Abstract
We evaluated the pharmacokinetics and efficacy of oral mycophenolate mofetil (MMF) for treatment of refractory graft-versus-host disease (GVHD). In a prospective study of acute GVHD, 9 of 19 patients (47%) had a response and 10 (53%) had no improvement. Survival at 6 and 12 months after the start of MMF was 37% and 16%, respectively. In a retrospective study of acute GVHD, 14 of 29 patients (48%) had a response and 15 (52%) had no improvement. Survival at 6 and 12 months was 55% and 52%, respectively. In a prospective study of chronic GVHD, the cumulative incidence of disease resolution and withdrawal of all systemic immunosuppressive treatment was 9%, 17% and 26% at 12, 24 and 36 months after starting MMF, respectively. Thirteen patients (59%) required additional systemic immunosuppressive treatment for chronic GVHD. Nine of the 42 patients (21%) in the prospective studies discontinued MMF treatment because of toxicity. Area under the curve plasma concentrations of mycophenolic acid appeared to be suboptimal among patients with acute GVHD but not among those with chronic GVHD. MMF can be used effectively for treatment of GVHD.
Keywords: MMF, HCT, acute and chronic GVHD
Introduction
Acute and chronic graft-versus-host disease (GVHD) are the most common early and late complications after allogeneic hematopoietic stem cell transplantation (HCT) and is a major cause of morbidity and non-relapse mortality [1,2]. Depending on the degree of recipient HLA mismatching, the source of the donor graft and other risk factors, 30 – 80% of graft recipients will develop acute GVHD, usually requiring systemic immunosuppressive treatment [3–6]. Overall response rates in the range of 24 – 55% have been reported after initial therapy with glucocorticoids or other immunosuppressive medications [7–12], and many patients require secondary therapy with additional immunosuppressive agents. Overall response rates in the range of 30 – 75% have been reported for a variety of agents used for treatment of steroid-resistant or steroid-refractory acute GVHD, although transplant-related mortality remains high, even when secondary treatment improves GVHD manifestations [13–21].
Depending on the stem cell source and the time of evaluation, approximately 30 – 65% of patients develop chronic GVHD after HCT, often requiring long-term glucocorticoid treatment [22, 23]. In a large retrospective study, the median duration of treatment for chronic GVHD was 23 months [24]. Both acute and chronic GVHD are associated with an increased risk of transplant-related mortality, often related to opportunistic infections [22, 25]. More effective and less toxic therapies and treatment strategies are needed to improve the management of GVHD and decrease morbidity and non-relapse mortality.
Mycophenolate mofetil (MMF) is the 2(4-morpholine) ethyl ester of mycophenolic acid (MPA). MMF is rapidly absorbed following oral administration and hydrolyzed to the active metabolite, MPA [26]. MPA selectively and reversibly inhibits inosine monophosphate dehydrogenase (IMPDH), blocking the de novo pathway of purine synthesis in T and B lymphocytes [27–29]. Preclinical studies in allogeneic transplant models have shown that MMF has activity in preventing graft rejection and GVHD [30–33]. After transplantation of unrelated DLA-mismatched marrow in dogs, MMF synergizes with cyclosporine (CSP) to prevent GVHD and improve survival [34]. Phase III clinical studies after kidney transplantation have shown that MMF is effective in the prevention of graft rejection [35–38]. Studies using MMF for treatment of acute and chronic GVHD following HCT have reported efficacy in the range of 30 – 90% [39–48].
Two Phase II clinical trials and a retrospective review were conducted to evaluate the safety and efficacy of oral MMF for treatment of acute and chronic GVHD. Since gastrointestinal toxicity from the conditioning regimen or gut involvement from acute GVHD might affect the absorption of oral MMF, plasma levels of MPA were measured in order to assess differences between acute and chronic GVHD and to determine the optimal dosing of MMF for treatment of GVHD.
Patients and Methods
Patient enrollment in these two prospective clinical trials began in November 1995 and was completed in December 1997. The Institutional Review Board (IRB) at Fred Hutchinson Cancer Research Center (FHCRC) approved the trials, and all patients signed consent documents. Patients were approached sequentially for enrollment in each of these trials, provided they met the eligibility criteria summarized below. The original plan was to enroll 20 patients in each study.
For purposes of comparison and validation, results were retrospectively reviewed for all patients who received MMF for secondary therapy of acute GVHD after initial treatment with prednisone following HCT with myeloablative conditioning regimens between 2000 and 2005. This analysis was also approved by the FHCRC IRB.
Patients
The primary therapy for acute GVHD was prednisone or methylprednisolone, initially at 2 mg/kg/day, in addition to continued administration of cyclosporine (CSP) originally prescribed for prophylaxis. Patients with acute GVHD were eligible for the acute GVHD study (AGVHD Group) if GVHD manifestations worsened after 3 days of primary therapy, showed no improvement after 7 days, or persisted for more than 14 days. Patients with skin or gastrointestinal involvement had to have biopsy-proven evidence of GVHD.
Initial treatment for chronic GVHD was prednisone at 1 mg/kg/day, in addition to continued administration of CSP or tacrolimus (TAC). Patients with extensive chronic GVHD were eligible for the chronic GVHD study (CGVHD Group) if GVHD manifestations 1) worsened or showed no improvement after at least 2 months of initial treatment, 2) persisted after at least 9 months of treatment in patients with “high risk” chronic GVHD (progressive onset or platelet count < 100,000/mm3 at onset), 3) or for at least 18 months of treatment in patients with “standard risk” chronic GVHD, or 4) if GVHD manifestations recurred during a taper of steroid doses.
Patients were excluded from participation in either study if they were unable to tolerate oral therapy, if the neutrophil count was less than 1500/uL or if they had evidence of recurrent malignancy. Patients with a serum creatinine concentration >2.0 mg/dL were excluded from participation in the acute GVHD study, and those with a creatinine clearance <30 ml/min were excluded from the chronic GVHD study.
Treatment Plan and Supportive Care
All study patients received the oral formulation of MMF at an initial dose of 1 gram twice daily for adults and 20 mg/kg/day twice daily for patients who weighed less than 50 kilograms. For patients in the AGVHD Group, the dose of MMF could be increased to 1.5 grams twice daily if GVHD manifestations had worsened at study day 7 or had not improved by study day 14. The protocol recommended adjustments in the dose of MMF if hematopoietic or gastrointestinal toxicity was suspected. Recommendations were to decrease the MMF dose by 50% if the neutrophil count decreased to <1000/uL, and to discontinue administration of MMF if the neutrophil count decreased to <750/uL. Additional recommendations were to decrease the dose of MMF if patients developed gastrointestinal problems not caused by GVHD and to discontinue administration of MMF if problems persisted afterwards.
For patients in the AGVHD Group, MMF was administered for 35 days. Treatment with MMF was discontinued if GVHD resolved or there was no response by study day 35. Treatment with MMF could be continued beyond 35 days in patients with a partial response and in those with recurrent GVHD after treatment with MMF was discontinued. Glucocorticoid doses were tapered at the discretion of the attending physician. For patients in the retrospective AGVHD Group, the decision to start MMF therapy, the dosing and route of administration, and the duration of therapy were determined by the attending physician.
For treatment of CGVHD, MMF was administered for 9–12 months. Treatment with MMF was discontinued if GVHD manifestations resolved. If GVHD manifestations improved but persisted after 9–12 months, treatment with MMF was continued for another 9–12 months. If GVHD manifestations showed no improvement or worsened after 2 months, treatment with MMF was discontinued. In the absence of toxicity, administration of CSP or TAC was continued during the initial period of treatment with MMF.
All protocol patients received antifungal prophylaxis with fluconazole. All cytomegalovirus (CMV)-seropositive patients had weekly testing for reactivation of CMV, and pre-emptive therapy with ganciclovir was initiated, when appropriate. Patients received prophylaxis against Pneumocystis pneumonia with trimethoprim (TMP)/sulfamethoxazole (SMX). Dapsone was given to patients who could not tolerate TMP/SMX.
Pharmacokinetics
Plasma levels of MPA, the active metabolite of MMF, were measured on days 1, 7 and 35 after the start of MMF therapy. Day 1 sampling was started before administration of the first dose of MMF. Specimens were collected immediately before and at 1, 2, 4, 8, and 12 hours after ingestion of MMF. Blood was collected in tubes containing EDTA. Blood was centrifuged at 4° C and the plasma was collected. Plasma was stored at −70 C and shipped to the University of Alberta, Canada, for analysis. MPA plasma levels were quantified by HPLC and peak plasma concentration (Cmax) and area-under-the-curve (AUC) were determined as described previously [49].
Evaluation of response
The evaluation and grading of acute and chronic GVHD has been described previously [1,2,50]. Treatment responses in the AGVHD Group were categorized as a complete response, a partial response or a treatment failure. A complete response was defined as the absence of any symptoms related to GVHD, with no additional agents needed to control the disease. A partial response was defined as improvement of at least one stage in severity of acute GVHD in one organ without deterioration in any other organ. Treatment failure was defined as the absence of improvement, deterioration of acute GVHD in any organ by at least one stage, the development of GVHD manifestations in a previously unaffected organ, or the use of any additional agents to control the disease. Patients were scored for best response at any time after starting treatment with MMF, with follow-up censored at the onset of any subsequent systemic immunosuppressive therapy. This criterion was also used to assess response to treatment with MMF for patients in the retrospective AGVHD Group.
Responses in the CGVHD Group were measured according to two outcomes. Failure was defined as the use of any additional agents to control GVHD within 3 years after starting treatment with MMF, including resumption of treatment with agents used previously or the substitution of one calcineurin inhibitor for the other. Success was defined as the discontinuation of all systemic immunosuppressive therapy without recurrent malignancy within 12, 24 and 36 months after starting treatment with MMF. Medications used to treat chronic GVHD were abstracted from records of evaluations in Seattle and from correspondence with the referring physician. In both groups, follow-up for evaluation of GVHD was censored at the onset of recurrent malignancy.
Results
Patient and Transplant Characteristics
Nineteen patients with acute GVHD and 23 patients with chronic GVHD were enrolled in the two prospective studies, and the retrospective analysis included 29 patients (Table 1). Compared to the prospective AGVHD Group, the patients in the retrospective AGVHD Group were older (median age, 40 years versus 20 years), were more likely to have low-risk hematologic malignancies (52% versus 32%), had a higher proportion of unrelated (93% versus 53%) and HLA-mismatched donors (45% versus 37%), and a higher proportion received peripheral blood stem cell grafts (69% versus 21%). Approximately half of the patients in the CGVHD Group had unrelated donors. Most patients in the CGVHD Group had low-risk hematologic diseases at the time of transplant and received bone marrow grafts. Three patients in the CGVHD Group and one in the prospective AGVHD Group had previous allogeneic HCT. None of the patients in the retrospective acute GVHD Group had previous HCT.
Table 1.
Patient and Transplant Characteristics According to Treatment Group
| AGVHD |
|||
|---|---|---|---|
| Characteristic | Prospective Group (n = 19) | Retrospective Group (n = 29) | CGVHD Group (n = 23) |
| Patient age, median years (range) | 20 (4 – 54) | 40 (2 – 61) | 27 (2 – 57) |
| Disease risk, n (%)1 | |||
| Low | 6 (32) | 15 (52) | 15 |
| High | 13 (68) | 14 (48) | 8 |
| Donor type and recipient HLA-matching, n (%) | |||
| Related donor | |||
| HLA-matched | 5 (26) | 2 (7) | 10 |
| HLA-mismatched | 4 (21) | 0 (0) | 3 |
| Unrelated donor | |||
| HLA-matched | 7 (37) | 14 (48) | 4 |
| HLA-mismatched | 3 (16) | 13 (45) | 6 |
| Stem cell source, n (%)2 | |||
| Marrow | 16 (84) | 9 (31) | 21 |
| Peripheral blood stem cells | 4 (21) | 20 (69) | 2 |
| Conditioning regimen, n (%) | |||
| Cyclophosphamide/TBI | 6 (32) | 14 (48) | 13 |
| Busulfan/cyclophosphamide | 0 (0) | 10 (35) | 6 |
| Busulfan/TBI | 5 (26) | 0 (0) | 0 |
| Other | 8 (42) | 5 (17) | 4 |
| GVHD prophylaxis, n (%) | |||
| Cyclosporine/methotrexate | 15 (78) | 23 (80) | 16 |
| Tacrolimus/methotrexate | 0 (0) | 3 (10) | 0 |
| Cyclosporine/prednisone | 2 (11) | 0 (0) | 2 |
| Other | 2 (11) | 3 (10) | 5 |
Low-risk disease included chronic myelogenous leukemia in chronic phase; acute leukemia in first remission; refractory anemia without excess blasts; and lymphoma in first remission, first untreated relapse or second remission. Three patients with thalassemia, Chediak-Higashi syndrome and paroxysmal nocturnal hemoglobinuria were included in the low risk category.
One patient in the prospective AGVHD Group received peripheral blood stem cells and marrow.
Treatment Response AGVHD Groups
In the prospective study, the median onset of acute GVHD was 10 days after HCT (range 5 – 30) (Table 2). MMF therapy was started at a median of 42 days after transplantation and 31 days after the diagnosis of acute GVHD. The median duration of MMF administration was 35 days (range 3 – 416). Seventeen patients received glucocorticoids as initial treatment for acute GVHD and two patients received CSP. Three patients had received one additional systemic immunosuppressive therapy for acute GVHD before starting treatment with MMF. In this group, 6 of 19 patients (31%) had complete resolution of acute GVHD, 3 (16%) had partial responses, and 10 (53%) had no improvement. In 3 patients, treatment with MMF was stopped after 3 days because of progressive GVHD. One of these patients had no further treatment, and the other 2 were treated with antithymocyte globulin. All 3 died within 12 days. At the start of treatment with MMF, 12 patients (63%) had Grade II GVHD, 5 (26%) had Grade III GVHD and 2 (11%) had Grade IV GVHD. Eight of the 12 patients (67%) with Grade II GVHD at the start of MMF therapy had a complete or partial response, compared to only 1 of the 7 patients (14%) with Grades III–IV GVHD. All but one of the 14 patients surviving beyond 100 days after HCT developed chronic GVHD.
Table 2.
Acute GVHD Characteristics and Treatment Responses
| Characteristic | Prospective Group (n = 19) | Retrospective Group (n = 29) |
|---|---|---|
| Onset of acute GVHD, median day after HCT (range) | 10 (5 – 30) | 11 (5 – 34) |
| Acute GVHD grade at start of MMF therapy, n (%) | ||
| I | 0 (0) | 3 (10) |
| II | 12 (63) | 22 (76) |
| III | 5 (26) | 4 (14) |
| IV | 2 (11) | 0 (0) |
| Organ involvement at the start of MMF therapy, n (%) | ||
| Skin | 13 (68) | 22 (76) |
| Gastrointestinal | 10 (53) | 11 (38) |
| Hepatic | 6 (32) | 7 (24) |
| Start of MMF therapy, median day (range) | ||
| From HCT | 42 (27 – 89) | 52 (22 – 131) |
| From diagnosis of acute GVHD | 31 (14 – 79) | 42 (13 – 120) |
| From start of primary therapy | 24 (9 – 68) | 35 (4 – 121) |
| Clinical response, n (%) | ||
| Complete response | 6 (31) | 9 (31) |
| Partial response | 3 (16) | 5 (17) |
| Treatment failure | 10 (53) | 15 (52) |
| Subsequent chronic GVHD, n (%) | ||
| Yes | 13 (93) | 22 (88) |
| No | 1 (7) | 3 (12) |
| Not evaluated | 5 | 4 |
In comparing the retrospective AGVHD Group with the prospective AGVHD Group, the main differences were a lower incidence of Grade III–IV GVHD at the onset of MMF therapy (14% versus 37%), and longer median times from HCT to the onset of treatment with MMF (52 days versus 42 days), from initial diagnosis of GVHD to the onset of treatment with MMF (42 days versus 31 days), and from the onset of steroid treatment to the onset of treatment with MMF (35 days versus 24 days) (Table 2). Patients in the retrospective AGVHD Group had a lower incidence of gastrointestinal (38% versus 53%) and hepatic (24% versus 32%) GVHD. There were no differences in the time of onset of GVHD or the clinical response to MMF. In this group, 9 of 29 patients (31%) had complete response, 5 (17%) had partial response, and 15 (52%) had no improvement. Despite the differences in GVHD severity and interval times at onset of MMF therapy, the response rates in the two AGVHD groups were virtually identical. Seventy-nine percent of the patients in the restrospective AGVHD Group were initially treated with the oral formulation of MMF. All patients in the prospective AGVHD Group were treated exclusively with the oral formulation.
Treatment Response CGVHD Group
Treatment with MMF was started at a median of 20 months after HCT (Table 3). MMF was administered for a median of 12 months (range 3 – 99). Sixteen of the 23 patients (70%) had received at least one systemic immunosuppressive therapy for chronic GVHD in addition to glucocorticoids and the original prophylactic calcineurin inhibitor before starting treatment with MMF. Seven patients had received tacrolimus, six had received azathioprine, and 4 had received thalidomide. When MMF therapy was started, most patients were receiving combination immunosuppressive therapy with a calcineurin inhibitor plus glucocorticoids. Nine patients had chronic GVHD involving two organs and 10 had chronic GVHD affecting 3 or more organs, most often involving the skin and the oral cavity.
Table 3.
Chronic GVHD Characteristics and Treatment Responses
| Characteristic | CGVHD Group (n = 23) |
|---|---|
| Months from transplantation to start of MMF, median (range) | 20 (4 – 91) |
| Number of prior chronic GVHD therapies, n | |
| 1 | 13 |
| 2 | 3 |
| None | 7 |
| Affected sites at the start of treatment with MMF, n | |
| Skin | 18 |
| Oral | 18 |
| Eyes | 9 |
| Gastrointestinal tract | 4 |
| Lung | 4 |
| Liver | 4 |
| Vagina | 1 |
| Number of sites involved at the start of treatment with MMF, n | |
| 1 | 4 |
| 2 | 9 |
| 3 | 6 |
| More than 3 | 4 |
| Immunosuppressive therapy (IS) at study enrollment, n | |
| CSP or tacrolimus plus glucocorticoids | 14 |
| Glucocorticoids alone | 4 |
| CSP or tacrolimus alone | 2 |
| Other | 3 |
| Months of treatment with MMF, median (range)2 | 12 (3 – 99) |
| Patients who discontinued all immunosuppressive treatment, n (%)1 | |
| 12 months | 2 (9) |
| 24 months | 4 (17) |
| 36 months | 6 (26) |
| Months to discontinuation of immunosuppressive treatment, median (range) | 22 (9 – 35) |
| Patients requiring additional treatment for GVHD after MMF, n (%)2 | 13 (59) |
Two patients could not be evaluated at 12 months, 9 at 24 months and 9 at 36 months.
One patient was lost to follow-up and could not be evaluated.
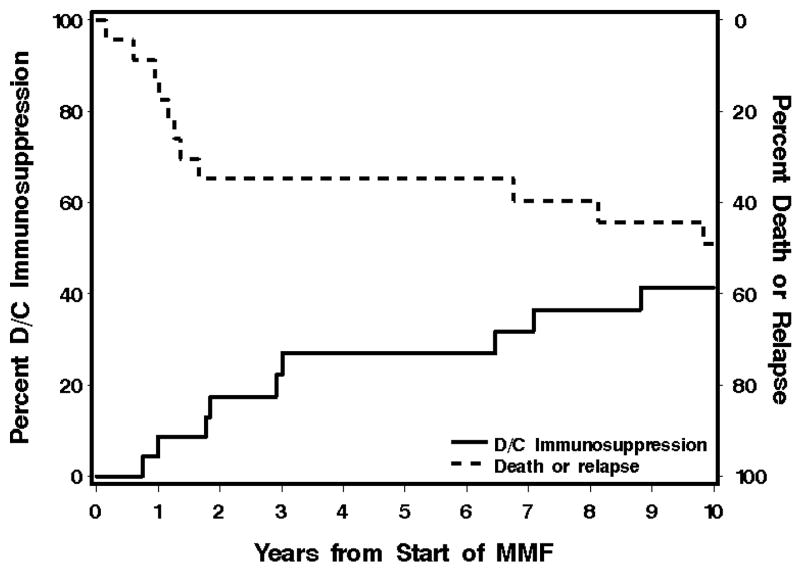
At 12, 24 and 36 months after the start of MMF therapy 2 (9%), 4 (17%) and 6 (26%) patients, respectively, had discontinued all immunosuppressive therapy after resolution of chronic GVHD at 9 – 35 (median, 22) months after starting treatment with MMF (Figure 2). Two patients could not be evaluated at 12 months. One had died, and the other had recurrent malignancy. At 24 and 36-months, 7 additional patients could not be evaluated. One was lost to follow-up, one had recurrent malignancy, and 5 had died. None of the patients who died or had recurrent malignancy had discontinued immunosuppressive treatment after resolution of GVHD. Thirteen of the 22 patients (59%) with follow-up information required additional therapy for chronic GVHD within three years after starting treatment with MMF. One patient was lost to follow-up and could not be evaluated.
Figure 2.
Overall survival and duration of immunosuppression in the CGVHD Group.
Toxicity and Early Discontinuation of MMF
Eight patients in the prospective AGVHD Group discontinued treatment with MMF before day 35 (Table 4). Three had progressive GVHD and discontinued treatment with MMF after 3 days, and one other patient died on day 29. Administration of MMF was discontinued in 4 patients because of neutropenia (n = 2), abdominal pain (n = 1) or pulmonary infiltrate (n = 1). Doses of MMF were reduced in 3 other patients because of neutropenia or presumed gastrointestinal toxicity. In the CGVHD Group, 11 patients discontinued administration of MMF prematurely due to gastrointestinal discomfort (n = 4), neutropenia (n = 1), lack of efficacy (n = 4) or recurrent malignancy (n = 2). Doses of MMF were reduced in one patient because of presumed gastrointestinal toxicity. Altogether, 9 of the 42 patients (21%) in the two prospective studies discontinued MMF treatment because of toxicity.
Table 4.
Reasons for Early Discontinuation of Treatment with MMF
| Reason | Prospective AGVHD Group (n = 19) | CGVHD Group (n = 23)* |
|---|---|---|
| Death | 1 | 0 |
| Cytopenia | 2 | 1 |
| Gastrointestinal toxicity | 1 | 4 |
| Pulmonary infiltrates | 1 | 0 |
| Uncontrolled GVHD | 3 | 4 |
| Recurrent malignancy | 0 | 2 |
One patient was lost to follow-up
Pharmacokinetics
Pharmacokinetic testing was completed in twelve patients in the prospective AGVHD Group and 18 in the CGVHD Group. On day 2 (median; range, 1–10) after starting treatment with MMF, the median MPA-AUC was 15.8 (range, 7.6–25.3; mean 17.1 +/−5.5) μg × hr/mL in the AGVHD Group, compared to 49.9 (range, 19.2–202.8; mean 62.0 +/− 47.9) μg × hr/mL on day 2 (median; range, 1–13) in the CGVHD Group (Wilcoxon, p<0.0001)). The median Cmax was 4.8 (range, 1.8–8.6; mean 5.1 +/− 2.3) μg/mL in the AGVHD Group compared to 12.0 (range, 5.8–24.7; mean 12.8 +/− 5.7) in the CGVHD Group (Wilcoxon, p<0.0001).
Outcome
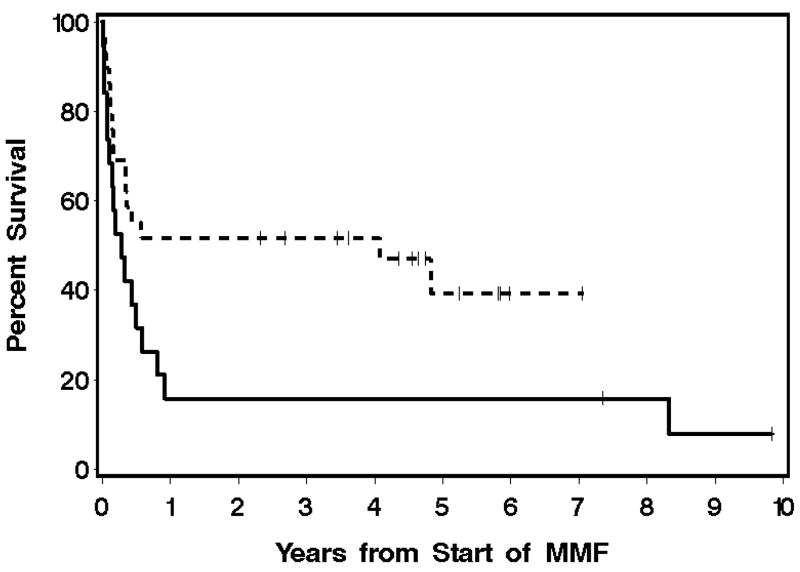
In the prospective AGVHD Group, 7 (37%) and 3 (16%) patients were alive at 6 and 12 months, respectively, after starting treatment with MMF. Two patients (11%) died with recurrent malignancy on days 119 and 182, respectively, after starting treatment with MMF. These results contrast with survival rates of 55% and 52% at 6 and 12 months, respectively, in the retrospective AGVHD Group (Figure 1). Two patients (7%) died with recurrent malignancy respectively on days 126 and 158 after starting treatment with MMF. In the CGVHD Group, 22 patients (96%) were alive one year after starting treatment with MMF (Figure 2). Twelve of 23 (52%) patients with follow-up information remain alive at 7.9 – 10.6 (median, 9.5) years after starting treatment with MMF. One of these patients is still being treated with immunosuppressive medications at the time of last follow-up. Three patients had recurrent malignancy after treatment with MMF therapy. Causes of death for all prospective study patients are listed in Table 5. In the retrospective group, fungal infection was implicated in six of seven patients who died with infection, compared to 3 of 6 patients who died with infection in the prospective group. Overall, the causes of death between the groups were not different.
Figure 1.
Overall survival in the prospective (solid line) and retrospective (dashed line) AGVHD Groups.
Table 5.
Causes of Death
| Cause | Prospective AGVHD Group (n = 19) | Retrospective AGVHD Group (n = 29) | CGVHD Group (n = 23) |
|---|---|---|---|
| Respiratory failure (not infection) | 1 | 2 | 5 |
| Infection | 6 | 7 | 3 |
| Recurrent malignancy | 3 | 1 | 0 |
| Cardiopulmonary | 2 | 0 | 1 |
| GVHD | 4 | 5 | 1 |
| Multi-organ failure | 1 | 0 | 1 |
| Secondary malignancy | 0 | 1 | 0 |
Discussion
A requirement for additional therapy beyond glucocorticoids to treat acute or chronic GVHD identifies a group of patients with generally poor outcomes. Historically, antithymocyte globulin has been commonly used to treat refractory acute GVHD [14,20,21,51,52], but a variety of new immunosuppressive agents have been evaluated more recently [15–19,52,53,54]. Depending on the definition of response and time of evaluation, response rates range from 30–75%, but survival has been poor, even in studies with high response rates. Survival rates are difficult to evaluate in these studies because many reports did not show Kaplan-Meier estimates of survival across time. Mortality was commonly associated with uncontrolled GVHD, often complicated by opportunistic infection.
A few reports have described the use of MMF for treatment of refractory acute GVHD in small numbers of patients [40,41,46–48]. Comparisons with our experience are complicated by differences in patient selection, the degree of immunosuppression at the beginning of treatment with MMF, definitions of response and the timing of evaluations. It is unknown whether the patients described in these reports were treated with the parenteral formulation of MMF. In our studies only the oral formulation of MMF was used. The overall combined response and survival rates among the 42 patients included in these reports is 48% and 38%, respectively. In our prospective study of acute GVHD, the clinical response rate was 47%, but survival at 6 and 12 months after starting treatment with MMF was only 37% and 16%, respectively. Only 2 patients died with recurrent hematologic malignancy. In the retrospective AGVHD Group, the clinical response rate was nearly identical, although the 6 and 12 month survival rates were 55% and 52%, respectively. Two patients died with recurrent hematologic malignancy.
The early onset of acute GVHD in the prospective and retrospective studies of acute GVHD was somewhat unusual and could raise some question about the accuracy of the initial diagnosis, especially since engraftment syndrome can mimic acute GVHD. Engraftment syndrome generally resolves during short-term treatment with high-dose glucocorticoids, whereas GVHD generally resolves more slowly. The persistence of inflammatory manifestations despite initial glucocorticoid treatment suggests that our patients had GVHD rather than engraftment syndrome.
The poor 6 and 12 month survival rates in the prospective acute GVHD study, which was completed in the mid-1990s, prompted a retrospective review of more recent experience with the use of MMF for treatment of steroid-resistant acute GVHD, since MMF is now widely used for secondary treatment of acute GVHD. Reasons for the difference in survival between the two AGVHD groups are not entirely clear. Although the median age of the prospective AGVHD Group was lower and a higher proportion had HLA-matched related donors, this group also contained higher proportions of patients with high-risk malignancies and Grades III – IV GVHD at the start of MMF therapy. Three patients in the prospective AGVHD Group had progressive GVHD requiring additional immunosuppressive therapy and died within 12 days after starting treatment with MMF. The shorter interval times from HCT, diagnosis of GVHD and initiation of prednisone treatment to onset of treatment with MMF suggest more aggressive progression of GVHD and greater glucocorticoid resistance in the prospective cohort compared to the retrospective cohort. The similarity of response rates in the two groups, however, argues against this supposition. The favorable survival among patients in the retrospective AGVHD Group may reflect improved management of GVHD, longer experience with the use of MMF, and the availability of the intravenous formulation, although most patients in the retrospective group were treated initially with the oral formulation.
One could argue that differences in survival between the two AGVHD groups might be attributable to advances in transplantation during the years when these groups were treated. Patients in the prospective group had HCT in 1995–1997, while patients in the retrospective group had HCT in 2000–2005. The latter group might have benefited from improved primary treatment regimens, such as combination therapy with busulfan and cyclophosphamide. Ten patients (35%) in the retrospective AGVHD Group received this combination of chemotherapy, compared to none in the prospective AGVHD Group. The proportion of patients in the retrospective AGVHD Group who received growth factor-mobilized blood cells was higher in the retrospective AGVHD group than in the retrospective AGVHD group (69% versus 21%). The use of mobilized blood cells likely resulted in earlier neutrophil engraftment and may have influenced immune reconstitution. Newer antibiotic therapies, particularly for the treatment for fungal infections, were more readily available to patients in the recent cohort. This potential advantage was not validated, however, since the proportions of patients who died with infections were not strikingly different in the two groups.
A variety of agents have been used to treat refractory chronic GVHD [55–59], and response rates with the use of MMF have been approximately 40 – 90% [42–45]. Lopez et al. [43] reported on 34 patients who received MMF therapy for treatment of chronic GVHD. Responses were observed in 9 of 10 patients who received MMF for initial treatment of chronic GVHD and in 18 of 24 patients who received MMF as secondary treatment for persistent or progressive chronic GVHD. At a median follow-up of 24 months, 22 of the 30 patients (73%) treated with glucocorticoids at the start of MMF therapy were able to reduce the dose of glucocorticoids, although only one patient was able to discontinue glucocorticoid treatment. The number of patients requiring additional agents to treat chronic GVHD after starting MMF therapy is not reported. Twenty-nine patients (85%) were alive at the time of the analysis. Addition of MMF to a calcineurin inhibitor and glucocorticoids produced 17 complete responses (65%) among 26 pediatric patients with refractory chronic GVHD [45]. Treatment with MMF was started at a median of 14 months after HCT, and all patients with improvement were able to discontinue treatment with MMF within 3 years. The number of patients who were able to discontinue all immunosuppressive therapy is not reported. Twenty-two patients (85%) were alive at a median of 4.7 years after HCT.
In the current study, most patients had received a variety of agents in addition to a calcineurin inhibitor and glucocorticoids for management of chronic GVHD before beginning treatment with MMF. Also, the median time from HCT to the beginning of treatment with MMF was nearly 20 months, suggesting that this group of patients may have had more resistant chronic GVHD. Despite these unfavorable risk factors, the 96% 1-year survival of the CGVHD Group compares favorably with the results of other studies reporting the use of MMF for first or second-line treatment of chronic GVHD. The 5-year survival rate was 74%, which is similar to patients with newly diagnosed “standard risk” chronic GVHD [60]. The rate of discontinuation of all immunosuppressive treatment at 1-, 2- and 3-years after the start of MMF in the CGVHD Group compares favorably with historical results. Even more impressive is the observation that the 26% 3-year cumulative incidence of discontinued immunosuppressive treatment after resolution of chronic GVHD was comparable to historical results in patients with newly diagnosed chronic GVHD [24].
The ability to administer treatment over a prolonged period of time and the availability of an intravenous and oral formulation are advantages for the use of MMF, allowing for multi-agent therapy, particularly for chronic GVHD. Although treatment with MMF was scheduled to stop after 35 days in the acute GVHD study, 6 patients continued treatment for more than 35 days. In the CGVHD Group, the median duration of MMF therapy was 11.6 months. Consistent with previous reports, most patients showed good tolerance to MMF therapy, although 4 patients in the AGVHD Group and 5 in the CGVHD Group discontinued treatment with MMF therapy prematurely because of suspected toxicity generally related to gastrointestinal complaints and neutropenia. The rate of discontinuation reflects, in part, the fact that safety concerns were of prime importance in these early studies investigating the use of MMF for treatment of GVHD. Since experience was limited, investigators had a low threshold in deciding to discontinue administration of MMF. As the use of MMF has expanded, for both treatment and prophylaxis, dose adjustments are more likely the first step to managing potential toxicities, before discontinuing administration of MMF. A direct causal link between MMF treatment and the toxicities observed in these studies could not always be made. In some cases, adverse effects had multiple causes, particularly when administration of MMF was discontinued because of cytopenia or gastrointestinal complaints, which occur frequently after HCT even in the absence of treatment with MMF.
At the time these studies were conducted, only the oral formulation of MMF was available. Pharmacokinetic analysis indicates that the AUC and Cmax of MPA were lower in patients in the prospective AGVHD Group compared to the CGVHD Group, possibly due to reduced bioavailability or interference with the enterohepatic recirculation. Ten of 19 patients in the AGVHD Group had evidence of gastrointestinal GVHD when MMF therapy was started. Low levels of MPA and decreased bioavailability after administration of oral MMF have been noted previously in acute GVHD prevention studies [61,62]. In a study evaluating MPA levels after the administration of oral MMF for initial treatment of acute GVHD, Kiehl et al. [63] reported lower MPA levels among patients with gastrointestinal GVHD compared to those with skin GVHD. This difference was not noted in our small sampling of patients who had pharmacokinetic sampling performed. They also observed that trough plasma MPA concentrations were higher among patients with improvement in GVHD manifestations than among those who had no improvement.
The standard dose of MMF at our center for treatment of acute GVHD is 30 mg/kg/day in divided doses, or approximately 1 gram twice daily. In renal transplant studies, the AUC of MPA after oral administration predicts allograft rejection among patient receiving cyclosporine [64,65]. In these pharmacokinetic studies, an increased MMF dose correlated with an increased AUC of MPA. van Gelder et al. [64] showed that after kidney transplantation, day 7 mean MPA AUC levels of 17, 27 and 43.9 μg × hr/mL resulted in biopsy-proven acute rejection rates of 28, 15 and 12 %, respectively. Similarly, Hale et al. [65] found that an MPA AUC of 15, 25 and 40 μg × h/mL yielded efficacy rates of 50%, 75% and 90% following kidney transplantation. The median MPA AUC within 10 days after the start of MMF in our prospective AGVHD study was only 15.8 μg × hr/mL. The MPA AUC among patients with chronic GVHD was consistent with results from solid organ transplant recipients.
Oral MMF can be used successfully for treatment of refractory acute and chronic GVHD. The long-term survival rate in the prospective AGVHD Group was low, although more recent experience using MMF as secondary treatment is more encouraging. The difference should be interpreted with caution, since the improved outcome in the more recent cohort of patients is based on a retrospective analysis. The challenge remains to control the response of donor cells against recipient alloantigens, while at the same time allowing protective immune responses against pathogens. Results with the use of MMF for treatment of steroid-refractory chronic GVHD have encouraged the development of phase III trials to establish the benefits and risks of MMF or MPA for initial treatment of chronic GVHD.
Acknowledgments
This research was supported by grants CA18029, CA15704 and HL36444 from the National Institutes of Health, Department of Health and Human Services, Bethesda, MD.
References
- 1.Sullivan KM. Graft-versus-host disease. In: Blume KG, Forman SJ, Appelbaum FR, editors. Thomas’ Hematopoietic Cell Transplantation. 3. Blackwell Publishing Ltd; Malden, MA: 2004. pp. 635–664. [Google Scholar]
- 2.Sullivan KM, Agura E, Anasetti C, Appelbaum F, Badger C, Bearman S, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28:250–259. [PubMed] [Google Scholar]
- 3.Bensinger WI, Martin PJ, Storer B, Clift R, Forman SJ, Negrin R, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med. 2001;344:175–181. doi: 10.1056/NEJM200101183440303. [DOI] [PubMed] [Google Scholar]
- 4.Hansen JA, Gooley TA, Martin PJ, Appelbaum F, Chauncey TR, Clift RA, et al. Bone marrow transplants from unrelated donors for patients with chronic myeloid leukemia. N Engl J Med. 1998;338:962–968. doi: 10.1056/NEJM199804023381405. [DOI] [PubMed] [Google Scholar]
- 5.Kernan NA, Bartsch G, Ash RC, Beatty PG, Champlin R, Filipovich A, et al. Analysis of 462 transplantations from unrelated donors facilitated by the National Marrow Donor Program. N Engl J Med. 1993;328:593–602. doi: 10.1056/NEJM199303043280901. [DOI] [PubMed] [Google Scholar]
- 6.Martin PJ, McDonald GB, Sanders JE, Anasetti C, Appelbaum FR, Deeg HJ, et al. Increasingly frequent diagnosis of acute gastrointestinal graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2004;10:320–327. doi: 10.1016/j.bbmt.2003.12.304. [DOI] [PubMed] [Google Scholar]
- 7.Martin PJ, Schoch G, Fisher L, Byers V, Anasetti C, Appelbaum FR, et al. A retrospective analysis of therapy for acute graft-versus-host disease: Initial treatment. Blood. 1990;76:1464–1472. [PubMed] [Google Scholar]
- 8.Weisdorf D, Haake R, Blazar B, Miller W, McGlave P, Ramsay N, et al. Treatment of moderate/severe acute graft-versus-host disease after allogeneic bone marrow transplantation: an analysis of clinical risk features and outcome. Blood. 1990;75:1024–1030. [PubMed] [Google Scholar]
- 9.Hings IM, Severson R, Filipovich AH, Blazar BR, kersey JH, Ramsay NKC, et al. Treatment of moderate and severe acute GVHD after allogeneic bone marrow transplantation. Transplantation. 1994;58:437–442. doi: 10.1097/00007890-199408270-00008. [DOI] [PubMed] [Google Scholar]
- 10.MacMillan ML, Weisdorf DJ, Wagner JE, DeFor TE, Burns LJ, Ramsay NKC, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8:387–394. doi: 10.1053/bbmt.2002.v8.pm12171485. [DOI] [PubMed] [Google Scholar]
- 11.Lee SJ, Zahrieh D, Agura E, MacMillan ML, Maziarz RT, McCarthy PL, et al. Effect of up-front daclizumab when combined with steroids for the treatment of acute graft-versus-host disease: results of a randomized trial. Blood. 2004;104:1559–1564. doi: 10.1182/blood-2004-03-0854. [DOI] [PubMed] [Google Scholar]
- 12.Roy J, McGlave PB, Filipovich AH, Miller WJ, Blazar BR, Ramsay NK, et al. Acute graft-versus-host disease following unrelated donor marrow transplantation: failure of conventional therapy. Bone Marrow Transplant. 1992;10:77–82. [PubMed] [Google Scholar]
- 13.Martin PJ, Schoch G, Fisher L, Byers V, Appelbaum FR, McDonald GB, et al. A retrospective analysis of therapy for acute graft-versus-host disease: Secondary treatment. Blood. 1991;77:1821–28. [PubMed] [Google Scholar]
- 14.MacMillan ML, Weisdorf DJ, Davies SM, DeFor TE, Burns LJ, Ramsay NKC, et al. Early antithymocyte globulin therapy improves survival in patients with steroid-resistant acute graft-versus-host disease. Biol Blood Marrow Transplant. 2002;8:40–46. doi: 10.1053/bbmt.2002.v8.pm11858189. [DOI] [PubMed] [Google Scholar]
- 15.Couriel D, Saliba R, Hicks K, Ippoliti C, de Lima M, Hosing C, et al. Tumor necrosis factor-α blockade for the treatment of acute GVHD. Blood. 2004;104:649–654. doi: 10.1182/blood-2003-12-4241. [DOI] [PubMed] [Google Scholar]
- 16.Bolanos-Meade J, Jacobsohn DA, Margolis J, Ogden A, Guillaume Wientjes M, Boyd JC, et al. Pentostatin in steroid-refractory acute graft-versus-host disease. J Clin Oncol. 2005;22:2661–2668. doi: 10.1200/JCO.2005.06.130. [DOI] [PubMed] [Google Scholar]
- 17.Ho VT, Zahrieh D, Hochberg E, Micale E, Levin J, Reynolds C, et al. Safety and efficacy of denileukin diftitox in patients with steroid refractory acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104:1224–1226. doi: 10.1182/blood-2004-01-0028. [DOI] [PubMed] [Google Scholar]
- 18.Antin JH, Weinstein HJ, Guinan EC, McCarthy P, Bierer BE, Gilliland DG, et al. Recombinant human interleukin-1 receptor antagonist in the treatment of steroid-resistant graft-versus-host disease. Blood. 1994;84:1342–1348. [PubMed] [Google Scholar]
- 19.McCaul KG, Nevill TJ, Barnett MJ, Toze CL, Currie CJ, Sutherland HJ, et al. Treatment of steroid-resistant acute graft-versus-host disease with rabbit antithymocyte globulin. J Hematother Stem Cell Res. 2000;9:367–374. doi: 10.1089/15258160050079470. [DOI] [PubMed] [Google Scholar]
- 20.Khoury H, Kashyap A, Adkins DR, Brown RA, Miller G, Vij R, et al. Treatment of steroid-resistant acute graft-versus-host disease with anti-thymocyte globulin. Bone Marrow Transplant. 2001;27:1059–1064. doi: 10.1038/sj.bmt.1703032. [DOI] [PubMed] [Google Scholar]
- 21.Arai S, Margolis J, Zahurak M, Anders V, Vogelsang GB. Poor outcome in steroid-refractory graft-versus-host disease with antithymocyte globulin treatment. Biol Blood Marrow Transplant. 2002;8:155–160. doi: 10.1053/bbmt.2002.v8.pm11939605. [DOI] [PubMed] [Google Scholar]
- 22.Lee SJ, Klein JP, Barnett J, Ringden O, Antin JH, Cahn JY, et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002;100:406–414. doi: 10.1182/blood.v100.2.406. [DOI] [PubMed] [Google Scholar]
- 23.Goerner M, Gooley T, Flowers MED, Sullivan KM, Kiem HP, Sanders JE, et al. Morbidity and mortality of chronic GVHD after hematopoietic stem cell transplantation from HLA-identical siblings for patients with aplastic or refractory anemias. Biol Blood Marrow Transplant. 2002;8:47–56. doi: 10.1053/bbmt.2002.v8.pm11858190. [DOI] [PubMed] [Google Scholar]
- 24.Stewart BL, Storer B, Storek J, Deeg HJ, Storb R, Hansen JA, et al. Duration of immunosuppressive treatment for chronic graft-versus-host disease. Blood. 2004;104:3501–3506. doi: 10.1182/blood-2004-01-0200. [DOI] [PubMed] [Google Scholar]
- 25.Socie G, Stone JV, Wingard JR, Weisdorf D, Henslee-Downey PJ, Bredeson C, et al. Long-term survival and late deaths after allogeneic bone marrow transplantation. Late Effects Working Committee of the International Bone Marrow Transplant Registry. N Engl J Med. 1999;341:14–21. doi: 10.1056/NEJM199907013410103. [DOI] [PubMed] [Google Scholar]
- 26.Lee WA, Gu L, Miksztal AR, et al. Bioavailability improvement of mycophenolic acid through amino ester derivatization. Pharm Res. 1990;7:161–166. doi: 10.1023/a:1015828802490. [DOI] [PubMed] [Google Scholar]
- 27.Eugui EM, Almquist SJ, Muller CD, Allison AC. Lymphocyte-selective cytostatic and immunosuppressive effects of mycophenolic acid in vitro: role of deoxyguanosine nucleotide depletion. Scand J Immunol. 1991;33:161–173. doi: 10.1111/j.1365-3083.1991.tb03746.x. [DOI] [PubMed] [Google Scholar]
- 28.Eugui EM, Mirkovich A, Allison AC. Lymphocyte-selective antiproliferative and immunosuppressive activity of mycophenolic acid and its morpholinoethyl ester (RS-61443) in rodents. Transplant Proc. 1991;23(Suppl 2):15–18. [PubMed] [Google Scholar]
- 29.Eugui EM, Mirkovich A, Allison AC. Lymphocyte-selective antiproliferative and immunosuppressive effects of mycophenolic acid in mice. Scand J Immunol. 1991;33:175–183. doi: 10.1111/j.1365-3083.1991.tb03747.x. [DOI] [PubMed] [Google Scholar]
- 30.Platz KP, Sollinger HW, Hullett Eckhoff DE, Eugui EM, Allison AC. RS-61433 a new, potent immunosuppressive agent. Transplantation. 1991;51:27–31. doi: 10.1097/00007890-199101000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Hao L, Lafferty KJ, Allison AC, Eugui EM. RS-61443 allows islet allografting and specific tolerance induction in adult mice. Transplant Proc. 1990;22:876–879. [PubMed] [Google Scholar]
- 32.D’Alessandro AM, Rankin MA. Heterotopic rat intestinal transplantation: effect of cyclosporine and RS-61443 on graft-vs-host disease and rejection. Transplant Proc. 1994;26:1611–1612. [PubMed] [Google Scholar]
- 33.Smit WM, Wagemans MAJ, Jansen CL, Horn GJVD, Surachno JS. Prevention of graft-versus-host disease by RS-61443 in two different rodent models. Transplantation. 1993;55:221–223. [PubMed] [Google Scholar]
- 34.Yu C, Seidel K, Nash RA, Deeg HJ, Sandmaier BM, Barsoukov A, et al. Synergism between mycophenolate mofetil and cyclosporine in preventing graft-versus-host disease among lethally irradiated dogs given DLA-nonidentical unrelated marrow grafts. Blood. 1998;91:2581–2587. [PubMed] [Google Scholar]
- 35.European Mycophenolate Mofetil Cooperative Study Group. Placebo-controlled study of mycophenolate mofetil combined with cyclosporine and corticosteroids for prevention of acute rejection. Lancet. 1995;345:1321–1325. [PubMed] [Google Scholar]
- 36.Sollinger HW for the U.S. Renal Transplant Mycophenolate Mofetil Study Group. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. Transplantation. 1995;60:225–232. doi: 10.1097/00007890-199508000-00003. [DOI] [PubMed] [Google Scholar]
- 37.The Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group. A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. Transplantation. 1996;61:1029–1037. [PubMed] [Google Scholar]
- 38.The Mycophenolate Mofetil Renal Refractory Rejection Study Group. Mycophenolate mofetil for the treatment of refractory, acute, cellular renal transplant rejection. Transplantation. 1996;61:722–729. [PubMed] [Google Scholar]
- 39.Basara N, Blau WI, Romer E, Rudolphi M, Bischoff M, Kirsten D, et al. Mycophenolate mofetil for the treatment of acute and chronic GVHD in bone marrow transplant patients. Bone Marrow Transplant. 1998;22:61–65. doi: 10.1038/sj.bmt.1701281. [DOI] [PubMed] [Google Scholar]
- 40.Takami A, Mochizuki K, Okumura H, Ito S, Suga Y, Yamazaki H, et al. Mycophenolate mofetil is effective and well tolerated in the treatment of refractory acute and chronic graft-versus-host disease. Int J Hematol. 2006;83:80–85. doi: 10.1532/IJH97.05111. [DOI] [PubMed] [Google Scholar]
- 41.Baudard M, Vincent A, Moreau P, Kergueris MF, Harousseau JL, Milpied N. Graft-versus-host disease mycophenolate mofetil for the treatment of acute and chronic GVHD is effective and well tolerated but induces a high risk of infectious complications: a series of 21 BM or PBSC transplant patients. Bone Marrow Transplant. 2002;30:287–295. doi: 10.1038/sj.bmt.1703633. [DOI] [PubMed] [Google Scholar]
- 42.Busca A, Locatelli F, Marmont F, Audisio E, Falda M. Response to mycophenolate mofetil therapy for refractory chronic graft-versus-host disease. Haematologica. 2003;88:837–839. [PubMed] [Google Scholar]
- 43.Lopez F, Parker P, Nademanee A, Rodriguez R, Al-Kadhimi Z, Bhatia R, et al. Efficacy of mycophenolate mofetil in the treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11:307–313. doi: 10.1016/j.bbmt.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 44.Soydan EA, Arat M, Topcuoglu P, Ozcan M, Arslan O, Idilman R, et al. The effect of mycophenolate mofetil in steroid refractory or dependent chronic graft-versus-host disease. Bone Marrow Transplant. 2003;31(Suppl 1):S135. (abstr. P561) [Google Scholar]
- 45.Yusuf U, Sanders JE, Stephan V. Mycophenolate mofetil (MMF) as salvage treatment for steroid-refractory chronic graft-versus-host disease (GVHD) in children. Blood. 2001;98(Suppl 1):398A. (abstr. 1672) [Google Scholar]
- 46.Abhyankar S, Godder K, Christiansen N, Chiang KY, DeRienzo S, O’Neal W, et al. Treatment of resistant acute and chronic graft versus host disease with mycophenolate mofetil. Blood. 1998;92(Suppl 1):340b. (abstr. 4467) [Google Scholar]
- 47.Kim JG, Sohn SK, Kim DH, Lee NY, Suh JS, Lee KS, et al. Different efficacy of mycophenolate mofetil as salvage treatment for acute and chronic GVHD after allogeneic stem cell transplant. Eur J Haematol. 2004;73:56–61. doi: 10.1111/j.1600-0609.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- 48.Krejci M, Doubek M, Buchler T, Brychtova Y, Vorlicek J, Mayer J. Mycophenolate mofetil for the treatment of acute and chronic steroid-refractory graft-versus-host disease. Ann Hematol. 2005;84:681–685. doi: 10.1007/s00277-005-1070-0. [DOI] [PubMed] [Google Scholar]
- 49.Langman LJ, LeGatt DF, Yatscoff RW. Pharmacodynamic assessment of mycophenolic acid-induced immunosuppression by measuring IMP dehydrogenase activity. Clin Chem. 1995;41:295–299. [PubMed] [Google Scholar]
- 50.Przepiorka D, Weisdorf D, Martin PJ, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus conference of acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 51.Dugan MJ, Defor TE, Steinbuch M, Filipovich AH, Weisdorf DJ. ATG plus corticosteroid therapy for acute graft-versus-host disease: predictors of response and survival (Review) Ann Hematol. 1997;75:41–46. doi: 10.1007/s002770050310. [DOI] [PubMed] [Google Scholar]
- 52.MacMillan ML, Couriel D, Weisdorf DJ, Schwab G, Havrilla N, Fleming TR, et al. A phase 2/3 multicenter randomized trial of ABX-CBL versus ATG as secondary therapy for steroid-resistant acute graft-versus-host disease. Blood. 2007;109:2657–2662. doi: 10.1182/blood-2006-08-013995. [DOI] [PubMed] [Google Scholar]
- 53.Przepiorka D, Kernan NA, Ippoliti C, Papadopoulos EB, Giralt S, Khouri I, et al. Daclizumab, a humanized anti-interleukin-2 receptor alpha chain antibody, for treatment of acute graft-versus-host disease. Blood. 2000;95:83–89. [PubMed] [Google Scholar]
- 54.Benito AI, Furlong T, Martin PJ, Anasetti C, Appelbaum FR, Doney K, et al. Sirolimus (rapamycin) for the treatment of steroid-refractory acute graft-versus-host disease. Transplantation. 2001;72:1924–1929. doi: 10.1097/00007890-200112270-00010. [DOI] [PubMed] [Google Scholar]
- 55.Jurado M, Vallejo C, Perez-Simon JA, Brunet S, Ferra C, Balsalobre P, et al. Sirolimus as part of immunosuppressive therapy for refractory chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13:701–706. doi: 10.1016/j.bbmt.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 56.Parker PM, Chao N, Nademanee A, O’Donnell MR, Schmidt GM, Snyder DS, et al. Thalidomide as salvage therapy for chronic graft-versus-host disease. Blood. 1995;86:3604–3609. [PubMed] [Google Scholar]
- 57.Marcellus DC, Altomonte VL, Farmer ER, Horn TD, Freemer CS, Grant J, et al. Etretinate therapy for refractory sclerodermatous chronic graft-versus-host disease. Blood. 1999;93:66–70. [PubMed] [Google Scholar]
- 58.Seaton ED, Szydlo RM, Kanfer E, Apperley JF, Russell-Jones R. Influence of extracorporeal photopheresis on clinical and laboratory parameters in chronic graft-versus-host disease and analysis of predictors of response. Blood. 2003;102:1217–1223. doi: 10.1182/blood-2002-11-3351. [DOI] [PubMed] [Google Scholar]
- 59.Zaja F, Bacigalupo A, Patriarca F, Stanzani M, Van Lint MT, Fili C, et al. Treatment of refractory chronic GVHD with rituximab: a GITMO study. Bone Marrow Transplant. 2007;40:273–277. doi: 10.1038/sj.bmt.1705725. [DOI] [PubMed] [Google Scholar]
- 60.Martin PJ, Carpenter PA, Sanders JE, Flowers MED. Diagnosis and clinical management of chronic graft-versus-host disease. Int J Hematol. 2004;79:221–228. doi: 10.1532/ijh97.03176. [DOI] [PubMed] [Google Scholar]
- 61.Bornhauser M, Schuler U, Porksen G, Naumann R, Geissler G, Thiede C, et al. Mycophenolate mofetil and cyclosporine as graft-versus-host disease prophylaxis after allogeneic blood stem cell transplantation. Transplantation. 1999;67:499–504. doi: 10.1097/00007890-199902270-00001. [DOI] [PubMed] [Google Scholar]
- 62.Nash RA, Johnston L, Parker P, McCune JS, Storer B, Slattery JT, et al. A phase I/II study of mycophenolate mofetil in combination with cyclosporine for prophylaxis of acute graft-versus-host disease after myeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2005;11:495–505. doi: 10.1016/j.bbmt.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 63.Kiehl MG, Shipkova M, Basara N, Blau IW, Schutz E, Armstrong VW, et al. Mycophenolate mofetil in stem cell transplant patients in relation to plasma level of active metabolite. Clin Biochem. 2000;33:203–208. doi: 10.1016/s0009-9120(00)00053-9. [DOI] [PubMed] [Google Scholar]
- 64.van Gelder T, Hilbrands LB, Yanrenterghem Y, Weimar W, de Fijter JW, Squifflet JP, et al. A randomized double-blind, multicenter plasma concentration conrolled study of the safety and efficacy of oral mycophenolate mofetil for the prevention of acute rejection after kidney transplantation. Transplantation. 1999;68:261–266. doi: 10.1097/00007890-199907270-00018. [DOI] [PubMed] [Google Scholar]
- 65.Hale MD, Nicholls AJ, Bullingham RES, Hene R, Hoitsma A, Squifflet JP, et al. The pharmacokinetic-pharmacodynamic relationship for mycophenolate mofetil in renal transplantation. Clin Pharmacol Ther. 1998;64:672–683. doi: 10.1016/S0009-9236(98)90058-3. [DOI] [PubMed] [Google Scholar]