Abstract
Hepatitis C in prison populations is now a major public health problem, and large numbers of correctional facilities have no comprehensive management program, often because of formidable projected costs and tightening budget constraints. The North Dakota Department of Corrections and Rehabilitation has operated a management and therapy program since 2002 using consensus interferon and ribavirin with 45% cost savings. The program has provided excellent sustained viral responses: 54.2% for genotype 1 hepatitis C, 75% for genotypes 2 and 3, and 63.6% overall.
DETECTION AND MANAGEMENT of hepatitis C in the US prison population is a major public health problem, as evidenced by the estimate that more than one third1 of the approximately 5 400 000 people in the United States with active hepatitis C enter correctional facilities yearly.2 In North Dakota (population 642 000), the true incidence and prevalence of hepatitis C is not known; however, 25% of all positive antibody tests for hepatitis C reported yearly by the North Dakota Department of Health originate from the North Dakota Department of Corrections and Rehabilitation (ND DOCR).
In spite of guidelines published by the Federal Bureau of Prisons in 2005,3 state correctional facilities have only begun to formulate comprehensive management policies,4,5 a task now complicated by shrinking correctional budgets. Prisons offer an ideal setting for the treatment of hepatitis C6,7 because maximum compliance, which is necessary for achieving a sustained viral response, can be assured. Limited information is available on the outcome of correctional screening and treatment programs,4 most of which now use pegylated interferon and weight-based ribavirin. There is limited published data on the use of consensus interferon combined with ribavirin despite its lower cost and theoretical advantages. Studies exist that suggest the usefulness of this product in noncorrectional settings,8–10 but, to our knowledge, ours is the first report of consensus interferon combined with weight-based ribavirin for the treatment of hepatitis C in a US prison system.
Type 1 α-interferons (IFNs) are mostly made up of proteins containing 166 amino acids with 14 subtypes, all of which have antiproliferative and antiviral activities. Analysis of the subtypes has revealed a high degree of conservation for amino acids in certain positions. Minor amino acid changes result in major changes in binding affinity to cellular receptors; receptor affinity is directly correlated with biologic potency. A synthetic type 1 α-IFN has been manufactured with the most common amino acids at each position for the 14 subtypes, resulting in a protein having the highest binding affinity of all α-IFN molecules. The biopotency of this “consensus interferon,”11 which is also known as IFN alfacon-1, is 10-fold higher than that of all the natural variants.12 Its use in the treatment of hepatitis C has been limited by failure of attempts to prolong the half-life in vivo.
The 2 commercial interferons, PegIFNα-2a and PegIFNα-2b, now commonly used for hepatitis C therapy differ by only 1 amino acid. The significant difference is the configuration and weight of the polyethylene glycol polymers attached (by a process called pegylation) to the active interferon, which prolong the half-life. The pegylation process results in a 60% to 90% loss in the biologic activity of IFN, evidenced by comparison of the dosing of the pegylated products (150–180 μg weekly) with the dosing of the nonpegylated products (15 μg 3 times a week).
DESCRIPTION OF PROGRAM
The ND DOCR screens all prisoners entering the system for hepatitis C; the prevalence rate among the average census of 1400 prisoners (13%) is consistent with published rates for other prison systems (12%–31%).4 A pretreatment screening program, which is designed to maximize an inmate's chances of completing therapy, determines eligibility (see the box on the next page). This pretreatment screening program includes education and screening for continuing substance abuse, immunization for hepatitis A and B, and screening for the presence of hepatitis C and HIV. There is no limit to the number of prisoners undergoing hepatitis C therapy provided they meet the eligibility criteria. The screening criteria pertain only to the primary treatment of hepatitis C. The treatment program is funded by the state legislature under the ND DOCR budget and has received no funding from any other source.
CRITERIA FOR DETERMINING ELIGIBILITY FOR HEPATITIS C THERAPY USING CONSENSUS INTERFERON AND RIBAVIRIN: NORTH DAKOTA DEPARTMENT OF CORRECTIONS AND REHABILITATION, JULY 2002–NOVEMBER 2008
Pre-treatment Screening Criteria
Age 18-60 years.
Confirmation of hepatitis C RNA with genotype.
Immunization and serology confirming immunity to hepatitis A and B.
Hemoglobin A1c < 9%.
Adequately treated heart disease.
Adequately treated thyroid disease.
Absence of renal disease.
Absence of decompensated cirrhosis with ascites.
Hemoglobin > 10 grams.
Absence of autoimmune disease.
Absence of life-threatening nonhepatic disease.
Satisfactory clinic compliance with screening appointments.
If HIV postive, a CD4 (Helper T-cell) count > 200.
Two negative alcohol and drug tests 6 months apart.
Prison stay certain for at least 14 months for genotype 2,3 once treatment begins.
Prison stay certain for at least 20 months for genotype 1 once treatment begins.
Female inmates: recent negative pregnancy test and documentation of counseling in avoiding pregnancy until 6 months after treatment is complete.
Compliant with drug and alcohol treatments recommendations.
Absence of severe axis I diagnosis or psychiatric clearance for therapy.
No body piercing or tattoos for 6 months.
Source. North Dakota Department of Corrections and Rehabilitation.13
METHODS AND RESULTS
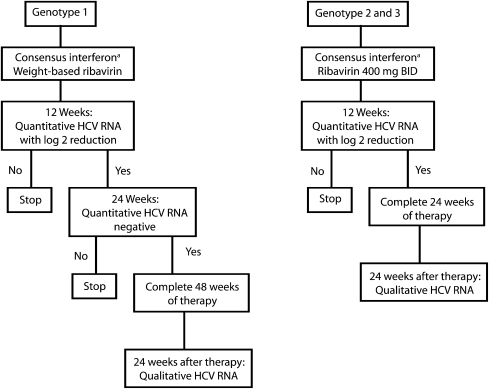
The ND DOCR treatment protocol (Figure 1) follows current National Institutes of Health (NIH) guidelines14 for primary therapy of hepatitis C, with the exception of replacing weekly pegylated interferon administration with 3-times-weekly consensus interferon administration. The other treatment criteria listed in the box on the next page are to ensure that inmates will complete treatment before their prison terms are completed. Table 1 shows a 1-day snapshot of the treatment final outcome among current inmates who were positive for hepatitis C antibody.
FIGURE 1.
Treatment protocol for hepatitis C therapy using consensus interferon and ribavirin: North Dakota Department of Corrections and Rehabilitation, July 2002–November 2008.
Note. BID = twice daily; MCG = micrograms.
a15 mcg subcutaneously three times a week.
TABLE 1.
Disposition of Inmates Positive for Hepatitis C Antibody on December 1, 2008: North Dakota Department of Corrections and Rehabilitation
| No. or No. (%) | |
| No. inmates with hepatitis C antibody | 146 |
| No. eligible to enter screening | 91 |
| Disposition | |
| Comorbidity precluded treatment | 4 (4.4) |
| Declined treatment | 6 (6.6) |
| Disqualified for noncompliance | 8 (8.8) |
| Negative viral load or biopsy | 24 (26.4) |
| Aged > 60 y | 1 (1.1) |
| Pending completion of screening | 15 (16.5) |
| Eligible for treatment | 33 (36.3) |
KEY FINDINGS
▪ Exposure to traffic has multiple impacts on the health of community residents.
▪ Collaborative, community-based participatory research that combines community knowledge with scientific expertise can engage community members, public agencies, academics, and decision-makers in understanding, and taking steps to mitigate, the health impacts of transportation planning decisions.
▪ A comprehensive qualitative and quantitative assessment of traffic health impacts on air quality, environmental noise, and traffic hazards can support community understanding of environmental health risks and provide evidence that serves as a catalyst for reducing negative traffic-related health exposures and disparities.
Almost all patients with genotype 1 hepatitis C are biopsied before treatment begins. Very few genotype 2 cases are biopsied. Patients with genotype 3 sometimes are biopsied, depending on whether a higher fibrosis score is suspected during the initial workup. The decision on treatment duration is made primarily on the basis of fibrosis score and the absence of detectable virus after the fourth week of therapy. Genotype 3 patients who begin treatment are assigned to either 24 or 48 weeks of therapy.
In order to optimize response, once therapy begins, no interruption can occur. Decisions to stop treatment are based on viral load at 12 and 24 weeks.15 For patients with genotype 1, if virus is detected at 24 weeks, the probability of a sustained viral response is so low that therapy is stopped; if virus is detected at 12 weeks, treatment continues provided viral RNA is either undetectable or has dropped 100-fold (2 logs) from baseline. To maximize the probability of a sustained viral response, dose reductions for hematologic side effects are not allowed. Because depression and psychiatric deterioration can significantly complicate management of therapy,16 every patient is initially screened with the Center for Epidemiologic Studies Depression Scale index.17 All inmates with positive screenings and all those with established Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, axis I diagnoses are cleared for therapy by a psychiatrist, with monthly follow-up when indicated.
Prison inmates undergoing therapy are treated exactly as private patients, except that instead of once-weekly injections of pegylated interferon, consensus interferon (15 mcg) is administered subcutaneously 3 times a week. For genotype 1 hepatitis C, ribavirin dosage is based on body weight, with a maximum dose of 1400 mg per 24 hours, administered orally in 2 doses. The dose for genotype 2 or 3 is usually a standard 400 mg administered orally twice daily.
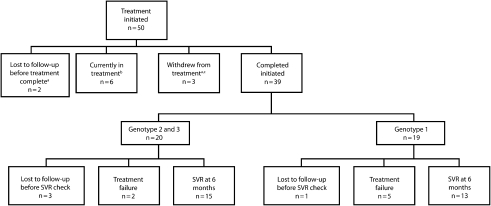
From July 2002 through November 2008, data for 50 patients were collected (Figure 2). Patient characteristics are listed in Table 2, along with characteristics of all ND DOCR inmates for comparison purposes. The liver biopsy rate was 56%; rates of metavir fibrosis were 37% for stage 1, 27% for stage 2, 36% for stage 3, and 0% for stage 4. On December 1, 2008, six inmates were in treatment, 2 inmates were discharged early or transferred, and 3 were withdrawn from treatment. The remaining 39 inmates who completed treatment were as follows: of 19 with genotype 1, 13 had a sustained viral response; of 20 with genotype 2 or 3, 15 had a sustained viral response. Response rates calculated by intention-to-treat analysis (but not including the 6 inmates then in treatment) were 54.2% for genotype 1 and 75% for genotypes 2 and 3, with an overall sustained viral response of 63.6%. These rates compare favorably with those of clinical trials18–20 using pegylated interferon, which reported sustained viral responses of 40% to 50% for genotype 1 and 70% to 80% for genotypes 2 and 3, with an overall sustained viral response of 40% to 55%. Table 3 shows comorbid medical conditions preceding or arising during the treatment period as well as initiation or adjustment of medications to treat these conditions. Antidepressant adjustment was required for 30% of inmates, and thyroid replacement was necessary for 14%.
FIGURE 2.
Outcomes of hepatitis C therapy using consensus interferon and ribavirin: North Dakota Department of Corrections and Rehabilitation, July 2002–November 2008.
Note. SVR = sustained virologic response; EVR = early virologic response.
aAll genotype 1.
bFour genotype 1, one genotype 3 and one genotype 4.
cOne inmate quit voluntarily, one inmate discontinued due to illegal drug use, and one inmate failed EVR.
TABLE 2.
Demographic Characteristics of Inmates: North Dakota Department of Corrections and Rehabilitation, July 2002–November 2008
| Characteristic | Inmates Treated for Hepatitis C | All Inmates |
| No. inmates | 50 | 1467 |
| Mean age, y | 40 | 31 |
| Male, % | 98 | 89 |
| Race/ethnicity, % | ||
| White | 76 | 66 |
| Native American | 10 | 22 |
| Hispanic | 10 | 5 |
| African American | 4 | 6 |
| Asian | 0 | 0.2 |
| Weight, kg, mean ±SD | 94.4 ± 15.7 | NA |
| Viral load, log/mL | 6.22 ± 0.71 | NA |
| Genotype 1, % | 56 | NA |
| Had biopsy, % | 56 | NA |
Note. NA = not available.
TABLE 3.
Comorbid Medical Conditions Preceding or Arising During Treatment with Consensus Interferon and Ribavirin for Hepatitis C: North Dakota Department of Corrections and Rehabilitation, July 2002–November 2008
| Comorbid Condition | No. (%) |
| Psychiatric | |
| Axis I disorder diagnosed before initiation of therapy | 24 (48) |
| Taking antidepressants at initiation of therapy | 22 (44) |
| Taking antipsychotics at initiation of therapy | 6 (12) |
| Antidepressant adjustment during therapy | 15 (30) |
| Antipsychotic adjustment during therapy | 1 (2) |
| Endocrine: thyroid replacement started during therapy | 7 (14) |
| Hematologic | |
| Erythropoietin started during therapy | 1 (2) |
| GCSF started during therapy | 0 |
Note. GCSF = granulocyte colony-stimulating factor.
DISCUSSION
Reported response rates in previous papers have included those who withdrew prior to completion of treatment as well as those who were lost to follow-up. This practice assumes that all those lost to follow-up did not achieve a sustained viral response. Given the relatively small sample size we report, this results in a negative bias for a sustained viral response, because most inmates lost to follow-up did complete therapy.
Early experience at the ND DOCR in treating hepatitis C with pegylated interferons was associated with prohibitive expense. This is not a unique problem.21–23 The use of consensus interferon and pretreatment screening have reduced the costs of effective therapy. Consensus interferon is 40% to 50% cheaper than pegylated interferons.24 The average cost for medication is $10 900 for genotype 1 and $5300 for genotype 2 or 3. Use of consensus interferon has avoided many of the hematologic and psychiatric side effects associated with standard therapy.
The success of this approach is likely multifactorial, but tight control maintained over medication adminstration is a major contributor. This therapy is essentially directly observed therapy. Prescreening is also a factor, because it intentionally selects for patients with a higher likelihood of successful completion. Racial distribution in the ND DOCR population is dissimilar to that of other states, so caution is advised when our results are generalized to other prison populations. However, the previously cited studies by Sjogren et al.9,10 included a more typical racial mix without loss of efficacy.
Pegylated interferons are used in other prison programs, for 2 reasons: (1) consultants formulating treatment programs are not aware of the advantages of consensus interferon, and (2) consensus interferon would not be the agent of choice outside the prison setting because pegylated interferons are more convenient for patients and, because they are recommended by the NIH as standard treatment and the administered drug levels are more constant than with consensus interferon, they are preferred by physicians.
Consensus interferon is less expensive than pegylated interferons and also saves money by limiting the need for growth factors. Use of consensus interferon could save millions of dollars for large correctional systems. A formal comparison trial with much larger numbers is needed to verify our results.
Acknowledgments
Kathleen Bachmeier, RN, MS, director of medical services for the North Dakota Department of Corrections and Rehabilitation and auditor for the American Correctional Association, was instrumental in conceiving the hepatitis C management and treatment program. She secured the original North Dakota legislative funding that made the program possible.
Human Participant Protection
This project was reviewed by the University of North Dakota institutional review board and approved as exempt.
References
- 1.Hammett TM. HIV/AIDS and other infectious diseases among correctional inmates: transmission, burden, and appropriate response. Am J Public Health 2006;96(6):974–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C in the United States, 1988 through 1994. N Engl J Med 1999;341(8):556–562 [DOI] [PubMed] [Google Scholar]
- 3.Guidelines for the prevention and treatment of hepatitis C and cirrhosis Federal Bureau of Prisons—Clinical Practice Guidelines. 2009. Available at: http://www.bop.gov/news/PDFs/hepatitis_c.pdf. Accessed October 22, 2009
- 4.Sterling R, Hofmann C, Luketic V, et al. Treatment of chronic hepatitis C virus in the Virginia Department of Corrections: can compliance overcome racial differences to response? Am J Gastroenterol 2004;99(5):866–872 [DOI] [PubMed] [Google Scholar]
- 5.Tan J, Joseph T, Saab S. Treating hepatitis C in the prison population is cost-saving. Hepatology 2008;48(5):1387–1395 [DOI] [PubMed] [Google Scholar]
- 6.Farley J, Vasdev S, Fischer B, et al. Feasibility and outcome of HCV treatment in a Canadian federal prison population. Am J Public Health 2005;95(10):1737–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen S, Spaulding A, Osei A, et al. Treatment of chronic hepatitis C in a state correctional facility. Ann Intern Med 2003;138(3):187–190 [DOI] [PubMed] [Google Scholar]
- 8.Fattovich G, Zagni I, Minola E, et al. A randomized trial of consensus interferon in combination with ribavirin as initial treatment for chronic hepatitis C. J Hepatol 2003;39(5):843–849 [DOI] [PubMed] [Google Scholar]
- 9.Sjogren M, Sjogren R, Holtzmuller K, et al. Interferon alfacon-1 and ribavirin versus interferon alfa-2b and ribavirin in the treatment of chronic hepatitis C. Dig Dis Sci 2005;50(4):727–732 [DOI] [PubMed] [Google Scholar]
- 10.Sjogren M, Sjogren R, Lyons M, et al. Sustained virologic response rates from a randomized trial of HCV genotype-1 subjects treated with either consensus IFN and ribavirin or pegylated interferon alfa-2b and ribavirin. Dig Dis Sci 2007;52(6):1540–1547 [DOI] [PubMed] [Google Scholar]
- 11.Fish EN, Harrison SA, Hassanein T. The role of consensus interferon in the current treatment of chronic hepatitis C viral infection. Gastroenterol Hepatol (New York) 2008;4(9 suppl. 18):1–10 [PMC free article] [PubMed] [Google Scholar]
- 12.Alberti A. Interferon alfacon-1: a novel interferon for the treatment of chronic hepatitis C. BioDrugs 1999;12(5):343–357 [DOI] [PubMed] [Google Scholar]
- 13.Hepatitis A, B, and C prevention programs Adult correctional facilities, North Dakota Department of Corrections and Rehabilitation. Available at: http://www.hepprograms.org/adult/adult14.asp. Accessed December 2008
- 14.Maru D, Bruce R, Basu S, Altice F. Clinical outcomes of hepatitis C treatment in a prison setting: feasibility and effectiveness for challenging treatment populations. Clin Infect Dis 2008;47(7):952–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis G, Wong J, McHutchison J, et al. Early virological response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C. Hepatology 2003;38(3):645–652 [DOI] [PubMed] [Google Scholar]
- 16.Neri S, Pulvirenti D, Bertino G. Psychiatric symptoms induced by antiviral therapy in chronic hepatitis C: comparison between interferon-alpha-2a and interferon-alpha-2b. Clin Drug Investig 2006;26(11):655–662 [DOI] [PubMed] [Google Scholar]
- 17.Radloff LS. The CES-D scale: a self report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401 [Google Scholar]
- 18.Manns M, McHutchison J, Gordon S, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001;358(9286):958–965 [DOI] [PubMed] [Google Scholar]
- 19.Fried M, Shiffman M, Reddy R, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002;347(13):975–982 [DOI] [PubMed] [Google Scholar]
- 20.McHutchinson JG, Lawitz EJ, Shiffman ML, Muir AJ, et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of Hepatitis C infection. N Engl J Med 2009;361(6):580–593 [DOI] [PubMed] [Google Scholar]
- 21.Del Rio R, Post A, Singer M. Cost-effectiveness of hematologic growth factors for anemia occurring during hepatitis C combination therapy. Hepatology 2006;44(6):1598–1606 [DOI] [PubMed] [Google Scholar]
- 22.Muir A, McHutchison J. Growth factors during HCV therapy may be “cost effective,” but are they “effective”? Hepatology 2006;44(6):1400–1403 [DOI] [PubMed] [Google Scholar]
- 23.Hoffman-LaRoche FDA filing for a license application (2002) Pegasys 180mcg weekly with rebavirin for patients with chronic hepatitis C, Table 26 and 27. Available at: http://www.fda.gov/ohrms/dockets/ac/02/briefing/3909B1_01_Hoffman-LaRoche.pdf. Accessed October 22, 2009
- 24.Infergen 15 μg dose vial pricing. 2008. Available at: www.drugstore.com. Accessed July 8, 2008.