Abstract
The Wistar-Kyoto (WKY) rat has been proposed as an animal model of depressive behavior and exhibits hyper-responsiveness to stressful stimulation when compared to other rat strains. We have demonstrated that WKY rats consume 200% more alcohol under naïve conditions as compared to their outbred counterparts, Wistar (WIS) rats. The present study was designed to understand the influence of stress and alcohol consumption on central dopamine type-2 (D2) receptor sites in these two behaviorally distinct rat strains. The first part of this study examined the effects of chronic stress on alcohol consumption, while the second part examined the binding of [125I]-Iodosulpiride to D2 receptors in control, stressed or stress and alcohol co-treated WKY compared to WIS rats. Exposure to chronic stress led to an increase in the amount of alcohol consumed by both rat strains, with WKY rats consuming significantly more alcohol than WIS rats with or without stress exposure. Quantitative autoradiography experiments showed that chronic stress increased D2 receptor binding in the caudate putamen (CPu), nucleus accumbens (NAc), substantia nigra (SN) and ventral tegmental area (VTA) of WKY rats, and reduced receptor binding in the CPu and SN of WIS rats. Compared to the stressed-animals, WKY rats co-treated with stress and alcohol demonstrated a reduction in D2 receptor sites in the cell body regions (SN and VTA), while WIS rats showed no changes in receptor binding. The observed changes in D2 receptor sites may indicate altered DA neurotransmission following stress and alcohol exposure. Since stressed WKY rats consumed more alcohol, it is possible that consumption of alcohol reverses the stress-induced D2 receptor alterations in the cell body regions, suggestive of a self medicating phenotype.
Keywords: Stress, Alcohol, Wistar-Kyoto rat, Doamine-2 receptor
INTRODUCTION
Stress has been shown to contribute to the induction of various psychiatric disorders such as depression and alcohol dependency (Anisman and Zacharko 1990). Exposure to stressful life events has been long associated with increased alcohol use in humans and psychological stress is a common risk factor for both depression and alcohol dependence (Volpicelli 1987; Pettinati 2004). Clinical studies demonstrate that treatment with antidepressant drugs reduces depressive symptoms and drinking in alcoholic patients, suggesting a similarity in the biological nature and pathways involved in these two disorders (Brown et al., 1997; Cornelius et al., 1997).
The mesolimbic dopamine (DA) pathway originating in the ventral tegmental area (VTA) projects to the nucleus accumbens (NAc) and plays a role in reinforcement or motivational behavior, while the nigrostriatal DA pathway originating in the substantia nigra (SN) projects to the dorsal striatum and plays a prominent role in expression of motor behavior (Fallon and Moore 1978; Carli 1985; Carr and White 1985; Di Chiara and Imperato 1988). Both pathways are activated during appetitive behaviors such as feeding, drinking and copulation, although stimulation of the nigrostriatal pathway is not as great in magnitude (Hernandez and Hoebel 1988; Young et al., 1992, Pfaus et al., 1995; Wilson et al., 1995; Mirenowicz and Schultz 1996). DA neurotransmission in the VTA-NAc circuit plays a major role in defensive responses toward aversive stimuli, responses toward rewarding stimuli such as alcohol and other drugs of abuse as well as depression-like behavior in animal models (Di Chiara and Imperato 1988; Abercrombie et al., 1989; Imperato et al., 1991; Yoshimoto et al., 1991; Nestler and Carlezon 2006). It has been noted that alterations in reward and motivational processes, at the level of dopamine DA neurotransmission, may be implicated in both stress and alcohol dependence (Cabib and Puglishi-Allegra 1996; Wise 1996). For example, stress has been shown to influence several behavioral patterns mediated by the DA system, such as locomotor activity, motivational behavior, sexual activity and sensitization to drugs of abuse (Cabib et al., 1988; Di Chiara 1995; Wise 1996; Pani et al., 2000). The mesolimbic and nigrostriatal DA neurons respond to various acute stressors by increasing DA synthesis, release, and metabolism, while chronic stress has been noted to produce deficits in extracellular DA levels (Cabib and Puglishi-Allegra 1996; Gambarana et al., 1999). In turn, DA deficits may lead to failure in coping mechanisms and behavioral depression under a stressful situation (Kapur and Mann 1992). Evidence also indicates that DA neurotransmission plays an important role in the reinforcing effects of alcohol. For example, local, systemic and self-administration of ethanol increases extracellular DA levels in the NAc, an area especially sensitive to rewarding substances (DiChiara and Imperato 1988; Yoshimoto et al., 1991). Pre-treatment with stress increases drug induced DA release, suggesting a greater level of reward associated with drug use under stressful conditions (Kalivas and Stewart 1991). The mesencephalic DA neurons, especially those projecting from the VTA to the NAc, are considered to be principal substrates of drug reinforcement (Nestler 1992; Wise 1996). In recent studies microinjections of DA type-2 (D2) receptor antagonists into the VTA or NAc were shown to decrease alcohol responding in rats (Czachowski et al., 2001; Eiler and June 2007). Altogether, these studies indicate a role for D2 receptors in modulating the effects of stress and alcohol on central DA neurotransmission.
The Wistar-Kyoto (WKY) rat has been proposed as an animal model of depressive behavior by our laboratory (Paré and Redei 1993; Tejani-Butt et al., 1994) as well as others (Redei et al., 1994; Lopez-Rubalcava and Lucki 2000; Allard et al., 2004). Studies have noted that WKY rats differ from other strains in their behavioral, physiological, and neuroendocrine responsiveness to environmental as well as pharmacological challenges (Redei et al., 1994; Pardon et al., 2002; Tejani-Butt et al., 2003). For example, WKY rats are hyper-responsive to stress, as indicated by a greater susceptibility to stress-ulcers and higher levels of plasma adrenocorticotropic hormone (ACTH) in response to restraint stress (Redei et al., 1994). Since naïve WKY rats have a higher density of norepinephrine (NE) transporter sites in limbic regions compared to control strains (Tejani-Butt et al., 1994), we hypothesized that increased reuptake of NE into the presynaptic neuron would decrease synaptic availability and produce a net deficit of NE in specific brain areas in this rat strain. In support of this hypothesis, WKY rats demonstrate an attenuated noradrenergic response to stressful stimulation, as measured by either a reduction in Fos activation or tyrosine hydroxylase mRNA in the locus coeruleus, reflecting a lack of an appropriate stress-coping strategy in these animals (Pardon et al., 2002; Ma and Morilak 2004). In addition to disturbances in the noradrenergic system, WKY rats also demonstrate strain differences in D1 receptor, D2 receptor and DA transporter sites that are suggestive of reduced basal DA levels in several limbic brain regions (Jiao et al., 2003; Yaroslavsky et al., 2006; Novick et al., 2008).
WKY rats consume 200% more alcohol over a 24 day experimental period compared to their out bred counterparts (Jiao et al., 2006). Since multiple reports suggest an effect of both alcohol and stress on D2 receptor sites (Cabib et al., 1998; Sari et al., 2006), the present study investigated the effects of stress-alcohol interactions on D2 receptor binding in WKY compared to WIS rats. The first part of the study examined the consequence of 24 days of stress on alcohol consumption in WKY and WIS rats, while the second part used an autoradiographic analysis to measure the effects of stress or stress-alcohol co-treatment on the binding of [125I]-Iodosulpiride to D2 receptors in these two rat strains.
METHODS
All experimental protocols were reviewed and approved by the Veterans Medical Center, Perry Point, MD VAH Institutional Review Committee for the use of animal subjects.
Animals
Male WIS and WKY rats (230–270 grams) were used in this study. Rats were assigned into control (n=6/strain), stress (n=8/strain) and stress and alcohol (n=8/strain) groups. All groups were equated on the basis of body weight. WKY rats were raised at the Veterans Medical Center, Perry Point, MD from a breeding stock initially obtained from Charles River Laboratories (Kingston, NY). Animals were individually housed at 22°C and placed on a 12-hr light/dark cycle, with lights on between 06:00 and 18:00h. Animals were allowed to acclimate for two weeks prior to the start of the experiment and were handled daily for one week prior to the start of the experiment. Food and water were kept available during the whole day.
Procedure
The chronic stress group received various stressors for a 24 day experimental period. The stressors included scrambled foot shock, shaker stress, cold swim restraint, heat stress, food deprivation and reversed light dark cycle and were modified from the Katz procedure (1981) (Tejani-Butt et al., 1994). The stress-alcohol group received the same stress procedure as described above, but had free access to water or alcohol and the control group received no stress and no alcohol throughout the experimental period.
To elicit voluntary alcohol consumption, the alcohol preference method as described by Sandbak and Murison (1996) was used with minor modifications. During the first seven day period (day1 to 7) a 3% alcohol solution was presented to the animals followed by a 5% solution during the second seven day period (day 8 to 14), and finally a 7% solution throughout the remaining time (day 15 to 24). Both alcohol and water were freely available throughout the experimental period until rats were sacrificed. The position of the tubes was switched daily (left/right) and no additional flavors were added to either bottle in order to eliminate the possibility of location and flavor preference. Body weight and alcohol consumption measurements were recorded daily at the same time on each day. Alcohol consumption was recorded in terms of grams alcohol per kilogram body weight. On day 25, WKY and WIS rats from the control, stress and stress-alcohol groups were sacrificed by rapid decapitation between 0800 and 1000, the brains removed and stored at −80°C till use. Brains were then sectioned (16μm) at −18°C in a cryostat microtome according to the Brain Atlas of Paxinos and Watson (1998) and mounted on gelatin-coated microscope slides for further QAR analysis. It is important to note that since the first part of the study was designed to specifically measure the behavioral effects of stress on alcohol consumption in WKY and WIS rats, an additional set of animals from both strains underwent the identical alcohol consumption paradigm as described above without the concurrent stress exposure. Since, these animals were used solely as a behavioral control for that specific experiment; their brains were not processed for D2 receptor binding in our QAR experiments.
[125I]-Iodosulpiride binding assay
D2 receptors were labeled with [125I]-Iodosulpiride using the method of Stefanski et al., with minor modifications as described below (2002). To label D2 receptors, duplicate sections were pre-incubated in a buffer solution [50mM Tris-HCL, 120 mM NaCl, 5mM KCl, 1mM MgCl2, 2mM CaCl2] for 30 minutes. Sections were then incubated in the same buffer solution containing 0.1 nM [125I]-Iodosulpiride (2000 Ci/mmol, Amersham Biosciences) and 5 nM PD 28,907 (to block D3 receptors) at room temperature for 30 minutes. To determine non-specific binding, adjacent sections were incubated in 0.1 nM [125I]-Iodosulpiride, 5 nM PD 28,907 and 1 uM domperidone for 30 minutes. Following incubation, the sections were rinsed twice in cold buffer for five minutes each, followed by a dip in cold deionized water. The slides were then dried at room temperature, transferred into cassettes, and exposed to Kodak BioMax MS film along with [3H] standards. The exposure time for plate 12 was four days and for plate 42 it was seven days. Films were developed using Kodak GBX developer.
Quantification
The films were analyzed using a computerized brain image analysis system (Brain 3.0). Data were expressed as fmol/mg brain protein. Statistical analysis was performed with Sigma Stat for Windows. The values represent mean +/− SEM (6–8 animals). Differences between means in individual regions were analyzed using two-way analysis of variance (ANOVA), with strain (two levels) and treatment (three levels) as independent variables. Where significant main effects (strain/treatment/strain-treatment) were reported (p<0.05), a post hoc Tukey’s test, with HSD P<0.05, was conducted to locate significant differences between strain-treatment groups. A two-way ANOVA with strain (two levels) and treatment (two levels) as independent variables, was used to determine the effects of stress on drinking behavior in the two rat strains, followed by post hoc Tukey test, with HSD P<0.05 to locate significant differences (p<0.05).
RESULTS
Alcohol Drinking Behavior
As graphed in Figure 1, average 24-day alcohol consumption was increased in stressed-WKY and WIS rats compared to non-stressed animals from their respective strains, F(1,24) =6.11, Tukey HSD test p< 0.05. A significant strain difference was also measured, with the average alcohol consumption being significantly greater in both non-stressed and stressed WKY compared to WIS rats (Tukey HSD test, p<0.05).
Figure 1.
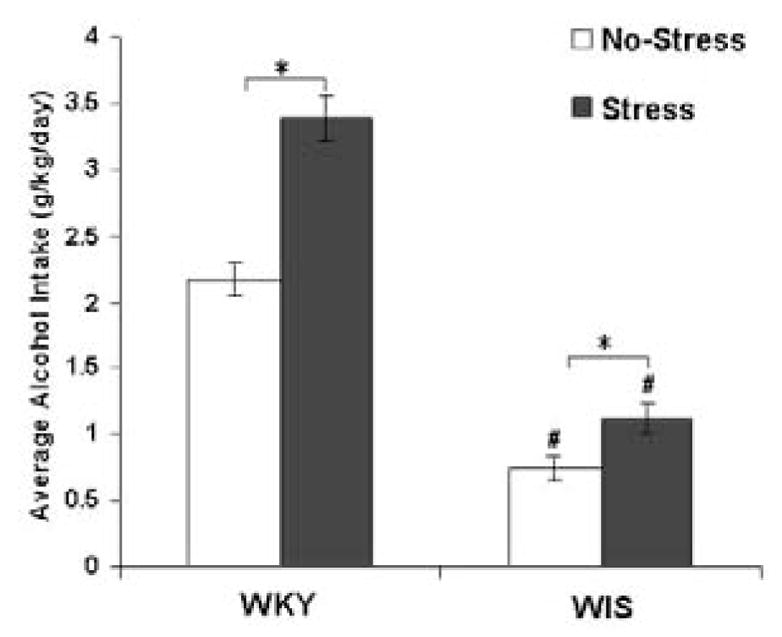
Average alcohol consumption in WKY and WIS rats over the 24-day experimental period. Each point represents mean ± SEM from 6–8 animals/group * Represents significant effect within strain, p<0.05. # Represents a significant strain difference within treatment group, p<0.001.
WKY-Wistar-Kyoto, WIS-Wistar
Effects of Stress and Alcohol on D2 Receptor Binding
Caudate Putamen (CPu)
A significant main effect was found in the CPu of WKY versus WIS rats with stress and alcohol exposure, F (2, 36) = 5.23, p<0.05. In WKY rats, the binding of [125I]-Iodosulpiride to D2 receptor sites was increased following 24 days of stress exposure in the CPu when compared to the control group (Tukey HSD test, p<0.05) (Figure 2). When alcohol was given at the same time of stress exposure, D2 receptor binding was found to be unchanged compared to control WKY rats. In contrast, stress exposure produced an opposite effect in WIS rats. The binding of [125I]-Iodosulpiride to D2 receptor sites was reduced in the CPu of WIS rats when compared to the control group (Tukey HSD test, p<0.05). However, the addition of alcohol to the stress procedure did not result in any changes in receptor binding in both rat strains.
Figure 2.
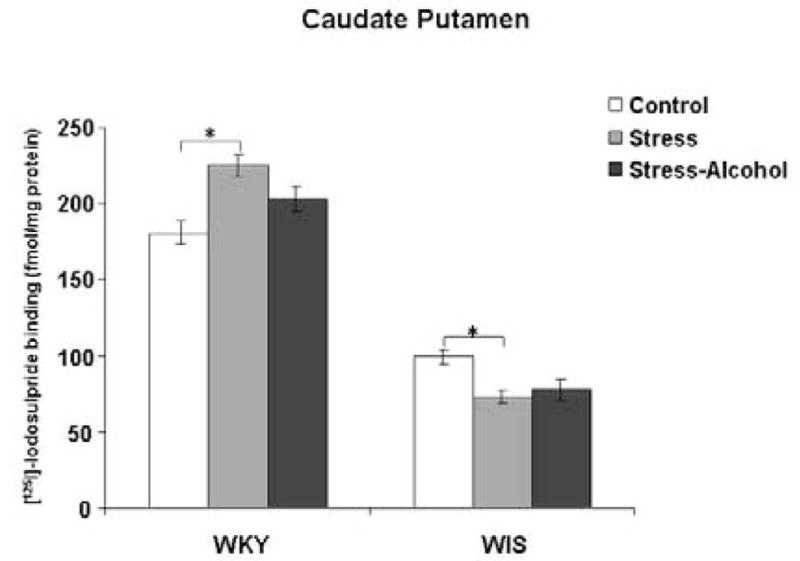
Binding of [125I]-Iodosulpiride to dopamine-2 receptor sites in the CPu of WKY and WIS rats. Data expressed as fmol/mg brain protein and reported as mean ± SEM of duplicate sections from 6–8 animals per group. * Represents significant differences between treatment groups within strain, p<0.05.
WKY-Wistar-Kyoto, WIS-Wistar, CPu-caudate putamen
Nucleus Accumbens (NAc)
Two-way ANOVA identified a main effect of stress and alcohol exposure in the NAc of WKY versus WIS rats, F (2, 36) = 4.11, p<0.05. In WKY rats, stress increased the binding of [125I]-Iodosulpiride to D2 receptor sites in the NAc compared to the control group (Tukey HSD test, p<0.05), with no changes in receptor binding observed following stress and alcohol co-treatment (Figure 3). In contrast, no differences in D2 receptor binding were measured in the WIS rat strain.
Figure 3.
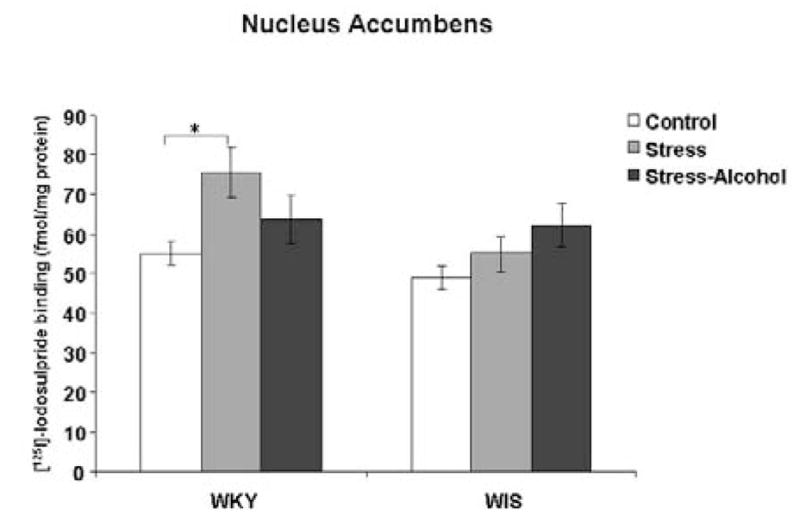
Binding of [125I]-Iodosulpiride to dopamine-2 receptor sites in the NAc of WKY and WIS rats. Data expressed as fmol/mg brain protein and reported as mean ± SEM of duplicate sections from 6–8 animals per group. * Represents significant differences between treatment groups within strain, p<0.05.
WKY-Wistar-Kyoto, WIS-Wistar, NAc-nucleus accumbens
Substantia Nigra (SN)
A significant main effect was measured in the SN of WKY versus WIS rats with stress and alcohol exposure, F (2, 36) = 11.7, p<0.001. In the SN of WKY rats, stress exposure increased the binding of [125I]-Iodosulpiride to D2 receptor sites compared to the control group (Tukey HSD test, p<0.001), while stress and alcohol co-treatment significantly reduced D2 receptor binding compared to the WKY stress group (Tukey HSD test, p<0.05) (Figure 4). In contrast, D2 receptor binding was reduced with stress exposure in WIS rats (Tukey HSD test, p<0.001), with no changes in D2 receptor binding occurring with the addition of alcohol to the stress procedure.
Figure 4.
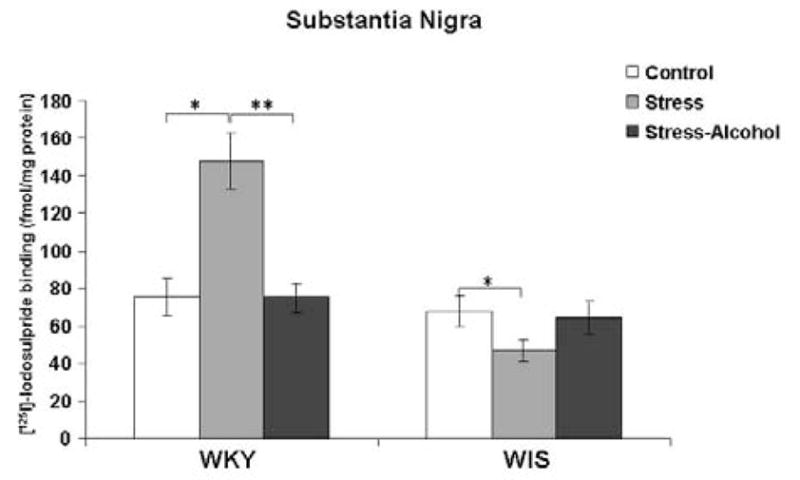
Binding of [125I]-Iodosulpiride to dopamine-2 receptor sites in the SN of WKY rats. Data expressed as fmol/mg brain protein and reported as mean ± SEM of duplicate sections from 6–8 animals per group. * Represents significant differences within strain compared to the control group, p<0.001. ** Represents significant differences within strain compared to the stress group, p<0.001.
WKY-Wistar-Kyoto, WIS-Wistar, SN-substantia nigra
Ventral Tegmental Area (VTA)
A significant main effect was measured with stress and alcohol exposure in the VTA of WKY and WIS rats, F (2, 36) = 10.2, p<0.001. In the VTA of WKY rats, stress exposure increased the binding of [125I]-Iodosulpiride to D2 receptor sites compared to the control group (Tukey HSD test, p<0.001), while stress and alcohol co-treatment significantly reduced D2 receptor binding compared to the WKY stress group (Tukey HSD test, p<0.001) (Figure 5). In contrast, no differences in D2 receptor binding were measured in the WIS rat strain.
Figure 5.
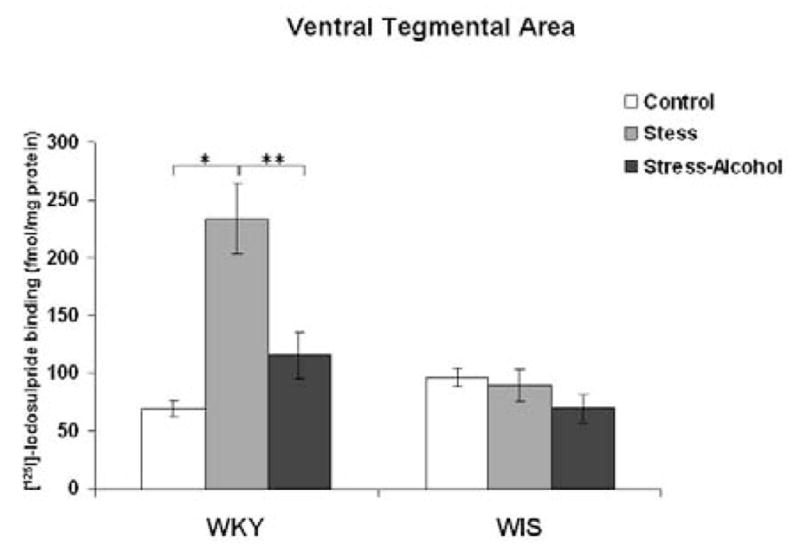
Binding of [125I]-Iodosulpiride to dopamine-2 receptor sites in the VTA of WKY and WIS rats. Data expressed as fmol/mg brain protein and reported as mean ± SEM of duplicate sections from 6–8 animals per group. * Represents significant differences within strain compared to the control group, p<0.001. ** Represents significant differences within strain compared to the stress group, p<0.001.
WKY-Wistar-Kyoto, WIS-Wistar, VTA-ventral tegmental area
DISCUSSION
Effects of Stress on Alcohol Drinking Behavior
Previous studies have noted an exaggerated neurochemical, hormonal and behavioral stress response in WKY rats and have proposed this rat strain as an appropriate animal model of depressive behavior (Paré 1989, 1992, 1994; Redei et al., 1994; Tejani-Butt et al., 1994, 2003; De La Garza and Mahoney 2004). More recently, we have reported that WKY rats exhibit significant strain differences in DA receptor sites in comparison to control strains, suggesting a basal difference in DA neurotransmission in these animals (Jiao et al., 2003; Yaroslavsky et al., 2006; Novick 2008). Accordingly, De La Garza and Mahoney have reported a decrease in DA levels in the pre-frontal cortex and an increase in DA turnover in the striatum and NAc of WKY rats under basal conditions (2004). More recently we have shown that WKY rats voluntarily consume 200% more alcohol than WIS rats (Jiao et al., 2006). Since stress has been shown to increase alcohol consumption in humans as well as rodent models, we examined the effects of chronic stress on alcohol consumption as well as examined the binding of [125I]-Iodosulpiride to D2 receptors in control, stressed or stress and alcohol co-treated WKY compared to WIS rats.
The current results demonstrate that both WKY and WIS rats voluntarily consume more alcohol over a 24 day schedule of novel stressors compared to non-stressed animals. These data are in agreement with a previous report by Lynch and colleagues who demonstrated that restraint stress led to an increase in alcohol consumption in WIS rats (Lynch et al., 1999). However, compared to WIS rats, WKY rats consume significantly more alcohol with or without stressful situations. Paré et al., (1999) have suggested an attenuation of depressive and anxious behaviors in WKY rats following alcohol consumption. This is based on the observation that WKY rats show decreased response latency in the Open Field Test and increased total entries as well as time spent in the open arms of the Elevated Plus-Maze following alcohol consumption (Paré et al., 1999). In past studies, Khantzian had proposed that individuals with substance use disorders take drugs as a means of coping with painful or threatening emotions (1990). At the same time, the tension reduction hypothesis of alcohol consumption suggested that increased drinking behavior is related to alcohol’s effects on reducing tension and/or anxiety (Kalodner et al., 1989; Young et al., 1990). For the last decade, Koob and colleagues have explored the theory of negative reinforcement, which suggests a genetic vulnerability for pathologies such as anxiety and depression, that are relieved by alcohol self administration (1993 (1998). In light of these studies, our findings suggest that both rat strains are consuming greater amounts of alcohol during stressful exposure due to the anxiolytic properties of alcohol, with WKY rats consume almost 300% more alcohol than WIS rats due to their inherent stress-sensitive phenotype.
Effects of Stress and Alcohol on D2 Receptor Binding in Wistar-Kyoto Rats
Evidence from various sources suggests that DA neurotransmission may play an intricate part in the ability to cope with a stressful situation (Katz et al., 1981; Kapur and Mann 1992; Pani et al., 2000). Moreover, stress induced disturbances in DA function have been linked to multiple pathological states including alcohol dependence, anxiety and depression (Piazza and Le Moal 1998; Millan 2003; Nestler and Carlezon 2006). Since alterations in receptor binding may be a direct or indirect result of changes in monoamine levels in certain brain regions, measurements of receptor density provide a useful indication of the status of a particular monoamine pathway. The present study measured D2 receptor density in control, stressed or stress and alcohol co-treated WKY and WIS rats. The binding of [125I]-Iodosulpiride to D2 receptor sites was increased in the CPu, NAc, SN and VTA regions of stressed compared to non-stressed WKY rats. D2 receptor sites located on the somata and dendrites of DA neurons in SN and VTA are autoreceptors and provide inhibitory feedback by modulating DA release, synthesis and/or firing rate; while D2 receptors located in the CPu and NAc are post-synaptic Gi-protein coupled receptors primarily located on non-dopaminergic neurons (O’Hara et al., 1996; Cragg and Greenfield 1997; Mercuri et al., 1997). The observed increase in D2 autoreceptor density in the cell body areas (SN and VTA) following stress exposure may represent increased inhibition of extracellular DA levels, thus leading to the observed D2 receptor up-regulation in the terminal regions (CPu and NAc) as one consequence of depressed DA levels in areas projecting from the SN and VTA. Accordingly, several studies have reported reduced DA output in the NAc as a result of chronic unavoidable stress (Gambarana et al, 1999; Scheggi et al., 2002). Our results are supported by studies that report similar increases in central D2 receptor densities in chronically stressed rodent models (Cabib et al., 1998; Djouma et al., 2006). Overall, our results suggest that in WKY rats, stress may lead to changes in the nigrostriatal and mesolimbic DA pathways, as measured by an increase in D2 receptor binding in all brain regions examined. Moreover, these changes in D2 receptor binding may contribute to the stress-sensetive phenotype previously observed in this rat strain (Paré 1989; Paré and Redei 1993; Redei et al., 1994).
While stress increased the binding of [125I]-Iodosulpiride to D2 receptor sites in the cell body regions (VTA and SN) of WKY rats, stress and alcohol co-treated animals showed a reduction in D2 receptor binding in these two regions compared to animals exposed to stress alone. Our data suggest that the addition of alcohol to the stress procedure may have prevented the stress-induced increase in D2 receptor binding. Coupled with evidence suggesting that alcohol increases DA levels in several brain regions, the observed reduction in autoreceptor D2 sites in the VTA and SN may reflect an elevation in DA levels following the addition of alcohol to the stress procedure (Gesa et al., 1985; Di Chiara and Imperato 1988). At the post-synaptic level, the lack of changes in D2 receptor binding in the stress and alcohol co-treated group may represent a “normalization” of DA levels in the CPu and NAc. Our results suggest that in WKY rats, increased alcohol consumption under stressful stimulation may serve to ameliorate the stress-induced consequences on D2 receptor binding in major DA cell body regions such as the VTA and SN.
Effects of Stress and Alcohol on D2 Receptor Binding in Wistar Rats
The pattern of alterations in WIS rats was different from what was observed in WKY rats following chronic stress as well as following stress-alcohol co-treatment. In contrast to the increase in D2 receptor binding in WKY rats, chronic stress reduced receptor binding in the CPu and SN brain regions, suggesting a facilitation of DA release in the nigrostriatal pathway in response to stressful stimuli. Studies have shown that DA levels increase during a stressful situation and this increase is correlated with an animals’ ability to cope with the stressor at hand (Cabib and Pulglisi-Allegra 1996). In previous reports, WIS rats were more resistant to helpless or immobile behaviors following stressors when compared to WKY rats (Paré 1994). Thus, the opposite changes in DA receptor binding following stress, as observed in WIS rats, may be related to the reported stress-resilience of this rat strain. In the present study, WIS rats did consume more alcohol under stress compared to non-stress conditions; however, the addition of alcohol to the stress procedure did not alter D2 receptor binding compared to the stress group. These effects may be partially due to the lower amounts of alcohol consumed by WIS rats.
Conclusion
In summary, the present study demonstrates that stressed-WKY rats consume more alcohol than stressed-WIS rats. The addition of alcohol to the chronic stress procedure appeared to reverse the stress-induced increases in D2 receptor sites in the SN and VTA of WKY rats. These findings suggest a role for D2 receptor sites in mediating the anxiolytic and rewarding properties of alcohol in the WKY rat strain, and lend further support for the use of this rat strain to examine the mechanisms involved in stress-alcohol interactions.
Acknowledgments
This study was supported by USPHS Grant AA 015921 to S.T-B, and research funds from the Office of Research and Development, Medical Research Service, Department of Veteran Affairs (W. P.). The authors wish to thank Dr. William P. Paré and Dr. Xilu Jiao for their help with this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie ED, Keefe KA, DiFrischa DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens and medial frontal cortex. J Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Allard JS, Tizabi Y, Shaffery JP, Trouth CO, Manaye K. Stereological analysis of the hypothalamic hypocretin/orexin neurons in an animal model of depression. Neuropeptides. 2004;38(5):311–315. doi: 10.1016/j.npep.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Anisman H, Zacharko RM. Multiple neurochemical and behavioral consequences of stressors: implications for depression. Pharmacol Ther. 1990;46:119–136. doi: 10.1016/0163-7258(90)90039-5. [DOI] [PubMed] [Google Scholar]
- Brown RA, Evans M, Miller IW, Burgess ES, Mueller TI. Cognitive-behavioral treatment for depression in alcoholism. J Consult Clin Psychol. 1997;65:715–726. doi: 10.1037//0022-006x.65.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib S, Kempf E, Schleef C, Mele A, Puglisi-Allegra S. Different effects of acute and chronic stress on two dopamine-mediated behaviors in the mouse. Physiol Behav. 1988;43:223–227. doi: 10.1016/0031-9384(88)90242-9. [DOI] [PubMed] [Google Scholar]
- Cabib S, Pulglisi-Allegra S. Stress, depression and the mesolimbic dopamine system. Psychopharmacology (Berl) 1996;128:331–342. doi: 10.1007/s002130050142. [DOI] [PubMed] [Google Scholar]
- Cabib S, Giardino L, Calza L, Zanni M, Mele A, Puglisi-Allegra S. Stress promotes major changes in dopamine receptor densities within the mesoaccumbens and nigrostriatal systems. Neuroscience. 1998;84(1):193–200. doi: 10.1016/s0306-4522(97)00468-5. [DOI] [PubMed] [Google Scholar]
- Carli M, Evenden JL, Robbins TW. Depletion of unilateral striatal dopamine impairs initiation of contra lateral actions and not sensory attention. Nature. 1985;313:679–682. doi: 10.1038/313679a0. [DOI] [PubMed] [Google Scholar]
- Carr GD, White NM. Anatomical disassociation of amphetamine’s rewarding and aversive effects: an intracranial microinjection study. Psychopharmacology Berl. 1986;89:679–682. doi: 10.1007/BF00174372. [DOI] [PubMed] [Google Scholar]
- Cornelius JR, Salloum IM, Ehler JG, Jarrett PJ, Cornelius MD, Perel JM, Thase ME, Black A. Fluoxetine in depressed alcoholics: a double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1997;54:700–705. doi: 10.1001/archpsyc.1997.01830200024004. [DOI] [PubMed] [Google Scholar]
- Cragg SJ, Greenfield SA. Differential autoreceptor control of somatodendritic and axon terminal dopamine release in substantia nigra, ventral tegmental area and striatum. J Neurosci. 1997;17:5738–5746. doi: 10.1523/JNEUROSCI.17-15-05738.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czachowski CL, Chappell AM, Samson HH. Effects of raclopride in the nucleus accumbens on ethanol seeking and consumption. Alc Clin Exp Res. 2001;10:1431–1440. doi: 10.1097/00000374-200110000-00005. [DOI] [PubMed] [Google Scholar]
- De La Garza R, Mahoney JJ. A distinct neurochemical profile in WKY rats at baseline and in response to acute stress: implications for animal models of anxiety and depression. Brain Res. 2004;1021:209–218. doi: 10.1016/j.brainres.2004.06.052. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. The role of dopamine in drug abuse viewed from the perspective of its role on motivation. Drug Alcohol Depend. 1995;38:95–137. doi: 10.1016/0376-8716(95)01118-i. [DOI] [PubMed] [Google Scholar]
- Gambarana C, Masi F, Tagliamonte A, Scheggi S, Ghiglieri O, De Montis MG. A chronic stress which impairs reactivity in rats also decreases dopaminergic transmission in the nucleus accumbens: a microdialysis study. J Neurochem. 1999;72:2039–2046. doi: 10.1046/j.1471-4159.1999.0722039.x. [DOI] [PubMed] [Google Scholar]
- Djouma E, Card K, Lodge DJ, Lawrence AJ. The CRF1 receptor antagonist, antalarmin, reverses isolation induced up-regulation of dopamine D2 receptors in the amygdala and nucleus accumbens of fawn hooded rats. Eur J Neurosci. 2006;23:3319–3327. doi: 10.1111/j.1460-9568.2006.04864.x. [DOI] [PubMed] [Google Scholar]
- Eiler WJA, June HL. Blockade of GABAA receptors within the extended amygdala attenuates D2 regulation of alcohol-motivated behaviors in the ventral tegmental area of alcohol-preferring (P) rats. Neuropsychopharmacology. 2007;52:1570–1579. doi: 10.1016/j.neuropharm.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1978;180:545–580. doi: 10.1002/cne.901800310. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG. Food reward and cocaine increase extra cellular dopamine in the nucleus accumbens as measured by micro dialysis. Life Sci. 1988;42:1705–1712. doi: 10.1016/0024-3205(88)90036-7. [DOI] [PubMed] [Google Scholar]
- Imperato A, Puglisi-Allegra S, Casolini P, Angelucci L. Changes in dopamine and acetylcholine release during and following stress are independent of the pituitary-adrenocortical axis. Brain Res. 1991;538:111–117. doi: 10.1016/0006-8993(91)90384-8. [DOI] [PubMed] [Google Scholar]
- Jiao X, Paré WP, Tejani-Butt SM. Strain differences in the distribution of dopamine transporter sites in rat brain. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:913–919. doi: 10.1016/S0278-5846(03)00150-7. [DOI] [PubMed] [Google Scholar]
- Jiao X, Paré WP, Tejani-Butt SM. Alcohol consumption alters dopamine transporter sites in Wistar-Kyoto rat brain. Brain Res. 20061073–1074:175–182. doi: 10.1016/j.brainres.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug-and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16(3):223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kalodner CR, Delucia JL, Ursprung AW. An examination of the tension reduction hypothesis: the relationship between anxiety and alcohol in college students. Addict Behav. 1989;14:646–654. doi: 10.1016/0306-4603(89)90007-5. [DOI] [PubMed] [Google Scholar]
- Kapur S, Mann JJ. Role of the dopaminergic system in depression. Biol Psychiatry. 1992;32:1–17. doi: 10.1016/0006-3223(92)90137-o. [DOI] [PubMed] [Google Scholar]
- Katz RJ, Roth KA, Carroll BJ. Acute and chronic stress effects on open field activity in the rat: Implications for a model of depression. Neurosci Biobehav Rev. 1981;5:247–251. doi: 10.1016/0149-7634(81)90005-1. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of affective disorders: focus on heroin and cocaine dependence. Am J Psychiatry. 1990;142:1259–1264. doi: 10.1176/ajp.142.11.1259. [DOI] [PubMed] [Google Scholar]
- Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, Merlo-Pich E, Weiss F. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res. 1998;22(1):3–9. [PubMed] [Google Scholar]
- Koob GF, Markou A, Weiss F, Schulteis G. Opponent process and drug dependence: neurobiological mechanisms. Semin Neurosci. 1993;5:351–358. [Google Scholar]
- Lopez-Rubalcava C, Lucki I. Strain differences in the behavioral effects of antidepressant drugs in the rat forced swim test. Neuropsychopharmacology. 2000;22:191–199. doi: 10.1016/S0893-133X(99)00100-1. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Kushner MG, Rawleigh JM, Fiszdon J, Carroll ME. The effects of restraint stress on voluntary alcohol consumption in rats. Exp Clin Psychopharmacol. 1999;7:318–323. doi: 10.1037//1064-1297.7.4.318. [DOI] [PubMed] [Google Scholar]
- Ma S, Morilak Induction of FOS expression by acute immobilization stress is reduced in locus coeruleus and medial amygdale of Wistar-Kyoto rats compared to Sprague-Dawley rats. Neuroscience. 2004;124(4):963–72. doi: 10.1016/j.neuroscience.2003.12.028. [DOI] [PubMed] [Google Scholar]
- Mercuri NB, Saiardi A, Bonci A, Picetti R, Calabresi P, Bernardi G, Borrelli E. Loss of autoreceptor function in dopaminergic neurons from dopamine D2 receptor deficient mice. Neuroscience. 1997;79:323–327. doi: 10.1016/s0306-4522(97)00135-8. [DOI] [PubMed] [Google Scholar]
- Millan M. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70:83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379:449–451. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular mechanisms of drug addiction. J Neurosci. 1992;12(7):2439–2450. doi: 10.1523/JNEUROSCI.12-07-02439.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Phsychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Novick A, Yaroslavsky I, Tejani-Butt S. Strain differences in the expression of D1 receptors in Wistar-Kyoto (WKY) and Wistar (WIS) rats. Life Sci. 2008;83(1–2):74–78. doi: 10.1016/j.lfs.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara CM, Uhland-Smith A, O’Malley KL, Todd RD. Inhibition of dopamine synthesis by dopamine D-2 and D-3 but not D-4 receptors. J Pharmacol Exp Ther. 1996;277:186–192. [PubMed] [Google Scholar]
- Pani L, Porcella A, Gesa GL. The role of stress in the pathophysiology in the dopaminergic system. Mol Psychiat. 2000;5:14–21. doi: 10.1038/sj.mp.4000589. [DOI] [PubMed] [Google Scholar]
- Pardon MC, Gould GG, Garcia A, Phillips L, Cook MC, Miller SA, Mason PA, Morilak DA. Stress reactivity of the brain noradrenergic system in three rat strains differing in their neuroendocrine and behavioral responses to stress: Implications for susceptibility to stress-related neuropsychiatric disorders. Neuroscience. 2002;115(1):229–42. doi: 10.1016/s0306-4522(02)00364-0. [DOI] [PubMed] [Google Scholar]
- Paré WP. Stress ulcer susceptibility and depression in Wistar-Kyoto rats. Physiol Behav. 1989;46:993–998. doi: 10.1016/0031-9384(89)90203-5. [DOI] [PubMed] [Google Scholar]
- Paré WP. Learning behavior, escape behavior, and depression in an ulcer susceptible rat strain. Intergr Physiol Behav Sci. 1992;27:130–141. doi: 10.1007/BF02698502. [DOI] [PubMed] [Google Scholar]
- Paré WP, Redei E. Depressive behavior and stress ulcer in Wistar-Kyoto Rats. J Physiol (Paris) 1993;89:229–238. doi: 10.1016/0928-4257(93)90010-q. [DOI] [PubMed] [Google Scholar]
- Paré WP. Open field, learned helplessness, defensive burying and forced-swim tests in WKY rats. Physiol Behav. 1994;55:433–439. doi: 10.1016/0031-9384(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Paré AMT, Parè WP, Kluczynski J. Negative affect and voluntary alcohol consumption in Wistar-Kyoto (WKY) and Sprague-Dawley Rats. Physiol Behav. 1999;67(2):219–225. doi: 10.1016/s0031-9384(99)00054-2. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Cordinates. Academic Press; New York: 1998. [Google Scholar]
- Pettinati HM. Antidepressant treatment of co-occurring depression and alcohol dependence. Biol Psychiatry. 2004;56:785–792. doi: 10.1016/j.biopsych.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Damsma G, Wenkstern D, Fibiger HC. Sexual activity increases dopamine transmission in the nucleus accumbens and striatum of female rats. Brain Res. 1995;693:21–30. doi: 10.1016/0006-8993(95)00679-k. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19(2):67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Redei E, Paré WP, Aird F, Kluczynski J. Strain differences in hypothalamic pituitary adrenal activity and stress ulcer. Am J Physiol. 1994;266:353–360. doi: 10.1152/ajpregu.1994.266.2.R353. [DOI] [PubMed] [Google Scholar]
- Sandbak T, Murison R. Voluntary alcohol consumption in rats: relationships to defensive burying and stress gastric erosions. Physiol Behav. 1996;59:983–989. doi: 10.1016/0031-9384(95)02173-6. [DOI] [PubMed] [Google Scholar]
- Sari Y, Bell RL, Zhou FC. Effects of chronic alcohol and repeated deprivations on dopamine D1 and D2 receptor levels in the extended amygdala of inbred alcohol-prefering rats. Alcohol Clin Exp Res. 2006;30(1):46–56. doi: 10.1111/j.1530-0277.2006.00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheggi S, Leggio B, Masi F, Grappi S, Gambarana C, Nanni G, Rauggi R, De Montis MG. Selective modifications in the nucleus accumbens of dopamine synaptic transmission in rats exposed to chronic stress. J Neurochem. 2002;83:895–903. doi: 10.1046/j.1471-4159.2002.01193.x. [DOI] [PubMed] [Google Scholar]
- Stefanski R, Lee S, Yasar S, Cadet JL, Goldberg SR. Lack of persistent changes in the dopaminergic system of rats withdrawn from methamphetamine self administration. Eur J Pharmacol. 202(439):59–68. doi: 10.1016/s0014-2999(02)01301-8. [DOI] [PubMed] [Google Scholar]
- Tejani-Butt SM, Paré WP, Yang J. Effect of repeated novel stressors on depressive behavior and brain norepinephrine receptor system in Sprague-Dawley and Wistar-Kyoto rats. Brain Res. 1994;649:27–35. doi: 10.1016/0006-8993(94)91045-6. [DOI] [PubMed] [Google Scholar]
- Tejani-Butt SM, Kluczynski J, Paré WP. Strain-dependent modification of behavior following antidepressant treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:7–14. doi: 10.1016/s0278-5846(02)00308-1. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR. Uncontrollable events and alcohol drinking. Br J Addict. 1987;82:381–392. doi: 10.1111/j.1360-0443.1987.tb01494.x. [DOI] [PubMed] [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- Yaroslavsky I, Colletti M, Jiao X, Tejani-Butt S. Strain differences in the distribution of dopamine (D2 and D3) receptor sites in rat brain. Life Sci. 2006;79(8):772–776. doi: 10.1016/j.lfs.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Wilson C, Nomikos GG, Collu M, Fibiger HC. Dopaminergic correlates of motivated behavior; importance of drive. J Neurosci. 1995;15:5169–5178. doi: 10.1523/JNEUROSCI.15-07-05169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto K, McBride WJ, Lumeng L, Li T-K. Alcohol stimulates the release of dopamine and serotonin in the nucleus accumbens. Alcohol. 1991;9:17–22. doi: 10.1016/0741-8329(92)90004-t. [DOI] [PubMed] [Google Scholar]
- Young AM, Joseph MH, Gray JA. Increased dopamine release in vivo in nucleus accumbens and caudate nucleus of the rat during drinking: a microdialysis study. Neuroscience. 1992;48:871–876. doi: 10.1016/0306-4522(92)90275-7. [DOI] [PubMed] [Google Scholar]
- Young RM, Oei TP, Knight RG. The tension reduction hypothesis revisited: an alcohol expectancy perspective. Br J Addict. 1990;85:31–40. doi: 10.1111/j.1360-0443.1990.tb00621.x. [DOI] [PubMed] [Google Scholar]


