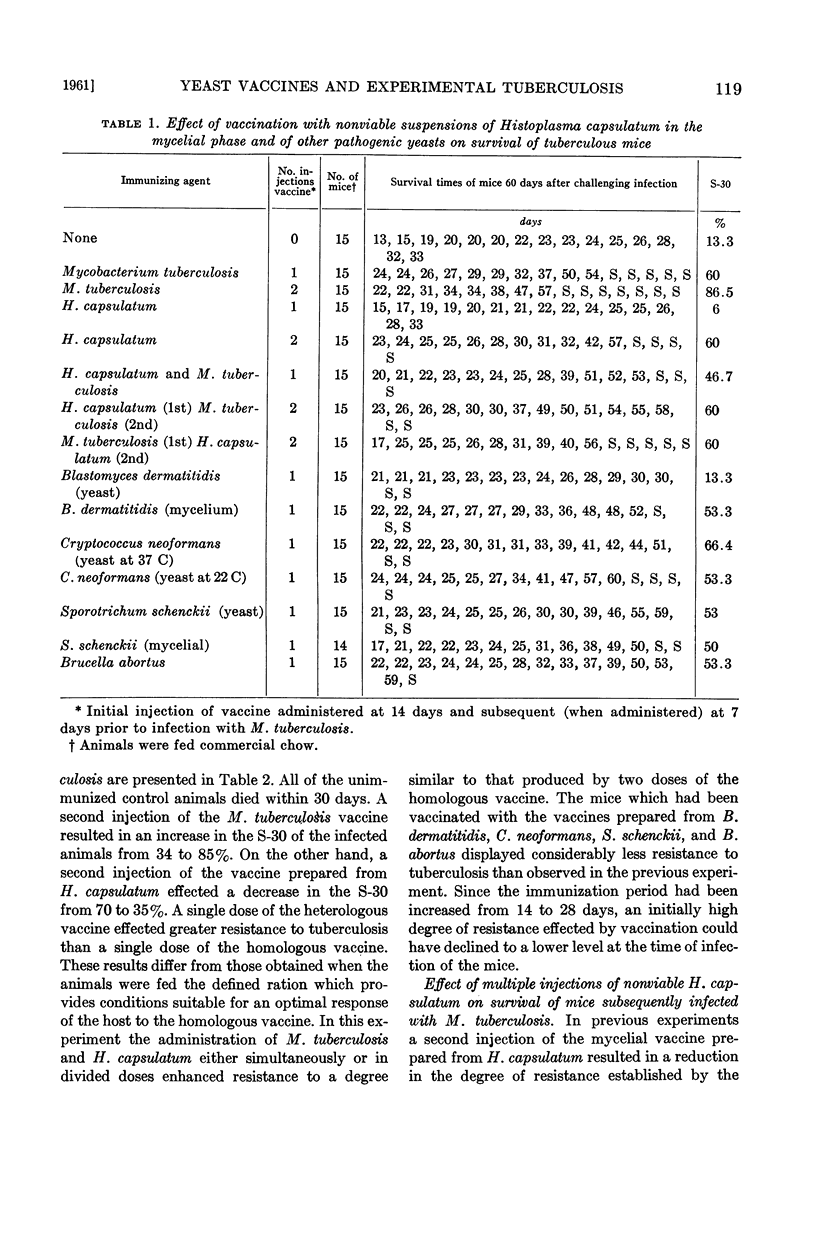
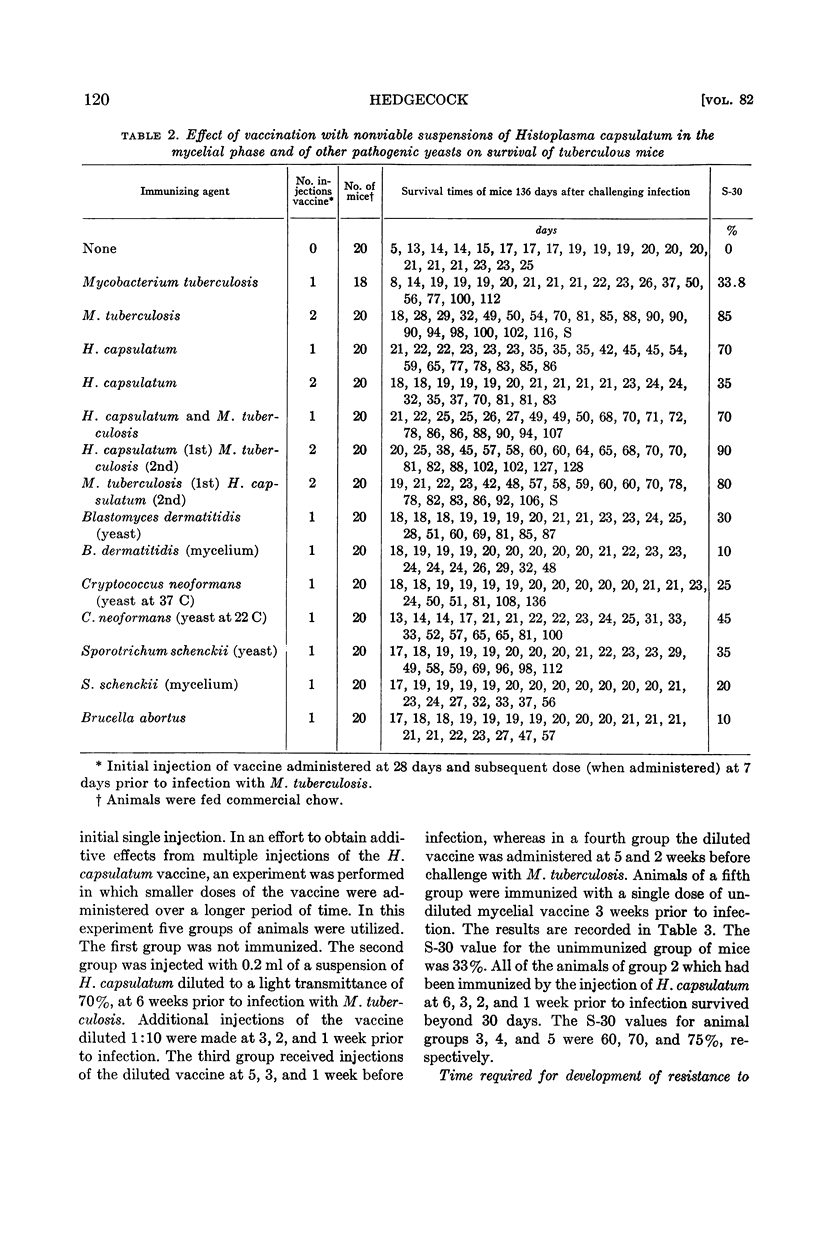
Abstract
Hedgecock, Loyd W. (Veterans Administration Hospital, Kansas City, Mo.). Effect of vaccines prepared from Histoplasma capsulatum and other yeasts on experimental tuberculosis. J. Bacteriol. 82:115–123. 1961.—Resistance to experimental tuberculosis was enhanced by the injection of a nonviable vaccine prepared from Histoplasma capsulatum in the mycelial phase but not by the organism in the yeast phase. Acquired resistance was established within 7 days or less and usually maintained for at least 29 days. When both mycelium vaccine of H. capsulatum and a vaccine prepared from tubercle bacilli were utilized in vaccination, the results were additive (in terms of acquired resistance) provided that an interval of 3 weeks was maintained between injection of each of the vaccines.
Resistance to tuberculosis was also demonstrable 14 days after the injection of nonviable preparations of Brucella abortus and Cryptococcus neoformans, as well as by yeast and mycelial vaccines of Blastomyces dermatitidis and Sporotrichum schenckii. Resistance decreased significantly when the animals were challenged 29 days after vaccination, with the exception of those injected with the yeast phase of B. dermatitidis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DUBOS R. J., SCHAEDLER R. W. Effects of cellular constituents of mycobacteria on the resistance of mice to heterologous infections I. Protective effects. J Exp Med. 1957 Nov 1;106(5):703–717. doi: 10.1084/jem.106.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBOS R. J., SCHAEDLER R. W. Reversible changes in the susceptibility of mice to bacterial infections. I. Changes brought about by injection of pertussis vaccine or of bacterial endotoxins. J Exp Med. 1956 Jul 1;104(1):53–65. doi: 10.1084/jem.104.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS D. G., PERKINS F. T. The ability of pertussis vaccine to produce in mice specific immunity of a type not associated with antibody production. Br J Exp Pathol. 1954 Aug;35(4):322–330. [PMC free article] [PubMed] [Google Scholar]
- HEDGECOCK L. W. Effect of dietary fatty acids and protein intake on experimental tuberculosis. J Bacteriol. 1955 Oct;70(4):415–419. doi: 10.1128/jb.70.4.415-419.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEDGECOCK L. W. The effect of diet on the inducement of acquired resistance by viable and nonviable vaccines in experimental tuberculosis. Am Rev Tuberc. 1958 Jan;77(1):93–105. doi: 10.1164/artpd.1958.77.1.93. [DOI] [PubMed] [Google Scholar]
- LANDY M., PILLEMER L. Increased resistance to infection and accompanying alteration in properidin levels following administration of bacterial lipopolysaccharides. J Exp Med. 1956 Sep 1;104(3):383–409. doi: 10.1084/jem.104.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYKA W. Enhancement of resistance to tuberculosis in mice experimentally infected with brucella abortus. Am Rev Tuberc. 1956 Feb;73(2):251–265. doi: 10.1164/artpd.1956.73.2.251. [DOI] [PubMed] [Google Scholar]
- PILLEMER L., ROSS O. A. Alterations in serum properdin levels following injection of Zymosan. Science. 1955 May 20;121(3151):732–733. doi: 10.1126/science.121.3151.732. [DOI] [PubMed] [Google Scholar]
- POST G. W., JACKSON A., GARBER P. E., VEACH G. E. Pulmonary sporotrichosis. Dis Chest. 1958 Oct;34(4):455–459. doi: 10.1378/chest.34.4.455. [DOI] [PubMed] [Google Scholar]
- ROWLEY D. Rapidly induced changes in the level of non-specific immunity in laboratory animals. Br J Exp Pathol. 1956 Jun;37(3):223–234. [PMC free article] [PubMed] [Google Scholar]
- SALVIN S. B. Endotoxin in pathogenic q fungi. J Immunol. 1952 Jul;69(1):89–99. [PubMed] [Google Scholar]
- WEISS D. W. Vaccination against tuberculosis with nonliving vaccines. I. The problem and its historical background. Am Rev Respir Dis. 1959 Nov;80:676–688. doi: 10.1164/arrd.1959.80.5.676. [DOI] [PubMed] [Google Scholar]
- YOUMANS G. P., YOUMANS A. S. The measurement of the response of immunized mice to infection with Mycobacterium tuberculosis va. hominis. J Immunol. 1957 May;78(5):318–329. [PubMed] [Google Scholar]


