Abstract
The discovery that aerobic exercise increases adult hippocampal neurogenesis and can enhance cognitive performance holds promise as a model for regenerative medicine. This study adds two new pieces of information to the rapidly growing field. First, we tested whether exercise increases vascular density in the granular layer of the dentate gyrus, whole hippocampus, and striatum in C57BL/6J mice known to display procognitive effects of exercise. Second, we determined the extent to which new neurons from exercise participate in the acute neuronal response to high levels of running in B6D2F1/J (F1 hybrid of C57BL/6J female by DBA/2J male). Mice were housed with or without a running wheel for 50 days (runner vs. sedentary). The first 10 days, they received daily injections of BrdU to label dividing cells. The last 10 days, mice were tested for performance on the Morris water maze and rotarod and then euthanized to measure neurogenesis, c-Fos induction from running and vascular density. In C57BL/6J, exercise increased neurogenesis, density of blood vessels in the dentate gyrus and striatum (but not whole hippocampus), and enhanced performance on the water maze and rotarod. In B6D2F1/J, exercise also increased hippocampal neurogenesis but not vascular density in the granular layer. Improvement on the water maze from exercise was marginal, and no gain was seen for rotarod, possibly because of a ceiling effect. Running increased the number of c-Fos positive neurons in the granular layer by fivefold, and level of running was strongly correlated with c-Fos within 90 min before euthanasia. In runners, ~3.3% (±0.008 S.E.) of BrdU-positive neurons in the middle of the granule layer displayed c-Fos when compared with 0.8% (±0.001) of BrdU-negative neurons. Results suggest that procognitive effects of exercise are associated with increased vascular density in the dentate gyrus and striatum in C57BL/6J mice, and that new neurons from exercise preferentially function in the neuronal response to running in B6D2F1/J.
Keywords: exercise, wheel running, c-Fos, adult neurogenesis, angiogenesis, water maze, rotarod, C57BL/6J, DBA/2J
INTRODUCTION
An acute bout of aerobic physical activity, whether voluntary or forced, strongly and selectively increases neuronal activity in the hippocampus (Vanderwolf, 1969; Oddie and Bland, 1998; Bland et al., 2006). This is true even when animals are running in place, such as on a running wheel or on a treadmill (Hirase et al., 1999; Oladehin and Waters, 2001). It thus appears to occur independently from changes in place or spatial information (Bose and Recce, 2001; Bland et al., 2006). For example, the speed of voluntary wheel running is strongly correlated with the discharge frequency of pyramidal cells and interneurons in the hippocampus of rats (Czurko et al., 1999). In outbred Hsd/ICR mice, running increases c-Fos in the dentate gyrus by threefold. Moreover, the level of c-Fos induction is strongly correlated with the average speed of wheel running within the 90 min preceding euthanasia (Rhodes et al., 2003a).
Aerobic physical activity (either voluntary or forced) also massively increases neurogenesis in the dentate gyrus of mice and rats (van Praag et al., 1999b; Rhodes et al., 2003b; Uda et al., 2006; Leasure and Jones, 2008; Trejo et al., 2008). This results in increased total numbers of granular neurons (Rhodes et al., 2003b) and increased volume of the entire granule layer (Rhodes et al., 2003b; Clark et al., 2008). This discovery has generated great excitement because it represents a model for understanding how an effective regenerative system works in the adult mammalian brain (Cotman et al., 2007). This study aims to fill two gaps in the rapidly growing field.
It is known that adult hippocampal neurogenesis occurs in the inside layer of the dentate gyrus adjacent to the hilus, near blood vessels, and it has been hypothesized that exercise increases angiogenesis in this area to support neurogenesis (Palmer et al., 2000; Pereira et al., 2007). However, studies reporting direct measurements of angiogenesis or vascular density in the granular layer of the dentate gyrus in response to exercise are scarce. Although it is established that exercise increases vascular density in the motor cortex (e.g., Swain et al., 2003), cerebellum (e.g., Black et al., 1990), and striatum (e.g., Ding et al., 2006a), to the best of our knowledge, only three studies have reported changes in properties of blood vessels in the dentate gyrus in response to exercise. In the first study, van Praag et al. (2005) counted blood vessel fragments within sections of the whole dentate gyrus, and collected measurements on two individual vessel fragments per animal using Lectin stain. They found that voluntary wheel running did not change the number of fragments but increased surface area and perimeter of the individual vessels sampled in young, but not old male C57BL/6J mice. In a later study by the same group, they measured the percentage of the whole dentate gyrus covered by blood vessels in young animals housed with wheels for only 2 h per day (vs. animals never housed with wheels), and they found no difference between groups (van Praag et al., 2007). In the third study, Ekstrand et al. (2008) counted number of cells in the molecular layer of the dentate gyrus colabeled with both BrdU and rat-endothelial cell antigen-1 (indicating new vascular tissue) in adult, male, Wistar rats. They found approximately a threefold increase in the numbers of such cells (i.e., angiogenesis) in animals housed individually with running wheels when compared with single housed sedentary controls. Taken together these data suggest that exercise likely increases the area covered by vessels in the dentate gyrus, but a direct measurement of area fraction, specifically in the granular layer of the dentate gyrus (where neurogenesis occurs), to our knowledge, has not been reported.
Another gap in the literature relates to the functional significance of adult hippocampal neurogenesis (Kempermann, 2008). Recent studies have established that new neurons generated in adulthood display morphological and electrophysiological properties consistent with older granular neurons generated during early postnatal development (van Praag et al., 1999a; van Praag et al., 2002; Laplagne et al., 2006; Zhao et al., 2006; Toni et al., 2008). Moreover, several studies have reduced neurogenesis using a chemical toxin (e.g., Shors et al., 2002), irradiation (e.g., Meshi et al., 2006; Saxe et al., 2006; Clark et al., 2008), or genetic engineering approaches (e.g., Dupret et al., 2008) to see whether blockade of neurogenesis impairs learning and memory on behavioral tasks hypothesized to require hippocampus. However, results are mixed, and depend on the technique used to reduce neurogenesis, the behavioral task examined, genotype or species, as well as the timeframe between when neurogenesis is interrupted and behavior measured.
To circumvent some of these issues, a few recent studies have examined whether the new neurons are preferentially recruited into neuronal responses to a water maze experience (Jessberger and Kempermann, 2003; Kee et al., 2007) or repeated exposure to an enriched environment (Tashiro et al., 2007) as compared with older neurons using immunohistochemical detection of immediate early gene expression (e.g., c-Fos). The data suggest that there is a critical period when new neurons are more likely to show c-Fos induction than older neurons. However, relatively few scattered granule neurons display c-Fos immediate early gene activity in response to the water maze (Jessberger and Kempermann, 2003) or reexposure to an enriched environment (Tashiro et al., 2007). In comparison, a large number of neurons display c-Fos in response to wheel running (Rhodes et al., 2003a). It is not clear why such a large neuronal activation of the dentate gyrus takes place in response to acute bouts of wheel running. Nonetheless, an alternative hypothesis is that the new neurons are recruited into the function of the hippocampus in the neuronal response to wheel running itself (Rhodes et al., 2003b).
This study had two main objectives. The first was to determine the extent to which the volume fraction of blood vessels in the granular layer of the dentate gyrus, whole hippocampus, and whole striatum changes after chronic voluntary wheel running exercise in C57BL/6J mice. C57BL/6J mice were chosen for this analysis because they are well known to display enhanced behavioral performance from wheel running exercise, and hence serve as a useful model to explore neurobiological correlates of cognitive enhancement (van Praag et al., 1999a; van Praag et al., 2005; Clark et al., 2008). We predicted volume fraction of blood vessels would increase in all three areas.
The second objective was to determine the extent to which new neurons from exercise are recruited into the acute neuronal c-Fos induction from wheel running in the granular layer of the dentate gyrus. For this objective, we chose to study B6D2F1/J mice as opposed to C57BL/6J because the F1 hybrid mice run at higher levels and previous studies have established that level of running within 90 min prior to euthanasia is positively correlated with amount of c-Fos induction (Rhodes et al., 2003a). Moreover, differences in chronic levels of running are positively correlated with number of new neurons (Rhodes et al., 2003b). Therefore, we reasoned that by virtue of their higher levels of running, F1 hybrid mice would show larger numbers of new neurons and larger numbers of c-Fos positive cells than C57BL/6J, which would increase the probability of finding overlap between the two. We predicted that new neurons would be preferentially recruited into the neuronal response to wheel running in this strain (i.e., that BrdU-positive (BrdU+) neurons would display greater probability for c-Fos induction from running than BrdU-negative (BrdU−) neurons). To the best of our knowledge, B6D2F1/J mice have not been measured for effects of exercise on the water maze or rotarod. Therefore, another objective of this experiment was to test the extent to which the F1 strain displays benefits of exercise on behavioral tasks. Here, the prediction was not as clear. On the one hand, given that they run more than C57BL/6J, they would be expected to show higher levels of neurogenesis, and perhaps stronger procognitive effects. On the other hand, previous studies using mice selectively bred for high levels of wheel running found that the high runners did not display gains in performance from running even though they ran ~12 km/day and generated more neurogenesis than the unselected control lines that ran 4 km/day (Rhodes et al., 2003b).
MATERIALS AND METHODS
Animals
Mice from the C57BL/6J strain (n = 24 males) and B6D2F1/J strain (n = 6 females and 6 males) were used. B6D2F1/J mice are the hybrid offspring of a cross between a C57BL/6J female and a DBA/2J male. Individual animals within this strain share the feature with C57BL/6J that they are isogenic (meaning that all same sex members are genetically identical). However, they are heterozygous at all loci that differ between DBA/2J and C57BL/6J, and hence they have the potential to display what has been referred to as “hybrid vigor,” or enhanced performance (Livesay, 1930; Han et al., 2008).
Husbandry
Animals arrived at the Beckman Institute Animal facility from The Jackson Laboratory at 5 weeks of age. Upon arrival, they were housed four per cage by sex in standard polycarbonate shoebox cages with corncob bedding (Harlan Teklad 7097, Madison, WI). They were housed this way for 11 (experiment 1) or 14 days (experiment 2) until they were individually housed either in standard shoebox cages (without filter tops) or cages with wheels as described later. Rooms were controlled for temperature (21°C ± 1°C) and photoperiod (12:12 L/D; lights on at 7 am and off at 7 pm). Food (Harlan Teklad 7012) and water were provided ad libitum. The Beckman Institute Animal Facility is AAALAC approved. All procedures were approved by the University of Illinois Institutional Animal Care and Use Committee and adhered to NIH guidelines.
Experiment 1: C57BL/6J Males (n = 24)
The primary objective of this experiment was to determine the extent to which the volume fraction of blood vessels in the granular layer of the dentate gyrus, whole hippocampus, and striatum changes after chronic voluntary wheel running exercise in young male C57BL/6J mice. A secondary objective was to confirm that the vascular changes were associated with increased hippocampal neurogenesis and enhanced behavioral performance on the water maze, as previously reported for this strain (van Praag et al., 1999a; van Praag et al., 2005).
Mice (46 days old) were placed individually in cages either without (sedentary) or with running wheels (runners) for 50 days. All mice received daily injections of 50 mg/kg bromo-deoxyuridine (BrdU) to label dividing cells during the first 10 days. Note that mice were deliberately not housed in cages with locked wheels because mice climb in locked wheels and we wanted to keep physical activity to a minimum in the sedentary group (Koteja et al., 1999; Rhodes et al., 2000). Dimensions of running wheel cages were 36 cm × 20 cm × 14 cm (L W H) with a 23-cm diameter wheel mounted in the cage top (Respironics, Bend, OR). Dimensions of cages without wheels were 29 cm × 19 cm × 13 cm (L W H). Wheel rotations were monitored continuously in 1 min increments throughout the experiments via magnetic switches interfaced to a computer running VitalView software (Respironics, Bend, OR).
After 36 days of being housed with or without wheels, mice (age 82 days) were tested on two behavioral tasks, Morris water maze, then rotarod. Testing took place during the light phase of the light/dark cycle. Animals were returned to cages with or without wheels immediately after testing. Hence, runners had continuous access to running wheels throughout the behavioral testing period.
Water maze
Mice were trained on Morris water maze with two trials per day for 5 days. A trial lasted either 60 s or after the mouse reached the platform and remained on the platform for 10 s. If a mouse did not reach the platform in 60 s, it was gently guided there by hand. Mice were placed back in their cage and allowed to rest for 30 s between trials. One hour after training on day 5, the platform was removed and mice were tested with a probe trial (60 s).
The maze consisted of a circular tub, 150 cm diameter and 30 cm deep. A platform, made of white plastic mesh 8.5 cm square, was placed in the middle of one quadrant submerged 0.5 cm below the surface of the water. Crayola white tempera paint was added to the water to make the water sufficiently opaque to hide the platform from sight. White was chosen to provide contrast for video tracking from above (black mouse on white background). Water temperature was maintained at 25–26°C. Topscan (CleverSystems, Reston, VA) video tracking software was used to measure path length, swim speed, and duration spent in different quadrants of the maze.
Rotarod
After water maze, mice were tested for performance on a rotarod (AccuRotor Rota Rod Tall Unit, 63-cm fall height, 30-mm diameter rotating dowel; Accuscan, Columbus, OH). Animals were placed on the dowel starting at 0 rpm. The dowel was then accelerated at 60 rpm/min. A photobeam at the base stopped the timer automatically when a mouse fell off the dowel. This was repeated four consecutive trials per day for 3 days.
Experiment 2: B6D2F1/J (n = 12; 6 Males and 6 Females)
The primary objective of this experiment was to determine the extent to which new neurons from exercise are recruited into the neuronal c-Fos response to running in the dentate gyrus. A secondary objective was to determine the extent to which exercise changes morphology of the dentate gyrus (vascular density and neurogenesis) and behavioral performance in F1 mice.
The experiment proceeded in exactly the same way as experiment 1 except for the following. Mice were 49 days old instead of 46 days old when they were placed individually with or without running wheels. Mice were tested for behavioral performance after 42 (rather than 36) days of running. A smaller water maze was used (100 cm rather than 150 cm), with the same size platform (8.5 cm × 8.5 cm). The probe trial was given 24 h (rather than 1 h) after the final acquisition trial. The following day after the probe trial, the mice were trained on a visible platform version of the water maze (two trials per day for 3 days). The trials proceeded similar to the hidden version except a flag pole (19.5 cm) rose from the middle of the platform bearing a flag (black plastic) and the platform was moved to the middle of a different, randomly selected quadrant each trial (excluding the target quadrant from the hidden version). After behavioral testing, the mice were given 2 days to recover levels of wheel running. Then, they were euthanized at the height of their active period, between 2 and 3 h after the lights shut off in the animal rooms.
Immunohistochemistry
Following Clark et al. (2008), animals were anesthetized with 100 mg/kg sodium pentobarbital (ip) and then perfused transcardially with 4% paraformaldehyde in phosphate buffer solution (PBS). Brains were postfixed overnight and transferred to 30% sucrose in PBS. Brains were sectioned using a cryostat into 40-μm coronal sections and stored in tissue cryoprotectant at −20°C. Four separate 1-in-6 series of these sections (i.e., series of sections throughout the rostrocaudal extent of the brain with 240 μm increments separating each section) were stained in each of the following ways.
BrdU-DAB. Purpose: To detect BrdU+ (newly divided) cells in the dentate gyrus. Free floating sections were washed in tissue buffering solution (TBS) and then treated with 0.6% hydrogen peroxide. To denature DNA, sections were treated with 50% deionized formamide, 10% 20×SCC buffer, 2 N hydrochloric acid, and 0.1 M Boric acid. Sections were then treated with a solution of 0.3% Triton-X and 3% goat serum in TBS (TBS-X plus), and then incubated in primary antibody against BrdU made in rat (Accurate, Westbury, NY) at a dilution of 1:100 in TBS-X plus for 72 h at 4°C. Sections were then washed in TBS, treated with TBS-X plus for 30 min, and then incubated in secondary antibody against rat made in goat at 1:250 in TBS-X plus for 100 min at room temperature. Sections were then treated using the ABC system (Vector, Burlingame, CA) and stained using a diaminobenzidine kit (Sigma, St. Louis, MO).
c-Fos-DAB. Purpose: To detect c-Fos-positive (transcriptionally activated) cells in the dentate gyrus in response to wheel running. Free floating sections were washed in PBS and then treated with 0.5% hydrogen peroxide. Sections were then treated with a solution of 0.2% Triton-X and 5% goat serum in TBS (TBS-X plus) for 1 h, and then incubated in primary antibody against c-Fos made in rabbit (Calbiochem, San Diego, CA) at a dilution of 1:20,000 in PBS-X plus for 48 h at 4°C. Sections were then washed in PBS, treated with PBS-X plus for 60 min, and then incubated in secondary antibody against rabbit made in goat at 1:500 in TBS-X plus for 90 min at room temperature. Sections were then treated using the ABC system (Vector, Burlingame, CA) and stained using a diaminobenzidine kit (Sigma, St. Louis, MO).
Collagen IV-DAB. Purpose: To visualize blood vessels in brain sections. Following Franciosi et al. (2007), free floating sections were washed in PBS, followed by distilled water at 37°C. Sections were then treated with pepsin (1 mg/ml) in 0.2 N HCL at 37°C for 12 min for antigen retrieval, then washed in PBS for 15 min at 27°C. Sections were then transferred to TBS, treated with 0.5% hydrogen peroxide in TBS for 30 min, washed again in TBS, and then treated with 0.1% Triton-X and 3% goat serum in TBS. Sections were then incubated in primary antibody against collage IV at a dilution 1:300 in TBS-X plus for 72 h. Following incubation, sections were washed in TBS, treated with TBS-X plus for 1 h, and then incubated in secondary antibody against rabbit made in goat at 1:500 in TBS-X plus for 90 min. Finally, sections were treated using the ABC system (Vector, Burlingame, CA) and stained using a diaminobenzidine kit (Sigma, St. Louis, MO). Once mounted on slides, sections were lightly stained with methylene blue (1,000 ml water, 0.16 g methylene blue, 0.1 g Azure II, 1.7 g Na2HPO4, and 0.56 g KH2PO4).
Triple-fluorescent label. Purpose: Experiment 1, to determine the proportion of BrdU+ cells in the dentate gyrus that differentiated into neurons. Experiment 2, to determine the proportion of BrdU+ and BrdU− neurons in the dentate gyrus that expressed c-Fos. The procedure for BrdU-DAB was repeated except for the following. A cocktail was used for the primary antibody step, rat anti-BrdU (1:100; Accurate, West-bury, NY), mouse anti-NeuN (1:50; Chemicon, Billerica, MA), and rabbit anti-c-Fos (1:7,000; Calbiochem, San Diego, CA). Secondary antibodies made in goat were conjugated with fluorescent markers (Cy2 anti-rabbit, Cy3 anti-rat, Cy5 anti-mouse) at dilution 1:200 and also delivered as a cocktail. ABC and diaminobenzidine steps were omitted.
Image Analysis
BrdU-DAB and c-Fos-DAB
Following Clark et al. (2008), the entire granule layer (bilateral), represented in the 1-in-6 series, was photographed by systematically advancing the field of view of the Zeiss brightfield light microscope, and taking multiple photographs, via axiocam interfaced to computer, under 10× (total 100×) magnification. These photographs were then analyzed using ImageJ software to generate unbiased estimates of total number of labeled cells (BrdU or c-Fos) per cubic micrometer dentate gyrus sampled and area of the granular layer within the sections.
Blood vessels
The entire striatum, hippocampus, and dentate gyrus represented in a 1 in 6 series, unilaterally, were photographed by systematically advancing the field of view of the Zeiss brightfield light microscope under 20× (total 200×) magnification. A random sample of these photographs (12 per individual for dentate gyrus and 20 for striatum and whole hippocampus) was then microanalyzed to estimate vascular density. A 494 point grid was placed over each of these photographs using ImageJ software. The area of interest was outlined. Then, the total number of grid intersections that crossed over a blood vessel and the total number of grid intersections within the outlined region were counted manually and expressed as a ratio, equivalent to the area fraction (Sikorski et al., 2008). The measurements were repeated for eight individuals (using a different sample of photographs) to establish repeatability of individual measurements and measurement error. In addition, the area of the structures within sections was estimated by outlining the structures using Stereo Investigator software (version 7.5, MBF Bioscience, Williston VT).
Triple label
A Leica SP2 laser scanning confocal microscope (using a 40× oil HCX PL APO C5 objective with 1.25 numerical aperture, pinhole size 81.35 μm, 1-Air Unit) was used to determine the proportion of BrdU+ cells differentiated into neurons (NeuN+) (in both experiments) and to determine the proportion of neurons (BrdU+ or BrdU−) displaying c-Fos (in experiment 2). Each BrdU+ cell in the granular layer (represented in the 1-in-6 series) was microanalyzed by performing sequential line scans (line average 8) to establish colabeling with NeuN or c-Fos. In addition, the granular layer represented in the 1-in-6 series was focused on a single plane (randomly selected on the z-axis), and all the granular neurons (not labeled with BrdU, i.e., BrdU−) were counted as well as the number colabeled with c-Fos in that plane (BrdU−, NeuN+, c-Fos +).
Statistical Analysis
In experiment 1, we estimated the proportion of BrdU+ cells in the granular layer that also expressed NeuN mature neuronal marker. In experiment 2, we estimated the proportion of BrdU+ or BrdU− granular neurons colabeled with c-Fos. These proportion data were analyzed among the groups by logistic regression. For these analyses, the deviance is reported in place of the F statistics.
The sedentary group was compared to the runner group within strains using unpaired t-tests. In experiment 2, data were also analyzed using ANOVAs with sex, exercise treatment, and the interaction between sex and treatment as factors.
The water maze acquisition data and the rotarod data were analyzed within strains, using repeated measures ANOVA with day or trial as the within-subjects factor and exercise treatment (runner vs. sedentary) as the between-subjects factor. In experiment 2, sex and interactions between sex and treatment were also included in the analysis.
Pearson’s correlations between amount of running (e.g., km/h or km/day) and number of c-Fos positive cells or number of new neurons were analyzed using simple linear regression.
RESULTS
Wheel Running
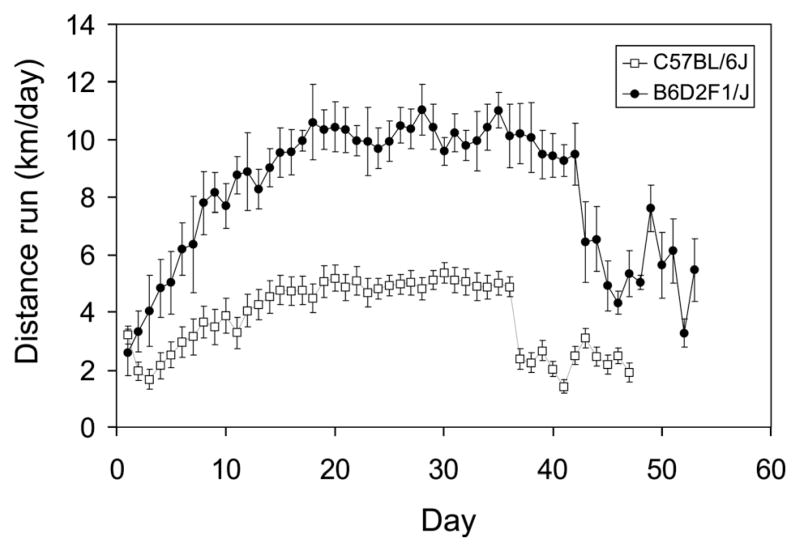
C57BL/6J males ran approximately half as far as B6D2F1/J males or females [Fig. 1; F(1,15) = 19.3, P < 0.001]. Running was similar between the sexes in B6D2F1/J. Average level of wheel running over the entire experiment was 3.8 km/day (±0.32 S.E.) in C57BL/6J males, whereas it was 8.1 (±0.54) and 8.4 (±0.71) km/day for B6D2F1/J males and females, respectively. Inspection of Figure 1 shows that in both genotypes, wheel running distance increased steadily for the first 20 days and thereafter maintained a plateau until behavioral testing when levels dropped by ~50%.
FIGURE 1.
Wheel running over the course of the study. Distance run (km/day) (± S.E.) shown separately for C57BL/6J (open symbol; n = 12 males) and B6D2F1/J mice (closed symbol; n = 3 males and 3 females). The first 10 days, mice received daily injections of 50 mg/kg BrdU to label dividing cells. The last 10 days, mice were tested on water maze then rotarod, during the light phase of the light/dark cycle when levels of wheel running are negligible.
Experiment 1: Neurovascular Adaptations to Exercise in C57BL/6J Mice
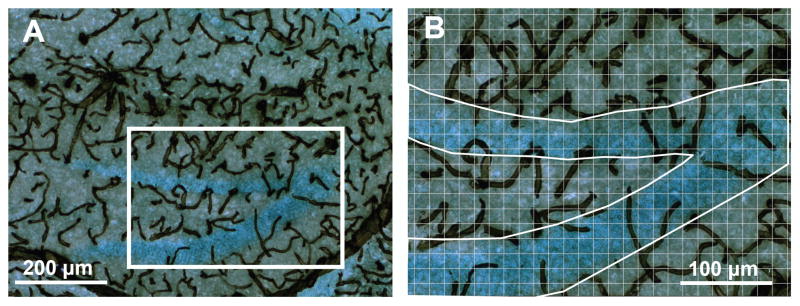
The collagen IV-DAB immunohistochemical procedure consistently stained vasculature in the brain across all regions and all individuals (Fig. 2). The sampling method (Fig. 2B; 12 photo- F2 graphs per animal for the dentate gyrus and 20 for the striatum and whole hippocampus) produced negligible measurement error. This was established by repeating the measurements for eight individuals (using a different sample of photographs). The Pearson’s correlation between duplicate measurements was greater than 0.98 for all three regions (all P < 0.0001).
FIGURE 2.
Blood vessels in the hippocampus. (A) A representative section stained with an antibody against collagen IV with diaminobenzidine as the chromogen, combined with a light Nissl stain to visualize blood vessels in and around the dentate gyrus (×100 total magnification). (B) The same section at ×200 total magnification with grid overlaid and region outlined to illustrate the method for estimating area fraction covered by vessels in the granular layer. Twelve or more such pictures per animal were analyzed to count the number of vertices intersecting with vascular tissue within defined brain regions. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Dentate gyrus
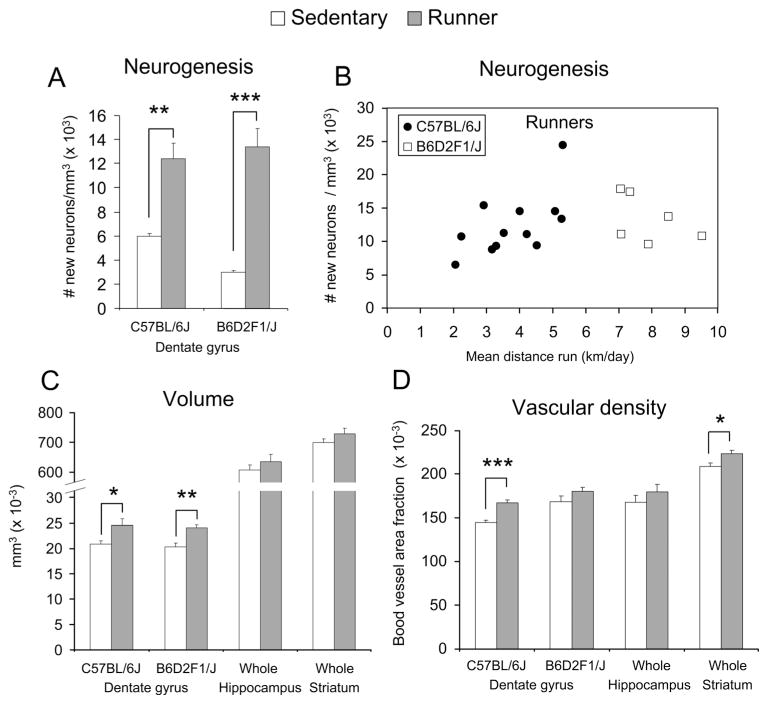
Wheel running increased the number of new neurons twofold (Fig. 3A) [t(22) = 4.3, P = 0.0003]. Level of running F3 was significantly correlated with number of new neurons among individuals whether running was expressed as the total distance during the first 10 days (R2 = 0.36, n = 12, P = 0.04), or total distance over the entire study (Fig. 3B, filled circles) (R2 = 0.45, n = 12, P = 0.02). In runners, a 94% (±1.7 S.E.) of BrdU cells in the granular layer differentiated into neurons (as indicated by double labeling with NeuN) when compared with 86% (±3.3) in sedentary mice [deviance(1,8) = 2.7, P = 0.03]. Volume of the granular layer increased by 18% (Fig. 3C) [t(22) = 2.6, P = 0.02], and density of blood vessels by 16% (Fig. 3D) [t(21) = 5.6, P < 0.0001].
FIGURE 3.
Neuroanatomical changes induced from exercise. (A) Average number of new neurons per cubic millimeter (±SE) shown separately for sedentary versus runners in the C57BL/6J and the B6D2F1/J genotypes. Open bars are sedentary, filled bars are the runners. (B) Number of new neurons per cubic millimeter in individual runners plotted against the average amount they ran over the course of the study in km/day. Filled circles are C57BL/6J and open boxes are B6D2F1/J. (C) Average volume (±SE) of the granular layer of the dentate gyrus in C57BL/6J and B6D2F1/J, the whole hippocampus in C57BL/6J, and the whole striatum in C57BL/6J shown separately for runners versus sedentary mice. (D) The same as C except for blood vessel area fraction. * indicates P < 0.05, **P < 0.01, ***P < 0.001.
Whole hippocampus
Exercise did not significantly change volume (Fig. 3C) or vascular density (Fig. 3D), but the trend was for a 5 and 7% increase in each variable, respectively.
Whole striatum
Exercise did not significantly change volume of the entire striatum (Fig. 3C) but the trend was for a 4% increase. Vascular density significantly increased by 7% (Fig. 3D) [t(21) = 2.5, P = 0.02].
Experiment 2: Functional Analysis of New Neurons in High-Running B6D2F1/J Mice
In the young adult B6D2F1/J mice (both sexes), wheel running increased neurogenesis 4.5-fold (Fig. 3A) [t(10) = 6.8, P < 0.0001]. Level of running was not significantly correlated with number of new neurons among individuals (Fig. 3B, open squares). The percentage of BrdU cells double labeled with NeuN was 95% (±1.0) in runners and 83% (± 2.8) in sedentary mice [deviance(1,10) = 7.3, P < 0.0001]. Volume of the granular layer increased by 18% (Fig. 3C) [t(10) = 3.8, P = 0.004]. There was a nonsignificant trend for density of blood vessels to increase by 7% (Fig. 3D). Effects of sex or interactions between sex and treatment were not significant for any of the variables.
c-Fos induction from wheel running in F1 mice
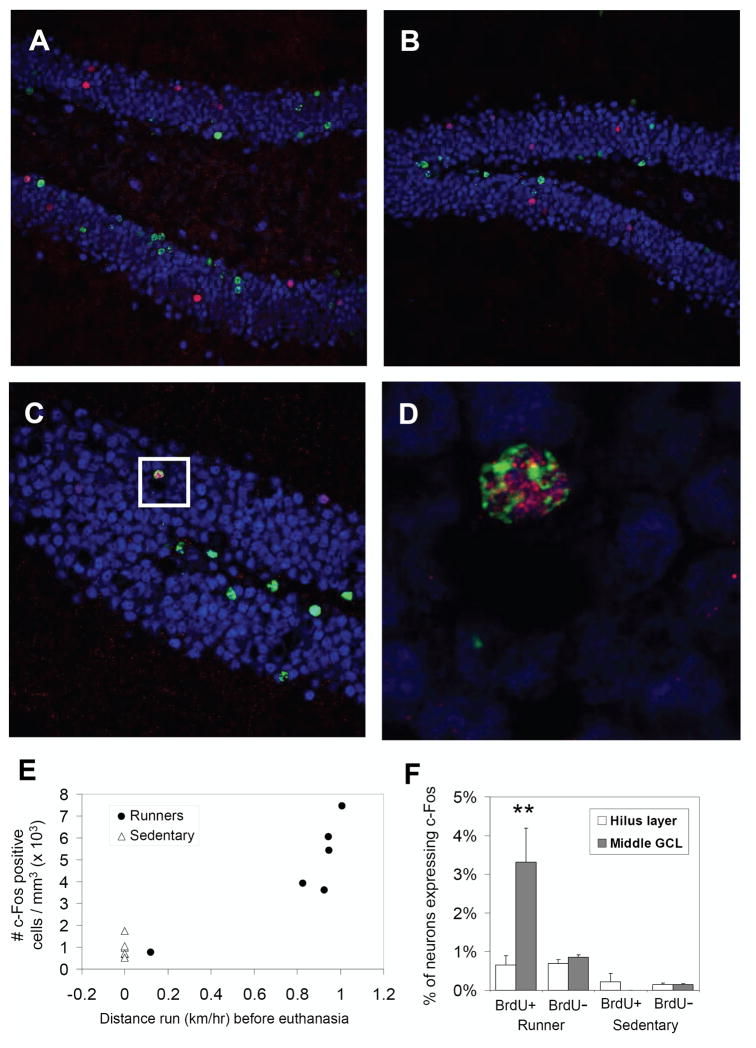
Running significantly increased the number of c-Fos positive F4 cells in the dentate gyrus by approximately fivefold (Fig. 4E) [t(10) = 3.7, P = 0.004]. Sedentary mice had an average of 950 (±177 S.E.) c-Fos positive cells per cubic millimeter dentate gyrus, whereas runners had 4,533 (±951) cells. The Pearson’s correlation (r) between level of running within 90 min before euthanasia and number of c-Fos positive cells among the six individuals was 0.78. This was marginally not significant because of the small sample size (P = 0.07). However, the correlation was even stronger (r = 0.87) and significant (P = 0.03) for distance run the first 60 of the 90-min period (Fig. 4E). One individual female mouse happened to run very little within this period (0.1 km/h) and had very few c-Fos positive cells (753 mm−3). All the other animals ran an order of magnitude farther (range from 1.0 to 1.4 km/h) and numbers of c-Fos cells were also much higher (they ranged from 3,612 to 7,446 mm−3). This mouse was not a low runner on previous days. In fact, across the entire study, this individual ran the most (an average of 9.5 km/day). There was no evidence that this mouse was sick at the time of sampling, and the mouse ran normal levels on the previous day. Hence, we believe that the low running within the 90-min sampling window on the final day was a chance event for this individual. When this mouse was excluded from the analysis, the correlation between level of running and number of c-Fos cells remained high, despite the low sample size and reduced range among the runners (n = 5, R2 = 0.64). With this low runner removed, the mean level of c-Fos cells per cubic millimeter in the runner group increased to 5,290 (±644).
FIGURE 4.
New neurons are recruited into the c-Fos induction from wheel running in B6D2F1/J mice. (A–C) Representative sections from B6D2F1/J runners triple labeled for BrdU (green), NeuN (blue), and c-Fos (red) sampled 2 h after the lights shut off in the animal room. (D) The area within the white box in panel C, zoomed in, showing a cell that was triple labeled, indicating an episode where a new neuron displayed c-Fos in response to running. (E) Number of c-Fos positive cells shown for each individual sedentary animal (open triangles) or runner (filled circles) plotted against the amount they ran within a 60 min window starting 90 min before the animals were removed from their wheels and euthanized. Note one runner ran very little (~0.1 km/h) within this window relative to the others. The sedentary animals were assigned a value of zero for distance run. (F) The percentage of neurons (i.e., NeuN positive cells) in the granular layer displaying c-Fos shown separately for sedentary (far right) vs. runner groups (left), BrdU positive (BrdU+, new neurons) versus BrdU negative (BrdU−, unlabeled with BrdU) neuronal populations, and for cells lining the inside of the granule cell layer adjacent to the hilus (open bars labeled “Hilus layer”) versus cells in outer layers (gray bars, labeled “Middle GCL”). Standard error bars estimated from logistic regression are shown. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Phenotypic analysis of NeuN-positive cells for coexpression of c-Fos
We examined a total of 2,151 BrdU+ neurons (new neurons) and 73,722 BrdU− neurons (neurons unlabeled with BrdU) in the granule cell layer of the dentate gyrus to determine the proportion expressing c-Fos (Figs. 4A–D). The BrdU+ cells were concentrated in the inside layer, adjacent to the hilus where 77% of the 2,151 cells were located. In contrast, the c-Fos cells were evenly distributed from the inside layer (adjacent to the hilus, where 15% of the cells were located) to the outer edge of the granular layer (away from the hilus) where the remaining 85% were located (Fig. 4F).
In sedentary mice, the percentage of c-Fos positive neurons was very low, between 0 and 0.2% (±0.002 S.E.; see Fig. 4F) and was similar for BrdU+ versus BrdU− neurons and similar for the inside layer versus the outer portions of the granule cell layer. In runners, the percentage of c-Fos cells was higher [deviance(1,22) = 182.9, P < 0.0001] ranging from 0.7 (±0.002) to 3.3% (±0.008) depending on BrdU labeling and location in the granule layer (Fig. 4F). This was reflected by a significant main effect of BrdU labeling [i.e., BrdU+ vs. BrdU−, deviance(1,22) = 4.4, P = 0.04], a significant main effect of granule cell region [inside layer adjacent to the hilus vs. layers away from the hilus, deviance(1,21) = 6.6, P = 0.01] and a significant interaction between BrdU labeling and region [deviance(1,20) = 9.2, P = 0.002] in the logistic regression. BrdU+ cells in the outer portions of the granular layer (i.e., not adjacent to the hilus) were five times more likely to display c-Fos than BrdU+ cells in the inside layer or when compared with BrdU− cells in either region (Fig. 4F). The percentage of c-Fos cells among BrdU+ cells collapsed across region (i.e., considering the entire granule cell layer without separating inside hilus layer from the rest) was 1.4% (±0.003) when compared with 0.8% (±0.001) for BrdU− cells throughout the granular layer.
Behavioral Performance
Experiment 1: C57BL/6J males
Water maze (150 cm diameter, 8.5 cm square platform)
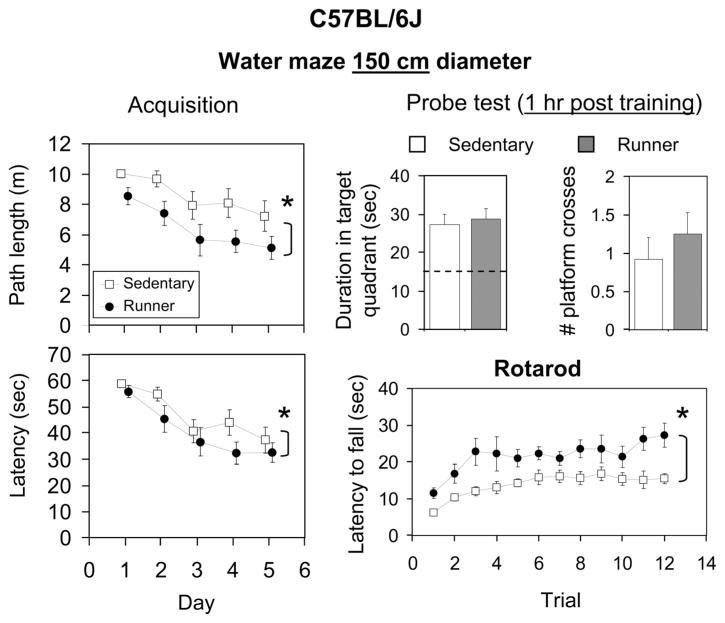
Acquisition. Both runners and sedentary mice learned the water maze as indicated by significantly decreased path length [F(4,88) = 6.8, P < 0.0001] and latency [F(4,88) = 11.9, P < 0.0001] to reach the hidden platform across days (Fig. 5). Runners displayed shorter path lengths [F(1,88) = 10.9, P = 0.001] and latency [F(1,88) = 4.6, P = 0.04] when compared with sedentary mice across days. The interaction between exercise treatment and day was not significant. No differences in swim speed were detected between the groups.
FIGURE 5.
Effects of exercise on behavioral performance in C57BL/6J mice. Runners (filled symbols, filled bars) are shown separately from the sedentary group (open symbols, open bars). For water maze (150 cm diameter), acquisition, mean path length (m), and latency (s) across days are shown. For the water maze probe trial (1 h after the last training trial), mean duration in the target quadrant (s) and number of crossings through the platform location are shown. For rotarod, mean latency (s) to fall on each of 12 trials (four trials per day for 3 days) are shown. Standard error bars are shown throughout.
Probe test (1 h post training). One hour after the final acquisition trial, both runners and sedentary animals displayed significantly more time in the target quadrant than any other quadrant (all P < 0.0001), and on an average, animals crossed through the platform location at least one time. No significant differences were detected between runners and sedentary mice for duration or number of crosses through the hidden platform.
Rotarod
Both runners and sedentary mice learned the rotarod as indicated by a significant increase in latency to fall off as the trials progressed [F(11,253) = 5.6, P < 0.0001]. Runners performed better than sedentary mice across all trials [F(1,253) = 25.2, P < 0.0001; a difference of ~9 s]. No interaction between exercise treatment and trial was detected.
Experiment 2: B6D2F1/J mice
Water maze (100 cm diameter, 8.5 cm square platform)
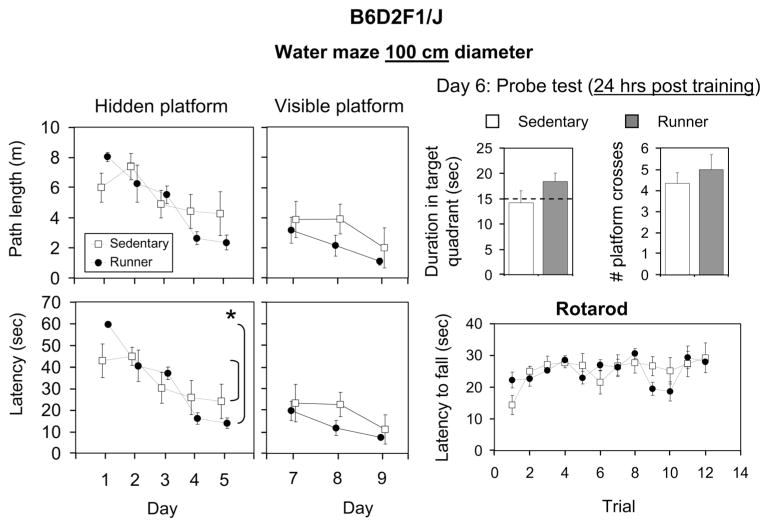
Acquisition. Both runners and sedentary mice learned the water maze as indicated by significantly decreased path length [F(4,40) = 10.0, P < 0.0001] and latency [F(4,40) = 19.3, P < 0.0001] to reach the hidden platform across days (Fig. 6). Runners displayed a steeper learning curve, as reflected by a significant interaction between exercise treatment and day for latency [F(4,40) = 3.5, P = 0.02]. For path length, the interaction was not significant but the trend was in the same direction [F(4,40) = 2.4, P = 0.07]. No main effect of exercise treatment was detected. No differences in swim speed were detected between the groups.
FIGURE 6.
Effects of exercise on behavioral performance in B6D2F1/J mice. Runners (filled symbols, filled bars) are shown separately from the sedentary group (open symbols, open bars). For water maze (100 cm diameter), the hidden platform acquisition (days 1–5) and visible platform acquisition (days 7–9) are shown as mean path length (m) and latency (s) across the days. For the probe test (on day 6, 24 h after the last hidden platform training trial), mean duration in the target quadrant (s) and number of crossings through the platform location are shown. For rotarod, mean latency (s) to fall on each of 12 trials (four trials per day for 3 days) are shown. Standard error bars are shown throughout.
Probe test (24 h post training). Twenty-four hours after the final acquisition trial, both runners and sedentary animals spent ~15 s in the target quadrant, the expected time based on random performance. On the other hand, both groups crossed through the location of the hidden platform on average between 4 and 5 times. No significant differences were detected between runners and sedentary mice for duration or number of crosses through the hidden platform.
Visible platform water maze
Both runners and sedentary mice learned the task as indicated by significantly decreased path length [F(2,20) = 4.9, P = 0.02] and latency [F(2,20) = 7.6, P = 0.004] to reach the visible platform across days. However, no effect of exercise or interaction between exercise treatment and day was observed.
Rotarod
Both runners and sedentary mice learned the rotarod as indicated by significantly increased latency to fall off as the trials progressed [F(11,110) = 3.2, P < 0.001]. Runners performed similar to sedentary mice. No interaction between exercise treatment and trial was detected.
DISCUSSION
The discovery that exercise increases the growth of new nervous tissue in the adult hippocampus has generated great interest and enthusiasm. If we can understand how an effective natural regenerative system works in the adult mammalian brain, then that holds promise for reverse engineering treatments for regenerative medicine. It is not surprising therefore, that research in this area has grown rapidly in recent years. This paper adds two new pieces of information to the growing literature. First, the results establish that in young adult male C57BL/6J mice, exercise increases the percentage of blood vessels in the granule layer of the dentate gyrus, whereas the change is smaller in the hippocampus and striatum. That suggests a specific role for the dentate gyrus in neuronal response to exercise (Pereira et al., 2007). The second novel piece of information is the discovery that new neurons, 7–8 weeks old, are preferentially recruited into the neuronal c-Fos induction from wheel running. This is important because it provides novel evidence for the functional significance of exercise-induced neurogenesis. It is possible that, in addition to extending plasticity for spatial learning and memory (van Praag et al., 1999a; van Praag et al., 2005; Kee et al., 2007), new neurons generated from exercise play a role in the function of the hippocampus in aerobic physical activity (Rhodes et al., 2003b).
Vascular Density
The 16% increase in vascular density (from exercise) in the granular layer of the dentate gyrus was greater than in the whole hippocampus (where the difference was not significant) suggesting that the changes are specific to the dentate gyrus (Fig. 3). The percentage increase in the granular layer was also greater than the increase in the striatum, where previous reports of changes in vascular tissue from exercise have been documented (Ding et al., 2004a,b, 2006b). These results support and extend van Praag et al. (2005) for the granular layer of the dentate gyrus. However, the 7% increase in vascular density observed in the striatum is dramatically different from the 10-fold increase reported in rats (Ding et al., 2004a,b, 2006b). The explanation for this difference is not clear. They examined the dorsolateral striatum, whereas we analyzed the whole striatum, so it could reflect regional differences. Also, they only counted capillaries less than 25 μm, whereas we counted all stained vascular tissue. It also may be due to species (rat vs. mouse). On the other hand, a 10-fold change in area covered by blood vessels seems extremely high based on our observations of repeatable individual differences in blood vessel density throughout the brain (e.g., Fig. 2A). In our experience, the various immunohistochemical methods available for visualizing vascular tissue (including antibodies against CD-31 or Laminin, and Lectin stains) can produce inconsistent results. Therefore, it is possible that the difference is due to incomplete staining in Ding et al. (2004a,b, 2006b) where Laminin was used (e.g., see Fig. 3B in Ding et al., 2004a).
The result that angiogenesis accompanies neurogenesis in the granular layer is not surprising because new tissue requires metabolic support (i.e., oxygen, nutrients, waste elimination). However, given that the new cells may increase the entire volume of the granular layer, it is not clear why the new vessels do not simply expand in proportion to the added nervous tissue producing more total vascular tissue without changing density. One possibility is that increased blood flow to this region (Pereira et al., 2007) associated with greater neuronal activation (Czurko et al., 1999; Rhodes et al., 2003a), resulted in greater perfusion of both old and new neurons.
Recruitment of New Neurons in the c-Fos Induction From Running
In experiment 2, we entertained the hypothesis that new neurons from running might play a role in the c-Fos induction from the wheel running itself (Rhodes et al., 2003b). We reasoned that if wheel running activated neurons in the granular layer (Rhodes et al., 2003a), and the neuronal activity contributed to the signaling for the generation of new neurons in that region, then the new neurons might be recruited into the neuronal response once they are mature. Results confirmed this hypothesis. New (BrdU labeled) neurons (7–8 weeks old) were significantly more likely to be recruited into the neuronal c-Fos response to running when compared with the population of unlabeled neurons (a combination of older neurons and new neurons unlabeled with BrdU) (Fig. 4F). Importantly, this difference was only for new neurons that had migrated into the granule cell layer away from where they were born (i.e., the inside layer adjacent to the hilus) (van Praag et al., 2002). These results suggest either that the new neurons located in the middle of the granule cell layer were more mature (i.e., labeled for BrdU earlier during the 10-day treatment) and hence more integrated into the circuit, or that only a subset of new neurons migrated into the interior of the granule cell layer, and those were the ones involved in running. Future research is needed to explore different age classes of new neurons to determine when maximum recruitment occurs into the c-Fos response to running.
The discovery that new neurons generated from exercise are preferentially recruited into neural activation from running is important because it provides new evidence for the functional significance of exercise-induced neurogenesis. Previous studies have suggested that new neurons from exercise play a role in extending plasticity for spatial learning and memory (van Praag et al., 1999a; Kee et al., 2007; Clark et al., 2008). Our data suggest it is also possible that new neurons generated from exercise play a role in the function of the dentate gyrus in the wheel running behavior itself. It is not clear why neuronal activity in the hippocampus is closely correlated with wheel running speed (Fig. 4E) (Czurko et al., 1999; Rhodes et al., 2003a) or why new neurons are needed to support that activation. It is possible that the new neurons replace older, dying or dead neurons, or that they are more plastic and therefore more likely to take on whatever function the hippocampus is playing (whether wheel running or solving a water maze).
Behavioral Performance
Previous studies have established that C57BL/6J genotype displays procognitive effects of exercise on the Morris water maze, although the effects have always been small and depend on the parameters used for the maze (van Praag et al., 1999a; van Praag et al., 2005; Clark et al., 2008). In this study, runners displayed significantly shorter path lengths and latencies to the hidden platform across days during acquisition, but they did not differ during the probe trial (see Fig. 5). In a previous study using C57BL/6J, we used a smaller maze (70 cm diameter with the same size platform as here) but similar parameters for the exercise treatment. In that study, differences between runners and sedentary mice were smaller for acquisition than reported here, but larger and significant for the probe trial (Clark et al., 2008). The larger maze used in this study (150 cm diameter) may have been more difficult for the animals. Evidence for this is that the animals appeared to remember the location of the target quadrant during the probe trial but they were unable to pinpoint the exact location of the hidden platform, as indicated by relatively few crossings (Fig. 5).
Although we observed a strong main effect in the C57BL/6J model, with runners performing better than sedentary mice across days during acquisition, we did not find a significant interaction between exercise treatment and day, implying that the slope of the learning curves were similar (i.e., the runner group had a constant advantage). On the other hand, on the very first trial (data not shown), none of the mice made it to the platform by themselves within the 1-min period, and average latency and path length to the platform were the same between the groups. The runner advantage began to emerge by the second trial, and therefore differences in the steepness of the learning curves may have been obscured by averaging the trials together on the first day. Taken together, results suggest that the exercise treatment used in this study resulted in benefits in behavioral performance on the water maze in the C57BL/6J genotype, and that the dimensions of the water maze can influence whether effects are seen during acquisition or in the probe test.
The rotarod data show that exercise can also improve behavioral performance on a task not hypothesized to involve the hippocampus in the C57BL/6J model. This is consistent with the growing knowledge that the benefits of exercise are broad and not limited to the hippocampus. The neuroanatomical variables that changed from exercise (Fig. 3) occurred in parallel with gain in performance on the water maze and the rotarod (Fig. 5). This makes it more difficult to identify causal connections between specific physiological responses such as neurogenesis and specific cognitive outcomes such as spatial memory enhancement. One way to tackle this problem is to directly manipulate the hypothesized substrates such as neurogenesis to determine whether the substrates are required for specific procognitive benefits of exercise (Clark et al., 2008).
The behavioral results for the F1 genotype were more complicated. The water maze was smaller (100 cm diameter), and the F1 sedentary mice started out performing slightly better than the runners on day 1. However, by the end of the 5 days of training, runners performed better than sedentary. Hence, over the 5 days of training, runners displayed a steeper learning curve than sedentary mice. The probe test occurred 24 h after the final training trial. Therefore, it is not clear whether the poor performance demonstrated by all the mice, as indicated by equal time spent in all quadrants, was a reflection of the mice forgetting the location of the platform or adopting an alternative strategy of random searching after realizing that the platform was gone. The large number of crossings is consistent with the latter explanation.
The visible platform data (Fig. 6) are difficult to interpret because the mice performed well on the first day (labeled day 7 on the graph in Fig. 6), leaving little room for improvement (i.e., ceiling effect). However, the statistically insignificant trend was for runners to perform better than sedentary mice, which would suggest that the steeper slope on the hidden version may have reflected a general performance gain rather than one specific for spatial ability. On the other hand, runners did not differ from sedentary mice for swim speed or rotarod performance, suggesting that their motor performance was similar. Taken together, in the F1 genotype, runners may have learned the water maze faster than sedentary mice, but more work is required to evaluate the extent to which the benefit is related to spatial learning and memory as opposed to other variables that can influence performance on these tasks.
Strain Comparisons
The purpose for using two different strains in this study was not to characterize genetic differences. C57BL/6J was chosen for experiment 1 because procognitive effects of exercise have already been established for this strain (van Praag et al., 1999a; van Praag et al., 2005; Clark et al., 2008). The B6D2F1/J strain was chosen for experiment 2 because they run more than C57BL/6J (Fig. 1), and we reasoned that they would be more likely to show overlap between numbers of new neurons and c-Fos positive cells in the granular layer because they would generate more of each. Because of these different experimental priorities, the two strains were studied in two separate experiments with slightly different parameters (see Materials and Methods section). Therefore, strong conclusions about genetic differences are not possible because the environment too was different. More than two genotypes are required anyway to draw statistical inferences about genetic correlations between physiology and behavior (Garland and Adolph, 1994). That being said, a few observations are worth noting. First, the F1 mice (both sexes) ran much more than the male C57BL/6J mice, as expected (Fig. 1). Also, level of running was correlated with number of new neurons among C57BL/6J runners, but not F1 (Fig. 3B). This is consistent with results of a selective breeding experiment where high running lines (that ran ~12 km/day) showed no correlation between amount of running and number of new neurons, whereas the control lines, that ran at moderate levels (~4 km/day), showed a strong correlation (Rhodes et al., 2003b). One explanation for this result is that high-running mice reach the physiological limit, ceiling, or capacity for generating new neurons from physical activity (Rhodes et al., 2003b).
Another interesting difference was that exercise improved performance on the rotarod in C57BL/6J, whereas exercise had no effect in the F1 (Fig. 5 vs. Fig. 6). High baseline performance in the sedentary group may have obscured performance gains in the F1 (i.e., ceiling effect). Results might have been different had we used different parameters for the rotarod (e.g., increased rate of acceleration, diameter of the dowel, etc.) (Rustay et al., 2003).
SUMMARY
There is growing evidence that the dentate gyrus is a major locus for phenotypic change induced from exercise in the brain (Christie et al., 2008; van Praag, 2008). Here, we extend this recent literature, by showing that density of blood vessels significantly increases in the granular layer of the dentate gyrus in the C57BL/6J mouse model. This increase in vasculature may support neurogenesis, neural activity associated with exercise, or as an inevitable consequence of increased blood flow to the region (Pereira et al., 2007). We also demonstrated that new neurons of 7–8 week age class are preferentially recruited into the neuronal response to wheel running in the B6D2F1/J high-running mouse model. This is important because it provides new evidence for the functional significance of exercise-induced neurogenesis. In addition to extending plasticity for spatial learning and memory, new neurons generated from exercise may play a role in the neuronal activation of the hippocampus associated with aerobic physical activity.
Acknowledgments
The Center For Healthy Minds at UIUC.
The authors thank Dack Shearer, Reid McClure, Donnell Parker, and Holly Fairfield for excellent animal care.
References
- Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci USA. 1990;87:5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland BH, Bird J, Jackson J, Natsume K. Medial septal modulation of the ascending brainstem hippocampal synchronizing pathways in the freely moving rat. Hippocampus. 2006;16:11–19. doi: 10.1002/hipo.20136. [DOI] [PubMed] [Google Scholar]
- Bose A, Recce M. Phase precession and phase-locking of hippocampal pyramidal cells. Hippocampus. 2001;11:204–215. doi: 10.1002/hipo.1038. [DOI] [PubMed] [Google Scholar]
- Christie BR, Eadie BD, Kannangara TS, Robillard JM, Shin J, Titterness AK. Exercising our brains: How physical activity impacts synaptic plasticity in the dentate gyrus. Neuromol Med. 2008;10:47–58. doi: 10.1007/s12017-008-8033-2. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155:1048–1058. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Czurko A, Hirase H, Csicsvari J, Buzsaki G. Sustained activation of hippocampal pyramidal cells by ‘space clamping’ in a running wheel. Eur J Neurosci. 1999;11:344–352. doi: 10.1046/j.1460-9568.1999.00446.x. [DOI] [PubMed] [Google Scholar]
- Ding Y, Li J, Luan X, Ding YH, Lai Q, Rafols JA, Phillis JW, Clark JC, Diaz FG. Exercise pre-conditioning reduces brain damage in ischemic rats that may be associated with regional angiogenesis and cellular overexpression of neurotrophin. Neuroscience. 2004a;124:583–591. doi: 10.1016/j.neuroscience.2003.12.029. [DOI] [PubMed] [Google Scholar]
- Ding YH, Luan XD, Li J, Rafols JA, Guthinkonda M, Diaz FG, Ding Y. Exercise-induced overexpression of angiogenic factors and reduction of ischemia/reperfusion injury in stroke. Curr Neurovasc Res. 2004b;1:411–420. doi: 10.2174/1567202043361875. [DOI] [PubMed] [Google Scholar]
- Ding YH, Ding Y, Li J, Bessert DA, Rafols JA. Exercise preconditioning strengthens brain microvascular integrity in a rat stroke model. Neurol Res. 2006a;28:184–189. doi: 10.1179/016164106X98053. [DOI] [PubMed] [Google Scholar]
- Ding YH, Li J, Zhou Y, Rafols JA, Clark JC, Ding Y. Cerebral angiogenesis and expression of angiogenic factors in aging rats after exercise. Curr Neurovasc Res. 2006b;3:15–23. doi: 10.2174/156720206775541787. [DOI] [PubMed] [Google Scholar]
- Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN, Piazza PV. Spatial relational memory requires hippocampal adult neurogenesis. PLoS ONE. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand J, Hellsten J, Tingstrom A. Environmental enrichment, exercise and corticosterone affect endothelial cell proliferation in adult rat hippocampus and prefrontal cortex. Neurosci Lett. 2008;442:203–207. doi: 10.1016/j.neulet.2008.06.085. [DOI] [PubMed] [Google Scholar]
- Franciosi S, De Gasperi R, Dickstein DL, English DF, Rocher AB, Janssen WG, Christoffel D, Sosa MA, Hof PR, Buxbaum JD, Elder GA. Pepsin pretreatment allows collagen IV immunostaining of blood vessels in adult mouse brain. J Neurosci Methods. 2007;163:76–82. doi: 10.1016/j.jneumeth.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland T, Jr, Adolph SC. Why not to do two-species comparative studies: Limitations on inferring adaptation. Physiol Zool. 1994;67:797–828. [Google Scholar]
- Han Z, Mtango NR, Patel BG, Sapienza C, Latham KE. Hybrid vigor and transgenerational epigenetic effects on early mouse embryo phenotype. Biol Reprod. 2008;79:638–648. doi: 10.1095/biolreprod.108.069096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirase H, Czurko A, Csicsvari J, Buzsaki G. Firing rate and theta-phase coding by hippocampal pyramidal neurons during ‘space clamping’. Eur J Neurosci. 1999;11:4373–4380. doi: 10.1046/j.1460-9568.1999.00853.x. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Kempermann G. Adult-born hippocampal neurons mature into activity-dependent responsiveness. Eur J Neurosci. 2003;18:2707–2712. doi: 10.1111/j.1460-9568.2003.02986.x. [DOI] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Kempermann G. The neurogenic reserve hypothesis: What is adult hippocampal neurogenesis good for? Trends Neurosci. 2008;31:163–169. doi: 10.1016/j.tins.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Koteja P, Garland T, Jr, Sax JK, Swallow JG, Carter PA. Behaviour of house mice artificially selected for high levels of voluntary wheel running. Anim Behav. 1999;58:1307–1318. doi: 10.1006/anbe.1999.1270. [DOI] [PubMed] [Google Scholar]
- Laplagne DA, Esposito MS, Piatti VC, Morgenstern NA, Zhao C, van Praag H, Gage FH, Schinder AF. Functional convergence of neurons generated in the developing and adult hippocampus. PLoS Biol. 2006;4:e409. doi: 10.1371/journal.pbio.0040409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leasure JL, Jones M. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience. 2008;156:456–465. doi: 10.1016/j.neuroscience.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Livesay EA. An experimental study of hybrid vigor or heterosis in rats. Genetics. 1930;15:17–54. doi: 10.1093/genetics/15.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, Malapani C, Moore H, Hen R. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci. 2006;9:729–731. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- Oddie SD, Bland BH. Hippocampal formation theta activity and movement selection. Neurosci Biobehav Rev. 1998;22:221–231. doi: 10.1016/s0149-7634(97)00003-1. [DOI] [PubMed] [Google Scholar]
- Oladehin A, Waters RS. Location and distribution of Fos protein expression in rat hippocampus following acute moderate aerobic exercise. Exp Brain Res. 2001;137:26–35. doi: 10.1007/s002210000634. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Koteja P, Swallow JG, Carter PA, Garland T. Body temperatures of house mice artificially selected for high voluntary wheel-running behavior: Repeatability and effect of genetic selection. J Therm Biol. 2000;25:391–400. doi: 10.1016/s0306-4565(99)00112-6. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Garland T, Jr, Gammie SC. Patterns of brain activity associated with variation in voluntary wheel-running behavior. Behav Neurosci. 2003a;117:1243–1256. doi: 10.1037/0735-7044.117.6.1243. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, van Praag H, Jeffrey S, Girard I, Mitchell GS, Garland T, Jr, Gage FH. Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel running. Behav Neurosci. 2003b;117:1006–1016. doi: 10.1037/0735-7044.117.5.1006. [DOI] [PubMed] [Google Scholar]
- Rustay NR, Wahlsten D, Crabbe JC. Assessment of genetic susceptibility to ethanol intoxication in mice. Proc Natl Acad Sci USA. 2003;100:2917–2922. doi: 10.1073/pnas.0437273100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski AM, Hebert N, Swain RA. Conjugated linoleic acid (CLA) inhibits new vessel growth in the mammalian brain. Brain Res. 2008;1213:35–40. doi: 10.1016/j.brainres.2008.01.096. [DOI] [PubMed] [Google Scholar]
- Swain RA, Harris AB, Wiener EC, Dutka MV, Morris HD, Theien BE, Konda S, Engberg K, Lauterbur PC, Greenough WT. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117:1037–1046. doi: 10.1016/s0306-4522(02)00664-4. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: A critical period during an immature stage. J Neurosci. 2007;27:3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo JL, Llorens-Martin MV, Torres-Aleman I. The effects of exercise on spatial learning and anxiety-like behavior are mediated by an IGF-I-dependent mechanism related to hippocampal neurogenesis. Mol Cell Neurosci. 2008;37:402–411. doi: 10.1016/j.mcn.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Uda M, Ishido M, Kami K, Masuhara M. Effects of chronic treadmill running on neurogenesis in the dentate gyrus of the hippocampus of adult rat. Brain Res. 2006;1104:64–72. doi: 10.1016/j.brainres.2006.05.066. [DOI] [PubMed] [Google Scholar]
- van Praag H. Neurogenesis and exercise: Past and future directions. Neuromol Med. 2008;10:128–140. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999a;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999b;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Lucero MJ, Yeo GW, Stecker K, Heivand N, Zhao C, Yip E, Afanador M, Schroeter H, Hammerstone J, Gage FH. Plant-derived flavanol (−)epicatechin enhances angiogenesis and retention of spatial memory in mice. J Neurosci. 2007;27:5869–5878. doi: 10.1523/JNEUROSCI.0914-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]