Abstract
Background
The factors governing latency in tuberculosis (TB) are not well understood but appear to include pathogen and host factors. We have used Tuberculin skin test positivity as a tool to study cytokine responses in latent TB.
Methods
To identify host factors important in maintenance of TST positivity, we examined mycobacteria-specific immune responses of tuberculin skin test positive (TST+; latent TB) or negative (TST−; healthy) individuals in South India where skin test positivity can define TB latency.
Results
While PPD- and Mycobacterium tuberculosis culture filtrate Ag-specific Th1 and Th2 cytokines were not significantly different between the two groups, the Th17 cytokines—IL-17 and IL-23—were significantly decreased in TST+ individuals compared with the TST− individuals. This Th17 cytokine modulation was associated with significantly increased expression of CTLA-4 and Foxp3. Although CTLA-4 blockade failed to restore full production of IL-17 and IL-23 in TST+ individuals, depletion of regulatory T cells significantly increased production of these cytokines.
Conclusion
TST positivity is characterized by increased activity of regulatory T cells and a coincident downregulation of the Th17 response.
Keywords: T cells, Bacterial, Cytokines, Inflammation, Tolerance/Suppression/Anergy
Although Mycobacterium tuberculosis (Mtb) infects ∼ 2 billion people worldwide, 90% of Mtb-infected individuals can resist overt disease (active tuberculosis [TB]) development and manifest only latent infection [1,2,3]. Latent TB is defined as persistent presence of live Mtb within an infected host without causing disease. It is characterized by a delayed-type hypersensitivity response to purified protein derivative (PPD) mediated by mycobacteria-specific T cells [4] although, in areas of low Mtb transmission, quantiferon assay positivity has been suggested to be a better predictor of latency [5]. In South India, however, the tuberculin skin test (TST) has been conclusively shown to be an accurate diagnostic tool for latent TB [6].
Latent TB can be maintained for the lifetime of the individual unless the immunologic balance between host and pathogen is perturbed, resulting in reactivation of Mtb and active disease [7]. The host and environmental factors involved in compromising the ability to contain latent infection are human immunodeficiency virus coinfection, malnutrition, aging, stress, Type 2 diabetes, use of immunosuppressive agents, and other genetic factors [7]. On the pathogen side, latency is thought to reflect a transition from replicating to nonreplicating dormant bacilli, with this transition being influenced by a variety of factors including oxygen deprivation and nitric oxide [7,8]. In vitro and in vivo models of latency combined with genome-wide transcriptome profiling have led to identification of Mtb genes highly expressed during latency called dosR or devR (dormancy) genes [7,8]; however, each of the host- and pathogen-related factors controlling resistance and/or susceptibility to TB awaits complete elucidation.
Based on murine models, immunity to Mtb requires Th1 responses and (to a lesser extent) Th17 responses. Thus, IL-12, IFN-γ, and TNF-α (and IL-17 and IL-23) all play important roles in induction and maintenance of protective immune responses against tuberculous disease [7,9,10,11]. Recently, a number of regulatory factors, including regulatory T cells (Treg), IL-10, TGFβ, CTLA-4, and PD-1, have been implicated in establishment of chronic viral, bacterial, and parasitic infections [12,13,14]. To study the roles of the major T cell subsets and potential regulatory factors, we examined Mtb antigen-specific induction of Th1, Th2, Th17, and Treg responses as well as expression of IL-10, TGFβ, CTLA-4, and PD-1 in TST positive or TST negative individuals. We observed that TST positivity is characterized by increased expression of Treg markers and coincident downregulation of Th17 cells, thus providing a novel mechanism by which Th17 responses are negatively regulated in human infection.
Methods
Study population
We studied a group of 25 individuals who were tuberculin skin test positive (TST+) and 25 who were TST negative (hereafter TST−) in Tamil Nadu, South India. Age distribution of the TST+ group was from 19 to 50 (median age of 32 years) and of the TST− group was from 15 to 48 (median age of 30 years). Each of the two groups comprised 15 men and 10 women. The first 30 patients were utilized for cytokine and mRNA studies, while the last 20 patients were utilized for blockade and depletion studies. All TST+ subjects had positive skin test reactivity to intradermal tuberculin (2 TU). A positive tuberculin skin test was defined as an induration at the site of inoculation of at least 12-mm diameter to account for the high prevalence of environmental mycobacteria. This cutoff was based on a rigorous multivariate analysis of 280,000 subjects followed in South India for 15 years in which 8–11 mm tuberculin skin test reaction to PPD-S had a risk for development of culture positive TB that was low (and no different from those with 0–7 mm) but where subjects with 12 mm or greater tuberculin skin test reaction, in comparison, had a 3-fold greater relative risk for development of active TB and a 6.l% lifetime risk [6]. All TST− subjects had tuberculin skin test reaction size < 5 mm. All subjects had normal chest radiographs. None of the subjects had pulmonary symptoms (cough, fever, chest pain, hemoptysis) or a positive sputum for Mtb by smear microscopy and culture. All subjects had been bacillus Calmette-Guérin (BCG) vaccinated at birth. All individuals were examined as part of a clinical protocol (NCT 01-I-N261) approved by Institutional Review Boards both of the National Institute of Allergy and Infectious Diseases and of the Tuberculosis Research Center; informed consent was obtained from all participants.
Isolation of peripheral blood mononuclear cells (PBMC)
Heparinized blood was collected and PBMC isolated by Ficoll diatrizoate gradient centrifugation (LSM; ICN Biomedicals). Erythrocytes were lysed using ACK lysis buffer (Biosource International). Cells were then washed and cultured in RPMI-1640 (BioWhittaker) supplemented with 20 mM glutamine (BioWhittaker), 10% heat-inactivated FCS (Harlan Bioproducts for Science), and 50 μg/ml of gentamycin (Mediatech).
Antigens
Mycobacterial PPD (Statens Serum Institute, Copenhagen, Denmark) and culture filtrate antigen from M. tuberculosis H37 Rv (hereafter Mtb CFA; kind gift of Dr. P. Selvaraj, Tuberculosis Research Center, India) were used as antigenic stimuli, and anti-CD3 antibody was used as positive control. Final concentrations were 10 μg/ml for PPD and Mtb CFA and 5 μg/ml for anti-CD3.
In vitro culture
PBMC were cultured with PPD or Mtb CFA or anti-CD3 in 24-well tissue culture plates (Corning) at concentrations of 5 × 106/well. After 24 h, culture supernatants were collected and analyzed for cytokines. For depletion of Treg, we positively selected CD4+CD25+ T cells using the Treg isolation kit from Miltenyi-Biotec and used the negatively selected cells for culture. For CTLA-4 blocking experiments, we cultured cells in the presence of CTLA-4 Ig (10 μg/ml) or control Ig (10 μg/ml) (Ancell).
Enzyme-linked immunosorbent assay (ELISA)
Levels of cytokines in the culture supernatants were measured using Bioplex multiplex cytokine assay system (Bio-Rad). The cytokines analyzed were IL-2, IFN-γ, TNF-α, IL-12, IL-4, IL-5, IL-10, IL-13, IL-17, IL-23, IL-6, and IL-1β. IL-23 ELISA was performed using the kit from R&D Systems.
RNA preparation
PBMC were lysed using the reagents of a commercial kit (QIAshredder; Qiagen). Total RNA was extracted according to the manufacturer's protocol (RNeasy mini kit; Qiagen), and RNA was dissolved in 50 μl of RNase-free water.
cDNA synthesis
RNA (1 μg) was used to generate cDNA using TaqMan reverse transcription reagents according to the manufacturer's protocol (Applied Biosystems Inc.). Briefly, random hexamers were used to prime RNA samples for reverse transcription using MultiScribe reverse transcriptase.
Real-time RT-PCR
Real-time quantitative RT-PCR was performed in an ABI 7500 sequence detection system (Applied Biosystems) using TaqMan Assays-on-Demand reagents for CTLA-4, PD-1, TGFβ, Foxp3, RORγt, and an endogenous 18s ribosomal RNA control. Relative transcripts were determined by the formula:
where CT is the threshold cycle during the exponential phase of amplification.
Statistical analysis
Comparisons were made using either the Mann-Whitney U test (unpaired comparisons) or the Wilcoxon signed rank test (paired comparisons). Spearman rank correlation was used to examine relationships between variables. All statistics were performed using GraphPad Prism version 5 for Windows (GraphPad Software, Inc.).
Results
TST positivity is not associated with alterations in Th1 or Th2 cytokines
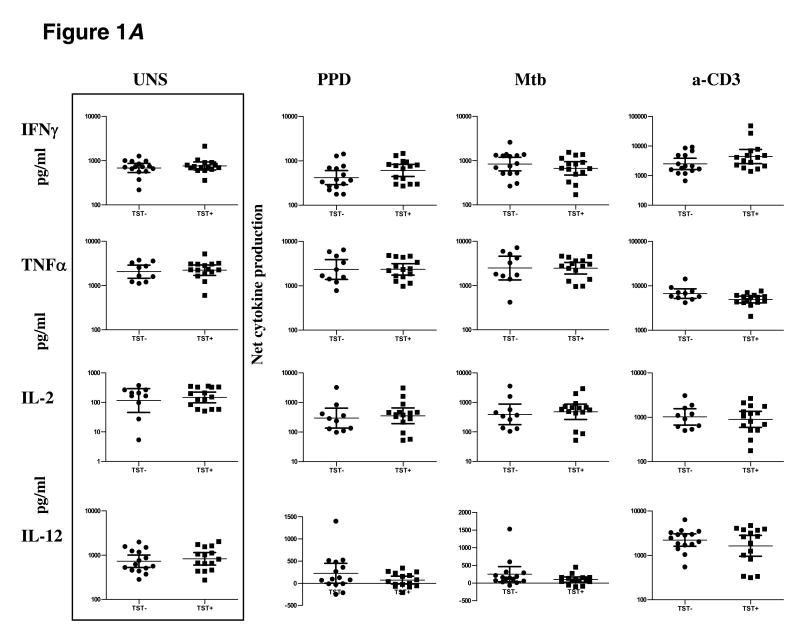
To determine the impact of TST positivity on Th1 responses, we stimulated PBMC from TST+ or TST− individuals with PPD or Mtb CFA or anti-CD3 for 24 h and measured levels of prototypical Th1 cytokines by ELISA (figure 1A). There were no intrinsic differences in levels of IFN-γ, TNF-α, IL-2, or IL-12 produced spontaneously or in response to anti-CD3 between the two groups. In addition, there were no differences in levels of IFN-γ (geometric mean [GM] of 608.2 pg/ml in TST+ vs. 418.5 pg/ml in TST− for PPD; GM of 669.1 pg/ml in TST+ vs. 835.7 pg/ml in TST− for Mtb CFA), TNF-α (GM of 2343 pg/ml in TST+ vs. 2329 pg/ml in TST− for PPD; GM of 2464 pg/ml in TST+ vs. 2488 pg/ml in TST− for Mtb CFA), IL-2 (GM of 353.1 pg/ml in TST+ vs. 296 pg/ml in TST− for PPD; GM of 479.8 pg/ml in TST+ vs. 392.6 pg/ml in TST− for Mtb CFA), and IL-12 (GM of 28.99 pg/ml in TST+ vs. 48.45 pg/ml in TST− for PPD; GM of 15.43 pg/ml in TST+ vs. 147.7 pg/ml in TST− for Mtb CFA) in response to PPD or Mtb CFA between the two groups.
Figure 1.
TST positivity is not associated with alteration in Th1 or Th2 cytokines. PBMC from TST+ (n = 15) and TST− (n = 10–15) patients were stimulated with PPD (10 μg/ml) or Mtb CFA (10 μg/ml) or anti-CD3 (5 μg/ml) for 24 h, and levels of A Th1 cytokines IL-2, IFN-γ, TNF-α, and IL-12 as well as B Th2cytokines IL-4, IL-5, and IL-13 were measured by ELISA. Results are shown as net cytokine production over media control. P values were calculated using the Mann-Whitney test and only statistically significant P values are shown.
To determine the impact of TST positivity on mycobacterial antigen-specific Th2 responses, we stimulated PBMC from TST+ or TST− individuals with PPD or Mtb CFA or anti-CD3 for 24 h and measured levels of IL-4, IL-5, and IL-13 above that produced spontaneously. As with the Th1 cytokines, there were no significant alterations in spontaneous or anti-CD3-stimulated production of IL-4, IL-5, or IL-13 (figure 1B). In addition, as can be seen in figure 1B, PPD and Mtb CFA induced no significant alterations in production of IL-4, IL-5 and IL-13 between the two groups.
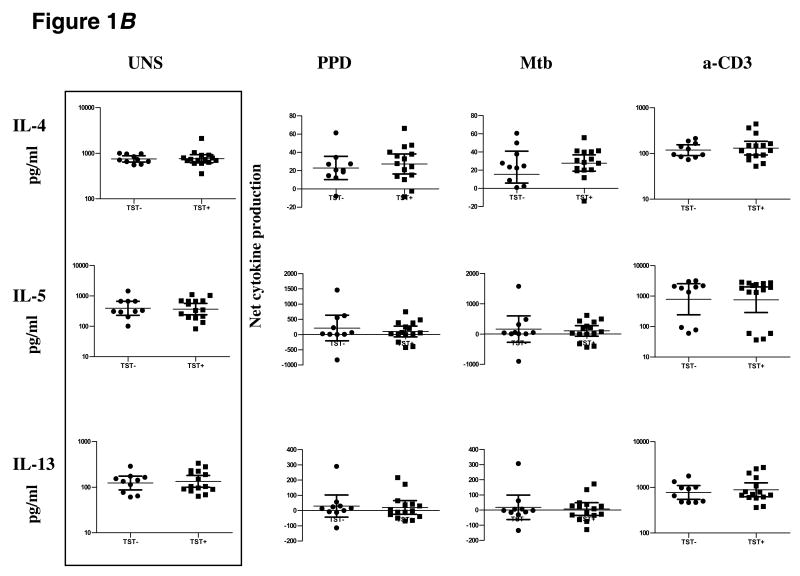
TST positivity is associated with downregulation of IL-17 and IL-23 production
To determine the impact of TST positivity on mycobacterial antigen-specific Th17 responses, we stimulated PBMC from TST+ or TST− individuals with PPD or Mtb CFA or anti-CD3 for 24 h and measured levels of IL-17 and its inducing cytokines, IL-23, IL-6, and IL-1β. There were no significant differences between the two groups in the spontaneous or anti-CD3-stimulated production of IL-17, IL-23, IL-6, or IL-1β (figure 2). In contrast, as shown in figure 2, in response to both PPD and Mtb CFA, TST+ individuals produced significantly less IL-17 (GM of 105.2 pg/ml in TST+ vs. 823.7 pg/ml in TST− for PPD [P < .0001]; GM of 186.3 pg/ml in TST+ vs. 937 pg/ml in TST− for Mtb CFA [P < .0001]) and IL-23 (GM of 167.9 pg/ml vs. 920.9 pg/ml for PPD [P = .0002]; GM of 143.7 pg/ml vs. 1146 pg/ml for Mtb CFA [P = .0014]) than did those in the TST− group . There were no significant differences in production of IL-6 and IL-1β in response to PPD or Mtb CFA between the TST+ and TST− groups.
Figure 2.
TST positivity is associated with downregulation of antigen-specific IL-17 and IL-23. PBMC from TST+ (n = 15) and TST− (n = 10–15) patients were stimulated with PPD (10 μg/ml), Mtb CFA (10 μg/ml), or anti-CD3 (5 μg/ml) for 24 h, and IL-17, IL-23, IL-6, and IL-1β cytokine levels were measured by ELISA. Results are shown as net cytokine production over media control. P values were calculated using the Mann-Whitney test and only statistically significant P values are shown.
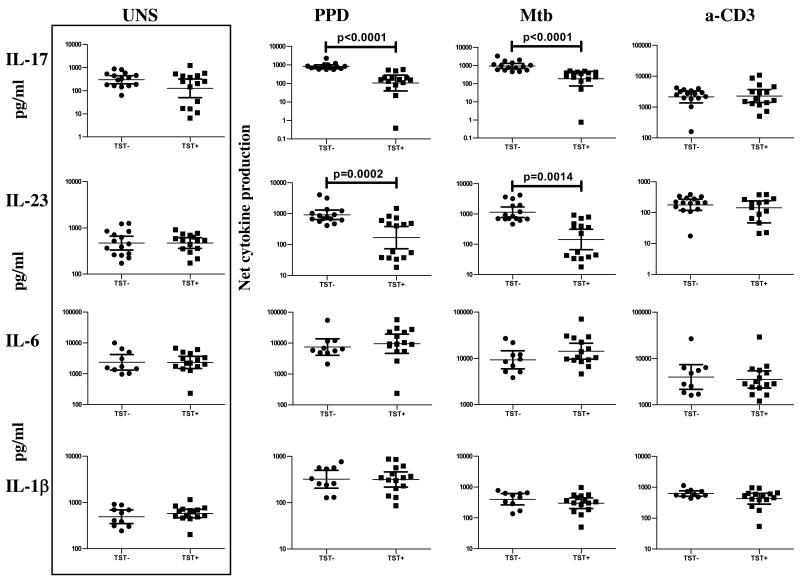
TST positivity is associated with enhanced Foxp3 and diminished RORγt expression
Because Treg and Th17 cells exhibit mutually reciprocal inhibition in murine T cells and as Foxp3 and RORγt are the transcription factors driving Treg and Th17 cell differentiation, respectively [15,16], we isolated RNA from cells stimulated with PPD, Mtb CFA, or anti-CD3 and estimated RNA levels of Foxp3 and RORγt by quantitative RT-PCR. While no differences in baseline or anti-CD3-induced Foxp3 or RORγt expression were observed, PPD and Mtb CFA induced a significantly higher expression of Foxp3 (GM mean fold change of 1.897 in TST+ compared with .7604 in TST− for PPD [P = .0007] and GM of 4.302 vs. .7105 for Mtb CFA [P = .0006]) and significantly lower expression of RORγt (GM mean fold change of .9443 in TST+ compared with 2.463 in TST− for PPD [P < .0001] and GM of .9491 vs. 2.630 for Mtb CFA [P= .0002]) in TST+ compared with TST− individuals (figure 3). Furthermore, there was a striking inverse relationship between expression of Foxp3 following PPD and Mtb stimulation and that of RORγt (r = -.7937; P < .0001 for PPD and r = -.7005; P = .0006 for Mtb by Spearman rank), suggesting that induction of Treg expression was modulating Th17 cells in the context of mycobacterial antigen stimulation.
Figure 3.
TST positivity is associated with upregulation of Foxp3 mRNA and downregulation of RORγt. A PBMC from TST+ (n = 10) and TST− (n = 10) patients were stimulated with PPD (10 μg/ml), Mtb CFA (10 μg/ml), or anti-CD3 (5 μg/ml) for 24 h, and Foxp3 and RORγt mRNA levels were measured by real time RT-PCR. Results are shown as fold change over media control. P values were calculated using the Mann-Whitney test. B Correlation between PPD- and Mtb-induced levels of Foxp3 and RORγt mRNA is shown as X-Y scatter plots. P values were calculated using the Spearman rank correlation and only statistically significant P values are shown.
TST positivity is not associated with changes in the expression/production of TGFβ and IL-10
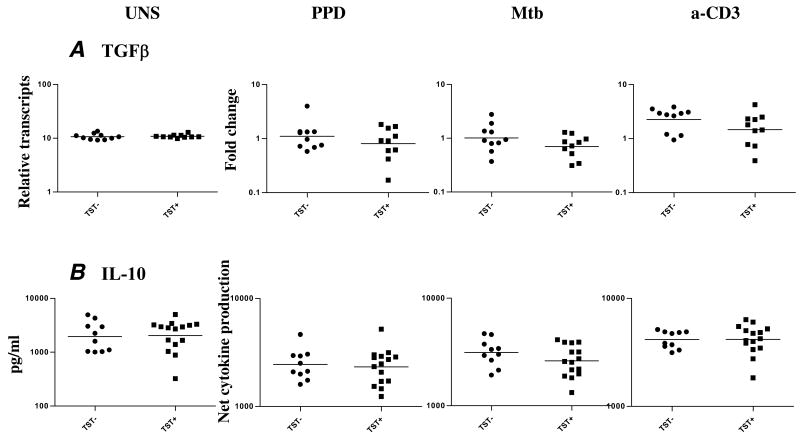
Because TGFβ and IL-10 are associated with downregulation of immune responses, we examined expression patterns of TGFβ and IL-10 in latent TB. We examined expression of TGFβ by RT-PCR and production of IL-10 by ELISA. As shown in figure 4, we observed no significant differences in expression of both at baseline or following mycobacterial antigen or anti-CD3 stimulation.
Figure 4.
TST positivity is not associated with changes in the expression of TGFβ or IL-10. PBMC from TST+ (n = 10–15) and TST− (n = 10) patients were stimulated with PPD (10 μg/ml), Mtb CFA (10 μg/ml), or anti-CD3 (5 μg/ml) for 24 h, and TGFβ mRNA and IL-10 cytokine levels were measured by real-time RT-PCR and ELISA, respectively. Results are shown as fold change or as net cytokine production over media control. P values were calculated using the Mann-Whitney test.
TST positivity is associated with increased expression of CTLA-4 but not PD-1
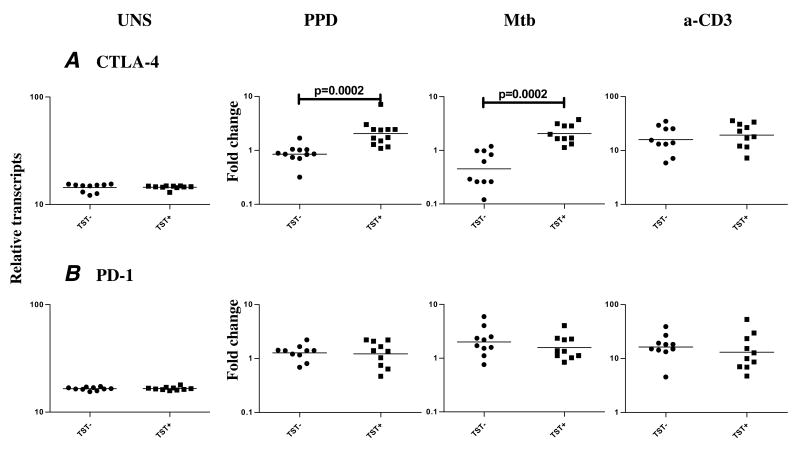
Because CTLA-4 and PD-1 have been shown to be involved in regulation of immune responses in active TB [17,18], we isolated RNA from cells stimulated with PPD, Mtb CFA, or anti-CD3 and measured expression of CTLA-4 and PD-1. As shown in figure 5, we observed increased expression of PPD- and Mtb CFA-induced CTLA-4 (GM mean fold change of 2.052 in TST+ compared with .4525 in TST− for PPD [P = .0002] and GM of 2.048 vs. .8518 for Mtb CFA [P = .0002]) but not PD-1 (GM mean fold change of 1.225 in TST+ compared with 1.276 in TST− for PPD and GM of 1.577 vs. 2.012 for Mtb CFA) mRNA in the TST+ individuals compared with TST− subjects. No difference in baseline or anti-CD3-induced levels of CTLA-4 and PD-1 mRNA was observed.
Figure 5.
TST positivity is associated with upregulation of CTLA-4 but not PD-1 mRNA. PBMC from TST+ (n = 10) and TST− (n = 10) patients were stimulated with PPD (10 μg/ml), Mtb CFA (10 μg/ml), or anti-CD3 (5 μg/ml) for 24 h, and CTLA-4 and PD-1 levels were measured by real-time RT-PCR. Results are shown as fold change over media control. P values were calculated using the Mann-Whitney test and only statistically significant P values are shown.
Depletion of Treg significantly enhances antigen-specific Th1 and Th17 responses in TST positive individuals
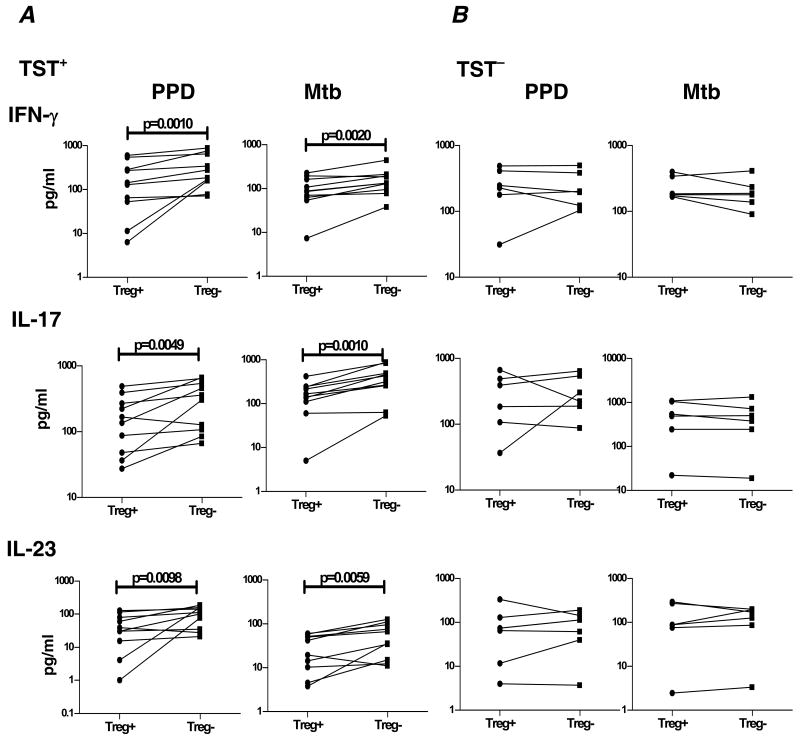
To determine the functional relationship between increased expression of Foxp3 and downregulated Th17 responses observed in TST+ individuals, we cultured cells from these patients with PPD or Mtb CFA following depletion of CD4+CD25+ T cells and measured levels of IFN-γ, IL-17, and IL-23 by ELISA (figure 6A). Depletion of CD4+CD25+ T cells completely abrogated induction of Foxp3 mRNA in antigen-stimulated cultures (data not shown). We also observed significantly increased (2–3 fold) PPD- and Mtb CFA-induced production of IFN-γ (GM of 253.1 pg/ml in the absence of Treg vs. 100.6 pg/ml in the presence of Treg for PPD [P= .0010]; GM of 135.3 pg/ml vs. 79.26 pg/ml for Mtb CFA [P= .0020]), IL-17 (GM of 248 pg/ml vs. 126.3 pg/ml for PPD [P = .0049]; and 294.8 pg/ml vs. 118.5 pg/ml for Mtb CFA [P = .0010]), and IL-23 (GM of 80.11 pg/ml vs. 26.36 pg/ml for PPD [P = .0098]; GM of 41.85 pg/ml vs. 21.18 pg/ml for Mtb CFA [P = .0059]) after Treg depletion in TST+ individuals.
Figure 6.
CD4+CD25+ T cell depletion causes upregulation of IFN-γ, IL-17, and IL-23 production in TST+ individuals. PBMC production of IFN-γ, IL-17, and IL-23 following 24-h stimulation with PPD (10 μg/ml) or Mtb CFA (10 μg/ml) in the presence of CD4+CD25+ T cell depletion in TST+ (A) or TST− (B) individuals is shown as line graphs with each line representing a single individual (n = 6–10). Results are shown as net cytokine production over media control. P values were calculated using the Wilcoxon signed rank test and only statistically significant P values are shown.
To ensure that Treg depletion was not just enhancing Th1 and Th17 immune responses nonspecifically, we cultured cells from TST− individuals and examined production of IFN-γ, IL-17, and IL-23 by ELISA (figure 6B) following removal of Treg. We observed no significant changes in TST- and Mtb CFA-induced production of IFN-γ, IL-17 and IL-23 in the presence or absence of Treg in TST− individuals. Collectively, these data suggest that Treg play an important role in downregulation of mycobacterial antigen-specific Th1 and Th17 responses in TST+ individuals.
CTLA-4 blockade does not reverse antigen-specific Th1 and Th17 downregulation in TST positivity
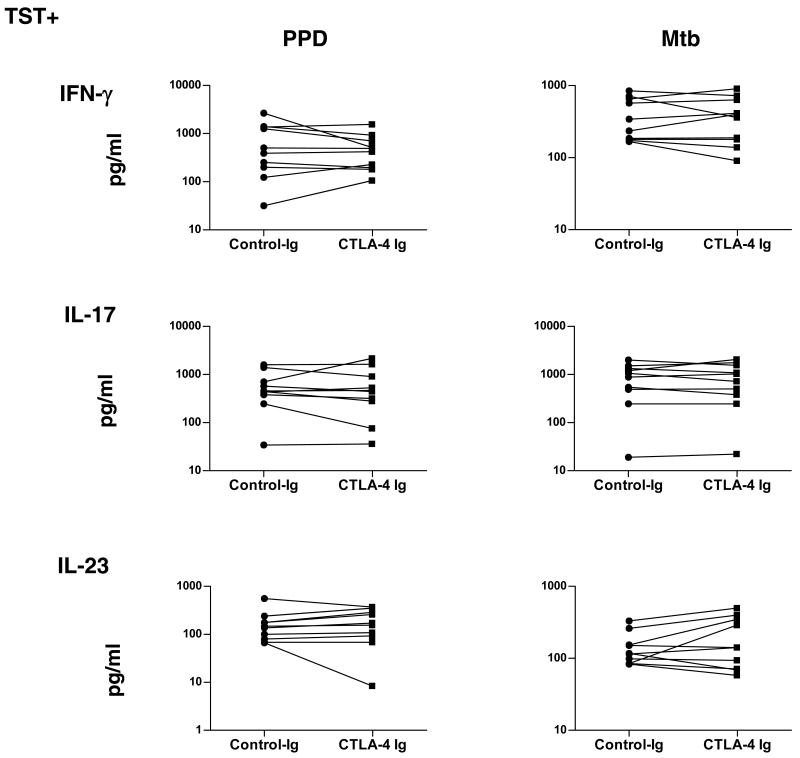
To determine the relationship between increased expression of CTLA-4 and downregulated Th17 responses observed in TST+ individuals, we cultured cells from these patients with PPD or Mtb CFA in the presence of CTLA-4 Ig or control Ig (figure 7) and measured levels of IFN-γ, IL-17, and IL-23 by ELISA. As shown in figure 7, we observed no significant changes in PPD- and Mtb CFA-induced production of IFN-γ, IL-17, and IL-23 after CTLA blockade. Collectively, these data suggest that CTLA-4 is not playing a major role in downregulation of mycobacterial antigen-specific Th17 responses.
Figure 7.
CTLA-4 blockade does not alter the production of IFN-γ, IL-17, or IL-23 in TST+ individuals. PBMC production of IFN-γ, IL-17, and IL-23 following 24-h stimulation with PPD (10 μg/ml) or Mtb CFA (10 μg/ml) in the presence of CTLA-4 Ig or control Ig is shown as line graphs with each line representing a single TST+ individual (n = 10). Results are shown as net cytokine production over media control. P values were calculated using the Wilcoxon signed rank test.
Discussion
Infection with Mtb can produce a variety of outcomes, including active infection—manifesting either as primary fulminant TB or as chronic, slowly progressing TB—and latent infection, which occurs when the initial infection is controlled but not completely eliminated [9]. Active TB is characterized by increased Treg and decreased Th1 responses, both factors contributing to establishment of active infection [19,20,21]; however, the roles of Treg and Th1/Th2/Th17 cells in latent TB have not been elucidated. Because latent TB afflicts the overwhelming majority (90%) of the nearly 2 billion TB-infected people, the study of factors influencing development of latent infection is of great importance. To this end, we studied two groups of individuals from the same community—one group positive for the tuberculin skin test reaction and the other not. Our study of antigen-driven T cell responses in these individuals unravels several interesting factors governing TST skin positivity as discussed below.
T cell differentiation into Th1 and Th2 lineages based on their cytokine profile and transcription factor expression has served as the basis of our understanding the pathogenesis of a variety of infectious and allergic diseases [22]. With the advent of newer techniques, T cell differentiation has expanded into several subsets, including Treg, Th17 cells, and polyfunctional T cells, among others [14,23,24,25,26]. Th1 cells are essential for resistance to TB both in mice and in humans. Deficiencies in the IL-12-IFN-γ-Stat1 pathway leads to disseminated mycobacterial infection in humans and to abrogation of resistance in mice [27,28]. In addition, TNF-α, another Th1 cytokine, is of almost equal importance, as treatment with biologics (e.g., anti-TNF-α antibody) for inflammatory disorders such as rheumatoid arthritis has caused reactivation of TB in some individuals [29]. We observed no differences in expression of Th1 cytokines at both baseline and following antigen-specific stimulation between the TST+ and TST- individuals. .Although induction of antigen-specific IFN-γ production is usually a hallmark of TB infection, it has been previously shown that no significant differences in IFN-γ expression were observed when healthy but potentially exposed individuals are separated on the basis of TST reactivity [30,31]. In addition, another potential explanation might be that all individuals in the study had been BCG vaccinated at birth and, although memory T cell-IFN-γ responses to mycobacterial antigen wane with time, this response can be significantly higher than in BCG-unvaccinated individuals [32]. Absence of differences in Th1 response between the two groups in our study suggests that development of latent TB is not primarily dependent on Th1 responses.
Increased Th2 responses have also been postulated to play a role in susceptibility to TB, as IL-4 and IL-13 can undermine Th1-mediated immunity and drive inappropriate alternative activation of macrophages [33]. Our study reveals that Th2 cytokines play a very small role in TST positivity, as both TST+ and TST− individuals had equivalent (and low) Th2 responses both at baseline and following antigen stimulation.
We also examined induction of Th17 responses in TST+ and TST- individuals. Induction of IL-17, a potent inflammatory cytokine induced by Mtb infection, is dependent, in general, on the presence of IL-1β, IL-6, TGFβ, IL-21, and IL-23. In response to Mtb, IL-17 is largely dependent on IL-23 [10,24] with Th17 differentiation requiring induction of transcription factor RORγt [16,34]. Although Th17 cells are not as important as Th1 cells in mediating protection against primary Mtb infection, IL-17 appears to be critical in induction of Mtb-specific memory response and mediation of protection against challenge infections and during vaccinations [35,36,37,38]. In addition, the IL-23-IL17 axis has been found to be important in human immune response to TB [31,39,40,41]. Our finding that TST+ individuals have significantly impaired IL-17 and IL-23 production suggests that lack of Th17 upregulation is a main feature of TST positivity. In addition, diminished expression of RORγt suggests that lack of Th17 induction may be mediated at the transcriptional level.
A variety of factors has recently been discovered to play important roles in dampening immune responses in infectious diseases, autoimmunity, and tumor biology. The most important and well studied are Treg, characterized by surface expression of markers CD4 and CD25 and intracellular expression of Foxp3 [14]. In addition, other factors that act independently from or in concert with Treg have also been studied, including negative costimulatory molecules CTLA-4, PD-1, and BTLA; inhibitory cytokines IL-10 and TGFβ; metabolic enzyme IDO; ubiquitin ligases cbl-b and GRAIL; perforin- and/or granzyme B-dependent killing; and IL-2 consumption [42]. We examined the role of IL-10, TGFβ, and PD-1 and found no significant differences in their expression/production between TST+ and TST− individuals; however, we did observe significantly increased expression of Foxp3 (indicating enhanced Treg expression) and CTLA-4 (indicating enhanced co-inhibitory activity by T cells) in TST+ individuals. Inhibition of CTLA-4 interaction with its ligands CD80 and CD86 by CTLA-4-Ig administration had no effect on antigen-driven Th1 and Th17 responses, indicating that downregulated Th17 responses in TST+ individuals were not due to an effect of CTLA-4.
Treg are known regulators of Th17 responses in mice [43,44]. Indeed, reciprocal regulation of Th17 and Treg is an established feature of T cell differentiation and is governed by the presence of specific cytokines [43,44]. TGFβ is a known inducer of Treg as well as of Th17 cells [34,43,44,45,46,47,48]. Presence of accessory cytokines IL-6 or IL-21, however, skews development of Th17 cells at the expense of the Treg lineage [34,43,44,45,46,47,48]. This interdependent regulation of Th17 and Treg is less well characterized in humans than is the association of Th17 upregulation and Treg suppression that has been described in only a few human autoimmune and inflammatory disorders [49,50]. Our study reveals—in an infectious disease setting (latent TB as defined by TST positivity)—that there is a clear inverse relationship between Th17 cells and Treg and that depletion of CD4+CD25+ T cells reverses Mtb-specific Th17 inhibition in TST+ but not TST− individuals.
While we have measured cytokine responses by ELISA and mRNA expression of costimulatory and other molecules by RT-PCR, further evaluation by using surface and intracellular cytokine flow cytometry is necessary. Nevertheless, this study highlights the differential immune responses that characterize susceptibility to latent TB and suggests that the balance between Th17 responses and Treg might play a vital role in mediating resistance to latent infection. Elucidation of the mechanism by which Treg downregulate Th17 responses and by which lack of Th17 cells or Th17-mediated cytokines predisposes to latent TB in humans would provide valuable information in designing better therapeutics and vaccines for this highly prevalent infection.
Acknowledgments
We thank the Tuberculosis Research Center, Epidemiology Unit, Tiruvallur, for valuable assistance in recruiting the patients for this study and NIAID intramural editor Brenda Rae Marshall for assistance.
Because the authors are government employees and this is a government work, the work is in the public domain in the United States. Notwithstanding any other agreements, the NIH reserves the right to provide the work to PubMedCentral for display and use by the public, and PubMedCentral may tag or modify the work consistent with its customary practices. You can establish rights outside of the U.S. subject to a government use license.
Financial support: Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Abbreviations used in this paper
- BCG
bacillus Calmette-Guérin
- CFA
culture filtrate antigen
- GM
geometric mean
- Mtb
Mycobacterium tuberculosis
- PBMC
peripheral blood mononuclear cell
- PPD
purified protein derivative of TB
- TB
tuberculosis
- Treg
regulatory T cell
- TST
Tuberculin skin test
Footnotes
Potential conflicts of interest: none reported.
References
- 1.Comstock GW, Livesay VT, Woolpert SF. The prognosis of a positive tuberculin reaction in childhood and adolescence. Am J Epidemiol. 1974;99:131–138. doi: 10.1093/oxfordjournals.aje.a121593. [DOI] [PubMed] [Google Scholar]
- 2.Lillebaek T, Dirksen A, Baess I, Strunge B, Thomsen VO, et al. Molecular evidence of endogenous reactivation of Mycobacterium tuberculosis after 33 years of latent infection. J Infect Dis. 2002;185:401–404. doi: 10.1086/338342. [DOI] [PubMed] [Google Scholar]
- 3.Wayne LG, Sohaskey CD. Nonreplicating persistence of mycobacterium tuberculosis. Annu Rev Microbiol. 2001;55:139–163. doi: 10.1146/annurev.micro.55.1.139. [DOI] [PubMed] [Google Scholar]
- 4.Edwards PQ, Edwads LB. Story of the tuberculin test from an epidemiologic viewpoint. Am Rev Respir Dis. 1960;81(1):1–47. Pt 2. [PubMed] [Google Scholar]
- 5.Vinton P, Mihrshahi S, Johnson P, Jenkin GA, Jolley D, et al. Comparison of QuantiFERON-TB Gold In-Tube Test and tuberculin skin test for identification of latent Mycobacterium tuberculosis infection in healthcare staff and association between positive test results and known risk factors for infection. Infect Control Hosp Epidemiol. 2009;30:215–221. doi: 10.1086/595695. [DOI] [PubMed] [Google Scholar]
- 6.Radhakrishna S, Frieden TR, Subramani R. Association of initial tuberculin sensitivity, age and sex with the incidence of tuberculosis in south India: a 15-year follow-up. Int J Tuberc Lung Dis. 2003;7:1083–1091. [PubMed] [Google Scholar]
- 7.Lin MY, Ottenhoff TH. Not to wake a sleeping giant: new insights into host-pathogen interactions identify new targets for vaccination against latent Mycobacterium tuberculosis infection. Biol Chem. 2008 doi: 10.1515/bc.2008.057. [DOI] [PubMed] [Google Scholar]
- 8.Locht C, Rouanet C, Hougardy JM, Mascart F. How a different look at latency can help to develop novel diagnostics and vaccines against tuberculosis. Expert Opin Biol Ther. 2007;7:1665–1677. doi: 10.1517/14712598.7.11.1665. [DOI] [PubMed] [Google Scholar]
- 9.Kaufmann SH. How can immunology contribute to the control of tuberculosis? Nat Rev Immunol. 2001;1:20–30. doi: 10.1038/35095558. [DOI] [PubMed] [Google Scholar]
- 10.Khader SA, Cooper AM. IL-23 and IL-17 in tuberculosis. Cytokine. 2008;41:79–83. doi: 10.1016/j.cyto.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.North RJ, Jung YJ. Immunity to tuberculosis. Annu Rev Immunol. 2004;22:599–623. doi: 10.1146/annurev.immunol.22.012703.104635. [DOI] [PubMed] [Google Scholar]
- 12.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 13.Li MO, Flavell RA. Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10. Immunity. 2008;28:468–476. doi: 10.1016/j.immuni.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 15.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 16.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 17.Jurado JO, Alvarez IB, Pasquinelli V, Martinez GJ, Quiroga MF, et al. Programmed death (PD)-1:PD-ligand 1/PD-ligand 2 pathway inhibits T cell effector functions during human tuberculosis. J Immunol. 2008;181:116–125. doi: 10.4049/jimmunol.181.1.116. [DOI] [PubMed] [Google Scholar]
- 18.Kirman J, McCoy K, Hook S, Prout M, Delahunt B, et al. CTLA-4 blockade enhances the immune response induced by mycobacterial infection but does not lead to increased protection. Infect Immun. 1999;67:3786–3792. doi: 10.1128/iai.67.8.3786-3792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Wu CY. CD4+ CD25+ Treg cells inhibit human memory gammadelta T cells to produce IFN-gamma in response to M tuberculosis antigen ESAT-6. Blood. 2008;111:5629–5636. doi: 10.1182/blood-2008-02-139899. [DOI] [PubMed] [Google Scholar]
- 20.Roberts T, Beyers N, Aguirre A, Walzl G. Immunosuppression during active tuberculosis is characterized by decreased interferon- gamma production and CD25 expression with elevated forkhead box P3, transforming growth factor- beta, and interleukin-4 mRNA levels. J Infect Dis. 2007;195:870–878. doi: 10.1086/511277. [DOI] [PubMed] [Google Scholar]
- 21.Scott-Browne JP, Shafiani S, Tucker-Heard G, Ishida-Tsubota K, Fontenot JD, et al. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J Exp Med. 2007;204:2159–2169. doi: 10.1084/jem.20062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 23.Fontenot JD, Rudensky AY. Molecular aspects of regulatory T cell development. Semin Immunol. 2004;16:73–80. doi: 10.1016/j.smim.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 24.McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 26.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 27.Flynn JL. Lessons from experimental Mycobacterium tuberculosis infections. Microbes Infect. 2006;8:1179–1188. doi: 10.1016/j.micinf.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 28.Holland SM. Interferon gamma, IL-12, IL-12R and STAT-1 immunodeficiency diseases: disorders of the interface of innate and adaptive immunity. Immunol Res. 2007;38:342–346. doi: 10.1007/s12026-007-0045-8. [DOI] [PubMed] [Google Scholar]
- 29.Jacobs M, Samarina A, Grivennikov S, Botha T, Allie N, et al. Reactivation of tuberculosis by tumor necrosis factor neutralization. Eur Cytokine Netw. 2007;18:5–13. doi: 10.1684/ecn.2007.0083. [DOI] [PubMed] [Google Scholar]
- 30.Liebeschuetz S, Bamber S, Ewer K, Deeks J, Pathan AA, et al. Diagnosis of tuberculosis in South African children with a T-cell-based assay: a prospective cohort study. Lancet. 2004;364:2196–2203. doi: 10.1016/S0140-6736(04)17592-2. [DOI] [PubMed] [Google Scholar]
- 31.Scriba TJ, Kalsdorf B, Abrahams DA, Isaacs F, Hofmeister J, et al. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J Immunol. 2008;180:1962–1970. doi: 10.4049/jimmunol.180.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weir RE, Gorak-Stolinska P, Floyd S, Lalor MK, Stenson S, et al. Persistence of the immune response induced by BCG vaccination. BMC Infect Dis. 2008;8:9. doi: 10.1186/1471-2334-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahnert A, Seiler P, Stein M, Bandermann S, Hahnke K, et al. Alternative activation deprives macrophages of a coordinated defense program to Mycobacterium tuberculosis. Eur J Immunol. 2006;36:631–647. doi: 10.1002/eji.200535496. [DOI] [PubMed] [Google Scholar]
- 34.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 36.Khader SA, Pearl JE, Sakamoto K, Gilmartin L, Bell GK, et al. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J Immunol. 2005;175:788–795. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- 37.Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol. 2007;178:3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 38.Wozniak TM, Ryan AA, Triccas JA, Britton WJ. Plasmid interleukin-23 (IL-23), but not plasmid IL-27, enhances the protective efficacy of a DNA vaccine against Mycobacterium tuberculosis infection. Infect Immun. 2006;74:557–565. doi: 10.1128/IAI.74.1.557-565.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dheda K, Chang JS, Lala S, Huggett JF, Zumla A, et al. Gene expression of IL17 and IL23 in the lungs of patients with active tuberculosis. Thorax. 2008;63:566–568. doi: 10.1136/thx.2007.092205. [DOI] [PubMed] [Google Scholar]
- 40.Pasquinelli V, Townsend JC, Jurado JO, Alvarez IB, Quiroga MF, et al. IFN-gamma Production during Active Tuberculosis Is Regulated by Mechanisms That Involve IL-17, SLAM, and CREB. J Infect Dis. 2009;199:661–665. doi: 10.1086/596742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng MY, Wang ZH, Yao CY, Jiang LN, Jin QL, et al. Interleukin 17-producing gamma delta T cells increased in patients with active pulmonary tuberculosis. Cell Mol Immunol. 2008;5:203–208. doi: 10.1038/cmi.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 44.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 47.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 48.Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng X, Yu X, Ding YJ, Fu QQ, Xie JJ, et al. The Th17/Treg imbalance in patients with acute coronary syndrome. Clin Immunol. 2008;127:89–97. doi: 10.1016/j.clim.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 50.Nistala K, Moncrieffe H, Newton KR, Varsani H, Hunter P, et al. Interleukin-17-producing T cells are enriched in the joints of children with arthritis, but have a reciprocal relationship to regulatory T cell numbers. Arthritis Rheum. 2008;58:875–887. doi: 10.1002/art.23291. [DOI] [PMC free article] [PubMed] [Google Scholar]