by faba bean (Vicia faba L.) as affected by sulfur fertilization and rhizobial inoculation in semi-arid southern Ethiopia. Journal of Plant Nutrition and Soil. Sciences. 2007;170:412–418. [Google Scholar]
- Hartwig UA. The regulation of symbiotic N2 fixation: a conceptual model of N feedback from the ecosystem to the gene expression level. Perspectives in Plant Ecology, Evolution and Systematics. 1998;1:92–120. [Google Scholar]
- Hausmann A, Samans B, Lill R, Mühlenhoff U. Cellular and mitochondrial remodeling upon defects in iron–sulfur protein biogenesis. Journal of Biological Chemistry. 2008;283:8318–8330. doi: 10.1074/jbc.M705570200. [DOI] [PubMed] [Google Scholar]
- Hänsch R, Mendel RR. Sulfite oxidation in plant peroxisomes. Photosynthesis Research. 2005;86:337–343. doi: 10.1007/s11120-005-5221-x. [DOI] [PubMed] [Google Scholar]
- Hoefgen R, Nikiforova VJ. Metabolomics integrated with transcriptomics: assessing systems response to sulfur-deficiency stress. Physiologia Plantarum. 2008;132:190–198. doi: 10.1111/j.1399-3054.2007.01012.x. [DOI] [PubMed] [Google Scholar]
- Høgh-Jensen HH. The effect of potassium deficiency on growth and N2-fixation in Trifolium repens. Physiologia Plantarum. 2003;119:440–449. [Google Scholar]
- Høgh-Jensen H, Schjoerring JK. Measurement of biological dinitrogen fixation in grassland: comparison of the enriched 15N dilution and the natural 15N abundance method at different nitrogen application rates and defoliation frequencies. Plant and Soil. 1994;166:153–163. [Google Scholar]
- Høgh-Jensen H, Schjoerring JK, Soussana JF. The influence of phosphorus deficiency on growth and nitrogen fixation of white clover plants. Annals of Botany. 2002;90:754–753. doi: 10.1093/aob/mcf260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmoker JL, Clarkson DT, Saker LR, Rooney JM, Purves JV. Sulphate deprivation depresses the transport of nitrogen to the xylem and osmotic osmotic hydraulic conductance of barley (Hordeum vulgare L.) roots. Planta. 1991;185:269–278. doi: 10.1007/BF00194070. [DOI] [PubMed] [Google Scholar]
- Krusell L, Krause K, Ott T, et al. The sulfate transporter SST1 is crucial for symbiotic nitrogen fixation in Lotus japonicus root nodules. The Plant Cell. 2005;17:1625–1636. doi: 10.1105/tpc.104.030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the heat bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lunde C, Zygadlo A, Simonsen HT, Nielsen PL, Blennow A, Haldrup A. Sulfur starvation in rice: the effect of photosynthesis, carbohydrate metabolism, and oxidative stress protective pathways. Physiologia Plantarum. 2008;134:508–521. doi: 10.1111/j.1399-3054.2008.01159.x. [DOI] [PubMed] [Google Scholar]
- Mechin V, Consoli L, Le Guilloux M, Damerval C. An efficient solubilization buffer for plant proteins focused in immobilized pH gradients. Proteomics. 2003;3:1299–1302. doi: 10.1002/pmic.200300450. [DOI] [PubMed] [Google Scholar]
- Nikiforova VJ, Freitag J, Kempa S, Adamik S, Hesse H, Hoefgen R. Transcriptome analysis of sulfur depletion in Arabidopsis thaliana: interlacing of biosynthesic pathways provides response specificity. The Plant Journal. 2003;33:633–650. doi: 10.1046/j.1365-313x.2003.01657.x. [DOI] [PubMed] [Google Scholar]
- Noquet C, Avice JC, Rossato L, Beauclair P, Henry MP, Ourry A. Effects of altered source–sink relationships on N allocation and vegetative storage protein accumulation in Brassica napus L. Plant Science. 2004;166:1007–1018. [Google Scholar]
- Olivera M, Tejera N, Iribarne, Ocana A, Lluch C. Growth, nitrogen fixation and ammonium assimilation in common bean (Phaseolus vulgaris): effect of phosphorus. Physiologia Plantarum. 2004;121:498–505. [Google Scholar]
- Pacyna S, Schulz M, Scherer HW. Influence of sulphur supply on glucose and ATP concentrations of inoculated broad beans (Vicia faba minor L.) Biology and Fertility of Soils. 2006;42:324–329. [Google Scholar]
- Parsons R, Stanforth A, Raven JA, Sprent JI. Nodule growth and activity may be regulated by feedback mechanism involving phloem nitrogen. Plant, Cell and Environment. 1993;16:125–136. [Google Scholar]
- Prosser IM, Purves JV, Saker LR, Clarkson DT. Rapid disruption of nitrogen metabolism and nitrate transport in spinach plants deprived of sulphate. Journal of Experimental Botany. 2001;52:113–121. [PubMed] [Google Scholar]
- Saito K. Sulfur assimilatory metabolism. The long and smelling road. Plant Physiology. 2004;136:2443–2450. doi: 10.1104/pp.104.046755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer HW. Sulphur in crop production: invited paper. European Journal of Agronomy. 2001;14:81–111. [Google Scholar]
- Scherer HW, Lange A. N2 fixation and growth of legumes as affected by sulphur fertilization. Biology and Fertility of Soils. 1996;23:449–453. [Google Scholar]
- Scherer HW, Pacyna S, Spoth KR, Schulz M. Low levels of ferredoxin, ATP, and leghemoglobin contribute to limited N2 fixation of peas (Pisum sativum L.) and alfalfa (Medicago sativa L.) under S deficiency conditions. Biology and Fertility of Soils. 2008;44:909–916. [Google Scholar]
- Schultze J, Drevon JJ. P-deficiency increases the O2 uptake per N2 in alfalfa. Journal of Experimental Botany. 2005;56:1779–1784. doi: 10.1093/jxb/eri166. [DOI] [PubMed] [Google Scholar]
- Schwedock JS, Long SR. Rhizobium meliloti genes involved in sulfate activation: the two copies of nodPQ and a new locus, saa. Genetics. 1992;132:899–909. doi: 10.1093/genetics/132.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. 3rd edn. New York: WH Freeman and Company; 2003. [Google Scholar]
- Touraine B, Boutin JP, Marion-Poll AM, Briat JF, Peltier G, Lobrréaux S. Nfu:2 a scaffold protein required for (4Fe-4S) and ferredoxin iron-sulphur cluster assembly in Arabidopsis chloroplasts. The Plant Journal. 2004;40:101–111. doi: 10.1111/j.1365-313X.2004.02189.x. [DOI] [PubMed] [Google Scholar]
- Tallec T, Diquélou S, Lemauviel S, Cliquet JB, Lesuffleur F, Ourry A. Nitrogen:sulfur ratio alters competition between Trifolium repens and Lolium perenne under cutting: production and competitive abilities. European Journal of Agronomy. 2008;29:94–101. [Google Scholar]
- Vadez V, Sinclair TR, Serraj R. Asparagine and ureide accumulation in nodules and shoots as feedback inhibitors of N2 fixation in soybean. Physiologia Plantarum. 2000;110:215–223. [Google Scholar]
- Van Der Kooij TAW, De Kok LJ, Haneklaus S, Schnug E. Uptake and metabolism of sulphur dioxide by Arabidopsis thaliana. New Phytologist. 1997;135:101–107. doi: 10.1046/j.1469-8137.1997.00619.x. [DOI] [PubMed] [Google Scholar]
- Varin S, Lemauviel-Lavenant S, Cliquet JB, Diquélou S, Michaelson-Yeates TPT. Functional plasticity of Trifolium repens L. in response to sulphur and nitrogen availability. Plant and Soil. 2009a;317:189–200. [Google Scholar]
- Varin S, Leveel B, Lemauviel-Lavenant S, Cliquet JB. Does the white clover response to sulphur availability correspond to phenotypic or ontogenetic plasticity? Acta Oecologica. 2009b;35:452–457. [Google Scholar]
- Wang W, Scali M, Vignani R, Spadafora A, Sensi E, Mazzuca S, Cresto M. Protein extraction for two-dimensional electrophoresis from olive leaf, a plant tissue containing high levels of interfering compounds. Electrophoresis. 2003;24:2369–2375. doi: 10.1002/elps.200305500. [DOI] [PubMed] [Google Scholar]
- Zanetti S, Hartwig UA, Nosberger J. Elevated atmospheric CO2 does not affect per se the perference for symbiotic nitrogen as opposed to mineral nitrogen of Trifolium repens L. Plant, Cell and Environment. 1998;21:623–630. [Google Scholar]
- Zhao FJ, Wood AP, McGrath SP. Effects of sulphur nutrition on growth and nitrogen fixation of pea (Pisum sativum L.) Plant and Soil. 1999;212:209–219. [Google Scholar]