Abstract
We review functional MRI and other neuroimaging studies of language skills in children from infancy to adulthood. These studies show developmental changes in the networks of brain regions supporting language, which can be affected by brain injuries or neurological disorders. Particular aspects of language rely on networks that lateralize to the dominant hemisphere; others rely on bilateral or non-dominant mechanisms. Multiple fMRI tasks for pediatric patients characterize functional brain reorganization that may accompany language deficits.
Keywords: Language development, fMRI, brain development, child language, pediatric neuroimaging
Left hemisphere lateralization of language is a well-established concept in functional neuroanatomy supported by the presence of structural brain asymmetries in anterior and posterior language areas [1-3]. Such asymmetries are present prenatally [4, 5] which is suggestive of genetic underpinning of the left-hemispheric language lateralization bias.
Consistent with this, lesional and functional magnetic resonance imaging (fMRI) studies in adults have clearly shown a left-hemisphere dominance for language: left perisylvian regions in the frontal, and temporal and parietal lobes contribute to networks supporting many components of language processing, including word recognition, syntax, and semantics [6-9]. In addition to this left-hemisphere specialization, processing prosodic information associated with speech has been shown to rely more on right-hemispheric mechanisms [10-13]. The developmental trajectory of these specialized networks is becoming more clearly understood through imaging studies in children. For example, a recent functional MRI study by Dehaene-Lambertz et al. [14] demonstrated that left hemisphere dominance of language function may already be present in infancy; another study [15] that utilized optical topography revealed that left-lateralization of language is already present in neonates. Also supporting the notion of early functional specialization are magnetoencephalography (MEG) results [16], showing that the left inferior frontal region discriminated speech sounds as early as 6 months of age. Redcay et al. [17] used fMRI during passive listening in sleeping toddlers and 3-year-olds and found that while both age groups activated superior temporal primary and secondary auditory cortices, the younger group showed increased activation in frontal, occipital, and cerebellar regions. The authors suggested that these regions were supporting the rapid language acquisition processes during that stage of development.
A number of other neuroimaging studies have focused on school-age children: for example, a recent fMRI study [18] suggested that 6-year-olds, during passive listening to sentences, display activation time courses that peak later than adults. Children also showed a longer time delay between temporal and frontal activation than adults. The authors suggest that this is evidence of slower and less automatic language processes. In a large cross-sectional group of typically developing children ages 5−18, Holland et al. demonstrated increasing specialization of language functions to the left hemisphere as age increases. However, lateralization changes were more closely tied to the period of acquisition for language tasks than to general maturation. The largest changes in lateralization occurred for skills that are acquired over a longer period of development (such as verb generation), since children continue to improve their ability to form and retrieve semantic associations into adulthood. Likewise, age-related changes in lateralization were observed in a story processing task, which required children to process syntactic structures that are mastered in mid-childhood (more details regarding this task are presented below). In contrast, changes in lateralization with age were smallest (and statistically non-significant) for tasks designed to reflect early-acquired language skills, such as word-picture matching with early-acquired vocabulary [19]. In this same group of participants, using a prosody recognition task, Plante et al. [20] showed a trend toward more rightward lateralization with age. A few other studies have also supported a right-hemisphere specialization for prosody in childhood [21, 22]. All of these imaging studies suggest that some degree of hemispheric specialization is present throughout development, but in younger children this specialization may be less well-defined.
Effects of neuropathology on functional MRI of language
In a clinical context, information regarding lateralization of language function is used in neurosurgical planning in both children and adults [5, 23-28], so that resection of the eloquent regions can be avoided. In addition, fMRI may reveal changes in the network of brain regions supporting language skills related to seizure activity or injury. For example, in adult patients with left middle cerebral artery (LMCA) infarctions functional recovery is marked by either increases in activation in either right hemisphere homologues of classical left hemisphere language cortex or in the areas adjacent the area damaged by the stroke. In children, studies suggest that early insult to the left hemisphere may induce right hemisphere reorganization similar to adults [29-34]. Jacola et al. [35] and later Tillema et al. [36] examined pediatric patients with LMCA strokes occurring in the perinatal period using fMRI and the verb generation task, and confirmed that these patients show preferential right hemisphere activation, supporting the idea that a major left-hemisphere injury early in development has made the full extent of left-lateralization (as shown in the normal population) very unlikely. Patients in one of these studies also had lower full-scale and verbal IQ, suggesting a either a relationship between atypical language organization in the brain and decreases in language skill or the effect of prenatal or perinatal stroke on general intelligence [35].
Brain injuries occurring later in development may also have an impact on language networks, but reduced neuroplasticity with age may result in decreased potential for reorganization. Previous studies have documented deficits in expressive and receptive language skills [37, 38] following traumatic brain injury (TBI) in children. Because language skills are undergoing rapid change during early childhood, they may be particularly vulnerable to disruption by brain trauma. Karunanayaka et al. [39] examined the effects of traumatic brain injury on the functional networks supporting language, attention, and working memory in children. Results revealed significantly different levels of fMRI activation in perisylvian language areas between the groups of children with history of traumatic brain injury vs. children with other, not cranial injury. These authors also found significant associations between the fMRI activation and performance on language-specific neuropsychological and Glasgow Coma Scale (GCS) score. In addition, a subtle rightward shift in lateralization in language regions was observed in TBI patients with subtle brain trauma not associated with any white matter tract injury suggesting that even minor injury may lead to significant brain plasticity.
The neural circuitry of language may also be affected by chronic neuropathology, in particular, seizure activity in epilepsy [23, 40, 41]. Unlike stroke or TBI patients whose injury occurs at a particular point in development, seizure activity produces a more protracted and chronic injury to the brain. Adult data suggest that chronic epilepsy can exert profound and long-lasting impact on language functions, resulting in decrease in language skills as measured by neuropsychological tests of language function and academic achievement [42-45]. As in other populations with localized brain injury, an atypical language activation pattern has been observed in many adult fMRI studies [23, 24, 40, 46-48]. Specifically, early onset of epilepsy originating from the left hemisphere may lead to a bilateral or even right-lateralized distribution of language functions [23, 40, 48-50].
Focusing on pediatric patients, Yuan et al. [41] used fMRI to examine language lateralization using a silent verb-generation task in 18 epilepsy patients and compared them to 18 age/gender/handedness-matched healthy participants. The children with epilepsy were much more likely to show atypical lateralization, and there was a trend toward increasing left-lateralization with age in healthy controls that was not observed in the epilepsy patients. In another study of patients with epilepsy, Breier et al. [51], using MEG in a group of patients ages 9−45 years, found that the degree of atypical language lateralization was correlated with lower scores on tests of reading and spelling in patients with left-hemisphere-onset partial seizures.
These results in children with stroke, TBI, and epilepsy suggest a relationship between changes in the functional neuroanatomy of language, and decreases in language skills in children with neuropathology. Functional MRI during language tasks is an effective methodology for visualizing these changes.
A battery of functional MRI language tasks for activation across hemispheres
In both adult and pediatric groups, as discussed above, a number of language tasks have been employed to identify language processing regions in the dominant hemisphere, such as verb generation [52-54] object naming, sentence comprehension, or single-word listening or reading [27, 55-58]. These fMRI studies, in general, revealed a left-lateralized language network of frontal and temporal cortices (the traditional Broca's and Wernicke's areas). Here, we focus on one of these left-lateralizing language comprehension tasks, semantic decision, along with two other tasks that examine additional aspects of language processing in order to engage bilateral and right-hemispheric language circuits. These tasks were chosen to be easy for children to complete, and to elicit activation in a relatively short scan time.
Participants and Methods
Participants in our implementations of the three language tasks were 19 children and young adults (12 females) ages 7−30 (ten participants younger than 13, four ages 13−17, five age 18 and up). All were right-handed native speakers of English with no history of neuropsychological or learning disorders. Not all of the participants completed all three of the language tasks successfully; they either exited early from the scanner (N = ...) or their data had to be removed from the analysis due to excessive motion (N = ...).
MRI methods
Three functional MRI paradigms were included in the scan session for each participant (details below). Scanning was performed on a 3T Bruker Biospec 30/60 MRI scanner (fMRI parameters: TR/TE = 2000/38 ms, FOV 25.6 × 25.6 cm, matrix 64 × 64, slice thickness = 5 mm, resulting in a voxel size of 4×4×5mm, 25 axial slices. For the story processing task the TR was 3000ms). MRI data analysis was performed using routines written in IDL (Interactive Data Language, (ITT Visual Information Solutions, Boulder, CO). The echo planar images (EPI) were corrected for Nyquist ghosts and geometric distortion using the multi-echo reference method, and retrospectively corrected for motion using a pyramid co-registration algorithm and spatially normalized into Talairach space. A general linear model and a paired t-test were implemented to identify voxels activated by each task for each participant. After Talairach transformation, random-effects analysis was performed to determine significant group activations.
Semantic decision/Tone decision (SDTD) task
In this task, participants hear single words and make a button-press response if the item has target semantic properties, alternating with a control tone decision task where participants responded with a button-press to a target sequence of tones. This and similar tasks are known to activate numerous brain areas involved in language processing, including left inferior frontal and prefrontal cortex, superior and middle temporal regions; adult studies have also elicited activation in medial temporal regions, angular and posterior cingulate [23, 27, 55, 56, 58]. The clinical utility of this fMRI task in presurgical evaluation has been established in adults: one fMRI study utilizing the SDTD task in patients with left temporal lobe epilepsy has already shown 100% sensitivity and 73% specificity of this task in predicting significant naming decline after epilepsy surgery when language was lateralized to the left hemisphere [27]. Other study recently showed that language lateralization determined with fMRI and the SDTD strongly correlated with results from verb generation fMRI as well as intracarotid amobarbital procedure (IAP; Wada test) [27]. In healthy children 5−10 years old, Balsamo et al. [59] found a left-lateralized pattern of activation using a similar task, and our preliminary data below confirm this pattern. Recently, Blumenfeld et al. [60] examined a semantic judgment task in a group of children ages 9−11 years, and found correlations between level of activation and performance accuracy on the task. This fMRI task is designed to identify patients’ dominant hemisphere for language and localize basic language processing regions, and therefore detect potential changes in this architecture resulting from epileptic activity or injury.
In the pediatric version of the task we have implemented, 16 participants ages 7−30 heard single words (animal names) and made a button-press response if the item had target semantic properties (i.e. the animal walks on four legs). This semantic decision task was presented in 5 40-second blocks alternating with tone decisions. Eight stimuli were included in each block and performance data from button-press responses was recorded. Areas of activation in this task in both groups included the cerebellum, left middle frontal gyrus and left inferior frontal gyrus. This left-lateralized pattern of activation is consistent with previous studies employing a similar task in healthy adults [23, 27, 55, 56].
Along with demonstrating lateralization to the dominant hemisphere, fMRI has shown that a number of language functions in children and adults are supported by bilateral networks, as discussed above. Including additional neuroimaging tasks that activate wider language networks offers a greater opportunity to understand possible changes in cortical language organization across hemispheres. In addition, multiple language tasks may better characterize the extent of eloquent cortex that may be near cortical regions targeted for surgical resection [23, 61].
Processing Spoken Stories
The ability to process an aurally-presented story is supported by a network of auditory and language-processing brain regions. Multiple aspects of language processing are engaged during this naturalistic speech task, including speech perception, word recognition, syntactic processing and discourse coherence. Due to the complexity of auditory input that comprises natural speech processing, listening to speech sounds relative to non-speech sounds engages bilateral primary and secondary auditory processing regions when observed with fMRI [63, 64]. Previous fMRI studies have examined the neural basis of story processing in children, using a block-periodic, passive listening design contrasting short stories with tone sequences [39, 65-67]. Cross-sectional studies of story comprehension in children ages 5−18 revealed multiple neural components and functionally connected regions that participate in the narrative comprehension.[65, 68] These authors postulated, based on results from 313 subjects and analysis via independent components analysis, that story comprehension involves several consecutive processes located in various anatomical and functional brain areas; it progresses through several consecutive steps that include bilateral acoustic processing (bilateral superior temporal gyri; BA 42), semantic processing (bilateral superior temporal gyri; BA 22 [8]), left-lateralized fronto-temporal language network activation (either related to covert speech generation,[54, 65, 69], syntactic processing at the sentence level,[70] or semantic decision and subvocal rehearsal, [71]) re-processing in the superior temporal gyrus (non-domain-specific integrative process) and, finally, higher-order semantic processing occurring in the bilateral angular gyri.[65]. So while not all the networks supporting the many processing components of the story processing task are bilateral, the majority are, and therefore this task should identify shifts in language lateralization contralateral to the hemisphere less affected by injury or epileptic activity.
Vannest et al. [40] compared a block-design passive listening story paradigm to an active-response version including on-line performance monitoring (where participants answered interspersed comprehension question about the story) and a sparse acquisition technique. Both passive listening and active-response story processing tasks resulted in similar patterns of activation in the primary auditory cortex, superior temporal gyrus bilaterally, and left inferior frontal gyrus supporting multiple aspects of language processing in children. There was increased activation in dorsolateral prefrontal cortex during the active-response task, which was likely associated with maintenance and manipulation of sentence segments in working memory across acquisition intervals in order to construct the complete story and answer interspersed questions [72].
For children that are able to perform an active response version of the Story Listening task, being able to map this more extensive network may be beneficial. However, a passive story listening task also reveals brain regions involved in language processing, producing the same degree of lateralization and an equally large effect size in the temporal language area. This paradigm, therefore, may be appropriate for younger children or others who cannot perform the comprehension task. Young pediatric participants, some of whom require sedation to undergo an MRI procedure, may not be capable of actively generating responses to language stimuli. A passive listening task has already been demonstrated to activate bilateral auditory processing areas in such patients [17, 73] and may still be used to yield meaningful measures of language activation and lateralization.
Our recent implementation of the passive story listening task in the group of children and young adults replicates these existing studies. This task, as in previous studies from our group [65, 68] was based on a periodic 30s on–off, block design. A different story, read by an adult female speaker, was presented during each 30s on period (active). Each 30-second story contained 10 sentences with simple and a variety of syntactic constructions, and was designed by a Speech-Language pathologist to be appropriate for children 5 and up (audio tracks of these stories are available for download at http://www.irc.cchmc.org/software/pedaudio.php). During the control epochs, 1s duration tones were presented at random frequencies (400− 2500 Hz) and intervals (1−3 s), to control for sublexical auditory processing. Five cycles of active and control stimuli were presented for a total scan time of 5 minutes.
Areas of activation in this group include bilateral superior and middle temporal regions in both groups which is consistent with a number of previous results in children and adults (refs).
Prosody discrimination
Discrimination of linguistic prosody (e.g., identifying statements versus questions when the lexical content is the same) has been shown to rely on bilateral frontal and temporal mechanisms [12, 13], though language lateralization with this task has not yet been extensively tested in children. Recently, Plante et al. [74] used a prosody-matching task in fMRI to show bilateral insular, inferior/middle frontal, precentral sulcus, superior temporal gyrus and anterior occipital cortex activations while Wartenburger et al. found right frontal activation using optical topography as a result of perceiving prosody [22].
In our pediatric-appropriate task [13], participants were presented with audiovisual sentence stimuli and indicated with a button press whether the sentence is a statement or question (the sentences did not include interrogative words, so the decision regarding whether the sentence is a question or statement must be based on prosody alone). Thirty sentences of each type were presented, counterbalanced for sentence type in blocks of 5. The prosody discrimination task alternated with a control semantic judgment task (indicate whether or not the sentence discussed a person). This control task included the same sentence materials as the active task, again arranged into blocks of 5 and counterbalanced so that identical sentence stimuli (appearing once in the prosody discrimination task and once in the semantic task) were separated by 6 blocks. This task was completed in approximately 10 minutes.
Areas of increased BOLD activation included right superior temporal gyrus, and right inferior frontal gyrus extending into middle frontal gyrus. This right-lateralized network of activation is consisted with other results in the literature employing similar tasks [13].
Discussion
For studies of language lateralization using fMRI in children, a combination of language tasks is advantageous. Use of a single language paradigm with fMRI may not adequately reveal hemispheric and regional organization of language, particularly in the developing brain. The fMRI tasks described above are designed to activate left-dominant, bilateral, and right-dominant language processing networks, making it possible to examine functional reorganization that may accompany language deficits in patients with brain injury or chronic seizures. This data may have clinical utility in surgical planning. Semantic decision, story listening, and prosody discrimination are tasks suitable for children and can be completed in a single scan session, producing activation patterns that span both hemispheres.
Some drawbacks to the fMRI technique for language localization are that it has relatively poor sensitivity for single subject studies. While overall patterns such as lateralization have proven repeatable in individuals, a high level of variability is present, both within and between participants, and this may make interpretation of individual subject data difficult. Use of fMRI data in combination with another imaging technique, such as magnetoencephalography, may offer the most precise localization. Also, the multiple-task approach discussed above assumes that children will be able to be compliant in the MRI scanner for the duration of 3 tasks. This may be difficult for younger children. Byars et al. [75] discuss this issue of compliance based on experience scanning children across a range of ages, and address some practical considerations in scanning children. As discussed above, the story listening task elicits activation even in sedated or sleeping children and may be useful in many cases. Transcranial doppler ultrasound or optical tomography may not require the level of participant compliance that fMRI involves but reliability of these techniques is not documented, and localization methods are poor at this stage [15, 76-78].
fMRI offers a non-invasive, relatively child-friendly method for visualizing the impact of ictal or anatomical lesions on the functional distribution of language in the brain , and developmental changes in typically-developing children have been characterized [79], offering an age- adjusted context in which to view neuropathology. When pediatric participants are able to complete multiple language tasks, left, right, and bilaterally distributed language networks can be most accurately assessed.
Figure 1.
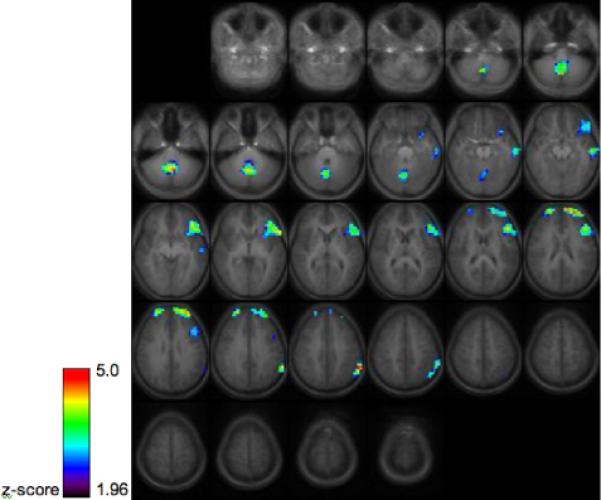
Group activation map for 16 healthy children and young adults performing the semantic decision task, thresholded at z=1.96, cluster size 30 (p<.05 corrected via Monte Carlo simulation). Images are in radiological orientation (the left hemisphere appears on the right side of the image.)
Figure 2.
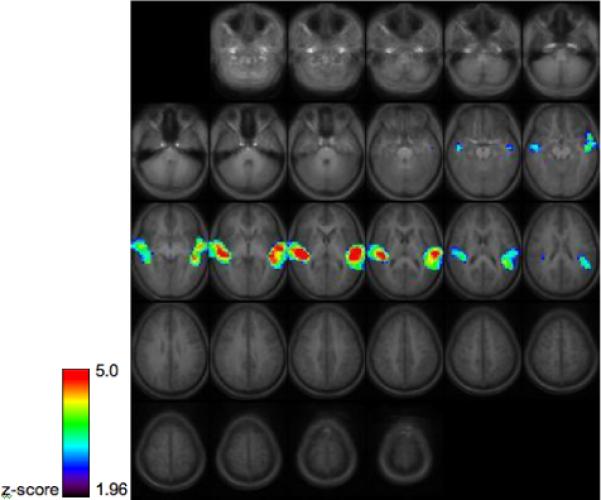
Group activation map for 14 healthy children and young adults performing the story processing task. Images are thresholded at z=1.96, cluster size 30 (p<.05 corrected via Monte Carlo simulation) Images are in radiological orientation (the left hemisphere appears on the right side of the image.)
Figure 3.
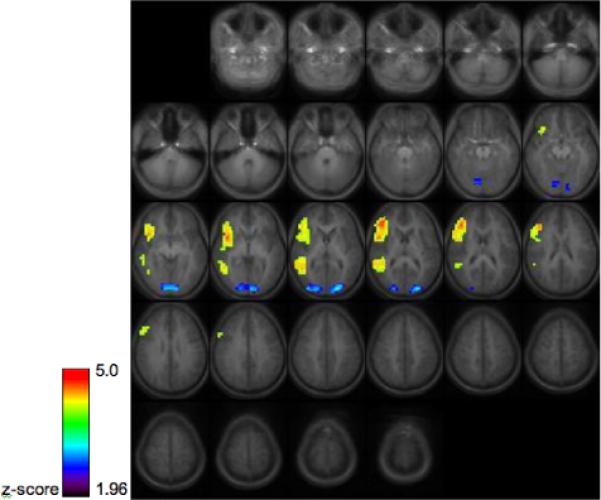
Group activation maps for 15 healthy children and young adults performing the prosody discrimination task, thresholded at z>1.96, cluster size 30 (p<.05 corrected via Monte Carlo simulation) Images are in radiological orientation (the left hemisphere appears on the right side of the image.)
References
- 1.Levistsky W, Geschwind N. Asymmetries of the right and left hemisphere in man. Transactions of the American Neurological Association. 1968;93:232–233. [PubMed] [Google Scholar]
- 2.Falzi G, Perrone P, Vignolo LA. Right-left asymmetry in anterior speech region. Archives of neurology. 1982;39:239–240. doi: 10.1001/archneur.1982.00510160045009. [DOI] [PubMed] [Google Scholar]
- 3.Albanese E, Merlo A, Albanese A, Gomez E. Anterior speech region. Asymmetry and weight-surface correlation. Archives of neurology. 1989;46:307–310. doi: 10.1001/archneur.1989.00520390073019. [DOI] [PubMed] [Google Scholar]
- 4.Chi JG, Dooling EC, Gilles FH. Left-right asymmetries of the temporal speech areas of the human fetus. Arch Neurol. 1977;34:346–348. doi: 10.1001/archneur.1977.00500180040008. [DOI] [PubMed] [Google Scholar]
- 5.Wada JA, Clarke R, Hamm A. Cerebral hemispheric asymmetry in humans. Cortical speech zones in 100 adults and 100 infant brains. Arch Neurol. 1975;32:239–246. doi: 10.1001/archneur.1975.00490460055007. [DOI] [PubMed] [Google Scholar]
- 6.Bookheimer S. Functional MRI of language: New approaches to understanding the cortical organization of semantic processing. Annual Review of Neuroscience. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- 7.Friederici AD. Towards a neural basis of auditory sentence processing. Trends in cognitive sciences. 2002;6:78–84. doi: 10.1016/s1364-6613(00)01839-8. [DOI] [PubMed] [Google Scholar]
- 8.Friederici AD, Ruschemeyer SA, Hahne A, Fiebach CJ. The role of left inferior frontal and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes. Cereb Cortex. 2003;13:170–177. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- 9.Martin RC. Language processing: Functional organization and neuroanatomical basis. Annual Review of Psychology. 2003;54:55–89. doi: 10.1146/annurev.psych.54.101601.145201. [DOI] [PubMed] [Google Scholar]
- 10.Meyer M, Alter K, Friederici AD, Lohmann G, von Cramon DY. FMRI reveals brain regions mediating slow prosodic modulations in spoken sentences. Hum Brain Mapp. 2002;17:73–88. doi: 10.1002/hbm.10042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zatorre RJ, Belin P, Penhune VB. Structure and function of auditory cortex: music and speech. Trends in cognitive sciences. 2002;6:37–46. doi: 10.1016/s1364-6613(00)01816-7. [DOI] [PubMed] [Google Scholar]
- 12.Baum SR, Dwivedi VD. Sensitivity to prosodic structure in left- and right-hemisphere-damaged individuals. Brain Lang. 2003;87:278–289. doi: 10.1016/s0093-934x(03)00109-3. [DOI] [PubMed] [Google Scholar]
- 13.Gandour J, Dzemidzic M, Wong D, et al. Temporal integration of speech prosody is shaped by language experience: an fMRI study. Brain & Language. 2003;84:318–336. doi: 10.1016/s0093-934x(02)00505-9. [DOI] [PubMed] [Google Scholar]
- 14.Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science (New York, NY. 2002;298:2013–2015. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- 15.Pena M, Maki A, Kovacic D, et al. Sounds and silence: an optical topography study of language recognition at birth. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11702–11705. doi: 10.1073/pnas.1934290100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imada T, Zhang Y, Cheour M, Taulu S, Ahonen A, Kuhl PK. Infant speech perception activates Broca's area: a developmental magnetoencephalography study. Neuroreport. 2006;17:957–962. doi: 10.1097/01.wnr.0000223387.51704.89. [DOI] [PubMed] [Google Scholar]
- 17.Redcay E, Haist F, Courchesne E. Functional neuroimaging of speech perception during a pivotal period in language acquisition. Developmental science. 2008;11:237–252. doi: 10.1111/j.1467-7687.2008.00674.x. [DOI] [PubMed] [Google Scholar]
- 18.Brauer J, Neumann J, Friederici AD. Temporal dynamics of perisylvian activation during language processing in children and adults. NeuroImage. 2008;41:1484–1492. doi: 10.1016/j.neuroimage.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmithorst VJ, Holland SK, Plante E. Object identification and lexical/semantic access in children: a functional magnetic resonance imaging study of word-picture matching. Hum Brain Mapp. 2007;28:1060–1074. doi: 10.1002/hbm.20328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plante E, Holland SK, Schmithorst VJ. Prosodic processing by children: an fMRI study. Brain Lang. 2006;97:332–342. doi: 10.1016/j.bandl.2005.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Homae F, Watanabe H, Nakano T, Asakawa K, Taga G. The right hemisphere of sleeping infant perceives sentential prosody. Neuroscience research. 2006;54:276–280. doi: 10.1016/j.neures.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Wartenburger I, Steinbrink J, Telkemeyer S, Friedrich M, Friederici AD, Obrig H. The processing of prosody: Evidence of interhemispheric specialization at the age of four. NeuroImage. 2007;34:416–425. doi: 10.1016/j.neuroimage.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Szaflarski JP, Holland SK, Jacola LM, Lindsell C, Privitera MD, Szaflarski M. Comprehensive presurgical functional MRI language evaluation in adult patients with epilepsy. Epilepsy Behav. 2008;12:74–83. doi: 10.1016/j.yebeh.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adcock JE, Wise RG, Oxbury JM, Oxbury SM, Matthews PM. Quantitative fMRI assessment of the differences in lateralization of language-related brain activation in patients with temporal lobe epilepsy. NeuroImage. 2003;18:423–438. doi: 10.1016/s1053-8119(02)00013-7. [DOI] [PubMed] [Google Scholar]
- 25.Benson RR, FitzGerald DB, LeSueur LL, et al. Language dominance determined by whole brain functional MRI in patients with brain lesions. Neurology. 1999;52:798–809. doi: 10.1212/wnl.52.4.798. [DOI] [PubMed] [Google Scholar]
- 26.Desmond J, Sum J, Wagner A, et al. Functional MRI Measurment of Language Lateralization in Wada-Tested Patients. Brain. 1995;118:1411–1419. doi: 10.1093/brain/118.6.1411. [DOI] [PubMed] [Google Scholar]
- 27.Sabsevitz DS, Swanson SJ, Hammeke TA, et al. Use of preoperative functional neuroimaging to predict language deficits from epilepsy surgery. Neurology. 2003;60:1788–1792. doi: 10.1212/01.wnl.0000068022.05644.01. [DOI] [PubMed] [Google Scholar]
- 28.Wada J, Rasmussen T. Intracarotid injection of sodium amytal for the lateralization of cerebral speech dominance. Jouranl of Neurosurgery. 1960;17:266–282. doi: 10.3171/jns.2007.106.6.1117. [DOI] [PubMed] [Google Scholar]
- 29.Muller RA, Watson CE, Muzik O, Chakraborty PK, Chugani HT. Motor organization after early middle cerebral artery stroke: a PET study. Pediatric neurology. 1998;19:294–298. doi: 10.1016/s0887-8994(98)00049-6. [DOI] [PubMed] [Google Scholar]
- 30.Brizzolara D, Pecini C, Brovedani P, Ferretti G, Cipriani P, Cioni G. Timing and type of congenital brain lesion determine different patterns of language lateralization in hemiplegic children. Neuropsychologia. 2002;40:620–632. doi: 10.1016/s0028-3932(01)00158-0. [DOI] [PubMed] [Google Scholar]
- 31.Booth JR, MacWhinney B, Thulborn KR, Sacco K, Voyvodic JT, Feldman HM. Developmental and lesion effects in brain activation during sentence comprehension and mental rotation. Dev Neuropsychol. 2000;18:139–169. doi: 10.1207/S15326942DN1802_1. [DOI] [PubMed] [Google Scholar]
- 32.Papanicolaou AC, Simos PG, Breier JI, et al. Brain plasticity for sensory and linguistic functions: a functional imaging study using magnetoencephalography with children and young adults. J Child Neurol. 2001;16:241–252. doi: 10.1177/088307380101600403. [DOI] [PubMed] [Google Scholar]
- 33.Staudt M, Grodd W, Niemann G, Wildgruber D, Erb M, Krageloh-Mann I. Early left periventricular brain lesions induce right hemispheric organization of speech. Neurology. 2001;57:122–125. doi: 10.1212/wnl.57.1.122. [DOI] [PubMed] [Google Scholar]
- 34.Lidzba K, Staudt M, Wilke M, Grodd W, Krageloh-Mann I. Lesion-induced right-hemispheric language and organization of nonverbal functions. Neuroreport. 2006;17:929–933. doi: 10.1097/01.wnr.0000221841.12632.d6. [DOI] [PubMed] [Google Scholar]
- 35.Jacola LM, Schapiro MB, Schmithorst VJ, et al. Functional magnetic resonance imaging reveals atypical language organization in children following perinatal left middle cerebral artery stroke. Neuropediatrics. 2006;37:46–52. doi: 10.1055/s-2006-923934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tillema JM, Byars AW, Jacola LM, et al. Cortical reorganization of language functioning following perinatal left MCA stroke. Brain Lang. 2008;105:99–111. doi: 10.1016/j.bandl.2007.07.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson VA, Morse SA, Klug G, et al. Predicting recovery from head injury in young children: a prospective analysis. J Int Neuropsychol Soc. 1997;3:568–580. [PubMed] [Google Scholar]
- 38.Morse S, Haritou F, Ong K, Anderson V, Catroppa C, Rosenfeld J. Early effects of traumatic brain injury on young children's language performance: a preliminary linguistic analysis. Pediatr Rehabil. 1999;3:139–148. doi: 10.1080/136384999289405. [DOI] [PubMed] [Google Scholar]
- 39.Karunanayaka PR, Holland SK, Yuan W, et al. Neural substrate differences in language networks and associated language-related behavioral impairments in children with TBI: A preliminary fMRI investigation. NeuroRehabilitation. 2007;22:355–369. [PMC free article] [PubMed] [Google Scholar]
- 40.Springer JA, Binder JR, Hammeke TA, et al. Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain. 1999;122:2033–2046. doi: 10.1093/brain/122.11.2033. [DOI] [PubMed] [Google Scholar]
- 41.Yuan W, Szaflarski JP, Schmithorst VJ, et al. fMRI shows atypical language lateralization in pediatric epilepsy patients. Epilepsia. 2006;47:593–600. doi: 10.1111/j.1528-1167.2006.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Austin JK, Huberty TJ, Huster GA, Dunn DW. Academic achievement in children with epilepsy or asthma. Dev Med Child Neurol. 1998;40:248–255. doi: 10.1111/j.1469-8749.1998.tb15457.x. [DOI] [PubMed] [Google Scholar]
- 43.Austin JK, Huberty TJ, Huster GA, Dunn DW. Does academic achievement in children with epilepsy change over time? Dev Med Child Neurol. 1999;41:473–479. [PubMed] [Google Scholar]
- 44.Hermann BP, Seidenberg M, Schoenfeld J, Davies K. Neuropsychological characteristics of the syndrome of mesial temporal lobe epilepsy. Arch Neurol. 1997;54:369–376. doi: 10.1001/archneur.1997.00550160019010. [see comment] [DOI] [PubMed] [Google Scholar]
- 45.Schoenfeld J, Seidenberg M, Woodard A, et al. Neuropsychological and behavioral status of children with complex partial seizures. Dev Med Child Neurol. 1999;41:724–731. doi: 10.1017/s0012162299001486. [see comment] [DOI] [PubMed] [Google Scholar]
- 46.Gaillard WD, Balsamo L, Xu B, et al. Language dominance in partial epilepsy patients identified with an fMRI reading task. Neurology. 2002;59:256–265. doi: 10.1212/wnl.59.2.256. [see comment] [DOI] [PubMed] [Google Scholar]
- 47.Billingsley RL, McAndrews MP, Crawley AP, Mikulis DJ. Functional MRI of phonological and semantic processing in temporal lobe epilepsy. Brain. 2001;124:1218–1227. doi: 10.1093/brain/124.6.1218. [DOI] [PubMed] [Google Scholar]
- 48.Brazdil M, Zakopcan J, Kuba R, Fanfrdlova Z, Rektor I. Atypical hemispheric language dominance in left temporal lobe epilepsy as a result of the reorganization of language functions. Epilepsy Behav. 2003;4:414–419. doi: 10.1016/s1525-5050(03)00119-7. [DOI] [PubMed] [Google Scholar]
- 49.Saltzman J, Smith ML, Scott K. The impact of age at seizure onset on the likelihood of atypical language representation in children with intractable epilepsy. Brain Cogn. 2002;48:517–520. [PubMed] [Google Scholar]
- 50.Saltzman-Benaiah J, Scott K, Smith ML. Factors associated with atypical speech representation in children with intractable epilepsy. Neuropsychologia. 2003;41:1967–1974. doi: 10.1016/s0028-3932(02)00323-8. [DOI] [PubMed] [Google Scholar]
- 51.Breier JI, Castillo EM, Simos PG, et al. Atypical language representation in patients with chronic seizure disorder and achievement deficits with magnetoencephalography. Epilepsia. 2005;46:540–548. doi: 10.1111/j.0013-9580.2005.48904.x. [DOI] [PubMed] [Google Scholar]
- 52.Holland SK, Plante E, Weber Byars A, Strawsburg RH, Schmithorst VJ, Ball WS., Jr. Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage. 2001;14:837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- 53.Szaflarski JP, Schmithorst VJ, Altaye M, et al. A longitudinal functional magnetic resonance imaging study of language development in children 5 to 11 years old. Ann Neurol. 2006;59:796–807. doi: 10.1002/ana.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Szaflarski JP, Holland SK, Schmithorst VJ, Byars AW. fMRI study of language lateralization in children and adults. Hum Brain Mapp. 2006;27:202–212. doi: 10.1002/hbm.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. Journal of Neuroscience. 1997;17:353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Binder JR, Swanson SJ, Hammeke TA, et al. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology. 1996;46:978–984. doi: 10.1212/wnl.46.4.978. [DOI] [PubMed] [Google Scholar]
- 57.Lee SS, Dapretto M. Metaphorical vs. literal word meanings: fMRI evidence against a selective role of the right hemisphere. Neuroimage. 2006;29:536–544. doi: 10.1016/j.neuroimage.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 58.Szaflarski JP, Binder JR, Possing ET, McKiernan KA, Ward BD, Hammeke TA. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology. 2002;59:238–244. doi: 10.1212/wnl.59.2.238. [DOI] [PubMed] [Google Scholar]
- 59.Balsamo LM, Xu B, Grandin CB, et al. A functional magnetic resonance imaging study of left hemisphere language dominance in children. Archives of neurology. 2002;59:1168–1174. doi: 10.1001/archneur.59.7.1168. [DOI] [PubMed] [Google Scholar]
- 60.Blumenfeld HK, Booth JR, Burman DD. Differential prefrontal-temporal neural correlates of semantic processing in children. Brain and language. 2006;99:226–235. doi: 10.1016/j.bandl.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilke M, Lidzba K, Staudt M, Buchenau K, Grodd W, Krageloh-Mann I. An fMRI task battery for assessing hemispheric language dominance in children. Neuroimage. 2006;32:400–410. doi: 10.1016/j.neuroimage.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 62.**Szaflarski JP, Holland SK, Jacola LM, Lindsell C, Privitera MD, Szaflarski M. Comprehensive presurgical functional MRI language evaluation in adult patients with epilepsy. Epilepsy Behav. 2008;12:74–83. doi: 10.1016/j.yebeh.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Belin P, Zatorre RJ. Adaptation to speaker's voice in right anterior temporal lobe. Neuroreport: For Rapid Communication of Neuroscience Research. 2003;14:2105–2109. doi: 10.1097/00001756-200311140-00019. [DOI] [PubMed] [Google Scholar]
- 64.Poeppel DaH G. Towards a new functional anatomy of language. Cognition. 2004;92:1–12. doi: 10.1016/j.cognition.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 65.Schmithorst VJ, Holland SK, Plante E. Cognitive modules utilized for narrative comprehension in children: a functional magnetic resonance imaging study. Neuroimage. 2006;29:254–266. doi: 10.1016/j.neuroimage.2005.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmithorst VJ, Holland SK, Plante E. Development of effective connectivity for narrative comprehension in children. Neuroreport. 2007;18:1411–1415. doi: 10.1097/WNR.0b013e3282e9a4ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vannest J, Karunanayaka P, Altaye M, Schmithorst VJ, Plante EM, Eaton K, Rasmussen J, Holland SK. Comparison of fMRI data from passive listening and active-response story processing tasks in children. Journal of Magnetic Resonance Imaging. doi: 10.1002/jmri.21694. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karunanayaka PR, Holland SK, Schmithorst VJ, et al. Age-related connectivity changes in fMRI data from children listening to stories. Neuroimage. 2007;34:349–360. doi: 10.1016/j.neuroimage.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 69.Szaflarski J, Schmithorst V, Altaye M, et al. Longitudinal fMRI study of narrative comprehension in children ages 5−11.. 12th Annual Meeting, Human Brain Mapping.; Florence, Italy. 2006. [Google Scholar]
- 70.Holland SK, Plante E, Weber Byars A, Strawsburg RH, Schmithorst VJ, Ball WS., Jr. Normal fMRI Brain Activation Patterns in Children Performing a Verb Generation Task. Neuroimage. 2001;14:837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- 71.Bullmore E, Horwitz B, Honey G, Brammer M, Williams S, Sharma T. How good is good enough in path analysis of fMRI data? Neuroimage. 2000;11:289–301. doi: 10.1006/nimg.2000.0544. [DOI] [PubMed] [Google Scholar]
- 72.Smith EE, Jonides J, Marshuetz C, Koeppe RA. Components of verbal working memory: evidence from neuroimaging. Proc Natl Acad Sci U S A. 1998;95:876–882. doi: 10.1073/pnas.95.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Patel AM, Cahill LD, Ret J, Schmithorst V, Choo D, Holland S. Functional magnetic resonance imaging of hearing-impaired children under sedation before cochlear implantation. Arch Otolaryngol Head Neck Surg. 2007;133:677–683. doi: 10.1001/archotol.133.7.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Plante E, Holland SK, Schmithorst VJ. Prosodic processing by children: an fMRI study. Brain & Language. 2006;97:332–342. doi: 10.1016/j.bandl.2005.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Byars AW, Holland SK, Strawsburg RH, et al. Practical aspects of conducting large-scale functional magnetic resonance imaging studies in children. J Child Neurol. 2002;17:885–890. doi: 10.1177/08830738020170122201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Knecht S, Deppe M, Ebner A, et al. Noninvasive determination of language lateralization by functional transcranial Doppler sonography: a comparison with the Wada test. Stroke. 1998;29:82–86. doi: 10.1161/01.str.29.1.82. [DOI] [PubMed] [Google Scholar]
- 77.Deppe M, Knecht S, Papke K, et al. Assessment of hemispheric language lateralization: a comparison between fMRI and fTCD. J Cereb Blood Flow Metab. 2000;20:263–268. doi: 10.1097/00004647-200002000-00006. [DOI] [PubMed] [Google Scholar]
- 78.Lohmann H, Drager B, Muller-Ehrenberg S, Deppe M, Knecht S. Language lateralization in young children assessed by functional transcranial Doppler sonography. Neuroimage. 2005;24:780–790. doi: 10.1016/j.neuroimage.2004.08.053. [DOI] [PubMed] [Google Scholar]
- 79.Holland SK, Vannest J, Mecoli M, et al. Functional MRI of language lateralization during development in children. Int J Audiol. 2007;46:533–551. doi: 10.1080/14992020701448994. [DOI] [PMC free article] [PubMed] [Google Scholar]


